Abstract
Context:
Various drugs affect body weight as a side effect.
Objective:
We conducted this systematic review and meta-analysis to summarize the evidence about commonly prescribed drugs and their association with weight change.
Data Sources:
MEDLINE, DARE, and the Cochrane Database of Systematic Reviews were searched to identify published systematic reviews as a source for trials.
Study Selection:
We included randomized trials that compared an a priori selected list of drugs to placebo and measured weight change.
Data Extraction:
We extracted data in duplicate and assessed the methodological quality using the Cochrane risk of bias tool.
Results:
We included 257 randomized trials (54 different drugs; 84 696 patients enrolled). Weight gain was associated with the use of amitriptyline (1.8 kg), mirtazapine (1.5 kg), olanzapine (2.4 kg), quetiapine (1.1 kg), risperidone (0.8 kg), gabapentin (2.2 kg), tolbutamide (2.8 kg), pioglitazone (2.6 kg), glimepiride (2.1 kg), gliclazide (1.8 kg), glyburide (2.6 kg), glipizide (2.2 kg), sitagliptin (0.55 kg), and nateglinide (0.3 kg). Weight loss was associated with the use of metformin (1.1 kg), acarbose (0.4 kg), miglitol (0.7 kg), pramlintide (2.3 kg), liraglutide (1.7 kg), exenatide (1.2 kg), zonisamide (7.7 kg), topiramate (3.8 kg), bupropion (1.3 kg), and fluoxetine (1.3 kg). For many other remaining drugs (including antihypertensives and antihistamines), the weight change was either statistically nonsignificant or supported by very low-quality evidence.
Conclusions:
Several drugs are associated with weight change of varying magnitude. Data are provided to guide the choice of drug when several options exist and institute preemptive weight loss strategies when obesogenic drugs are prescribed.
Background
Obesity is a worldwide epidemic and one of the most important public health concerns in developed nations (1). The World Health Organization estimates that, globally, over 200 million men and nearly 300 million women were obese in 2008 (2). In the United States, the lifetime risk of obesity has increased to approximately 25% (3), making it a major cause of morbidity and mortality (4–6).
Efforts to control this epidemic are dependent on stakeholder understanding of the factors that contribute to weight change and how these might be open to modification. According to the World Health Organization, over 75% of all cardiovascular disease mortality may be prevented with adequate changes in lifestyle (7). However, clinicians who prescribe medications that can impact weight can also modify these outcomes, if possible. Clinically, it would be useful to know the absolute effects on weight of various medications so that certain drugs can be avoided, replaced, or sought out as appropriate to a given situation. Current treatment guidelines are often limited by a lack of comparative data, such that it is impossible to determine the magnitude of the difference in weight change caused by two otherwise acceptable drugs.
An expert panel from The Endocrine Society is charged with developing clinical practice guidelines for the management of obesity. As part of a comprehensive management approach, medication choice can play a role. To aid in the development of the Society guidelines, we conducted this systematic review and meta-analysis aimed at summarizing the evidence about commonly prescribed drugs and their associations with weight loss or weight gain. The goal is to provide patients and clinicians with information that can inform and individualize treatment decisions.
Methods
Search and analysis methods, eligibility criteria, and the outcomes of interest were specified in advance in a protocol developed by study investigators with input from the expert panel from The Endocrine Society. This protocol is described in detail and has been published elsewhere (8).
To make this review of a large number of weight-affecting drugs feasible, and due to the availability of multiple systematic reviews (SRs) of these drugs, we conducted an umbrella (9) search strategy to identify eligible randomized controlled trials (RCTs). The umbrella strategy differs from a regular systematic search approach in that the umbrella strategy identifies studies from previous systematic reviews as opposed to the primary literature. To this extent, we searched for any systematic review that included RCTs comparing the drugs of interest to placebo. The list of the most relevant drugs was developed by members from The Endocrine Society. This list consisted of commonly prescribed drug families and specific drugs that have been associated with weight gain (obesogenic) or weight loss (leptogenic) (Supplemental Table 1, interventions list). Eligible SRs were used as a source to identify relevant RCTs. Considering that multiple published systematic reviews and meta-analyses (10–13) have already summarized and appraised the evidence supporting the efficacy of antiobesity drugs such as orlistat and phentermine, these drugs were not included in this summary.
