Abstract
Context and Objective:
Pheochromocytomas and paragangliomas (PGLs) are neuroendocrine tumors of sympathetic or parasympathetic paraganglia. Nearly 40% of PGLs are caused by germline mutations. The present study investigated the effect of genetic alterations on metabolic networks in PGLs.
Design:
Homogenates of 32 sporadic PGLs and 48 PGLs from patients with mutations in SDHB, SDHD, SDHAF-2, VHL, RET, and NF-1 were subjected to proton (1H) nuclear magnetic resonance (NMR) spectroscopy at 500 MHz for untargeted and HPLC tandem mass spectrometry for targeted metabolite profiling.
Results:
1H NMR spectroscopy identified 28 metabolites in PGLs of which 12 showed genotype-specific differences. Part of these results published earlier reported low complex II activity (P < .0001) and low ATP/ADP/AMP content (P < .001) in SDH-related PGLs compared with sporadics and PGLs of other genotypes. Extending these results, low levels of N-acetylaspartic acid (NAA; P < .05) in SDH tumors and creatine (P < .05) in VHL tumors were observed compared with sporadics and other genotypes. Positive correlation was observed between NAA and ATP/ADP/AMP content (P < .001) and NAA and complex II activity (P < .0001) of PGLs. Targeted purine analysis in PGLs showed low adenine in cluster 1 compared with cluster 2 tumors (SDH P < .0001; VHL P < .05) whereas lower levels (P < .05) of guanosine and hypoxanthine were observed in RET tumors compared with SDH tumors. Principal component analysis (PCA) of metabolites could distinguish PGLs of different genotypes.
Conclusions:
The present study gives a comprehensive picture of alterations in energy metabolism in SDH- and VHL-related PGLs and establishes the interrelationship of energy metabolism and amino acid and purine metabolism in PGLs.
Pheochromocytomas and paragangliomas (PGLs) are neuroendocrine tumors of the adrenal medulla and sympathetic or parasympathetic paraganglia (1). Nearly 40% of them are caused by germline mutations in tumor susceptibility genes that include von Hippel-Lindau (VHL), rearranged during transfection (RET), neurofibromatosis type 1 (NF-1); succinate dehydrogenase subunits A, B, C and D and assembly factor 2 (SDHA/B/C/D/AF2); transmembrane protein 127 (TMEM127); myc-associated factor X (MAX) (2); and the more-recently discovered hypoxia-inducible factor 2 α (HIF2α) (3) and fumarate hydratase (FH) (4). Gene expression profiling has revealed the presence of two clusters of PGLs: cluster 1 (VHL, SDH) and cluster 2 (RET, NF-1, TMEM127, and MAX), displaying signature of transcripts associated with angiogenesis and hypoxia and RNA synthesis and kinase signaling, respectively.
Previous studies by us and others demonstrate a genotype-specific alteration in respiratory chain enzyme activities (5–7). In comparison with other genotypes, VHL tumors show an overall decrease in respiratory chain enzyme function whereas SDH tumors demonstrate a decreased complex II and an attempted compensatory increase in the complex I, III, and IV activities, indicative of impaired mitochondrial function (6). Mitochondria are the metabolic hubs of the tumor, which, in addition to providing energy, generate reducing equivalents (NADH and NADPH) and intermediates for anabolic reactions. Thus, genotype-specific differences in the mitochondrial function could have a strong effect on intermediary metabolism of PGLs beyond aerobic glycolysis.
In this scenario, use of metabolomic techniques helps to discover and systematically analyze the metabolic fingerprint of the tumors, thus giving insights into alterations in the metabolic pathways that are involved in tumorigenesis (8). Proton (1H) nuclear magnetic resonance (NMR) spectroscopy is a cornerstone technology for untargeted metabolomics. It provides a holistic view of metabolism and has other advantages such as relatively simple sample preparation and rapid data acquisition. Moreover, it allows quantitation of a large number of metabolites using a single internal standard (9, 10).
In the present study, we used a combination of 1H NMR spectroscopy and liquid chromatography tandem mass spectrometry (LC-MS/MS) to investigate genotype-specific differences in the metabolic profile of 80 PGLs. 1H NMR spectroscopy was used for untargeted metabolite profiling and LC tandem MS was used to further investigate and/or validate the findings obtained from 1H NMR spectroscopy.
Materials and Methods
Patients
In the present study, tumors of patients with histologically proven PGLs evaluated at the Radboud University Medical Centre (RUMC), Nijmegen, The Netherlands (n = 52) and Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) (n = 24), National Institutes of Health, Bethesda, Maryland who underwent surgical resection between 1988 and 2012 and between 2003 and 2010 respectively, were included. Frozen primary tumors were used for the study. No metastatic tumors were used. The presence of germline mutations and large deletions in SDHB/C/D, RET, VHL, and, since 2011, in SDHA, SDHAF2, TMEM127, and MAX, was investigated using standard procedures. Data were collected under conditions of regular clinical care with ethical committee approval for the use of those data for scientific purposes at RUMC. The study was approved by the Institutional Review Board of NICHD, and all patients gave written informed consent before testing. The details of the patients' clinical characteristics, tumor location, and genotype are listed in Table 1. Tumors from patients in whom germline mutation could not be found in any of the PGL genes were considered “sporadic.” The extra-adrenal tumors were mainly abdominal (retroperitoneal) or thoracic in location.
