Abstract
Context:
Polycystic ovary syndrome (PCOS), a major cause of anovulatory infertility, is characterized by arrested follicular growth. Altered protein levels in the follicular fluid surrounding the ovum may reflect the molecular defects of folliculogenesis in these women.
Objective:
To identify differentially regulated proteins in PCOS by comparing the follicular fluid protein repertoire of PCOS with healthy women.
Methods:
The follicular fluid samples were collected from PCOS and normo-ovulatory women undergoing in vitro fertilization. Follicular fluid proteins were subjected to digestion using trypsin, and resultant peptides were labeled with isobaric tags for relative and absolute quantification reagents and analyzed by liquid chromatography tandem mass spectrometry. Differential abundance of selected proteins was confirmed by ELISA.
Results:
A total of 770 proteins were identified, of which 186 showed differential abundance between controls and women with PCOS. Proteins involved in various processes of follicular development including amphiregulin; heparan sulfate proteoglycan 2; tumor necrosis factor, α-induced protein 6; plasminogen; and lymphatic vessel endothelial hyaluronan receptor 1 were found to be deregulated in PCOS. We also identified a number of new proteins from follicular fluid, whose function in the ovary is not yet clearly established. These include suprabasin; S100 calcium binding protein A7; and helicase with zinc finger 2, transcriptional coactivator.
Conclusions:
Proteins indispensable for follicular growth were found to be differentially expressed in follicular fluid of women with PCOS, which may in part explain the aberrant folliculogenesis observed in these women.
Polycystic ovary syndrome (PCOS), typically characterized by oligo or anovulation, hyperandrogenemia, hyperinsulinemia, and polycystic ovaries, affects nearly 8% of women of reproductive age. These women are at risk of developing type 2 diabetes mellitus, coronary artery disease, and endometrial cancer (1).
Folliculogenesis, a highly coordinated event in the development and release of oocytes, is disrupted in PCOS. There is excess initial recruitment of primordial follicles for growth; however, subsequent development is arrested at the early preantral stage resulting in the formation of multiple cysts (1). Increased GnRH pulses in these women favor increased LH production, which along with excess insulin stimulates ovarian theca cells to produce more androgen resulting in cessation of follicular growth and dominant follicle selection, thus affecting ovulation. However, the precise molecular defects of follicular development in PCOS remain unknown.
Follicular fluid is produced in the growing antral follicles by secretions from granulosa and theca cells, and by the diffusion of plasma proteins (<500 kDa) through the basal membrane of the thecal vasculature (2). It provides the micro-environment for developing oocytes and contains several factors including proteins, steroids, polysaccharides, and metabolites that modulate oocyte developmental competence and ovulation. During follicular development, follicular fluid also serves as a medium for communication between oocyte and follicular cells. Follicular fluid composition may reflect any changes in the secretory processes of the ovarian cells and alterations in the plasma constituents due to pathological conditions. Proteome investigations of follicular fluid under multiple ovarian abnormalities have provided some information on ovarian pathophysiology underlying these disorders (3–5). Thus, we hypothesize that the protein expression profile of follicular fluid from women with PCOS can reflect the defects in the micro-environment of PCOS follicle. So far, only 2 studies have reported changes in the expression levels of a small number of proteins in the follicular fluid obtained from these women (6, 7). Knowing the complexity of this disorder, we reasoned that several other proteins might be altered in terms of abundance in the follicular fluid of women with PCOS, which have not yet been explored because of the limitations of the proteomics technology used in the previous studies.
Advances in quantitative proteomics strategy enabled determination of relative expression levels of a large number of proteins between healthy and diseased conditions. A number of chemical labeling strategies have been employed including Isotope Coded Affinity Tags, Tandem Mass Tags, and Isobaric Tags for Relative and Absolute Quantification (iTRAQ) in quantitative proteomics. These labeling strategies allow multiplexing and do not affect biochemical properties of the labeled peptides. iTRAQ technology involves labeling with different amine-specific isobaric tags that allows simultaneous identification and quantification of proteins from 4 to 8 different biological samples in a single experiment, thereby reducing inherent run to run variations observed in individual liquid chromatography mass spectrometry (LC-MS) analysis. As peptides are pooled from multiple samples after labeling, signals from each peptide become additive in MS and MS/MS scans, which allows more in-depth analysis of samples. The iTRAQ technology has been successfully used for the quantitative analysis of a number of biological samples including tissues, body fluids, and the secretome for the discovery of potential biomarkers and altered signaling pathways (8).
To understand the ovarian pathophysiology of PCOS in greater detail, we employed an iTRAQ-based quantitative proteomics approach to compare the proteome of follicular fluid obtained from women with PCOS with that of healthy controls undergoing in vitro fertilization (IVF).
Subjects and Methods
Study subjects and sample collection
Follicular fluid (∼5 mL) was collected from 26 women with PCOS and 26 regularly menstruating healthy women of similar age (Table 1) undergoing IVF in the INKUS IVF Clinic (Mumbai, India). PCOS subjects were diagnosed according to Rotterdam consensus criteria (9), and controls were undergoing IVF due to male factor infertility. All recruits underwent controlled ovarian hyperstimulation using a combination of GnRH agonist and recombinant FSH (long protocol). Follicular fluid was collected by transvaginal ultrasound-guided aspiration, 34 to 36 hours after human chorionic gonadotropin (10 000 IU) administration. Only macroscopically clear follicular fluid samples, indicating lack of blood contamination, were included in the study and processed as described previously (5). After oocyte retrieval, the cumulus granulosa cells were stripped and collected from five pairs of controls and women with PCOS and used for gene expression study. This work has been approved by the Institutional Ethics Committee of the National Institute for Research in Reproductive Health, and written informed consent was obtained from all participants.
Table 1.
