Abstract
Context:
Intermittent 3-month cyclic administration might optimize the anabolic potential of teriparatide (TPTD).
Objective:
To determine whether 3-month cyclical TPTD would produce a similar bone mineral density (BMD) response to daily therapy in treatment naive (Rx-naive) women and to confirm the results in alendronate (ALN)-treated (ALN-Rx) women over 24 months.
Design:
Subjects participated in a randomized open-label study for 2 years.
Setting:
Osteoporosis clinical research center.
Participants:
A total of 150 postmenopausal women with osteoporosis in two cohorts: 86 Rx-naive and 64 ALN-Rx.
Intervention:
Within cohorts, women were randomized to daily TPTD for 24 months or four 3-month TPTD cycles, each followed by 3 months off (12 mo total TPTD).
Main Outcomes:
BMD at 24 months.
Results:
In Rx-naive women, BMD increased in the lumbar spine (LS), total hip (TH), trochanter (Troch), and femoral neck (FN) in daily and cyclic groups (within groups, P < .0002, except cyclic FN, P = .13). Increases were 2-fold greater in daily vs cyclic groups (LS, 8.8 vs 4.8%; TH, 4.0 vs 2.1%; Troch, 5.6 vs 3.1%; and FN, 2.9 vs 1.2%; group differences, all P < .05). In daily vs cyclic groups, radius BMD declined (−4.2 vs −2.1%, respectively; both P < .01; group difference, P = .08) and total bone mineral increased modestly (1.4%, P = .18; vs 1.5%, P = .06; group difference, not significant). In ALN-Rx women, there were no group differences (daily vs cyclic: LS, 7.5 and 6.0%; TH, 3 and 2.5%; Troch, 3.7 and 3.3%; FN, 3 and 1.5%; within groups, P < .003; except cyclic FN, P = .2). In daily and cyclic groups, radius BMD decreased (−0.7% [not significant] and −1.4% [P < .05], respectively), and total bone mineral increased 2.3 and 3% (both P < .001).
Conclusion:
Cyclic TPTD over 2 years improves BMD similarly to daily treatment in women who remain on ALN, despite only 50% of the TPTD dose. However, there does not appear to be a BMD advantage to cyclic administration in treatment-naive women for up to 24 months.
PTH 1–34 (teriparatide; TPTD) stimulates bone formation and remodeling, with positive net bone balance resulting in increased bone mass, improved architecture, increased strength, and reduced risk of fracture (1–12). The biochemical response to TPTD is biphasic, with an initial brisk increase in markers of bone formation within days or weeks of initiating TPTD (2, 6, 10, 11). Bone formation marker levels usually peak within 1 year (varying for the specific marker measured) and begin to decline thereafter, despite continued treatment (12). Increases in markers of bone resorption are delayed until after 1 month, but also peak and decline during the second year of TPTD (2, 4, 10, 12). The increase in spine bone mineral density (BMD) after TPTD is also most rapid within the first 6 months, consistent with the biochemistry (2–4, 6, 10, 11). Furthermore, histomorphometric analyses of the iliac crest indicate that there is dramatic stimulation of bone formation and remodeling at 6 months (8, 13); however, after 18–36 months of TPTD, there is no evidence of ongoing stimulation of bone formation or remodeling, at least in cancellous bone (7, 14, 15). Thus, there appears to be tachyphylaxis associated with daily administration over this time.
The concept of administering TPTD cyclically was based on two hypotheses: first, that early direct stimulation of bone formation without prior resorption (modeling-based formation) (16) might be more important to ultimate BMD accrual than later activation of bone remodeling; and second, that repeated short cycles of TPTD might surmount the tachyphylaxis that develops after 6–15 months of daily therapy. A short cycle of TPTD could potentially dissociate the early modeling-based anabolic effect from the latter remodeling-based effect. To begin to test these hypotheses, we previously completed a trial examining the influence of 3-month TPTD cycles in women who had been treated with prior and ongoing alendronate (ALN) (5). Biochemical evidence of stimulation of bone formation was seen with each of three cycles over 15 months, and spine BMD increased similarly in the cyclic compared to the daily group, although only 60% of the total TPTD dose was administered.