Table 1.
Patient Characteristics
| Genotype | Patients, n | Age, y, Mean ± sd | Sex, M/F | Tumors, n | Tumor Location, A/E/HN | Tumor Volume, cm3 |
|---|---|---|---|---|---|---|
| Sporadic | 32 | 45.8 ± 13.9 | 15/17 | 32 | 29/3/0 | 227.7 ± 480.1 |
| SDHB | 9 | 31.6 ± 11.2 | 8/1 | 11 | 1/10/0 | 295.7 ± 335.5 |
| SDHD | 8 | 44 ± 10.5 | 6/2 | 8 | 1/1/6 | 27.9 ± 47.2 |
| SDHAF2 | 1 | 24 | 1/0 | 1 | 0/0/1 | 18.9 |
| VHL | 7 | 31.4 ± 10.5 | 6/1 | 7 | 6/1/0 | 70.9 ± 97.8 |
| RET | 12 | 37.7 ± 13.3 | 6/6 | 14 | 14/0/0 | 97.6 ± 179.4 |
| NF1 | 7 | 43.1 ± 18.9 | 4/3 | 7 | 7/0/0 | 115.7 ± 85.2 |
Abbreviations: A, adrenal; E, extra-adrenal; HN, head and neck; M, male; F, female.
Tumor tissue processing and sample preparation
Tumors were procured as early as possible, dimensions were recorded, and a small piece of the tumor was weighed, snap frozen, stored in liquid nitrogen, and used for experimental purposes. For histological confirmation, additional slices were stained with hematoxylin and eosin and re-evaluated by an independent pathologist (B.K.).
The tumor tissues were homogenized on ice in 10 μl of ice-cold distilled water per miligram tissue using a handheld Teflon/glass homogenizer. The samples were then centrifuged at 16 000 × g for 10 min at 4°C and the supernatants were subjected to ultrafiltration with Vivaspin Turbo 15, 10-kDa filters (Sartorius) for deproteinization.
1H NMR Spectroscopy
One-dimensional 1H NMR spectroscopy was performed to investigate the metabolic profile of PGLs. For this purpose, the total ultrafiltrate was made up to 700 μl with water, pH was adjusted to 2.5, and 20 μl of 20.2mM sodium 3-trimethylsilyl-2,2,3,3-tetradeuteropropionate (TSP; Aldrich) in D2O (Catalogue No. 435 767; Aldrich) was added to the samples. The samples were then placed in 5-mm NMR tubes and 1H NMR spectra were obtained using a Bruker 500 MHz spectrometer (pulse angle, 90°; delay time, 4 s; number of scans, 256). Water resonance was suppressed by gated irradiation centered on the water frequency. For resonance assignment, 2D corelation spectroscopy (COSY) NMR spectra were acquired for some of the samples. The spectral width in the F1 and F2 domains were 5500 Hz. A total of 2K data points were collected in t2, 256 t1 increments with 32 transient per increment were used. The relaxation delay was set to 2 seconds. Before the Fourier transformation, a sine-bell function was applied in both time domains. During the relaxation delay, the water resonance was presaturated.
Analysis of NMR spectra
The free-induction decays measured for these samples were processed using Topspin software (Topspin). Fourier transformation was applied on the free-induction decay of the samples and the resulting spectra were phase and baseline corrected. The chemical shifts in the spectra were referenced to the internal standard, TSP. Assignment of peak positions for compound identification was performed by comparing the peak positions in the spectra of PGLs with the reference spectral database of model compounds at pH, 2.5 using Amix version 3.9.14 (Bruker Biospin) (11, 12). Quantification of identified compounds was performed by manual integration of chosen peak(s) for a specific metabolite. Further confirmation of metabolite identification based on peak positions was obtained from 2D NMR.
HPLC-MS/MS
Deproteinized tumor tissue lysate was used for targeted measurement of purines using LC tandem mass spectrometry. Only PGLs with known hereditary mutations (n = 47, one VHL tumor was excluded for limited sample quantity) were included in this experiment. The samples were prepared by adding 100 μl of stable isotope internal standard solution containing dihydrouracil, uracil, uric acid, orotic acid, dihydrothymine, thymine, guanosine, and thymidine and 10 μl of 2% formic acid for acidification to 100 μl of deproteinized tumor tissue lysate. Five microliters of the sample thus prepared was injected into the HPLC-MS/MS system. The HPLC-MS/MS process is described in detail in Supplemental Information.
Multivariate statistical analysis
A total of 45 1H NMR spectra corresponding to 13 RET, 7 NF-1, 18 SDH, and 7 VHL tumors were considered for principal component analysis (PCA). Spectra from one RET and two SDH tumors were omitted in the analysis because the quality of the spectra was not suitable for PCA. PCA was also performed on purine concentrations normalized around mean from 20 SDH, 6 VHL, 14 RET, and 7 NF-1. For details see Supplemental Methods.