Search methods and selection of SRs
The first author (J.P.D.) searched MEDLINE, DARE, and the Cochrane Database of SRs for at least two SRs per drug through January 2013. An expert reference librarian (L.J.P.) provided assistance throughout the process.
When multiple SRs evaluated the same drug, we chose the one with the most recent search date. When more than two SRs shared a similar search date (<1 y apart), we chose the one with the largest number of included RCTs that were most clinically relevant to the typical application of the drug. For example, although sertraline has a therapeutic indication for eating disorders, its major clinical use is in depression and obsessive-compulsive disorder. When there was no clear difference in the frequency of the use of drugs by a specific condition, we included the SRs without considering this criterion (for example, beta blockers for myocardial infarction or for essential hypertension). When we found more than two SRs with no clear rationale to select one over the other, we included all of them (Supplemental Table 2, included SRs).
Eligibility criteria for RCT
We included parallel or crossover RCTs that enrolled adults (≥18 y old) and evaluated any drug listed in Supplemental Table 1 as an intervention as long as the length of treatment was at least 30 days. Studies that investigated combinations of drugs (except for the listed ones) were excluded. We also excluded studies that reported only subjective outcome measures (self-reported weight change). We elected not to include observational or quasi-randomized studies.
Agreement among the reviewers was measured using the κ statistic. A reference management system (DistillerSR) was used for study selection, providing real-time agreement statistics. The first author (J.P.D.) monitored the agreement between evaluators during the trial selection in order to discuss disagreements and clarify the protocol and selection criteria when needed. The whole selection team met with the first author and the senior author (M.H.M.) five times during the selection process.
Data extraction and management
Using a standardized, piloted, and web-based data extraction form and working in duplicate, we abstracted the following descriptive data from each study: full description of participants enrolled, the interventions received (dose, frequency, route), the monitoring for efficacy or adherence, and the measure of outcome (specifically defined as event rate or continuous measure and time frame). For studies with more than one follow-up period, we selected the longest.
When necessary, we calculated needed data elements from other reported statistics such as confidence intervals (CIs), P, or t values (14). When this was not possible, we imputed the missing values, such as standard deviation, from one large study (another RCT or a SR) with a similar population and intervention (15).
Assessment of risk of bias in included studies and confidence on the estimates
We assessed the methodological quality of RCTs using the Cochrane risk of bias tool to determine: how the randomization sequence was generated; how allocation was concealed; whether there were important imbalances at baseline; which groups were blinded (patients, caregivers, data collectors, outcome assessors, data analysts); what was the loss to follow-up; whether the analyses were by intention to treat; and how missing outcome data were handled. We also analyzed the adequacy of the outcome measurement process, assigning higher confidence to the RCTs that evaluated weight changes using a specific predefined protocol. No scoring system was derived for risk of bias assessment.
We used the GRADE framework (Grading of Recommendations, Assessment, Development and Evaluation) (16) to rate the confidence on the evidence supporting the weight change effect associated with each drug. This rating reflects our confidence in the pooled estimate. We rated the confidence downward based on methodological limitations, imprecision, indirectness, inconsistency, and reporting and publication biases. Factors that led to rating the confidence upward were large magnitude of effect and the presence of a dose-duration response gradient. The confidence in the estimates was rated as high (⊕⊕⊕⊕), moderate (⊕⊕⊕○), low (⊕⊕○○), or very low (⊕○○○).
Meta-analysis
We defined a clinically important weight change (for either weight gain or loss) as a change ≥ 2 kg or ≥ 5% from the baseline, defined by Stevens et al (17). The outcomes of this meta-analysis were: 1) absolute weight change (Abs WC)—weight loss or gain assessed as a continuous outcome expressed as a mean difference in the absolute weight change in kilograms or as a body mass index (BMI) change in kilograms/(meter)2; 2) percentage weight change (Per WC)—weight loss or gain assessed as a continuous outcome expressed as a mean difference in the relative weight change in kilograms or as a BMI change in kilograms/(meter)2 from the baseline weight or BMI; 3) weight gain/weight loss (WG/WL) ≥ 5%—an important change in weight of 5% or more as described by Stevens et al (17) (for example, 7 to 10%), either for weight gain or loss, from the baseline weight (this outcome is presented as a relative risk); and 4) any WG/WL—weight change rate assessed as a dichotomous outcome, defined as a number of patients with increased or decreased weight over the total number of patients in each group (this outcome is presented as a relative risk).