In the current study, we determine whether the effects of cyclic compared with daily TPTD are similar in patients with osteoporosis who have not been pretreated with ALN. Furthermore, we evaluate the differences between daily and cyclic TPTD over 2 years in a newly recruited cohort of women on prior and ongoing ALN.
Subjects and Methods
This was a randomized, open-label study in postmenopausal women with osteoporosis recruited concurrently into two parallel cohorts: women on ALN (70 mg/wk) for at least 1 year (ALN-Rx; n = 64), and women with minimal or no prior osteoporosis therapy (Rx-naive; n = 86). Within each cohort, volunteers were randomized to daily TPTD or cyclic TPTD given in 3-month cycles for a total treatment period of 24 months. TPTD was generously supplied by Eli Lilly.
Patient population
This study was approved by the Helen Hayes Hospital Institutional Review Board, and all participants provided informed consent. Volunteers were recruited from our Osteoporosis Clinic and BMD Screening Program and by advertisement and public speaking at educational programs and support groups.
Inclusion criteria
The study enrolled postmenopausal women (>45 y old) with osteoporosis defined by T-score ≤ −2.5 at lumbar spine (LS), total hip (TH), or femoral neck (FN), or by T-score ≤ −2 at any of these sites with a history of one or more osteoporosis-related fracture(s) or prevalent vertebral compression documented by spine radiograph. In the ALN-Rx arm, women had to have been on ALN for at least 1 year and be willing to continue throughout the trial.
Exclusion criteria
Women enrolled in the Rx-naive cohort could not have been on any antiresorptive agent for at least 6 months before enrollment and could not have used a bisphosphonate for more than 3 months within the previous 2 years. Use of any iv bisphosphonate resulted in exclusion. (This study was fully recruited before the marketing of denosumab).
Additional exclusions included more than two lumbar compression fractures or severe degenerative changes with fewer than two evaluable vertebrae, history of renal stone within the previous 5 years or multiple renal stones, hypercalcemia, elevated serum PTH or bone-specific alkaline phosphatase, clinically significant uric acid elevation, recent or active cancer, or radiation therapy.
At screening, BMD was measured, and lateral radiographs of the thoracolumbar spine were obtained. Blood samples were collected for serum calcium, bone-specific alkaline phosphatase, uric acid, creatinine, 25-hydroxyvitamin D [25(OH)D], and PTH. Dietary calcium intake was assessed, and each woman was provided with supplements to bring total intake to 1200 mg/d and with vitamin D3 supplements ≥ 1000 IU/d to maintain 25(OH)D levels ≥ 25 ng/mL. In those with lower levels, serum 25(OH)D was corrected before randomization.
Protocol
Treatment assignment
Volunteers within each cohort (Rx-naive and ALN-Rx) were randomly assigned to cyclic vs daily TPTD. In the ALN-Rx group, women continued ALN (70 mg/wk) throughout the 24-month trial. Daily TPTD was given as 20 μg/d sc for 24 months. Cyclic TPTD was given in 3-month cycles (3 months on TPTD 20 μg/d and 3 months off TPTD) for 24 months (12 mo total cumulative TPTD dose).
Self-injection techniques were taught, and technique was approved. Volunteers presented to the clinical research center every 3 months for assessment of compliance, side effects, and lab and BMD measurement when indicated. Compliance was assessed by review of subject injection diaries and by weighing returned TPTD pens.
Biochemical bone turnover markers
Fasting morning blood samples were obtained at baseline and every 3 months and analyzed for osteocalcin (OC), propeptide of type I procollagen (PINP), and cross-linked C-telopeptide (CTX) (Elecsys; Hoffmann-La Roche). Ranges for intra- and interassay coefficients of variation, based on the analysis of control samples with high, medium, and low concentrations were: OC, 0.5–1.1 and 2.4–4%; PINP, 1.6–2.5 and 1.9–3%; and CTX, 1.0–1.6 and 2.9–4.2%.