Confirmation of metabolites identified by 1H NMR spectroscopy
Independent targeted and quantitative HPLC and LC-MS/MS methods were used for confirmation of detection and quantitation of succinate and N-acetylaspartic acid (NAA) (see Supplemental Information).
Statistical methods
Statistical analyses were performed using SPSS version 18 (SPSS) and GraphPad Prism 6 software (GraphPad). The data was analyzed using independent samples Kruskal-Wallis test and Dunn's post test was used to compare the different genotypes. Statistical significance was accepted at P < .05.
Results
Overview of 1H NMR spectra of PGLs
NMR spectroscopy was performed in all 80 PGLs. The spectra showed resonances of 28 different metabolites (Figure 1 and Table 2). Among these are amino acids, sugars, intermediates of Krebs cycle, and glycolysis, catecholamines, organic acids, high-energy phosphates, creatine/creatinine, NAA, and N-acetylaspartylglutamic acid.
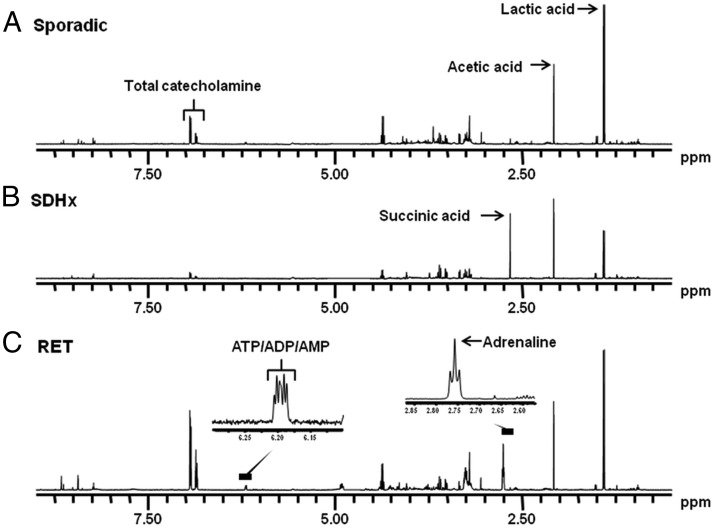
Figure 1. One-dimensional 500 MHz 1H-NMR spectra of PGLs at pH, 2.50.
A, Sporadic tumor showing normal tumor metabolites such as lactic acid, acetic acid, and catecholamines. B, SDHB tumor showing a high concentration of succinic acid (9.89 nmol/mg tissue). C, RET tumor showing high resonance of epinephrine and the overlap of the three doublets deriving from ATP/ADP/AMP.
Table 2.
NMR Assignment (pH, 2.50) and Occurrence for Metabolites Observed in PGLs of Different Genotypes
| Metabolite | δ 1Ha | Genotype |
||||
|---|---|---|---|---|---|---|
| SDH | VHL | RET | NF-1 | Sporadic | ||
| Acetic acid | 2.08 s | 2+ | 2+ | 2+ | 2+ | 2+ |
| N-Acetylaspartic acidb | 2.03 s | – | + | + | + | + |
| N-Acetylglutamylasparagineb | 2.05 s | – | – | + (n = 6)d | – | + (n = 4)d |
| 4-Aminobutyric acidb | 2.50 t | + | + | + | + | + |
| ATP/ADP/AMPb | 6.20c d | – | + | + | + | + |
| Citric acid | 2.92 AB | – | – | – | – | + (n = 4)d |
| Creatine | 3.05 s; 4.11 s | 2+ | + | 2+ | 2+ | 2+ |
| Creatinine | 3.13 s; 4.29 s | 2+ | + | 2+ | 2+ | 2+ |
| Epinephrineb | 2.73 t | – | – | 2+ | 2+ | 2+/− |
| Ethanol | 1.17 t; 3.64 q | 2+ | 2+ | 2+ | 2+ | 2+ |
| Formic acid | 8.24 s | + | + | + | + | + |
| Glutamate/Glutamineb | 2.17 m | + | + | + | + | + |
| Histidineb | 8.67 d | – | + (n = 1)d | + | – | – |
| 3-Hydroxybutyric acidb | 1.23 d | + | + | + | + | + |
| Inosineb | 6.09 d; 8.22 s | + | 2+ | 2+ | 2+ | 2+ |
| Inositol-myob | 3.27 t; 4.05 t | + | + | + | + | + |
| Inositol-scyllo | 3.34 s | + | + | + | + | + |
| Isoleucineb | 0.94 t; 1.02 d | + | + | – | + | + |
| Lactic acid | 1.41d; 4.36 q | 2++ | 2++ | 2++ | 2++ | 2++ |
| Lysineb | 1.72 m | + | + | + | + | + |
| Methanol | 3.35 s | 2+ | 2+ | 2+ | 2+ | 2+ |
| Methionineb | 2.12 s | + | + | + | + | + |
| Norepinephrineb | 6.90 m | + | + | 2+ | 2+ | 2+ |
| Pyruvate | 2. 37 s | + | + | + | + | + |
| Succinic acid | 2.67 s | 2++ | + | + | + | + |
| Sucroseb | 5.40 d | – | – | – | – | 2+ (n = 2)d |
| Threonineb | 1.33 d | + | + | + | + | + |
| Unknown | 2.14 s | + | – | – | – | – |
| Valineb | 1.00 d; 1.04 d | + | + | + | + | + |
+, Present in spectrum. +, low; 2+, medium 2++, high; based on peak heights.
