Abstract
Context:
Epidemiologic studies suggest that endogenous testosterone (T) levels in males may be implicated in cardiovascular disease (CVD), however further clarification is needed.
Objective:
We assessed the cross-sectional relationship between endogenous plasma T and mean carotid intima media thickness (cIMT), and the longitudinal relationship with incident clinical CVD events, cardiac mortality, and all-cause mortality using male participants in the Atherosclerosis Risk in Communities (ARIC) study.
Design:
This study involved a subset of men from visit 4 of the ARIC study.
Setting:
The study was conducted in a community based cohort.
Participants:
Males who provided a morning blood sample excluding those taking androgen therapy, with prevalent coronary heart disease (CHD), stroke, or heart failure (HF) (n = 1558).
Intervention:
None.
Main Outcome Measures:
Plasma T by liquid chromatography mass spectrometry and carotid IMT using high resolution B-mode ultrasound were obtained at visit 4. Incident CHD, HF, cardiac mortality, and all-cause mortality were identified by surveillance through 2010 (median 12.8 years).
Results:
Lower T was significantly associated with higher body mass index, greater waist circumference, diabetes, hypertension, lower HDL, and never smoking (P = 0.01). T was not associated with mean cIMT in unadjusted or adjusted analyses. Following multivariable adjustment, there was no association of quartile (Q) of T with incident CHD [hazard ratio (HR) = 0.87 (95% CI = 0.60–1.26) for Q1; 0.97 (95% CI = 0.69–1.38) for Q2; 0.97 (95% CI = 0.69–1.36) for Q3 compared to reference of Q4] or for incident HF [HR = 0.77 (95% CI = 0.46–1.29) for Q1; 0.72 (95% CI = 0.43–1.21) for Q2; 0.87 (95% CI = 0.53–1.42) for Q3 compared to reference of Q4]. Similarly there was no association of Q of T with mortality or cardiac-associated mortality.
Conclusions:
Low male plasma T is cross-sectionally associated with key CVD risk factors, but after adjustment there was no association with mean cIMT, incident cardiac events, or mortality. Our results are reassuring that neither high nor low T levels directly predict atherosclerosis, but are a marker for other cardiovascular risk factors.
Cardiovascular disease (CVD) is a leading cause of death, with earlier onset and possibly greater mortality risk seen in men compared to women (1). Sex hormones have been implicated as a possible explanation with a focus on testosterone (T), the predominant androgen produced in males. Unlike women who have complete loss of estrogen at the time of menopause, men have a decline in androgen production associated with aging (2), which is congruent with an increased risk of CVD. The Women's Health Initiative has shed light on the cardiovascular risk in women associated with loss of estrogen at menopause, and the risks and benefits of hormone replacement therapy in this population (3). But similar large-scale observational and intervention studies examining the long-term risk or benefit of T deficiency on CVD risk in men are limited.
Prior epidemiologic studies show an association of low T levels with higher mortality in males with and without coronary heart disease (CHD) after controlling for other cardiovascular risk factors (4). However recent prospective studies suggest this relationship is nonlinear, with low and high T associated with greater cardiac risk (5, 6). In contrast, retrospective pharmaco-epidemiologic studies of T replacement have raised controversy by suggesting possible increased mortality in those treated with T compared to those not treated, particularly within the first 90 days of filling the prescription, in the elderly and in younger men with prior CHD (7, 8). However, many have raised concerns about the methodology and statistical analysis of these studies and therefore further clarification is needed regarding the role of T therapy in cardiac risk (9).
Carotid intima media thickness (cIMT) as measured by b-mode ultrasound is considered a surrogate marker of atherosclerosis. A systematic review and meta-analysis found that cIMT was a strong predictor of future clinical ischemic cardiac and cerebrovascular events, despite heterogeneity among studies (10). Prior observational studies have suggested that low T is associated with higher carotid IMT in older men (11, 12) and in those with insulin resistance and diabetes mellitus (13). Congruently, a single center study of 50 men with metabolic syndrome or diabetes mellitus demonstrated significant improvement in cIMT in those subjects randomized to T injections for twelve months compared to placebo (14). However, few studies have looked at the association of endogenous T with both preclinical atherosclerosis and clinical atherosclerosis.
In the current study we analyzed data from the Atherosclerosis Risk in Communities (ARIC) cohort to assess the relationship between endogenous testosterone and atherosclerosis in males. We hypothesize that low T is independently associated with mean cIMT, incident CHD, HF, all-cause and cardiac-associated mortality.