Clinical Characteristics of Study Participantsa
| Parameters | Control (n = 26) | PCOS (n = 26) | P |
|---|---|---|---|
| Age, y | 32.00 ± 0.85 | 31.60 ± 0.93 | .72 |
| BMI, kg/m2 | 22.75 ± 0.56 | 26.63 ± 0.93 | .0002c |
| Administered rhFSH per day, IU/L | 342.8 ± 16.62 | 321.5 ± 24.02 | .41 |
| Duration of rhFSH, d | 9.95 ± 0.37 | 11.25 ± 0.31 | .005c |
| Number of oocytes retrieved | 12.35 ± 1.65 | 20.45 ± 1.80 | .0004c |
| P4, nmol/Lb | 9064 ± 168.9 | 9101 ± 344.1 | .92 |
| E2, nmol/Lb | 27.50 ± 0.5528 | 28.32 ± 0.5284 | .26 |
| T, nmol/Lb | 11.17 ± 1.122 | 18.27 ± 3.598 | .02c |
| SHBG, nmol/Lb | 243.70 ± 18.82 | 145.40 ± 19.64 | <.0001c |
Abbreviations: BMI, body mass index; E2, estradiol; P4, progesterone; rhFSH, recombinant human FSH; T, testosterone.
Results are expressed as mean ± SEM for each parameter. Comparison was done by unpaired t test.
Values were estimated in the follicular fluid collected on ovum pickup day.
P < .05 is statistically significant.
iTRAQ labeling and strong cation-exchange chromatography
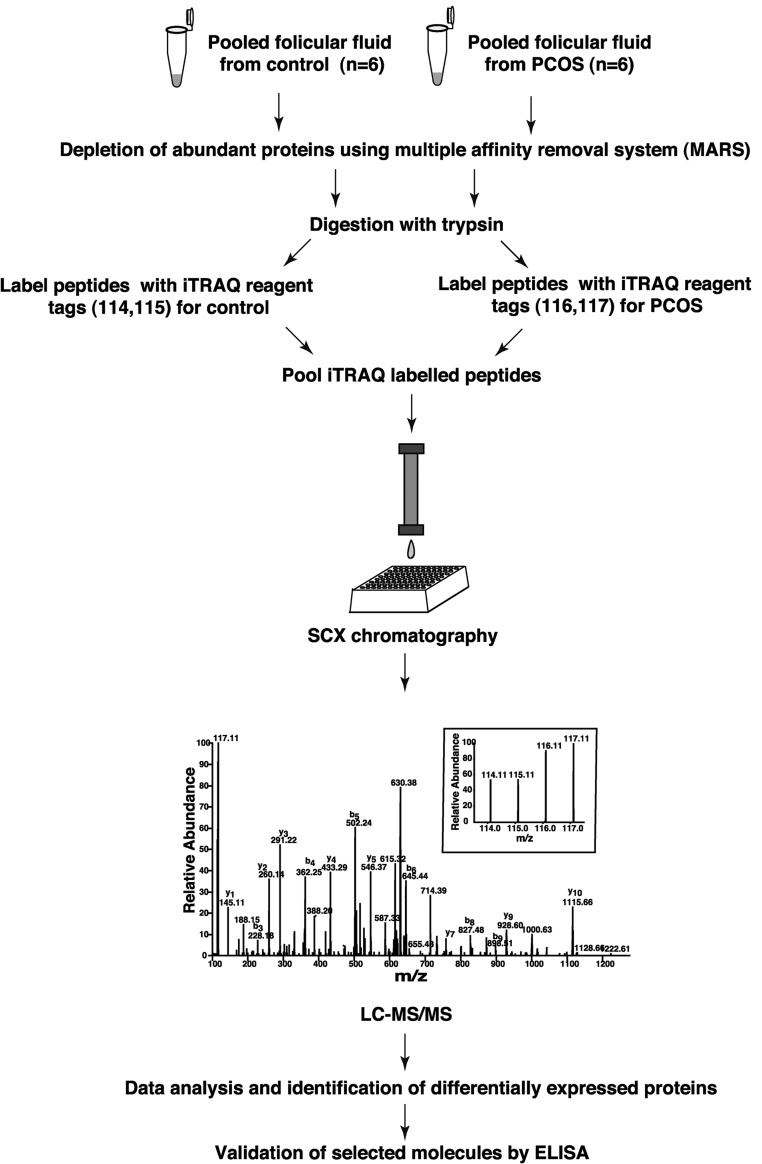
Protein estimation was done by Lowry's method. Six pooled PCOS and 6 control follicular fluid samples were separately immunodepleted of the 14 most abundant proteins using a multiple affinity removal system human 14 LC column (4.6 × 100 mm; Agilent Technologies) as described previously (5). The flow-through fraction was desalted and concentrated using 3-kDa cutoff filters (Amicon, Millipore). Protein samples were digested and iTRAQ labeled as described previously (10). Briefly, immunodepleted follicular fluid proteins from control and PCOS samples (200 μg each) were subjected to proteolytic digestion using trypsin (Promega) at 37°C for 12 hours. The peptide digest from the control and PCOS samples were split into equal halves to serve as technical replicates and labeled with 4-plex iTRAQ reagents according to manufacturer's instructions (iTRAQ Reagents Multiplex kit; Applied Sciex). The peptides from these technical replicates of the control sample were labeled with iTRAQ reagents, which yielded reporter ions of 114 and 115 mass to charge ratio (m/z) after MS/MS. Similarly, technical replicates of PCOS samples were labeled with iTRAQ reagents, which yielded reporter ions of 116 and 117 m/z. Labeled peptides were pooled and fractionated using strong cation-exchange chromatography (SCX) on a PolySULFOETHYL A column (PolyLC; pore size 200Å, bead size 5 μm, column dimensions 200 × 2.1 mm). Fractionated samples were desalted, vacuum dried, and stored at −80°C until further analysis.
LC-MS/MS analysis
Tandem MS analysis was carried out on a Fourier transform LTQ-Orbitrap Velos mass spectrometer (Thermo Fisher Scientific) interfaced with an Agilent 1200 series nano-LC system. The labeled peptides from each SCX fraction were enriched using a trap column (75 μm × 3 cm) and resolved on an analytical column (75 μm × 10 cm). Peptides were eluted using a linear gradient of 7% to 40% acetonitrile over 60 minutes. MS analysis was carried out in a data-dependent manner with survey scans acquired in the Orbitrap mass analyzer at a resolution of 60 000 at 400 m/z. During each duty cycle, the 20 most intense precursor ions from a survey scan were selected for MS/MS (resolution, 7500 at 400 m/z). Fragmentation was carried out using higher-energy collision-induced dissociation as the activation mode with 40% normalized collision energy. Ions selected for fragmentation were excluded for next 30 seconds from MS/MS analysis.