The precision of estimates of drug effects on weight is reflected in 95% CIs around such estimates. We extracted and evaluated outcomes by analyzing participants in the groups in which they were randomized (ie, intention to treat). For studies with loss to follow-up, we used the number of patients randomized as a denominator for the risk estimate, preserving randomization benefits in balancing prognosis of trial arms, realizing that this may underestimate the effect size (18).
We conducted random-effects meta-analysis using the DerSimonian and Laird (19) method to pool treatment effects from included studies. We used the I2 statistic (20) and Cochran's Q test to assess heterogeneity across studies. We assessed publication bias by the Begg adjusted rank correlation test and visual examination of funnel plots whenever there were at least 10 included studies with no considerable heterogeneity (defined as less than 70% of I2 according to the Cochrane collaboration) (14, 21, 22). Analysis was conducted using STATA version 12.0 (StataCorp).
Subgroup and sensitivity analysis
We planned to explore a few subgroup interactions to explain inconsistency in results across trials including subgroup analyses based on: 1) BMI status—obese (BMI ≥ 30 kg/m2) vs nonobese (BMI < 30 kg/m2); 2) risk of bias of the included studies (low and unclear risk of bias vs high risk of bias); and 3) gender (male vs female). Due to the lack of data about gender variation and BMI status within each included RCT, we could not perform these subgroup analyses. Most of the included studies presented serious or very serious methodological limitations; therefore, we did not perform a subgroup analysis based on the risk of bias.
We conducted a meta-regression to test whether the effect size (weight change) was affected by daily drug dose.
This review is reported in accordance with the recommendations set forth by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) work groups (23).
Results
Search results and study description
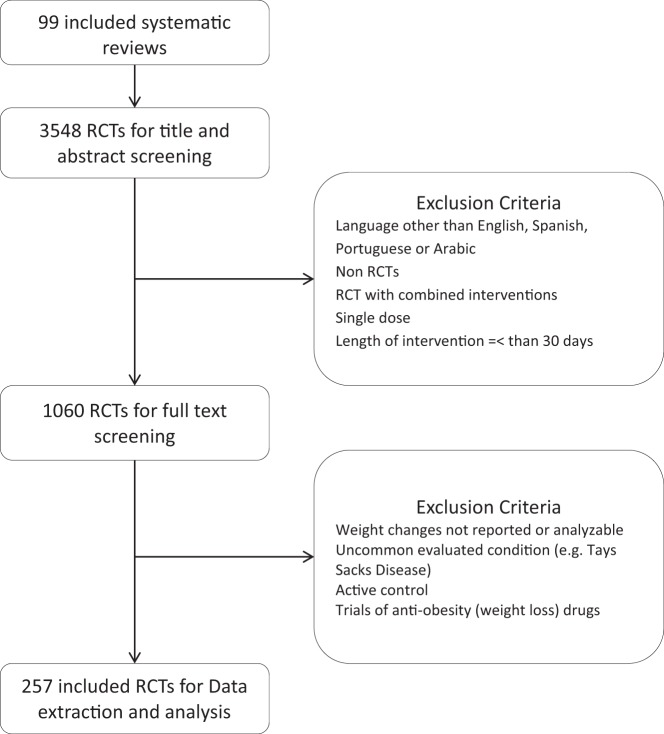
We identified 99 relevant SRs that included 3548 RCTs, which we reviewed and from which we selected 257 eligible RCTs (Figure 1). These RCTs evaluated weight change associated with 54 different drugs and enrolled more than 84 696 patients. During the full-text screening, reviewers had excellent agreement (average κ coefficient, 0.87). The main reasons for exclusion were nonrandomized study design and lack of weight change assessment.
Figure 1. The process of study selection.
In these trials, 46% of participants were men. Ages ranged from 22 to 88 years. Most RCTs (63%) exclusively enrolled obese patients; 23% enrolled only overweight patients. We found an underrepresentation of nonwhite populations in the included trials (Supplemental Table 3, study characteristics).