−, Absent in spectrum.
Chemical shifts (δ) in ppm relative to TSP; multiplicity are s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet; AB, AB system (second order coupling).
Spectrum not completely interpreted. Complete assignments of metabolites published by HMDB http://www.hmdb.ca/.
Overlap of three doublets.
Number of patients in whom the metabolites was found.
Genotype-specific differences in the metabolites as identified by 1H NMR spectroscopy
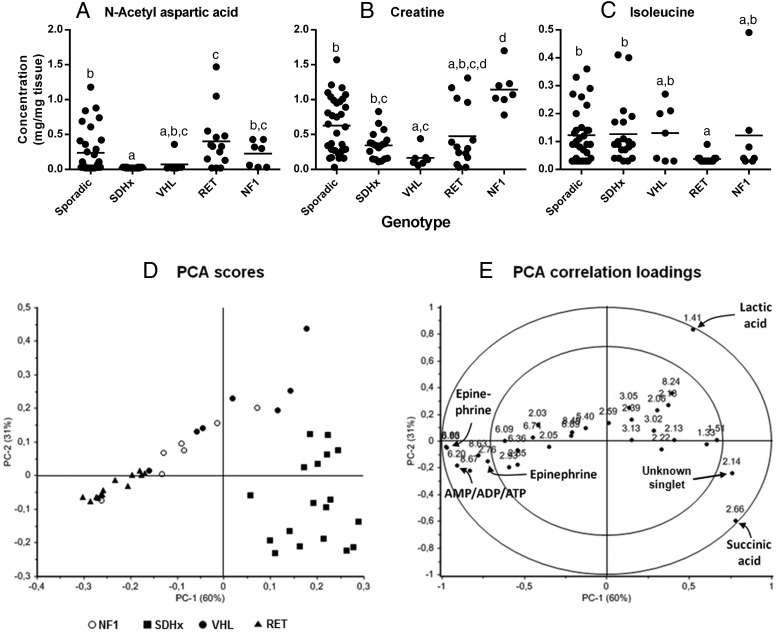
NAA content of the tumor tissue was estimated by integrating the area under the peak at 2.03 ppm. This peak was chosen as the multiplets at 2.94 and 4.72 overlapped with those of N-acetylaspartylglutamic acid. NAA was below detection limit in all the SDH and VHL tumors tested except one (Figure 2E, Supplemental Figure 4). The levels were higher in RET (P < .01), NF-1 (P < .05) and sporadic tumors (P < .05) when compared with SDH tumors (Figure 2E). NAA peaks were undetectable in 16 sporadics, 3 RET, and 3 NF-1 tumors. HPLC-MS/MS was used to validate the NAA content of the tumors and a significant correlation and linear relationship was observed between the two methods (Supplemental Figure 1).
Figure 2. Metabolite profiles and PCA analysis of 1H-NMR spectra of PGLs belonging to different genotypes.
Dot plot representing A, N-acetylaspartate; B, creatine; and C, isoleucine in mg/mg tumor tissue. Horizontal line represents the mean. Data sets having different alphabets above them are significantly different with data sets having the alphabet 'a' being the lowest (P < .05). In 40 tumor samples, N-acetylaspartate; in four tumor samples, creatine; and in 32 tumor samples, isoleucine were below detection limit. They have been represented as half the lowest detectable value. The PCA score plot, D, represents the first and second principle components and clearly discriminate between SDH, RET, and VHL tumors. The loading plot, E, shows the peak positions contributing to the maximum variances in principal components 1 and 2 in the outer circle indicating that the resonances of succinic acid, ATP/ADP/AMP, epinephrine, and the unknown singlet resonance at 2.14 ppm cause the separation between RET and SDH tumors in the PCA score plot.
Creatine concentrations in tumors were estimated by integrating the area under peak at 3.05 ppm as the singlet at 4.11 ppm had a lower signal-to-noise ratio and also was in a region with peaks arising from several other metabolites. Creatine levels were high in NF-1 tumors when compared with SDHD/AF2 head and neck PGLs (P < .01), VHL (P = .0001), and RET (P < .05) tumors (Supplemental Figure 4). VHL tumors had low creatine levels when compared with sporadic tumors (P < .05) (Figure 2F). Creatine peaks were undetectable in one sporadic and two RET-related PGLs.
Isoleucine levels in the tumor tissue were determined by integrating area under peaks for the doublet at 1.02 ppm as this doublet resonance had the best signal-to-noise ratio. Isoleucine peaks were consistently undetectable in RET tumors whereas in the tumors of other genotypes the levels were measurable (Figure 2D) although its levels were found to be undetectable in nine sporadic, five SDH, three VHL, and three NF-1 tumors.