Protein identification and quantification
The MS data were analyzed using Proteome Discoverer version 1.3 software (Thermo Fisher Scientific). MS/MS search was carried out using the Sequest (SCM build 59) search algorithm against the Human RefSeq49 database. Search parameters included trypsin as the protease with up to 1 missed cleavage, oxidation of methionine as a dynamic modification, methylthiolation at cysteine, and iTRAQ modification at the N terminus and lysine as static modifications. Precursor and fragment mass tolerance were set to 20 ppm and 0.1 Da, respectively. Peptide identifications were filtered with 1% false discovery rate threshold at the peptide level. Relative abundance of peptides in PCOS as compared with controls was calculated as the ratio of the sum of reporter ion intensities from technical replicates. Protein quantification was obtained by averaging the ratios of corresponding peptides. Differentially abundant proteins were identified with 1.5-fold change as the cutoff value. The differentially expressed proteins were categorized based on subcellular localization and molecular function using annotation in the Human Protein Reference Database (http://www.hprd.org/) (11).
Validation by ELISA
Differential abundance of selected proteins was further validated with follicular fluid samples collected from 20 control women and 20 women with PCOS that had not been used for iTRAQ labeling. Concentrations of heparin sulfate proteoglycan 2 (HSPG2) (USCN Life Science), amphiregulin (R&D Systems Inc), fibronectin 1 (FN1) (BioVendor Research and Diagnostic), and serpin peptidase inhibitor, clade A (α-1 antiproteinase, antitrypsin), member 1 (SERPINA1) (Promokine) in follicular fluid were measured by commercial ELISA kits as per the manufacture's protocol. Undepleted follicular fluid samples were diluted 1:2, 1:500, and 1:15 000 respectively, for performing HSPG2; amphiregulin; and FN1 ELISAs. The follicular fluid dilutions used for SERPINA1 ELISA were 1:40 000 for controls and 1: 100 000 for PCOS. Protein concentrations were determined by comparing the OD (450 nm) of samples with the standard curve and final concentrations were calculated by multiplying with respective dilution factors.
Results and Discussion
Altered expression of proteins in follicular fluid of women with PCOS
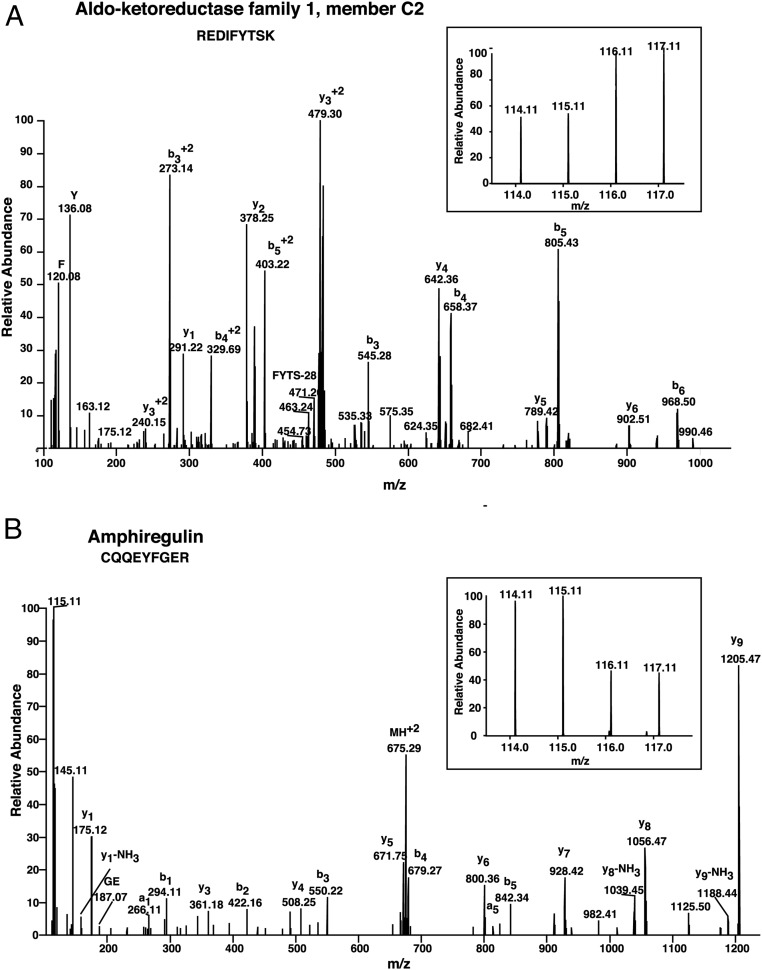
Follicular fluid samples from controls and women with PCOS of similar age were labeled with iTRAQ reagents and analyzed by LC-MS/MS (Figure 1). We identified a total of 770 proteins in follicular fluid, of which 186 were differentially expressed in PCOS (99 upregulated and 87 downregulated) (Supplemental Table 1). A list of differentially expressed peptides has been provided in Supplemental Table 2. Representative labeled MS/MS spectra of peptides from proteins aldo-keto reductase family 1, member C2 (AKR1C2) and amphiregulin are shown in Figure 2.
Figure 1. Schematic representation of the workflow of 4-plex iTRAQ-based strategy employed for comparison of the follicular fluid proteome from healthy controls and women with PCOS.
Figure 2. Representative MS/MS spectra of peptides from upregulated protein aldoketoreductse 1, member C2 (A) and downregulated protein amphiregulin (B).
Inset shows relative intensities of reporter ions.