Methodological quality of included studies and confidence in the estimates
We found at least one trial evaluating the effect on weight for 54 different drugs. The risk of bias was moderate or high in 191 of 257 (74%) of the trials. This was mainly due to the failure to report randomization methods or allocation concealment or the lack of following the intention-to-treat principle. Only 17 of 257 trials (7%) were open-label studies and did not blind the patients or investigator. The risk of bias for each individual trial is described in Supplemental Table 4 (methodological quality of included studies). The risk of bias was assessed overall for each comparison and was used to rate the quality of evidence. The confidence in the estimates was rated downward for most comparisons due to the risk of bias. The size and precision of the effect varied across drug classes. A few estimates were rated upward based on a large effect size and an exposure-response gradient. The complete data including the weight change and confidence rating process for each drug are shown in Supplemental Table 5.
Weight changes per drug class
Atypical antipsychotics
This drug class was associated with the most weight gain. Olanzapine was associated with the largest gain (2.4 kg), followed by quetiapine (1.1 kg) and risperidone (0.8 kg).
These three atypical antipsychotics reached their effect on weight in a median duration of 3 months. Quetiapine showed a dose response gradient suggesting higher weight gain with doses above 450 mg/d.
None of the drugs in this class were associated with weight loss. Aripiprazole was associated with weight gain (0.6 kg); however, the confidence in this estimate was very low. Ziprasidone may be a weight-neutral atypical antipsychotic; however, this estimate was associated with very low confidence (rated downward due to imprecision and risk of bias).
Anticonvulsants and mood stabilizers
Gabapentin was associated with a weight gain of 2.2 kg after 1.5 months of use. Divalproex was associated with increased risk of weight gain (of any magnitude), but not of clinically significant (≥5%) weight gain (relative risk, 2.8; 95% CI, 1.30, 6.02; and relative risk, 1.39; 95% CI, 0.38, 5.14, respectively; data insufficient to determine absolute weight difference). Carbamazepine was associated with weight gain of 1.0 kg; however, the confidence in this estimate is low.
Significant weight loss was noted with zonisamide (7.7 kg) and topiramate (3.8 kg).
Lithium and lamotrigine were not associated with a statistically significant effect on weight; therefore, they may be weight neutral.
Hypoglycemic agents
Agents associated with statistically significant weight gain were tolbutamide (2.8 kg), pioglitazone (2.6 kg), glimepiride (2.1 kg), gliclazide (1.8 kg), glyburide (2.6 kg), glipizide (2.2 kg), sitagliptin (0.55 kg), and nateglinide (0.3 kg).
Agents associated with statistically significant weight loss were metformin (1.1 kg; estimate heterogeneity was partially explained by the dose and duration of therapy), acarbose (0.4 kg), miglitol (0.7 kg), pramlintide (2.3 kg), and glucagon-like peptide-1 (GLP-1) agonists. Liraglutide doses of 1.2 μg or above used for a median of 6 months (range, 3–6.5 mo) demonstrated a weight loss of 1.7 kg. Exenatide was associated with a significant weight loss of 1.2 kg after 4 months of usage (range, 1–7 mo). Similar weight change was noted with exenatide 10, 15, and 20 μg. The weight loss with exenatide weekly dosing was 0.9 kg, and with daily dosing it was 1.3 kg (P value for difference = .7). Weight loss with GLP-1 agonists used < 3 months was 0.3 kg, 3–6 months was 1.3 kg, and > 6 months was 0.9 kg.
Data were also insufficient for other hypoglycemic agents such as rosiglitazone and repaglinide.
Antihypertensive agents
We found no evidence of a significant effect on weight for any of the listed antihypertensive agents. The estimates for all the included drugs under this class were inconclusive, with low or very low confidence.
Hormones
A pooled estimate from four RCTs of glucocorticoids used in rheumatoid arthritis suggested a weight increase of 4 to 8% (24). We also found one RCT (25) that compared glucocorticoids against sulfasalazine and demonstrated a weight gain of 1.7 kg after 1 year of treatment.
GH use was associated with a reduction of BMI by 0.59 kg/m2; however, data were too imprecise and heterogeneous to estimate a specific absolute weight change. The quality of evidence supporting weight change for T, leuprolide, and medroxyprogesterone was low; hence, they may be weight neutral.
Antihistamines
Data were only available on cyproheptadine, which was likely weight neutral.
Antidepressants
Weight gain was associated with the use of amitriptyline (1.8 kg) and mirtazapine (1.5 kg).