Materials and Methods
Study population
The ARIC study is a prospective investigation of CVD which included predominantly white and black males and females, age 45–64 years at baseline (1987–1989), sampled from four communities within the United States: Forsyth County, North Carolina; Minneapolis, Minnesota; Washington County, Maryland; and Jackson, Mississippi. The study was approved by the institutional review boards of all participating institutions. Informed consent was given by all participants enrolled in the study. Participants took part in a baseline visit which took place in 1987–1989 and was followed by three triennial visits through 1996–1998, with a fifth visit completed in 2011–2013. We considered visit 4 (1996–1998) to be our baseline for analysis and included male participants with carotid ultrasound imaging. Men taking androgen therapy and those with prevalent coronary heart disease (CHD), stroke, MI or heart failure (HF) were excluded.
Sex hormone measurements
Blood samples were obtained from male participants (1996–1998) and those drawn in the AM and with sufficient volume (>1 cm3) were frozen. Plasma total testosterone was performed by liquid chromatography mass spectrophotometry (Dr. Shalendar Bhasin's Lab, Boston University) in 2012. All samples obtained after 10:30 AM were excluded to correct for diurnal variation in testosterone levels. One T value >2000 mg/dL was excluded because this was felt to be nonphysiologically plausible and likely due to laboratory documentation error.
Carotid IMT
Carotid IMT was obtained at baseline using high resolution B-mode ultrasound. Our variable for analysis was the average IMT of the far wall across the left and right common carotid, carotid bifurcation, and internal carotid artery, respectively (six sites total). Missing values were imputed from sex- and race-specific multivariable linear models of mean IMT. Measurements of the far wall thickness show between reader reliability coefficients ranging from 0.78 to 0.93, and coefficients of variation ranging from 13.1 to 18.3% (15).
Cardiovascular events/mortality assessment
Cardiovascular events and deaths were identified by annual questionnaire or by continuous comprehensive surveillance. Surveillance events and hospitalizations were abstracted independently to confirm reproducibility and ensure whether the event met study criteria. Incident CHD was defined as a definite or probable myocardial infarction (MI), definite coronary death, or coronary revascularization procedure. Incident HF was defined as death from HF in any position on the death certificate or as the first hospitalization for HF with ICD 9 code 429 or ICD-10 code I50. All-cause mortality was defined as deaths from any cause. Cardiac-associated mortality was defined as definite fatal CHD or definite fatal MI.
Covariates
At baseline and follow up visit age, race/ethnicity, smoking status, medication use, and medical history were obtained. Because two of the field centers only enrolled participants of a single race, a composite variable of center and race was created. Sitting blood pressure (BP) was taken, and the average of two measurements was recorded. Anthropometry measures included height, weight, and waist circumference, with calculation of body mass index (BMI). Venipuncture was performed in the fasting state. Serum lipids included total cholesterol, triglycerides, and HDL measured by enzymatic assay, with LDL calculated by the Friedewald equation. Hypertension was defined as receiving antihypertensive medications or as a BP ≥140/90 mmHg. Diabetes mellitus was defined as receiving diabetic medications, self -reported physician diagnosis, a fasting glucose ≥126 mg/dL or a nonfasting glucose ≥200 mg/dL. Smoking status was defined as never, former or current.
Statistical analysis
Baseline characteristics were summarized using frequencies and percent, means and SDs, or medians and interquartile ranges (IQR; expressed as 25th-75th percentile). Plasma testosterone values were divided into quartiles. The Cochran-Armitage test for trend and general linear models regression were used to assess the cross-sectional association of quartile of T with cardiovascular risk factors listed above. Linear and logistic regression modeling was used to assess the association of quartiles of T with mean cIMT of the far wall. cIMT was dichotomized as ≤1.0 mm and >1.0 mm for the logistic regression analysis. The test for trend was based on the quartiles of T. Proportional hazards regression analysis was performed to assess the association of T quartiles with incident CHD, CHF, and mortality. Models were adjusted for age, race/center, BMI, waist circumference, smoking status, diabetes, hypertension, LDL, and HDL. To assess whether the results may be affected by survival bias, we stratified by age (53–59, 60–64, 65–69, and 70–74 years) and reanalyzed the data to see if the association of T quartiles with outcomes was consistent across age categories. All tests were two-sided and P < .05 or a 95% confidence interval (CI) not overlapping 1.0 was considered significant. All statistical analysis was conducted using SAS version 9.3 (SAS Institute, Inc.).