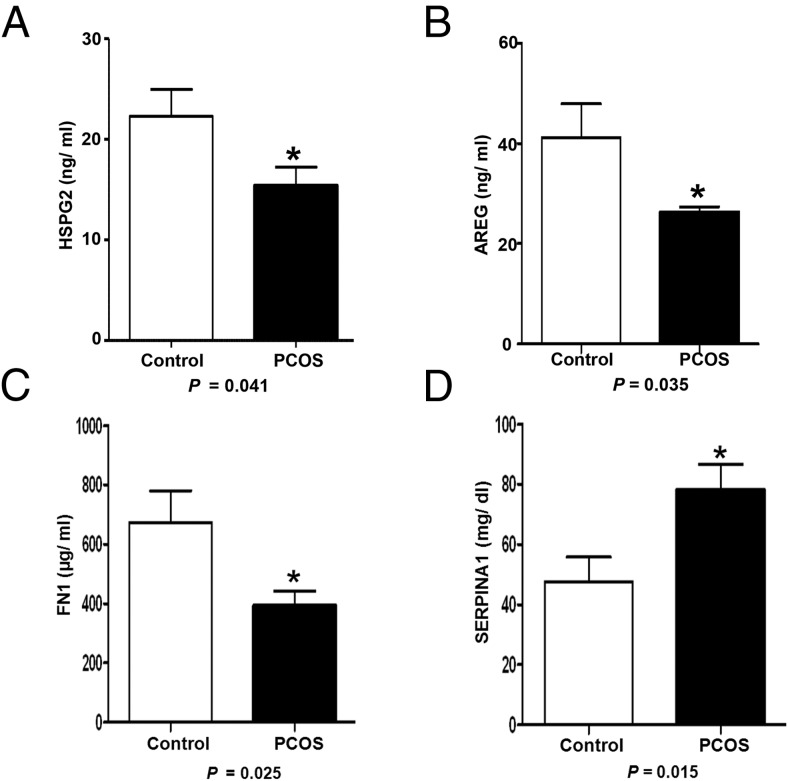
The validity of the approach employed in this study is further demonstrated by the identification and differential expression of several proteins in follicular fluid of women with PCOS, which are known to play a role in PCOS pathophysiology. These include TIMP metallopeptidase inhibitor 1; IGF binding proteins 2 to 4; IGF-2; coagulation factor II; super oxide dismutase 3 (SOD3); and plasminogen (PLG) (12, 13). Additionally, an altered expression of various proteins, not reported earlier in follicular fluid of women with PCOS, was observed. These include tumor necrosis factor, alpha-induced protein 6 (TNFAIP6); lymphatic vessel endothelial hyaluronan receptor 1 (LYVE1); CD14; syndecan-4; and amphiregulin, which are involved in the process of follicular maturation and ovulation. We also found a number of novel proteins in follicular fluid including TLC domain containing 1; mannosidase-α class 2A, member 2; suprabasin; S100 calcium binding protein A7; chromosome 3 open reading frame 52; and helicase with zinc finger 2, transcriptional coactivator, whose function in ovary is poorly defined. Differential expression of selected proteins was validated by ELISA (Figure 3).
Figure 3. Validation of expression of downregulated proteins HSPG2 (A), amphiregulin (AREG) (B), and FN1 (C) and upregulated protein SERPINA1 (D) by ELISA in 20 control and PCOS undepleted follicular fluid samples.
Statistical analyses were carried out using Student's t test using GraphPad Prism version 5 software. P < .05 is statistically significant. Values are given as mean ± SEM.
Insenser and Escobar-Morreale (14) recently reviewed the proteomics studies conducted in PCOS and discussed the challenges and guidelines for application of proteomics to PCOS research. Our study design complied with the majority of the issues discussed regarding recruitment of women with PCOS, sample size, and validation of proteomics data. As mentioned, the 2 previous studies on proteomics of follicular fluid in PCOS identified only 20 differentially expressed proteins of which we also detected SERPINA1; apolipoprotein A1; and haptoglobin (6, 7). Additionally, we identified 183 differentially expressed proteins, which have not been reported previously in PCOS follicular fluid by a proteomics approach. A study on ovarian biopsy samples from PCOS and control women confirmed differential expression of 69 proteins using a comparative 2-dimensional gel proteomics approach (15). However, there is no significant overlap between this data set and our study, which might be due to the differences in samples (follicular fluid/ovary), sample processing methods, and the sensitivity of the techniques employed.
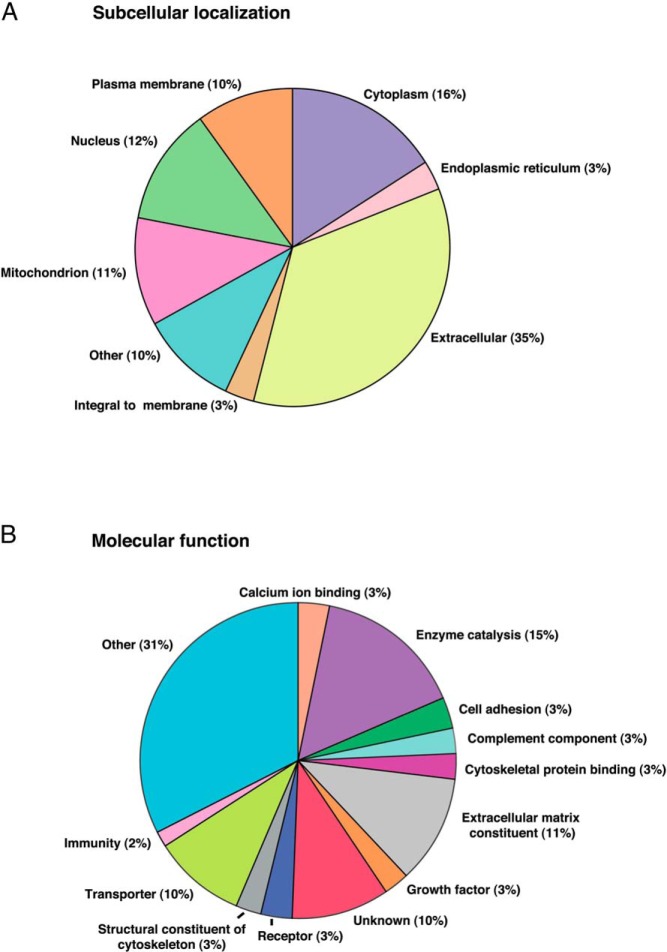
Human Protein Reference Database-based classification of 186 differentially expressed proteins in PCOS follicular fluid is provided in Figure 4. The majority of these proteins are extracellular in origin (Figure 4A). Proteins of plasma membrane and intracellular proteins including cytoplasmic, mitochondrial, and nuclear proteins were found to be altered in PCOS. Elevated levels of mitochondrial and nuclear proteins in follicular fluid of PCOS are likely due to alteration in cellular apoptosis in PCOS follicles. The classification of identified proteins based on molecular function (Figure 4B) revealed that the proteins involved in enzyme catalysis, extracellular matrix (ECM) components, growth factors, transporters, complement factors, and immune function are deregulated in follicular fluid of women with PCOS. Several classes of enzymes are known to be involved in follicular development (5). Derangement of a multitude of these enzymes indicates gross metabolic abnormalities in PCOS follicles, which might affect follicular growth. We also observed the downregulation of several ECM proteins which are the constituents of follicular basal lamina and cumulus-oocyte complex (COC) matrix. Their role in impairment of follicle development in PCOS is discussed below.