Weight loss was associated with the use of bupropion (1.3 kg) and fluoxetine (1.3 kg).
There was a nonclinically significant weight loss supported with low-quality evidence for sertraline, venlafaxine, and duloxetine and a nonsignificant weight change with the use of citalopram, escitalopram, paroxetine, and nortriptyline.
Discussion
Main findings
We conducted a systematic review and meta-analysis of 257 RCTs (54 different drugs; 84 696 patients enrolled). Our goal was to evaluate the weight change associated with the use of an a priori selected list of commonly used drugs.
We that found weight gain was associated with the use of amitriptyline, mirtazapine, olanzapine, quetiapine, risperidone, gabapentin, tolbutamide, pioglitazone, glimepiride, gliclazide, glyburide, sitagliptin, and nateglinide. Weight loss was associated with the use of metformin, acarbose, miglitol, pramlintide, liraglutide, exenatide, zonisamide, topiramate, bupropion, and fluoxetine. For many other remaining drugs (including antihypertensives and antihistamines), the weight change was either statistically nonsignificant or supported by low-quality evidence.
A key issue in interpreting data on drugs with estimates that are imprecise (not statistically significant) or estimates associated with low confidence rating is that there are two possibilities of inference: 1) these drugs may be weight neutral; or 2) these drugs cause a weight change that the current published literature cannot document with confidence. Thus, the absence of evidence (for a weight change) is not necessarily an evidence of absence (of a weight effect).
Limitations and strengths
The strengths of this review include the rigorous and comprehensive nature of the literature search. We attempted to identify at least two high-quality SRs for each evaluated drug. This umbrella approach (9) also made this systematic review feasible, a key concern that was realized from the outset.
Our findings are limited by the short-term follow-up of most of the included RCTs. Observational studies have a longer follow-up, but they are subject to a higher risk of bias typically, particularly when the outcome is weight change. These outcomes are subject to many cointerventions, confounders, and baseline prognostic imbalances. Therefore, we made the decision to consider only RCTs for this review.
We recognize that the evaluated drugs were chosen by the experts of The Endocrine Society based on their knowledge of the field. Although this process has its own limitations, it was felt to be an approach that closely met the needs of the guideline developers and clinicians in daily practice. To make this evidence synthesis feasible, The Endocrine Society task force had to choose a discrete list of drugs that had certain characteristics (suspected to affect weight, to have randomized evidence available, and to be commonly encountered in the practice). This list of drugs was chosen a priori. It is certainly plausible that other drugs not included here exert important weight changes.
Comparison with other reviews
Our findings are in general consistent with other systematic reviews that evaluated single drugs or drug classes (26–30) and faced similar challenges. The Agency for Healthcare Research and Quality commissioned a technology assessment report (29) comparing second-generation antidepressants, but a quantitative meta-analysis was not performed on the outcome of weight change. Our systematic review is conducted as a companion document to The Endocrine Society guideline on the pharmacological management of obesity. Analysis of weight change is challenging and can be complicated by nonlinear weight change during drug use. For example, our analysis of antidepressants showed weight gain with amitriptyline and mirtazapine and weight loss with bupropion and fluoxetine. However, the weight change is unclear for most other agents. Some RCTs showed statistically significant weight loss with duloxetine, (31), venlafaxine (32, 33), and sertraline (34), and other trials did not. A pooled analysis (35) of 10 RCTs showed that duloxetine-treated patients experienced weight loss after short-term treatment, followed by modest weight gain on longer-term treatment, and suggested similar weight changes among duloxetine, fluoxetine, and paroxetine. Therefore, for paroxetine, duloxetine, venlafaxine, and sertraline, the effect on weight remains unclear, and if differences between these three drugs exist, they are unlikely to be clinically significant.
An example of other nuances is observed when evaluating the association between GLP-1 agonists and weight loss. A recent RCT (36) suggested that long-acting GLP-1 agonists appear to be more effective on glycemic control given their effect on insulin production and suppression of glucagon, whereas shorter-acting ones may have a bigger effect on appetite and gastric emptying, and thus weight loss. We used such hypotheses to explore the observed heterogeneity in GLP-1 meta-analysis by conducting subgroup analyses based on dose, dosing interval, and duration of use. We found low-quality evidence suggesting a small amount of weight gain with sitagliptin. A network meta-analysis (27) showed no significant weight loss with sitagliptin (0.20 kg; 95% CI, −0.18, 60), and another meta-analysis of the head-to-head trials showed that patients on GLP-1 analogs lost 1.55 kg (95% CI, 1.12, 1.98) compared to those on sitagliptin (30).