Results
Baseline characteristics
A total of 1943 samples were assayed. Six were duplicate samples and we took the average of the two values. We excluded participants whose samples were drawn after 10:30 AM (n = 9), those taking androgen therapy (n = 4), and those with plasma T >2000 ng/dL (n = 1), prevalent CHD (n = 270), prevalent stroke (n = 39), prevalent MI (n = 35), and prevalent HF (n = 21). The remaining 1558 males had mean (SD) age = 63.1 (5.6) years and BMI = 28.18 (4.27) kg/m2. The median (IQR) of plasma total T was 377.6 (288.4–480.1) ng/dL.
Baseline demographic and clinical characteristics stratified by testosterone quartile are presented in Table 1. Testosterone levels were not significantly associated with age, race, LDL or use of lipid lowering medications. Participants with lower T had significantly higher BMI, greater waist circumference, higher prevalence of diabetes and hypertension, and lower HDL (all P for trend <.001). Participants with lower testosterone were less likely to be current smokers (P for trend = .002).
Table 1.
Demographic and Clinical Characteristics of All Men, Stratified by Testosterone Quartile
| Characteristic | All Participants (n = 1558) | Quartile 1 (n = 391) | Quartile 2 (n = 388) | Quartile 3 (n = 390) | Quartile 4 (n = 389) | P for Trend |
|---|---|---|---|---|---|---|
| Testosterone, ng/dL: | ||||||
| Mean (sd) | 402.3 (165.1) | 225.1 (57.7) | 333.1 (25.9) | 426.7 (28.5) | 624.8 (133.8) | |
| Median (IQRa) | 377.6 (288.4–480.1) | 237.6 (203.1–269.8) | 332.9 (310.0–355.8) | 424.9 (402.3–451.5) | 588.5 (531–671.9) | |
| Age in years, Mean (sd) | 63.1 (5.6) | 63.3 (5.6) | 62.8 (5.7) | 62.9 (5.7) | 63.4 (5.6) | .73 |
| Race/center, N (%) | .55b | |||||
| Forsyth County, NC: Black | 38 (2.4) | 8 (2.0) | 11 (2.8) | 9 (2.3) | 10 (2.6) | |
| Forsyth County, NC: White | 311 (20.0) | 70 (17.9) | 82 (21.1) | 81 (20.8) | 78 (20.0) | |
| Jackson, MS: Black | 197 (12.6) | 53 (13.6) | 35 (9.0) | 55 (14.1) | 54 (13.9) | |
| Minneapolis, MN: White | 558 (35.8) | 150 (38.4) | 140 (36.1) | 126 (32.3) | 142 (36.5) | |
| Washington County, MD: White | 454 (29.1) | 110 (28.1) | 120 (30.9) | 119 (30.5) | 105 (27.0) | |
| Body mass index, Mean (sd) | 28.2 (4.3) | 30.3 (4.5) | 28.9 (4.2) | 27.4 (3.6) | 26.2 (3.5) | <.001 |
| Waist circumference cm, Mean (sd) | 102.4 (11.2) | 108.0 (11.6) | 103.9 (11.2) | 100.3 (9.3) | 97.2 (9.6) | <.001 |
| Smoking status, N (%) | .002b | |||||
| Current | 240 (15.4) | 43 (11.0) | 61 (15.8) | 51 (13.1) | 85 (21.8) | |
| Former | 852 (54.7) | 231 (59.1) | 204 (52.7) | 223 (57.2) | 194 (49.9) | |
| Never | 465 (29.9) | 117 (29.9) | 122 (31.5) | 116 (29.7) | 110 (28.3) | |
| LDL cholesterol, mg/dL, Mean (sd) | 121.9 (31.6) | 119.9 (30.9) | 122.2 (30.7) | 123.7 (32.0) | 121.7 (32.8) | 0.34 |
| HDL cholesterol, mg/dL, Mean (sd) | 43.4 (12.9) | 39.4 (10.4) | 42.0 (12.3) | 44.4 (13.3) | 47.8 (14.0) | <.001 |
| Triglycerides, mg/dL, Mean (sd) | 139.1 (80.7) | 167.1 (90.4) | 147.6 (86.6) | 133.3 (73.9) | 108.5 (56.0) | <.001 |
| Lipid lowering medications, N (%) | 183 (11.8) | 47 (12.0) | 57 (14.7) | 47 (12.0) | 32 (8.2) | .21 |
| Fasting glucose, mg/dL, Mean (sd) | 109.7 (31.2) | 118.2 (40.8) | 112.2 (34.7) | 106.1 (20.5) | 102.3 (21.4) | <.001 |
| Diabetes, N (%) | 220 (14.2) | 90 (23.1) | 58 (15.0) | 40 (10.3) | 32 (8.2) | <.001 |
| Hypertension, N (%) | 647 (41.6) | 186 (47.8) | 169 (43.7) | 153 (39.2) | 139 (35.8) | <.001 |
Abbreviations: IQR, interquartile range; sd, standard deviation. Cut points for testosterone: quartile 1 ≤288.4 ng/dL, quartile 2 = 288.5–377.6 ng/dL, quartile 3 = 377.7–480.1 ng/dL, quartile 4 ≥480.2 ng/dL.