Figure 4. Categorization of differentially expressed proteins in follicular fluid of women with PCOS (Source: Human Protein Reference Database) A, Subcellular localization.
B, Molecular function.
Deregulation of basal lamina matrix proteins
Basal lamina matrix is composed of a lattice-type network of collagen type IV intertwined with a network of laminins stabilized by binding of entactin or nidogen to collagen and laminin. During follicle enlargement, the collagen content decreases while more laminin accumulates in the basal lamina (16). Recently, Irving-Rodgers et al (17) have shown that the phenotypes of the basal lamina matrix influence oocyte competence. Our findings of decreased levels of various basal lamina matrix proteins including collagens; laminins; secreted, protein, acidic cysteine-rich (SPARC); and HSPG2 in PCOS follicular fluid indicates basal lamina matrix composition is altered in PCOS. The glycoprotein HSPG2, also known as perlecan, is an integral component of basal lamina, which cross-links various ECM proteins. The expression of HSPG2 increases during early follicular growth that enables the binding of various growth factors, which are essential for follicular growth and differentiation (16). Decreased HSPG2 level in follicular fluid of women with PCOS (Figure 3A) may restrict the availability of these growth factors in the follicle, contributing to arrest of follicular growth. SPARC is a modulator of cell and ECM interaction, which regulates cell growth through interactions between the ECM and cytokines. It also plays a role in matrix mineralization, angiogenesis, and neoplastic transformation (18). The reduced level of SPARC in PCOS may affect matrix remodeling required for the expansion of the basal lamina during follicular growth.
Perturbation of cumulus oocyte complex matrix expansion
The formation of a COC matrix, its expansion, and mucification is critical for ovulation and fertilization. Epidermal growth factor (EGF)-like growth factors, mainly amphiregulin; epiregulin; and betacellulin, induce the transcription of several of the genes involved in COC matrix formation and expansion by mediating LH signal from mural to cumulus cells (19). We observed reduced expression of amphiregulin in PCOS, which is the most abundantly expressed EGF-like growth factor in follicular fluid. The estimated concentration of amphiregulin in follicular fluid was found to be significantly lower in PCOS than controls (Figure 3B). The downregulation of amphiregulin was also confirmed at the transcript level in granulosa cells of women with PCOS using semiquantitative real-time PCR (Supplemental Figure 1). This diminished amphiregulin may impair COC matrix expansion in PCOS. Supporting this assumption, we found downregulation of an amphiregulin-induced protein TNFAIP6 in PCOS. This protein is involved in maintaining COC matrix integrity by cross-linking structural backbones hyaluronan and inter-α-trypsin inhibitor. We also observed downregulation of α-1-microglobulin/bikunin precursor (AMBP), which is a component of the inter-α-trypsin inhibitor chain. Studies in animals showed that mice lacking AMBP or TNFAIP6 are infertile due to defects in the formation and expansion of COC matrix, respectively (20, 21). Diminished AMBP and TNFAIP6 in PCOS follicles might disrupt the proper organization and expansion of COC matrix architecture. Syndecan-4, an anticoagulant HSPG, whose expression normally increases in COC matrix during cumulus cell maturation and which plays an essential role in focal adhesion, was found to be downregulated in PCOS in our study. Additionally, syndecan-4 acts as a coreceptor for various growth factors including TGF-β; fibroblast growth factor 2 (FGF2); and vascular endothelial growth factor (VEGF) (22). Therefore, reduced syndecan-4 could deregulate the cell matrix interaction during assembly and expansion of COC matrix, as well as affect growth factor signaling in cumulus cells in PCOS.
Our study demonstrates the deregulation of a number of ECM proteins in follicular fluid of women with PCOS. The differential expression of these basal lamina matrix proteins in follicular fluid might be a reflection of altered composition of the matrix, which in turn may impede basal lamina expansion and selective passage of molecules across it, therefore affecting follicle development. Appropriate composition and organization of the COC matrix and its expansion is essential for maturation, release, and transport of oocytes through the oviduct for fertilization (20). Selection of oocytes during IVF in many species is determined by the extent of COC expansion (23). Deregulation of the proteins of COC matrix might affect the formation and expansion of the matrix, which is critical for fertility and thus explain the compromised oocyte developmental capacity and quality reported in women with PCOS (24).
Deregulation of complement coagulation cascade and angiogenesis
Complement proteins are part of innate immune system, which act in a cascade to trigger inflammation in response to injuries and pathogens. Folliculogenesis and ovulation are also considered to be controlled inflammatory processes. Transcriptome profiling of rat ovarian follicles and human granulosa cells has indicated that the complement system plays a role in follicle development as well as oocyte maturation (25, 26). We also report decreased levels of classical as well as alternate complement components including complement component 1, r subcomponent; complement component 1, q subcomponent, A chain; complement component 7; complement component 8, γ-polypeptide; and complement factor properdin in women with PCOS, whose effect on follicular development yet remains to be investigated.
Angiogenesis, initiated at the preantral stage of the folliculogenesis to provide oxygen and nutrients to the developing follicle, is a highly coordinated event balanced by a group of pro- and anti-angiogenic factors. Inadequate blood supply may be a limiting step for selection and maturation of dominant follicle and follicular fluid formation (27). The defects in angiogenesis may also lead to improper corpus luteum formation and luteal phase deficiency (28). Several ECM proteins and growth factors mainly VEGF; FGF2; and TGF-β; and proteases including matrix metalloproteases (MMPs); and plasmin, mediate follicular angiogenesis (29). We observed reduced levels of PLG, the precursor of plasmin, as well as overexpression of an inhibitor of PLG activator, SERPINA1 (30), in follicular fluid of women with PCOS (Figure 3D); all these may lead to low plasmin production. Plasmin, a serine protease, performs a number of functions including activation of VEGF; FGF2; and MMPs, which are the primary mediators of angiogenesis in the developing follicle and corpus luteum. MMPs further facilitate follicle wall rupture during ovulation (31–33). Additionally, by degrading laminin, proteoglycans, and fibronectins of follicle wall, plasmin helps in follicle wall expansion during folliculogenesis (34). Moreover, plasmin being a component of the fibrinolytic pathway catalyzes fibrin cleavage and clot dissolution. Thus, downregulation of PLG may explain the decreased global fibrinolytic capacity reported in PCOS (13). In addition, other angiogenic factors including signal peptide, CUB domain, EGF-like 1; angiotensinogen; S100 calcium binding protein A13; decorin; fms-related tyrosine kinase 4; LYVE1; and FN1 (Figure 3C), were detected to be low in women with PCOS. Although there are contradictory reports about angiogenesis in PCOS ovary, our finding of downregulation of a number of factors involved in angiogenesis suggests compromised angiogenesis in the follicles of women with PCOS. In support of this hypothesis, a previous study revealed that PCOS follicles appear less vascularized compared with normal follicles (35).