The as-yet unassigned singlet resonance at 2.14 ppm occurs in all SDH tumors. The concentration of the compound causing this resonance must be in the low micromolar range. Because the singlet partially coresonates with the glutamine multiplet resonance at 2.17 ppm it is hard to decide whether the 2.14-ppm singlet also is present in lower concentration in the other tumor types. Clearly, the concentration of the metabolite causing the 2.14-ppm singlet in the SDH tumors is significantly higher than in the other tumor types.
Correlation of NAA content with energy metabolism in PGLs
Positive correlations were observed between NAA and ATP/ADP/AMP content (R = 0.422, P < .001) and NAA content and activity of respiratory chain complex II (R = 0.465, P < .0001) in PGL tumors.
Multivariate statistical analysis of NMR results
Using PCA without scaling, tumors with mutations in SDHB, VHL, and RET could be well separated based on the variances in the peak positions pertaining to epinephrine, succinate, ATP/ADP/AMP, an unknown peak at 2.14 ppm, and lactic acid (Figure 2, D and E). High levels of epinephrine in RET tumors, increased succinate accumulation, and low levels of ATP/ADP/AMP in SDH tumors and higher levels of lactic acid in VHL tumors when compared with RET tumors are identified in the PCA as most discriminating features. NF-1 tumors from two patients were classified with VHL tumors because they contained higher lactic acid levels and epinephrine levels below the detection limit of NMR spectroscopy. The NF-1 tumors did not form a separate group because there was no single distinguishing metabolite detectable by 1H NMR spectroscopy for this group. To highlight the subtle differences and to minimize the effect of high intensity peaks, PCA was also performed after autoscaling, which revealed threonine (1.33 ppm) and alanine (1.51 ppm) peaks contributing to the separation of SDH tumors from the RET tumors.
Genotype-specific differences in purine metabolism in PGLs
Observations of consistent lack of ATP/ADP/AMP peaks in SDH tumors when compared with the other genotypes could probably be related to altered purine biosynthetic pathways in SDH related PGLs. Thus, purine concentrations were estimated using HPLC-MS/MS in PGL lysates prepared for 1H NMR spectroscopy.
Adenine levels were lower in SDH tumors when compared with RET and NF-1 tumors (P < .0001 and P < .05, respectively). Adenosine levels were lower in SDH (P < .01) and VHL (P < .05) tumors when compared with RET tumors in contrast with guanosine, which was higher in SDH tumors when compared with RET tumors (P < .05). Inosine levels were lower in cluster 1 tumors compared with those belonging to cluster 2 (P < .01) differing from hypoxanthine, which was higher in SDH tumors when compared with RET-related ones (P < .05, Table 3).
Table 3.
Overview of Purines (mg/mg tissue) Detected in PGLs
| Metabolites | Genotype |
|||
|---|---|---|---|---|
| Cluster 1 |
Cluster 2 |
|||
| SDH (n = 20) | VHL (n = 6) | RET (n = 14) | NF-1 (n = 7) | |
| Adenine | 0.68 ± 0.80a | 3.3 ± 4.11a,c | 16.18 ± 5.41b | 8.33 ± 6.95b,c |
| Adenosine | 1.33 ± 2.84a | 6.53 ± 12.67a,b | 3.59 ± 2.93b | 5.65 ± 5.67b |
| Guanine | 7.3 ± 6.73 | 2.9 ± 3.29 | 3.08 ± 1.29 | 2.01 ± 1.10 |
| Guanosine | 4.44 ± 5.26b | 4.51 ± 5.54a,b | 0.86 ± 0.9a | 1.79 ± 1.60a,b |
| Inosine | 23.35 ± 20.15a | 47.42 ± 36.10a,b | 40.26 ± 19.44a,b | 78.32 ± 67.70b |
| Hypoxanthine | 103.73 ± 48.28b | 116.16 ± 50.70a,b | 64.16 ± 19.46a | 96.49 ± 47.22a,b |
| Xanthine | 14.52 ± 15.58 | 20.22 ± 15.47 | 12.71 ± 6.60 | 15.96 ± 6.87 |
| Uric Acid | 64.25 ± 147.32 | 11.58 ± 4.21 | 31.76 ± 63.92 | 23.10 ± 28.38 |
Data are represented mean ± sd values with common superscripts are not significantly different.
: a Values are significantly lower than b values (where b is P < .05) when indicated after unique values.
No significant differences were observed between the two groups.
Multivariate statistical analysis of purines
Using PCA, the samples with mutations in SDH, RET, and NF-1 could be separated based on the differences in the concentrations of adenine, hypoxanthine, and inosine (Supplemental Figure 2). High levels of adenine in RET and hypoxanthine in SDH tumors emerge as the most discriminating features for these tumors in the PCA analysis.
Discussion
The present study extends the results of genotype-specific differences in metabolic profiles of PGLs from our previous publication using the same set of tumors (6). In the previous study using the same set of PGLs we reported decrease in complex II activity (P < .0001), increase in succinate content (P < .001), and decrease in ATP/ADP/AMP content (P < .001) in SDH tumors when compared with sporadic PGLs and PGLs of other genotypes. Further, we discussed the interrelationship of energy and catecholamine metabolism (6). In the present study, we have used multiple complementary analytical methods to further investigate genotype-specific differences in tumor metabolite profile. Here we describe genotype-specific differences in the contents of additional metabolites and demonstrate that these differences in metabolite profiles can be used to differentiate the genotypes using unsupervised statistical methods. The study thus gives a comprehensive picture of alterations in energy metabolism in SDH and VHL tumors. Further, interrelationships of genotype-specific alterations in energy, amino acid, and purine metabolism are established.