IQR expressed as the 25th–75th percentile.
P from χ-square test, not a test for trend.
Plasma T and cIMT
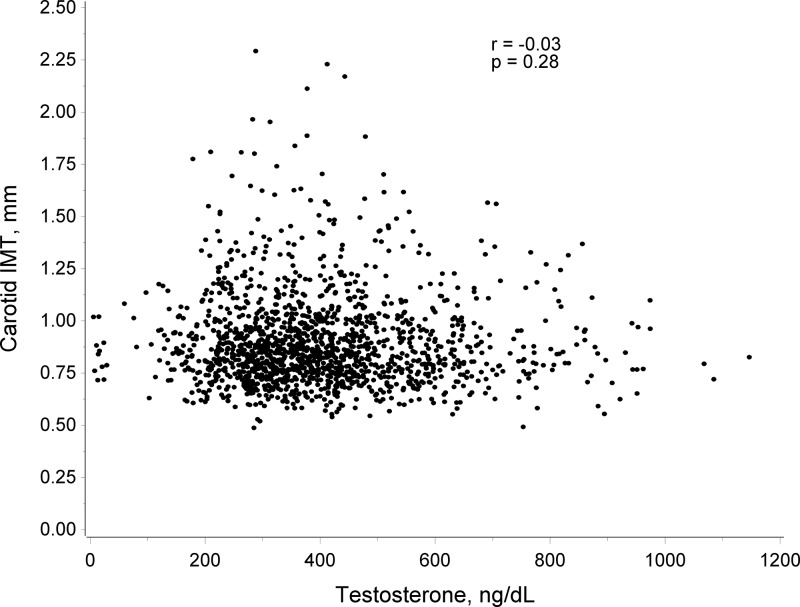
Participants had a mean (SD) cIMT of 0.90 mm (0.22). Mean (SD) cIMT values were 0.907 (0.231), 0.895 (0.22), 0.898 (0.22), 0.882 (0.21) mm across quartiles 1–4 of T, respectively (P for trend = 0.16) as demonstrated in Table 2 and Figure 1. After adjustment for traditional cardiovascular risk factors T quartiles were not associated cross-sectionally with cIMT (P for trend = 0.56). The results were similar and nonsignificant when data were stratified by age category to test for survival bias (results not shown). Logistic regression analysis to look at the association between testosterone quartile and mean cIMT >1.00 mm, was also not significant (unadjusted model P for trend = 0.84, adjusted model P for trend = 0.56).
Table 2.
Unadjusted and Adjusteda Associations of Testosterone Quartiles With Carotid IMT
| Testosterone Level | Carotid IMT (mm) Mean(sd) | Carotid IMT (mm) |
|
|---|---|---|---|
| Least Squares Means (95% CI) | P Value | ||
| Quartile 1 | 0.91 (0.22) | 0.912 (0.885–0.939) | .98 |
| Quartile 2 | 0.89 (0.22) | 0.913 (0.886–0.940) | .98 |
| Quartile 3 | 0.90 (0.22) | 0.930 (0.902–0.958) | .24 |
| Quartile 4 | 0.88 (0.21) | 0.912 (0.884–0.940) | |
Cut points for testosterone: quartile 1 ≤288.4 ng/dL, quartile 2 = 288.5–377.6 ng/dL, quartile 3 = 377.7–480.1 ng/dL, quartile 4 ≥480.2 ng/dL.