In many species, macrophages have been shown to accumulate in thecal layers during follicle growth and secrete various factors involved in follicular growth and development (36). We report downregulation of colony stimulating factor 1 receptor (CSF1R) and CD14 proteins in PCOS follicular fluid. CSF1R is a receptor for the growth factor-colony stimulating factor, which stimulates differentiation of monocytes to macrophages, and CD14 is a surface receptor for macrophages. Diminished expression of both of these proteins suggests poor macrophage infiltration in PCOS follicles (37, 38). Thus, a limited supply of blood-derived nutrients, hormones, and growth factors and low macrophage infiltration in the growing follicle as well as deregulation of the mechanism of follicular wall expansion may partially explain the arrest of follicular growth, impairment of dominant follicle selection, and defects in corpus luteum formation in these women.
The antioxidant enzymes paraoxonase 1; SOD3; and glutathione S-transferase α1 were found to be decreased in PCOS. Reactive oxygen species are generated in the growing follicle due to high metabolic activity. Excess reactive oxygen species can induce oxidative damage and cytotoxicity. It has been shown that oocyte maturation and meiotic spindle formation is significantly affected by increased oxidative stress (OS) in PCOS (39). Furthermore, in vitro studies showed that moderate OS induces hyperproliferation of rat theca cells (40). Thus, deregulation of antioxidant enzymes may increase OS, which may lead to thecal hyperplasia and augment androgen production in PCOS along with other factors. We also observed the altered expression of proteins involved in lipid transport and metabolism in PCOS.
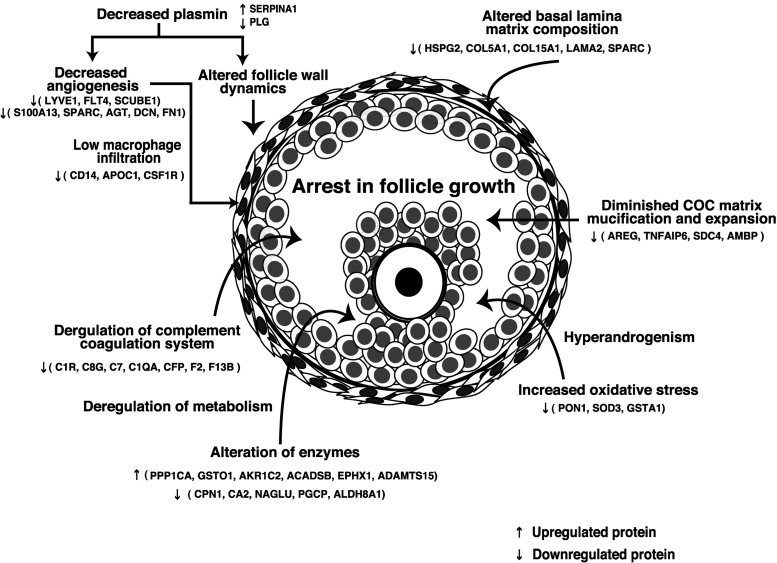
In the present study, by employing iTRAQ labeling and highly accurate MS/MS techniques, we attempted to understand the molecular events underlying follicular defects in PCOS. Proteins involved in ECM remodeling, complement coagulation cascade, fibrinolysis, vasculature development, angiogenesis, lipid transport, and metabolism were found to be deregulated in PCOS (Supplemental Table 3). These processes play an important role in the growth and maturation of the follicle as well as in ovarian steroidogenesis. Thus, cessation of follicle growth in PCOS might be a cumulative effect of deregulation of several biological processes as illustrated in Figure 5. Systematic evaluation of candidate molecules identified in our study would improve our ability to predict oocyte quality and probably the risk of development of PCOS and may also facilitate improved therapeutic interventions in the future.
Figure 5. Proposed mechanism for arrest in follicular growth in women with PCOS.
Abbreviations: ACADSB, acyl-CoA dehydrogenase, short/branched chain; ADAMTS15, ADAM metallopeptidase with thrombospondin type 1 motif, 15; AGT, angiotensin; AKR1C2, aldo-keto reductase family 1, member C2; ALDH8A1, aldehyde dehydrogenase 8 family, member A1; APOC1, apolipoprotein C-I; AREG, amphiregulin; C7, complement component 7; CA2, carbonic anhydrase II; CFP, complement factor properdin; C8G, complement component 8, γ-polypeptide; COL5A1, collagen, type V, α1; COL15A1, collagen, type XV, α1; CPN1, carboxypeptidase N, polypeptide 1; C1QA, complement component 1, q subcomponent, A chain; C1R, complement component 1, r subcomponent; DCN, decorin; EPHX1, epoxide hydrolase 1; F2, coagulation factor II (thrombin); F13B, coagulation factor XIII, B polypeptide; FLT4, fms-related tyrosine kinase 4; GSTA1-glutathione S-transferase α1; GSTO1, glutathione S-transferase ω1; LAMA2, laminin, α2; NAGLU, N-acetylglucosaminidase, α; PGCP, carboxypeptidase Q; PON1, paraoxonase1; PPP1CA, protein phosphatase 1, catalytic subunit, α isozyme; S100A13, S100 calcium binding protein A13; SCUBE1, signal peptide, CUB domain, EGF-like 1; SDC4, syndecan 4; TNFAIP6, TNF, α-induced protein 6.