A meta-analysis of RCTs of antipsychotics followed a Bayesian approach and provided rankings of these drugs in terms of weight gain. The ranking order, starting with the largest weight gain, was olanzapine, clozapine, risperidone, haloperidol, and aripiprazole (28). We opted to avoid ranking because it hides the quality of evidence and has multiple other limitations (37) and opted for presenting weight change estimates associated with each drug to facilitate shared decision making.
This systematic review is clinically relevant particularly because of the impact of weight on cardiovascular morbidity and mortality (38) and overall mortality (4), and it can help inform decisions about whether or not to use a specific drug (39). This systematic review represents a significant effort and is the product of an adequate balance between rigor and feasibility; it provides the best comparative evidence on weight effect for many different drugs. These data will be central for guideline developers, clinicians, and patients when choosing between different available therapies.
Implications for practice
The summarized evidence herein will help in treatment choice when several options are available. In addition, it may help with instituting preemptive weight loss strategies when prescribing certain drugs. For example, the large and quick effect of atypical antipsychotics on weight suggests that some preemptive weight management measures should be undertaken when prescribing these drugs. Several systematic reviews (40–43) have described such strategies. Physicians should not wait for a weight increase to recommend lifestyle modifications when prescribing a drug known to increase weight.
In addition to choosing a medication within a class, this review can also help patients with multiple comorbidities using drugs from multiple classes. Awareness of possible additive effects on weight from various drugs provides a rationale for developing preemptive strategies to prevent or address undesired weight affects.
The estimates presented in this review are only one factor to consider when choosing a drug; patients and clinicians will consider other factors relating to efficacy, adverse effects, cost, treatment burden, and patients goals.
Implications for research
There is underrepresentation of ethnic minorities in RCTs (44). The included studies in this review were no exception. We found that Blacks, Asians, and Hispanics were underrepresented in these datasets. Knowing the effect of various drugs on weight in these populations is particularly important because of the increased incidence and worse prognosis of several chronic conditions in minorities such as obesity, hypertension, chronic kidney disease, and diabetes (45–47).
Future studies that evaluate the effect of a drug on weight need to report the incidence of weight change in addition to the magnitude of weight change, should have longer follow-up, and should evaluate time trends to determine whether the effect is constant, linear, or prone to plateau over time. Evaluating these characteristics in meta-analyses of aggregate data is not reliable and requires individual patient data. The effect on weight after stopping the treatment remains largely unknown.
Conclusions
Several drugs are associated with weight change of varying magnitude. Data are provided to guide the choice of drug when several options exist and to institute preemptive strategies for weight management when drugs with known weight effects are prescribed.
Acknowledgments
This review was commissioned and funded by a contract from The Endocrine Society.
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This review was commissioned and funded by a contract from The Endocrine Society.
For article see page 342
- BMI
- body mass index
- CI
- confidence interval
- GLP-1
- glucagon-like peptide-1
- RCT
- randomized controlled trial
- SR
- systematic review.