Multivariable models adjusted for age, race/center, BMI, waist circumference, cigarette smoking, diabetes, hypertension, and LDL and HDL cholesterol.
Figure 1. Scatterplot of carotid IMT by testosterone level.
Plasma T and clinical CV events
Hazard ratios for incidence of CHD and CHF by quartile of testosterone are presented in Table 3. Median follow-up was 12.8 years for incident CHD (287 events) and 13.1 year for incident HF (140 events). Following multivariable adjustment, there was no association of quartile of T with incident CHD or incident CHF as demonstrated in Table 3 and Figure 2a. The results were similar and nonsignificant when data stratified by age category to test for survival bias (results not shown).
Table 3.
Testosterone and Incidence of Clinical Cardiovascular Events
| Testosterone Level | Incident Coronary Heart Disease |
Incident Congestive Heart Failure |
||
|---|---|---|---|---|
| Hazard Ratioa (95% CI) | P Value | Hazard Ratioa (95% CI) | P Value | |
| Quartile 1 | 0.87 (0.60–1.26) | .46 | 0.77 (0.46–1.29) | .32 |
| Quartile 2 | 0.97 (0.69–1.38) | .88 | 0.72 (0.43–1.21) | .22 |
| Quartile 3 | 0.97 (0.69–1.36) | .86 | 0.87 (0.53–1.42) | .58 |
| Quartile 4 | 1.0 | 1.0 | ||
Cut points for testosterone: quartile 1 ≤288.4 ng/dL, quartile 2 = 288.5–377.6 ng/dL, quartile 3 = 377.7–480.1 ng/dL, quartile 4 ≥480.2 ng/dL
Multivariable model adjusted for age, race/center, BMI, waist circumference, cigarette smoking, diabetes, hypertension, and LDL and HDL cholesterol.
Figure 2. (a) Cumulative incidence CHD by quartile of testosterone (T). Cut points for testosterone: quartile 1 ≤288.4 ng/dL, quartile 2 = 288.5–377.6 ng/dL, quartile 3 = 377.7–480.1 ng/dL, quartile 4 ≥480.2 ng/dL. (b) Cumulative incidence all-cause mortality by quartile of testosterone (T). Cut points for testosterone: quartile 1 ≤288.4 ng/dL, quartile 2 = 288.5–377.6 ng/dL, quartile 3 = 377.7–480.1 ng/dL, quartile 4 ≥480.2 ng/dL. (c) Cumulative incidence CHD mortality by quartile of testosterone (T). Cut points for testosterone: quartile 1 ≤288.4 ng/dL, quartile 2 = 288.5–377.6 ng/dL, quartile 3 = 377.7–480.1 ng/dL, quartile 4 ≥480.2 ng/dL.
Plasma T and incident all-cause mortality and cardiac-mortality
Hazard ratios for incidence of all-cause mortality and cardiac-associated mortality by quartile of T are presented in Table 4. There were 347 total deaths and 29 deaths attributed to cardiac causes. Following multivariable adjustment there was no association of quartile of T with incident all-cause mortality or cardiac mortality as demonstrated in Table 4 and Figure 2, b and c. The results were similar and nonsignificant when data were stratified by age category to test for survival bias (results not shown).
Table 4.
Adjusteda Associations of Testosterone Quartiles With All-Cause Mortality and Coronary Heart Disease Mortality
| Testosterone Level | All-Cause Mortality (347 events) |
CHD Mortality (29 events) |
||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| Quartile 1 | 0.96 (0.70–1.34) | .82 | 1.36 (0.45–4.08) | .59 |
| Quartile 2 | 0.99 (0.72–1.35) | .93 | 1.26 (0.43–3.70) | .67 |
| Quartile 3 | 1.00 (0.74–1.35) | .99 | 0.57 (0.16–1.99) | .38 |
| Quartile 4 | 1.0 | 1.0 | ||
Cut points for testosterone: quartile 1 ≤288.4 ng/dL, quartile 2 = 288.5–377.6 ng/dL, quartile 3 = 377.7–480.1 ng/dL, quartile 4 ≥480.2 ng/dL.
Multivariable models adjusted for age, race/center, BMI, waist circumference, cigarette smoking, diabetes, hypertension, and LDL and HDL cholesterol.
Discussion
Our study is one of the first to look cross-sectionally and prospectively at the association between endogenous plasma T and measures of atherosclerosis in men. Endogenous T was associated with many classical cardiovascular risk factors, but was not associated cross-sectionally with mean cIMT or prospectively with incident clinical cardiac outcomes, all-cause or cardiac mortality.