Acknowledgments
We thank Mrs Sushma Khavale from the National Institute for Research in Reproductive Health for her technical support. We thank the Department of Biotechnology for research support to the Institute of Bioinformatics, Bangalore.
This work was funded by the Department of Biotechnology (DBT), Government of India, under Grant BT/ PR10574/ MED/12/394/2008. We acknowledge the National Institute for Research in Reproductive Health and Indian Council of Medical Research (ICMR) for providing necessary support (NIRRH/MS/117/2014). We acknowledge the financial assistance provided by the DBT and ICMR, Government of India, to A.S.A. for pursuing her doctoral studies. T.S.K.P. is supported by a research grant on “Development of Infrastructure and a Computational Framework for Analysis of Proteomic Data” from the DBT. Harsha Gowda is a Wellcome Trust/DBT India Alliance Early Career Fellow. We thank Agilent Technologies for instrument support.
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was funded by the Department of Biotechnology (DBT), Government of India, under Grant BT/ PR10574/ MED/12/394/2008. We acknowledge the National Institute for Research in Reproductive Health and Indian Council of Medical Research (ICMR) for providing necessary support (NIRRH/MS/117/2014). We acknowledge the financial assistance provided by the DBT and ICMR, Government of India, to A.S.A. for pursuing her doctoral studies. T.S.K.P. is supported by a research grant on “Development of Infrastructure and a Computational Framework for Analysis of Proteomic Data” from the DBT. Harsha Gowda is a Wellcome Trust/DBT India Alliance Early Career Fellow. We thank Agilent Technologies for instrument support.
Footnotes
- AMBP
- α-1-microglobulin/bikunin precursor
- COC
- cumulus-oocyte complex
- CSF1R
- colony stimulating factor 1 receptor
- ECM
- extracellular matrix
- EGF
- epidermal growth factor
- FGF2
- fibroblast growth factor 2
- FN1
- fibronectin 1
- HSPG2
- heparin sulfate proteoglycan 2
- iTRAQ
- Isobaric Tags for Relative and Absolute Quantification
- IVF
- in vitro fertilization
- LC-MS
- liquid chromatography mass spectrometry
- LYVE1
- lymphatic vessel endothelial hyaluronan receptor 1
- MMP
- matrix metalloprotease
- m/z
- mass to charge ratio
- OS
- oxidative stress
- PCOS
- polycystic ovary syndrome
- PLG
- plasminogen
- SCX
- strong cation-exchange chromatography
- SERPINA1
- serpin peptidase inhibitor, clade A (α-1 antiproteinase, antitrypsin)
- SOD
- superoxide dismutase 3
- SPARC
- secreted protein acidic cysteine-rich
- TNFAIP6
- TNFα-induced protein 6
- VEGF
- vascular endothelial growth factor.
References
- 1. Diamanti-Kandarakis E. Polycystic ovarian syndrome: pathophysiology, molecular aspects and clinical implications. Expert Rev Mol Med. 2008;10:e3. [DOI] [PubMed] [Google Scholar]
- 2. Edwards RG. Follicular fluid. J Reprod Fertil. 1974;37:189–219. [DOI] [PubMed] [Google Scholar]
- 3. Kim YS, Kim MS, Lee SH, et al. . Proteomic analysis of recurrent spontaneous abortion: Identification of an inadequately expressed set of proteins in human follicular fluid. Proteomics. 2006;6:3445–3454. [DOI] [PubMed] [Google Scholar]
- 4. Jarkovska K, Kupcova Skalnikova H, et al. . Development of ovarian hyperstimulation syndrome: interrogation of key proteins and biological processes in human follicular fluid of women undergoing in vitro fertilization. Mol Hum Reprod. 2011;17:679–692. [DOI] [PubMed] [Google Scholar]
- 5. Ambekar AS, Nirujogi RS, Srikanth SM, et al. . Proteomic analysis of human follicular fluid: a new perspective towards understanding folliculogenesis. J Proteomics. 2013;87:68–77. [DOI] [PubMed] [Google Scholar]
- 6. Choi BC, K Y, Kim MS, Chung MK, Choi CH, Baek KH. Identification of overexpressed proteins by proteomic analysis using human follicular fluids derived from polycystic ovary syndrome (PCOS) patients. Fertil Steril. 2007;88:S180. [Google Scholar]
- 7. Dai G, Lu G. Different protein expression patterns associated with polycystic ovary syndrome in human follicular fluid during controlled ovarian hyperstimulation. Reprod Fertil Dev. 2012;24:893–904. [DOI] [PubMed] [Google Scholar]
- 8. Waldemarson S, Krogh M, Alaiya A, et al. . Protein expression changes in ovarian cancer during the transition from benign to malignant. J Proteome Res. 2012;11:2876–2889. [DOI] [PubMed] [Google Scholar]
- 9. Hu ZX, Qiao J, Li MZ, et al. . The differential expression profile of polycystic ovary syndrome associated genes [in Chinese]. Beijing Da Xue Xue Bao. 2004;36:600–604. [PubMed] [Google Scholar]
- 10. Chaerkady R, Kerr CL, Marimuthu A, et al. . Temporal analysis of neural differentiation using quantitative proteomics. J Proteome Res. 2009;8:1315–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goel R, Harsha HC, Pandey A, Prasad TS. Human Protein Reference Database and Human Proteinpedia as resources for phosphoproteome analysis. Mol Biosyst. 2012;8:453–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Qiao J, Feng HL. Extra- and intra-ovarian factors in polycystic ovary syndrome: impact on oocyte maturation and embryo developmental competence. Hum Reprod Update. 2011;17:17–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yildiz BO, Haznedarolu IC, Kirazli S, Bayraktar M. Global fibrinolytic capacity is decreased in polycystic ovary syndrome, suggesting a prothrombotic state. J Clin Endocrinol Metab. 2002;87:3871–3875. [DOI] [PubMed] [Google Scholar]
- 14. Insenser M, Escobar-Morreale HF. Proteomics and polycystic ovary syndrome. Expert Rev Proteomics. 2013;10:435–447. [DOI] [PubMed] [Google Scholar]