References
- 1. Barness LA, Opitz JM, Gilbert-Barness E. Obesity: genetic, molecular, and environmental aspects. Am J Med Genet A. 2007;143A:3016–3034. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization. Fact Sheet: Obesity and Overweight. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 3. Vasan RS, Pencina MJ, Cobain M, Freiberg MS, D'Agostino RB. Estimated risks for developing obesity in the Framingham Heart Study. Ann Intern Med. 2005;143:473–480. [DOI] [PubMed] [Google Scholar]
- 4. Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298:2028–2037. [DOI] [PubMed] [Google Scholar]
- 5. Prospective Studies Collaboration; Whitlock G, Lewington S, Sherliker P, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Preston SH, Stokes A. Contribution of obesity to international differences in life expectancy. Am J Public Health. 2011;101:2137–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Perk J, De Backer G, Gohlke H, et al. European guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J. 2012;33:1635–1701. [DOI] [PubMed] [Google Scholar]
- 8. Domecq JP, Prutsky G, Wang Z, et al. Drugs commonly associated with weight change: umbrella systematic review and meta-analysis (Protocol). Syst Rev. 2012;1:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smith V, Devane D, Begley CM, Clarke M. Methodology in conducting a systematic review of systematic reviews of healthcare interventions. BMC Med Res Methodol. 2011;11:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhou YH, Ma XQ, Wu C, et al. Effect of anti-obesity drug on cardiovascular risk factors: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2012;7:e39062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Smith SM, Meyer M, Trinkley KE. Phentermine/topiramate for the treatment of obesity. Ann Pharmacother. 2013;47:340–349. [DOI] [PubMed] [Google Scholar]
- 12. Dombrowski SU, Knittle K, Avenell A, Araújo-Soares V, Sniehotta FF. Long term maintenance of weight loss with non-surgical interventions in obese adults: systematic review and meta-analyses of randomised controlled trials. BMJ. 2014;348:g2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johansson K, Neovius M, Hemmingsson E. Effects of anti-obesity drugs, diet, and exercise on weight-loss maintenance after a very-low-calorie diet or low-calorie diet: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2014;99:14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration. Version 5.1.0. Updated March 2011 Hoboken, NJ: Wiley; 2011. [Google Scholar]
- 15. Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. J Clin Epidemiol. 2006;59:7–10. [DOI] [PubMed] [Google Scholar]
- 16. Swiglo BA, Murad MH, Schünemann HJ, et al. A case for clarity, consistency, and helpfulness: state-of-the-art clinical practice guidelines in endocrinology using the grading of recommendations, assessment, development, and evaluation system. J Clin Endocrinol Metab. 2008;93:666–673. [DOI] [PubMed] [Google Scholar]
- 17. Stevens J, Truesdale KP, McClain JE, Cai J. The definition of weight maintenance. Int J Obes (Lond). 2006;30:391–399. [DOI] [PubMed] [Google Scholar]
- 18. Montori VM, Guyatt GH. Intention-to-treat principle. CMAJ. 2001;165:1339–1341. [PMC free article] [PubMed] [Google Scholar]
- 19. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 20. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 21. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 22. Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ. 2006;333:597–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34. [DOI] [PubMed] [Google Scholar]
- 24. Da Silva JA, Jacobs JW, Kirwan JR, et al. Safety of low dose glucocorticoid treatment in rheumatoid arthritis: published evidence and prospective trial data. Ann Rheum Dis. 2006;65:285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Landewé RB, Boers M, Verhoeven AC, et al. COBRA combination therapy in patients with early rheumatoid arthritis: long-term structural benefits of a brief intervention. Arthritis Rheum. 2002;46:347–356. [DOI] [PubMed] [Google Scholar]
- 26. Robinson LE, Holt TA, Rees K, Randeva HS, O'Hare JP. Effects of exenatide and liraglutide on heart rate, blood pressure and body weight: systematic review and meta-analysis. BMJ Open. 2013;3(1)pii:e001986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Craddy P, Palin HJ, Johnson KI. Comparative effectiveness of dipeptidylpeptidase-4 inhibitors in type 2 diabetes: a systematic review and mixed treatment comparison. Diabetes Ther. 2014;5:1–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Klemp M, Tvete IF, Skomedal T, Gaasemyr J, Natvig B, Aursnes I. A review and Bayesian meta-analysis of clinical efficacy and adverse effects of 4 atypical neuroleptic drugs compared with haloperidol and placebo. J Clin Psychopharmacol. 2011;31:698–704. [DOI] [PubMed] [Google Scholar]