These results reflect strict adjustment for covariates which may affect cardiovascular risk and may be independently associated with testosterone status. It is well known that T declines with age (2), and that age is a risk factor used in many CHD risk prediction models. It is possible that the age range in our population was too narrow to find a significant difference. Endogenous T was otherwise negatively associated with traditional cardiac risk factors. Prior studies suggest that low T may be more prevalent in those with insulin resistance and diabetes due to decreased sex hormone binding globulins, suppression of pituitary gonadotrophins, as well as through excess inflammatory cytokines which suppress endogenous T production (16). With obesity and increased abdominal adiposity there are increased levels of aromatase which convert T to estradiol and which can further suppress pituitary gonadotrophins (17). In addition, low T may also predict insulin resistance and diabetes through changes in fat and muscle mass, effects on glucose transport and reduction in antioxidant activity (16). The association with low HDL and hypertension has been more variable. A cross sectional analysis in men 45–70 years old showed T correlated positively with HDL and four of five components of metabolic syndrome (18), while another study of males with a mean age of 52 years found no association between serum T and hypertension or HDL (19).
Our analysis contradicts prior cross-sectional studies that found a negative association of T with cIMT. In a sample of 239 males aged 40–70 participating in the Turku Aging Male Study, cIMT correlated positively with age, BP, BMI, total cholesterol, and LDL and correlated inversely with HDL, similar to our results. However, both common carotid IMT and carotid bulb IMT correlated inversely with serum T (P < .05) (11). In a large French population based cohort (Three-Cities) of males over the age of 65 years, total and bioavailable T inversely correlated with mean cIMT, before and after adjustment for other cardiac risk factors (20). However, in a larger Norwegian population cohort of greater than 1000 men with a mean age of 66 years, no association was seen cross-sectionally between T and cIMT or prospectively with progression in cIMT between 1994 and 2001 (21). A recent study of ARIC participants from baseline visit 1 found that a combination of cIMT and the presence of atherosclerotic plaque had a greater predictive value for clinical cardiac events than when evaluated as individual factors (22). However, the addition of plaque presence to cIMT actually reclassified more participants to a lower risk group of future cardiac event rather than higher risk group, and the effect was more pronounced in women than men. Given cIMT measurements were obtained of the far wall using the average of six sites distinct from areas of plaque, and with high inter-reader reliability and precision, we feel our results are valid. The variability in cIMT may represent earlier vascular changes associated with atherosclerosis (23), and is therefore a useful marker in assessing the early stages of atherosclerosis.
Our results suggest no relationship between endogenous T and incident clinical cardiac events, all-cause mortality and cardiac associated mortality. As of late 2013 there have been at least 12 observational studies which have assessed the role of endogenous T in the incidence of CVD, incident cardiovascular events, and CVD-associated mortality (24), which suggest lower T is associated with greater mortality (4) and weakly associated with incident CHD, taking into account the age of the study population and heterogeneity in methods (25). However, recent studies suggest a nonlinear relationship. A case-cohort analysis using the French Three City multicenter prospective cohort study of males over the age of 65 showed a “J-shaped association” between total testosterone and risk of ischemic CHD, before and after adjustment for traditional cardiac risk factors (6). Another recent study suggested a U shaped association between endogenous total T and all-cause mortality (5). Our results challenge and contrast these prior studies by suggesting in a large population of men without CVD that neither low nor high endogenous T are detrimental to CHD risk or mortality.
In comparison to prior studies, our study population is slightly younger and the age range is narrower, which should lead to more robust results and less influence from age-related comorbidities. In addition, our follow up (∼13 years) is much greater than most prior studies, which should also add to the validity of our results. Despite various modeling approaches to our data we were not able to find any relationship between plasma T and incident cardiovascular outcomes or cardiac associated mortality.
One explanation for our results is that the relationship between T and clinical atherosclerosis is mediated by other factors related to cardiac risk. Most of these, however, were adjusted for in our analysis and no significant association was found before or after adjustment. It is possible that no direct relationship exists between T and incident CVD, and that endogenous T levels are just a marker of illness and comorbidities, or that both low testosterone and CVD may occur concurrently as part of the aging process. There are no studies comparing levels of T pre- and post-treatment of various comorbidities related to cardiac disease. However, it is well known that acute and chronic illness suppresses T levels. Therefore, the focus may need to be on treating other comorbid conditions rather than focusing on treating T levels.