Metabolite profiling of tumors has been approached using two basic technological platforms, NMR or MS. In the present study, we have used multiple complementary analytical techniques which use both of these platforms. We used NMR as an untargeted metabolite-profiling technique allowing detection of the vast majority of proton-containing compounds in the low micromolar or higher concentration range. For targeted metabolite profiling, we used HPLC-MS/MS to test the presence of genotype-specific differences in the concentration of various purines in the tumors.
NMR-based metabolite profiling has the inherent disadvantage of limited low micromolar sensitivity, which limits the number of metabolites that could be identified. Thus, we were unable to generate a metabolomic profile of sufficient complexity for unbiased application of pathway analysis tools to identify genotype-specific dysfunctional metabolic pathways.
We used PCA as an unsupervised method for interpretation of NMR spectra and to classify the samples in genotype-specific groups on the basis of their metabolite profile. The peak positions contributing to maximum variability in the first three principal components included epinephrine, succinate, lactic acid, and high-energy phosphates of adenosine. This is explained by their respective roles in catecholamine and energy metabolism. Interestingly, high lactate levels observed in VHL tumors support the observations of high lactate dehydrogenase activity in these tumors by Favier et al (5). However, the results must be interpreted with caution because lactate levels in resected samples do not necessarily reflect the actual tumor metabolism. Despite alanine and threonine emerging as important variables after autoscaling, significant differences were not observed for these amino acids among the tumors of different genotypes when the concentrations of these amino acids were calculated based on the area under peak of the NMR spectra.
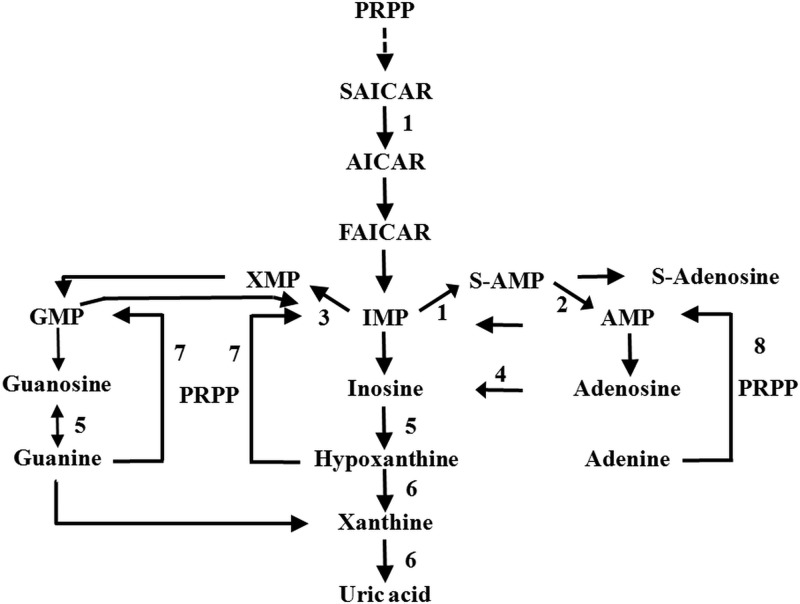
Reduced tumor ATP/ADP/AMP content as measured by 1H NMR spectroscopy suggested that there could be a reduced total adenylate, adenosine, and adenine content in the SDH tumors. This hypothesis was verified by assessing content of purines in PGLs using HPLC-MS/MS. Based on the results of this study, we hypothesize that in the de novo synthesis of the purine nucleotides, possibly the activity of inosine monophosphate (IMP) dehydrogenase is higher in SDH tumors whereas in the RET-related ones, adenylosuccinate synthase and/or lyase activities are higher. Thus, the IMP that is formed by the de novo pathway is committed to either of the purine biosynthetic branches in a genotype-specific way (Figure 3). In a similar situation, Pillwein et al (13) compared the normal brain tissue and glioblastomas for pool of purines and purine nucleotides and nucleosides and the activities of IMP dehydrogenase, hypoxanthine guanine phosphoribosyl transferase, and other enzymes. It was observed that total adenylates and guanylates were lower by 60% and 71% respectively and that incubation of such tumor sections with Tiazofurin, an IMP dehydrogenase inhibitor lead to a reduction in total guanylate pool. In addition, the enzyme hypoxanthine guanine phosphoribosyl transferase in the salvage pathway might have genotype-specific differences in substrate availability, which leads to the differences in their purine nucleotide profile. A recent report by Gottle et al (14) showed that increased purine nucleotide pools and energy reserve are observed post differentiation in rat pheochromocytoma cells (PC-12 subclones), which indirectly suggests that the lower purine pools are reflective of dedifferentiation. However, in contrast with the observations of Gottle et al (14) that undifferentiated cells have lower dopamine, SDH tumors have high dopamine levels. Thus, it is not clear whether purine pool has an effect on dopamine levels in hereditary PGLs. Further, using PCA of purine levels in PGLs, SDH, RET, and NF-1 tumors could be distinguished well. However, genotype-specific differences were not observed in levels of more downstream metabolites of purine biosynthetic pathway such as xanthine and uric acid, indicating lack of genotype-specific differences in further purine catabolism.