- 29. Gartlehner G, Hansen RA, Morgan LC, et al. Second-Generation Antidepressants in the Pharmacologic Treatment of Adult Depression: An Update of the 2007 Comparative Effectiveness Review. Rockville, MD: Agency for Healthcare Research and Quality; 2011. [PubMed] [Google Scholar]
- 30. Wang T, Gou Z, Wang F, Ma M, Zhai SD. Comparison of GLP-1 analogues versus sitagliptin in the management of type 2 diabetes: systematic review and meta-analysis of head-to-head studies. PLoS One. 2014;9:e103798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rynn M, Russell J, Erickson J, et al. Efficacy and safety of duloxetine in the treatment of generalized anxiety disorder: a flexible-dose, progressive-titration, placebo-controlled trial. Depress Anxiety. 2008;25:182–189. [DOI] [PubMed] [Google Scholar]
- 32. Montgomery SA, Tobias K, Zornberg GL, Kasper S, Pande AC. Efficacy and safety of pregabalin in the treatment of generalized anxiety disorder: a 6-week, multicenter, randomized, double-blind, placebo-controlled comparison of pregabalin and venlafaxine. J Clin Psychiatry. 2006;67:771–782. [DOI] [PubMed] [Google Scholar]
- 33. Nemeroff CB, Thase ME. A double-blind, placebo-controlled comparison of venlafaxine and fluoxetine treatment in depressed outpatients. J Psychiatr Res. 2007;41:351–359. [DOI] [PubMed] [Google Scholar]
- 34. Moscovitch A, Blashko CA, Eagles JM, et al. A placebo-controlled study of sertraline in the treatment of outpatients with seasonal affective disorder. Psychopharmacology (Berl). 2004;171:390–397. [DOI] [PubMed] [Google Scholar]
- 35. Wise TN, Perahia DG, Pangallo BA, Losin WG, Wiltse CG. Effects of the antidepressant duloxetine on body weight: analyses of 10 clinical studies. Prim Care Companion J Clin Psychiatry. 2006;8:269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dungan KM, Povedano ST, Forst T, et al. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomised, open-label, phase 3, non-inferiority trial. Lancet. 2014;384(9951):1349–1357. [DOI] [PubMed] [Google Scholar]
- 37. Benkhadra K, Wang Z, Murad MH. Network meta-analysis: introduction and an example that compares devices for PFO closure. Eur Heart J. 2014;pii:ehu343. [DOI] [PubMed] [Google Scholar]
- 38. Danielsen KK, Svendsen M, Maehlum S, Sundgot-Borgen J. Changes in body composition, cardiovascular disease risk factors, and eating behavior after an intensive lifestyle intervention with high volume of physical activity in severely obese subjects: a prospective clinical controlled trial. J Obes. 2013;2013:325464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Montori VM, Gandhi GY, Guyatt GH. Patient-important outcomes in diabetes–time for consensus. Lancet. 2007;370:1104–1106. [DOI] [PubMed] [Google Scholar]
- 40. Björkhem-Bergman L, Asplund AB, Lindh JD. Metformin for weight reduction in non-diabetic patients on antipsychotic drugs: a systematic review and meta-analysis. J Psychopharmacol. 2011;25:299–305. [DOI] [PubMed] [Google Scholar]
- 41. Das C, Mendez G, Jagasia S, Labbate LA. Second-generation antipsychotic use in schizophrenia and associated weight gain: a critical review and meta-analysis of behavioral and pharmacologic treatments. Ann Clin Psychiatry. 2012;24:225–239. [PubMed] [Google Scholar]
- 42. Ehret M, Goethe J, Lanosa M, Coleman CI. The effect of metformin on anthropometrics and insulin resistance in patients receiving atypical antipsychotic agents: a meta-analysis. J Clin Psychiatry. 2010;71:1286–1292. [DOI] [PubMed] [Google Scholar]
- 43. Fiedorowicz JG, Miller DD, Bishop JR, Calarge CA, Ellingrod VL, Haynes WG. Systematic review and meta-analysis of pharmacological interventions for weight gain from antipsychotics and mood stabilizers. Curr Psychiatry Rev. 2012;8:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bartlett C, Doyal L, Ebrahim S, et al. The causes and effects of socio-demographic exclusions from clinical trials. Health Technol Assess. 2005;9:iii–iv, ix,–x, 1–152. [DOI] [PubMed] [Google Scholar]
- 45. Sabanayagam C, Teo BW, Tai ES, Jafar TH, Wong TY. Ethnic differences in the association between blood pressure components and chronic kidney disease in middle aged and older Asian adults. BMC Nephrol. 2013;14:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sabanayagam C, Teo BW, Tai ES, Jafar TH, Wong TY. Ethnic variation in the impact of metabolic syndrome components and chronic kidney disease. Maturitas. 2013;74:369–374. [DOI] [PubMed] [Google Scholar]
- 47. Lanting LC, Joung IM, Mackenbach JP, Lamberts SW, Bootsma AH. Ethnic differences in mortality, end-stage complications, and quality of care among diabetic patients: a review. Diabetes Care. 2005;28:2280–2288. [DOI] [PubMed] [Google Scholar]