The strengths of our analysis include the use of a large sample of community dwelling men, comprehensive surveillance of all participants with appropriate adjudication of cardiac events, assay of plasma T using LCMS, and longitudinal follow up over 13 years. While some were excluded from our analysis due to prevalent CHD and HF, few were excluded due to androgen therapy, suggesting our sample was generally healthy and testosterone naive. In our analysis we limited those samples to those drawn before 10:30 AM to reduce confounding from natural variability in T levels throughout the day. The comprehensive surveillance and adjudication performed in ARIC allowed for longitudinal follow up with a large number of events with high validity.
One limitation in our analysis is that we only used a single sample for T assay. While clinical guidelines suggest the use of two values from separate time points to diagnose hypogonadal status, sex hormone assays at a single point may be adequate and representative of gonadal status (26). Our assay was also based on plasma samples, which limited our ability to assess sex hormone binding globulins or free testosterone. However, studies suggest plasma and serum testosterone are generally equivalent in males. The small number of events (n = 29) for the outcome of cardiac-associated deaths may limit our power to find an association with this outcome. Given the close surveillance and adjudication of all events, this is most likely due to the exclusion of participants who had prevalent CHD at baseline.
In conclusion, our study shows that endogenous T does correlate with other factors associated with cardiac risk, but does not correlate with a surrogate marker of atherosclerosis or incidence of future clinical cardiac events or mortality. Thus, T may be considered a marker of good health with no direct causal association with CHD. As suggested by others, there may be an optimal window for T levels, above or below which are associated with poor outcomes (24). Rather than treating T to a specific level, it may be more prudent to assess comorbidities and other cardiovascular risk factors and treat those accordingly to reduce cardiovascular risk.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts No. HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C. Testosterone assays were performed with support from Straken Pharmaceuticals Limited. The funding source had no role in the planning, analysis or writing of this manuscript.
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts No. HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C. Testosterone assays were performed with support from Straken Pharmaceuticals Limited. The funding source had no role in the planning, analysis or writing of this manuscript.
Footnotes
- BMI
- body mass index
- BP
- blood pressure
- CHD
- coronary heart disease
- CI
- confidence interval
- cIMT
- carotid intima media thickness
- CVD
- cardiovascular disease
- HF
- heart failure
- MI
- myocardial infarction.
References
- 1. Murphy SL, Xu J, Kochanek KD. Deaths: Final data for 2010. National Vital Statistics Reports. 2013. Vol. 61, No. 4. [PubMed] [Google Scholar]
- 2. Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR, Baltimore Longitudinal Study of Aging. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore longitudinal study of aging. J Clin Endocrinol Metab. 2001;86(2):724–731. [DOI] [PubMed] [Google Scholar]
- 3. Gurney EP, Nachtigall MJ, Nachtigall LE, Naftolin F. The women's health initiative trial and related studies: 10 years later: A clinician's view. J Steroid Biochem Mol Biol. 2014;142:4–11. [DOI] [PubMed] [Google Scholar]
- 4. Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Clinical review: Endogenous testosterone and mortality in men: A systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96(10):3007–3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yeap BB, Alfonso H, Chubb SA, et al. . In older men an optimal plasma testosterone is associated with reduced all-cause mortality and higher dihydrotestosterone with reduced ischemic heart disease mortality, while estradiol levels do not predict mortality. J Clin Endocrinol Metab. 2014;99(1):E9–E18. [DOI] [PubMed] [Google Scholar]
- 6. Soisson V, Brailly-Tabard S, Helmer C, et al. . A J-shaped association between plasma testosterone and risk of ischemic arterial event in elderly men: The french 3C cohort study. Maturitas. 2013;75(3):282–288. [DOI] [PubMed] [Google Scholar]