Figure 3. Purine metabolism pathway.
Enzymes involved are: 1) adenylosuccinate synthase, 2) adenylosuccinate lyase, 3) IMP dehydrogenase, 4) adenosine deaminase, 5) purine-nucleoside phosphorylase, 6) xanthine dehydrogenase, 7) hypoxanthine-guanine phosphoribosyltransferase, and 8) adenine phosphoribosyltransferase. Abbreviations: PRPP, 5-phospho-a-D-ribosyl-1-pyrophosphate; SAICAR, succinylaminoimidazole carboxamide ribotide; AICAR, aminoimidazole carboxamide ribotide; FAICAR, formylaminoimidazole carboxamide ribotide; S-AMP, adenylosuccinate; S-adenosine, succinyladenosine. Figure adopted from Wevers et al (21).
In this study, we report for the first time the presence of NAA in the peripheral nervous system. NAA is synthesized through acetylation of aspartate by L-Asparatate N-acetyl transferase, a membrane-bound enzyme, in the neuronal tissue (15). NAA is synthesized and exported from mitochondria and its synthesis is dependent on mitochondrial energy state. Mitochondrial NAA production is dependent on functioning of respiratory chain enzyme complexes and oxidative phosphorylation. Inhibition of these enzymes leads to significant decrease in mitochondrial NAA and ATP production in isolated rat brain mitochondria (16). This relates well with our observation of strong positive correlation between NAA content and respiratory chain complex II activity and NAA and ATP/ADP/AMP contents in PGLs. Further, we observed low NAA/total creatine ratio in tumors with compromised mitochondrial function (Supplemental Figure 3). Similar observations have also been made in patients with mitochondrial encephalomyopathies (17). Thus, the NAA levels in SDH and VHL tumors are directly linked to the energetic states of the tumor cell mitochondria.
In our studies we show the presence of high levels of lactate in some of the VHL tumors, which also show low NAA and total creatine levels. In similar observations, 1H NMR spectroscopic imaging of rat gliomas in vivo also demonstrated reduced NAA levels in tumors associated with high glycolytic activity (18). Also, it has been observed that in tissues with ischemic stroke there is increased anaerobic glycolysis as evidenced by increased levels of lactate in the lesion and the border zone brain tissue. Increased lactate levels in these tissues were associated with decreased NAA and total creatine levels. This was also associated with decreased glutamate and glutamine levels in the affected brain tissue (19). However, though we observed highly reduced NAA and reduced total creatine levels in tumors associated with increased aerobic glycolysis, the glutamate + glutamine levels were not different from other tumors. In SDH tumors, lower glutamate levels were observed compared with sporadic and VHL tumors by Imperiale et al (20) using 1H high-resolution magic angle spinning NMR spectroscopy in small tumor pieces. However, in the spectra of the present study obtained using homogenized tissue, glutamine and glutamate peaks were not clearly separated to verify the above discussed report. Thus, estimation of levels of glutamate and glutamine with other methods might help in giving more clarity.
Creatine serves as an energy reservoir and mode of energy transport within a cell by accepting a phosphate group in mitochondria through the activity of creatine kinase. Phosphorylated creatine can be shuttled to different cellular regions in need of high-energy phosphate group to regenerate ATP. In cells with low energetic status, phosphocreatine levels are low. The reduction in total creatine levels as observed by us in VHL tumors is suggestive of larger alterations in cellular energy metabolism. In particular, it reflects a lack of sufficient ATP levels to maintain stores of phosphocreatine, which might lead to efflux of cellular creatine stores. This might be reflected as low creatine levels in SDH and VHL tumors when compared with NF-1 tumors. Further, grouping of SDH tumors based on the tumor localization did not reveal location specific alterations in the metabolite levels. Thus, the tumor metabolism, as observed in the present study, tended to be influenced more in a genotype-specific way.
In conclusion, alterations in energy metabolism caused by hereditary mutations are associated with alterations in catecholamine, amino acid, and purine metabolism, which could in addition to energy metabolism be targeted in these tumors for diagnostics and therapeutics.
Acknowledgments
We thank Mr. Arno van Rooij and Ms. Hanneke Kwast for their help with the analysis of N-acetylaspartic acid and purines, respectively.
The work leading to these results has received funding from the European Union Seventh Framework Programme (FP7/2007–2013) under Grant No. 259735 (ENSAT CANCER).
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
The work leading to these results has received funding from the European Union Seventh Framework Programme (FP7/2007–2013) under Grant No. 259735 (ENSAT CANCER).
Footnotes
- IMP
- inosine monophosphate
- LC
- liquid chromatography
- MS
- mass spectrometry
- NAA
- N-acetylaspartic acid
- NICHD
- National Institute of Child Health and Human Development
- NMR
- nuclear magnetic resonance
- PCA
- principal component analysis
- PGLs
- pheochromocytomas and paragangliomas
- RUMC
- Radboud University Medical Centre
- TSP
- 3-trimethylsilyl-2,2,3,3-tetradeuteropropionate.