- 15. Ma X, Fan L, Meng Y, et al. . Proteomic analysis of human ovaries from normal and polycystic ovarian syndrome. Mol Hum Reprod. 2007;13:527–535. [DOI] [PubMed] [Google Scholar]
- 16. Rodgers RJ, Irving-Rodgers HF, Russell DL. Extracellular matrix of the developing ovarian follicle. Reproduction. 2003;126:415–424. [DOI] [PubMed] [Google Scholar]
- 17. Irving-Rodgers HF, Morris S, Collett RA, et al. . Phenotypes of the ovarian follicular basal lamina predict developmental competence of oocytes. Hum Reprod. 2009;24:936–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Irving-Rodgers HF, Rodgers RJ. Extracellular matrix in ovarian follicular development and disease. Cell Tissue Res. 2005;322:89–98. [DOI] [PubMed] [Google Scholar]
- 19. Zamah AM, Hsieh M, Chen J, et al. . Human oocyte maturation is dependent on LH-stimulated accumulation of the epidermal growth factor-like growth factor, amphiregulin. Hum Reprod. 2010;25:2569–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fülöp C, Szántó S, Mukhopadhyay D, et al. . Impaired cumulus mucification and female sterility in tumor necrosis factor-induced protein-6 deficient mice. Development. 2003;130:2253–2261. [DOI] [PubMed] [Google Scholar]
- 21. Zhuo L, Yoneda M, Zhao M, et al. . Defect in SHAP-hyaluronan complex causes severe female infertility. A study by inactivation of the bikunin gene in mice. J Biol Chem. 2001;276:7693–7696. [DOI] [PubMed] [Google Scholar]
- 22. Wathlet S, Adriaenssens T, Segers I, et al. . Cumulus cell gene expression predicts better cleavage-stage embryo or blastocyst development and pregnancy for ICSI patients. Hum Reprod. 2011;26:1035–1051. [DOI] [PubMed] [Google Scholar]
- 23. Yokoo M, Kimura N, Sato E. Induction of oocyte maturation by hyaluronan-CD44 interaction in pigs. J Reprod Dev. 2010;56:15–19. [DOI] [PubMed] [Google Scholar]
- 24. Dumesic DA, Padmanabhan V, Abbott DH. Polycystic ovary syndrome and oocyte developmental competence. Obstet Gynecol Surv. 2008;63:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nilsson EE, Savenkova MI, Schindler R, Zhang B, Schadt EE, Skinner MK. Gene bionetwork analysis of ovarian primordial follicle development. PLoS One. 2010;5:e11637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yoo SW, Bolbot T, Koulova A, et al. . Complement factors are secreted in human follicular fluid by granulosa cells and are possible oocyte maturation factors. J Obstet Gynaecol Res. 2013;39:522–527. [DOI] [PubMed] [Google Scholar]
- 27. Stouffer RL, Martínez-Chequer JC, Molskness TA, Xu F, Hazzard TM. Regulation and action of angiogenic factors in the primate ovary. Arch Med Res. 2001;32:567–575. [DOI] [PubMed] [Google Scholar]
- 28. Boutzios G, Karalaki M, Zapanti E. Common pathophysiological mechanisms involved in luteal phase deficiency and polycystic ovary syndrome. Impact on fertility. Endocrine. 2013;43:314–317. [DOI] [PubMed] [Google Scholar]
- 29. Pepper MS. Role of the matrix metalloproteinase and plasminogen activator-plasmin systems in angiogenesis. Arterioscler Thromb Vasc Biol. 2001;21:1104–1117. [DOI] [PubMed] [Google Scholar]
- 30. Tanaka N, Sekiya S, Takamizawa H, Kato N, Moriyama Y, Fujimura S. Characterization of a 54 kDa, alpha 1-antitrypsin-like protein isolated from ascitic fluid of an endometrial cancer patient. Jpn J Cancer Res. 1991;82:693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ferrara N. Binding to the extracellular matrix and proteolytic processing: two key mechanisms regulating vascular endothelial growth factor action. Mol Biol Cell. 2010;21:687–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ribatti D, Leali D, Vacca A, et al. . In vivo angiogenic activity of urokinase: role of endogenous fibroblast growth factor-2. J Cell Sci. 1999;112(Pt 23):4213–4221. [DOI] [PubMed] [Google Scholar]
- 33. Ebisch IM, Thomas CM, Wetzels AM, Willemsen WN, Sweep FC, Steegers-Theunissen RP. Review of the role of the plasminogen activator system and vascular endothelial growth factor in subfertility. Fertil Steril. 2008;90:2340–2350. [DOI] [PubMed] [Google Scholar]
- 34. Strickland S, Beers WH. Studies on the role of plasminogen activator in ovulation. In vitro response of granulosa cells to gonadotropins, cyclic nucleotides, and prostaglandins. J Biol Chem. 1976;251:5694–5702. [PubMed] [Google Scholar]
- 35. Järvelä IY, Sladkevicius P, Kelly S, Ojha K, Campbell S, Nargund G. Comparison of follicular vascularization in normal versus polycystic ovaries during in vitro fertilization as measured using 3-dimensional power Doppler ultrasonography. Fertil Steril. 2004;82:1358–1363. [DOI] [PubMed] [Google Scholar]
- 36. Russell DL, Robker RL. Molecular mechanisms of ovulation: co-ordination through the cumulus complex. Hum Reprod Update. 2007;13:289–312. [DOI] [PubMed] [Google Scholar]
- 37. Wu R, Van der Hoek KH, Ryan NK, Norman RJ, Robker RL. Macrophage contributions to ovarian function. Hum Reprod Update. 2004;10:119–133. [DOI] [PubMed] [Google Scholar]
- 38. Stanley ER, Berg KL, Einstein DB, et al. . Biology and action of colony–stimulating factor-1. Mol Reprod Dev. 1997;46:4–10. [DOI] [PubMed] [Google Scholar]
- 39. Chattopadhayay R, Ganesh A, Samanta J, Jana SK, Chakravarty BN, Chaudhury K. Effect of follicular fluid oxidative stress on meiotic spindle formation in infertile women with polycystic ovarian syndrome. Gynecol Obstet Invest. 2010;69:197–202. [DOI] [PubMed] [Google Scholar]
- 40. Kwintkiewicz J, Spaczynski RZ, Foyouzi N, Pehlivan T, Duleba AJ. Insulin and oxidative stress modulate proliferation of rat ovarian theca-interstitial cells through diverse signal transduction pathways. Biol Reprod. 2006;74:1034–1040. [DOI] [PubMed] [Google Scholar]