- 7. Vigen R, O'Donnell CI, Barón AE, et al. . Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310(17):1829–1836. [DOI] [PubMed] [Google Scholar]
- 8. Finkle WD, Greenland S, Ridgeway GK, et al. . Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One. 2014;9(1):e85805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Traish AM, Guay AT, Morgentaler A. Death by testosterone? We think not! J Sex Med. 2014;11(3):624–629. [DOI] [PubMed] [Google Scholar]
- 10. Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: A systematic review and meta-analysis. Circulation. 2007;115(4):459–467. [DOI] [PubMed] [Google Scholar]
- 11. Mäkinen J, Järvisalo MJ, Pöllänen P, et al. . Increased carotid atherosclerosis in andropausal middle-aged men. J Am Coll Cardiol. 2005;45(10):1603–1608. [DOI] [PubMed] [Google Scholar]
- 12. Muller M, van den Beld AW, Bots ML, Grobbee DE, Lamberts SW, van der Schouw YT. Endogenous sex hormones and progression of carotid atherosclerosis in elderly men. Circulation. 2004;109(17):2074–2079. [DOI] [PubMed] [Google Scholar]
- 13. Fukui M, Kitagawa Y, Nakamura N, et al. . Association between serum testosterone concentration and carotid atherosclerosis in men with type 2 diabetes. Diabetes Care. 2003;26(6):1869–1873. [DOI] [PubMed] [Google Scholar]
- 14. Aversa A, Bruzziches R, Francomano D, et al. . Effects of testosterone undecanoate on cardiovascular risk factors and atherosclerosis in middle-aged men with late-onset hypogonadism and metabolic syndrome: Results from a 24-month, randomized, double-blind, placebo-controlled study. J Sex Med. 2010;7(10):3495–3503. [DOI] [PubMed] [Google Scholar]
- 15. High-resolution B-mode ultrasound reading methods in the Atherosclerosis Risk in Communities (ARIC) cohort. The ARIC Study Group. J Neuroimaging. 1991;1(4):168–172. [PubMed] [Google Scholar]
- 16. Kalyani RR, Dobs AS. Androgen deficiency, diabetes, and the metabolic syndrome in men. Curr Opin Endocrinol Diabetes Obes. 2007;14(3):226–234. [DOI] [PubMed] [Google Scholar]
- 17. Schneider G, Kirschner MA, Berkowitz R, Ertel NH. Increased estrogen production in obese men. J Clin Endocrinol Metab. 1979;48(4):633–638. [DOI] [PubMed] [Google Scholar]
- 18. Grosman H, Rosales M, Fabre B, et al. . Association between testosterone levels and the metabolic syndrome in adult men. Aging Male. 2014;17:161–165. [DOI] [PubMed] [Google Scholar]
- 19. Kaplan SA, Meehan AG, Shah A. The age related decrease in testosterone is significantly exacerbated in obese men with the metabolic syndrome. What are the implications for the relatively high incidence of erectile dysfunction observed in these men? J Urol. 2006;176(4 Pt 1):1524–1527; discussion 1527–1528. [DOI] [PubMed] [Google Scholar]
- 20. Soisson V, Brailly-Tabard S, Empana JP, et al. . Low plasma testosterone and elevated carotid intima-media thickness: Importance of low-grade inflammation in elderly men. Atherosclerosis. 2012;223(1):244–249. [DOI] [PubMed] [Google Scholar]
- 21. Vikan T, Schirmer H, Njølstad I, Svartberg J. Endogenous sex hormones and the prospective association with cardiovascular disease and mortality in men: the Tromsø Study. Eur J Endocrinol. 2009;161(3):435–442. [DOI] [PubMed] [Google Scholar]
- 22. Nambi V, Chambless L, Folsom AR, et al. . Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: The ARIC (atherosclerosis risk in communities) study. J Am Coll Cardiol. 2010;55(15):1600–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Spence JD. Carotid plaque measurement is superior to IMT invited editorial comment on: Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: A meta-analysis-yoichi inaba, M.D., jennifer A. chen M.D., steven R. bergmann M.D., ph.D. Atherosclerosis. 2012;220(1):34–35. [DOI] [PubMed] [Google Scholar]
- 24. Ruige JB, Ouwens DM, Kaufman JM. Beneficial and adverse effects of testosterone on the cardiovascular system in men. J Clin Endocrinol Metab. 2013;98(11):4300–4310. [DOI] [PubMed] [Google Scholar]
- 25. Ruige JB, Mahmoud AM, De Bacquer D, Kaufman JM. Endogenous testosterone and cardiovascular disease in healthy men: A meta-analysis. Heart. 2011;97(11):870–875. [DOI] [PubMed] [Google Scholar]
- 26. Vermeulen A, Verdonck G. Representativeness of a single point plasma testosterone level for the long term hormonal milieu in men. J Clin Endocrinol Metab. 1992;74(4):939–942 [DOI] [PubMed] [Google Scholar]