References
- 1. Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet. 2005;366:665–675. [DOI] [PubMed] [Google Scholar]
- 2. Welander J, Söderkvist P, Gimm O. Genetics and clinical characteristics of hereditary pheochromocytomas and paragangliomas. Endocr Relat Cancer. 2011;18:R253–R276. [DOI] [PubMed] [Google Scholar]
- 3. Lorenzo FR, Yang C, Ng Tang Fui M, et al. A novel EPAS1/HIF2A germline mutation in a congenital polycythemia with paraganglioma. J Mol Med. 2013;91:507–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Castro-Vega LJ, Buffet A, De Cubas AA, et al. Germline mutations in FH confer predisposition to malignant pheochromocytomas and paragangliomas. Hum Mol Genet. 2014;23:2440–2446. [DOI] [PubMed] [Google Scholar]
- 5. Favier J, Brière JJ, Burnichon N, et al. The Warburg effect is genetically determined in inherited pheochromocytomas. PloS One. 2009;4:e7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rao JU, Engelke UF, Rodenburg RJ, et al. Genotype-specific abnormalities in mitochondrial function associate with distinct profiles of energy metabolism and catecholamine content in pheochromocytoma and paraganglioma. Clin Cancer Res. 2013;19:3787–3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rapizzi E, Ercolino T, Canu L, et al. Mitochondrial function and content in pheochromocytoma/paraganglioma of succinate dehydrogenase mutation carriers. Endocr Relat Cancer. 2012;19:261–269. [DOI] [PubMed] [Google Scholar]
- 8. Weljie AM, Jirik FR. Hypoxia-induced metabolic shifts in cancer cells: Moving beyond the Warburg effect. Int J Biochem Cell Biol. 2011;43:981–989. [DOI] [PubMed] [Google Scholar]
- 9. Wishart DS. Advances in metabolite identification. Bioanalysis. 2011;3:1769–1782. [DOI] [PubMed] [Google Scholar]
- 10. Weljie AM, Bondareva A, Zang P, Jirik FR. (1)H NMR metabolomics identification of markers of hypoxia-induced metabolic shifts in a breast cancer model system. J Biomol NMR. 2011;49:185–193. [DOI] [PubMed] [Google Scholar]
- 11. Engelke UF, Kremer B, Kluijtmans LA, et al. NMR spectroscopic studies on the late onset form of 3-methylglutaconic aciduria type I and other defects in leucine metabolism. NMR Biomed. 2006;19:271–278. [DOI] [PubMed] [Google Scholar]
- 12. Wevers RA, Engelke U, Wendel U, de Jong JG, Gabreëls FJ, Heerschap A. Standardized method for high-resolution 1H-NMR of cerebrospinal fluid. Clin Chem. 1995;41:744–751. [PubMed] [Google Scholar]
- 13. Pillwein K, Chiba P, Knoflach A, et al. Purine metabolism of human glioblastoma in vivo. Cancer Res. 1990;50:1576–1579. [PubMed] [Google Scholar]
- 14. Göttle M, Burhenne H, Sutcliffe D, Jinnah HA. Purine metabolism during neuronal differentiation: The relevance of purine synthesis and recycling. J Neurochem. 2013;127:805–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Patel TB, Clark JB. Synthesis of N-acetyl-L-aspartate by rat brain mitochondria and its involvement in mitochondrial/cytosolic carbon transport. Biochem J. 1979;184:539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bates TE, Strangward M, Keelan J, Davey GP, Munro PM, Clark JB. Inhibition of N-acetylaspartate production: Implications for 1H MRS studies in vivo. Neuroreport. 1996;7:1397–1400. [PubMed] [Google Scholar]
- 17. Mathews PM, Andermann F, Silver K, Karpati G, Arnold DL. Proton MR spectroscopic characterization of differences in regional brain metabolic abnormalities in mitochondrial encephalomyopathies. Neurology. 1993;43:2484–2490. [DOI] [PubMed] [Google Scholar]
- 18. Ziegler A, von Kienlin M, Décorps M, Rémy C. High glycolytic activity in rat glioma demonstrated in vivo by correlation peak 1H magnetic resonance imaging. Cancer Res. 2001;61:5595–5600. [PubMed] [Google Scholar]
- 19. van der Zijden JP, van Eijsden P, de Graaf RA, Dijkhuizen RM. 1H/13C MR spectroscopic imaging of regionally specific metabolic alterations after experimental stroke. Brain. 2008;131:2209–2219. [DOI] [PubMed] [Google Scholar]
- 20. Imperiale A, Moussallieh FM, Sebag F, et al. A new specific succinate-glutamate metabolomic hallmark in SDHx-related paragangliomas. PloS One. 2013;8:e80539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wevers RA, Engelke UF, Moolenaar SH, et al. 1H-NMR spectroscopy of body fluids: Inborn errors of purine and pyrimidine metabolism. Clin Chem. 1999;45:539–548. [PubMed] [Google Scholar]