Bone density
BMD of the LS, TH, trochanter (Troch), FN, and radius as well as total bone mineral (TBM; excluding the skull) were assessed by dual-energy x-ray absorptiometry at baseline and at 7.5, 15, and 24 months (GE/Lunar Vision). Coefficients of variation were: LS, 1.1%; TH, 1.5%; Troch, 1.5%; FN, 2.0%; and radius, 2.2%.
Clinical fractures
Clinical fractures excluded hands, feet, face, skull, and those associated with car accidents. Historical fractures were recorded at baseline. Fractures during the study were recorded as adverse events (AEs) or serious AEs and confirmed by review of x-ray reports.
Morphometric vertebral fractures
Prevalent and incident morphometric vertebral fractures were assessed by lateral thoracolumbar radiographs at baseline and 2 years. Optasia software (Optasia Medical) was used (by P.G.) to obtain six-point vertebral body contouring to calculate vertebral heights and assess the presence and degree of deformity. Fractures were reviewed and confirmed (by F.C.). All assessments were made blind to treatment assignment.
Safety assessments
Total serum calcium was assessed at every visit via standard automated chemistry. A preplanned algorithm for hypercalcemia (defined as ≥ 10.1 mg/dL, the upper limit for our laboratory) required stopping calcium supplements and repeating the measurement within 2 weeks. If hypercalcemia persisted, TPTD was to be withheld. BMD was assessed for ≥ 7.5% loss at LS or TH at any visit. If found, BMD was repeated, and if confirmed, the subject was to be withdrawn.
Statistical analysis
Statistical analyses were performed using SAS software, version 9.3 (SAS Institute, Inc). Descriptive characteristics for the study populations were compared between cyclic and daily groups, separately for the cohorts, using t tests for continuous variables and χ2 tests for categorical variables. Within-group BMD and bone turnover marker (BTM) changes were evaluated by paired t tests. A variable for slope of change in BMD was created for each subject for each skeletal site. General linear models were used to compare the slopes of BMD change between cyclic and daily groups within cohorts. Baseline BMD, BTMs, weight, body mass index (BMI), age, and years from menopause were evaluated as potential confounders.
Results
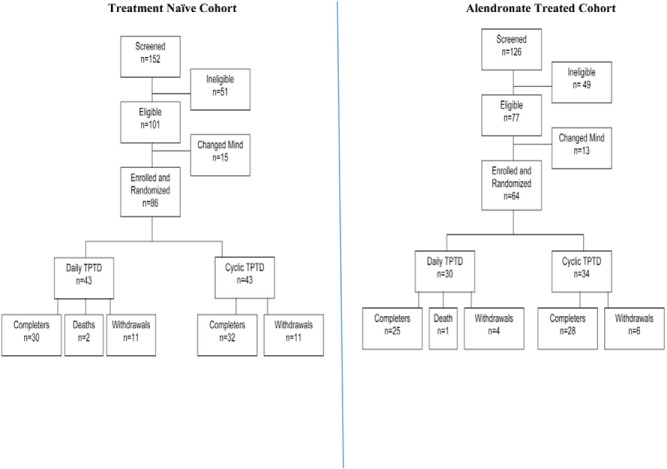
Figure 1 illustrates the derivation of the subjects and the number screened, enrolled, withdrawn, and completed. Reasons for discontinuation are also indicated. There were three deaths thought to be unrelated to study medication: one in the ALN-Rx group (septicemia), and two in the Rx-naive group (one due to cardiac arrest and one home death of unknown etiology). Overall, discontinuations were similar in cyclic and daily arms within cohorts. Seventy-two percent of the Rx-naive women and 83% of ALN-Rx women completed the study.
Figure 1. Overall study flow including subject selection and randomization, withdrawals, and completers for the Rx-naive and ALN-Rx cohorts.
Baseline characteristics (Table 1)
Table 1.
Baseline Characteristics of Rx-Naive and ALN-Rx Cohorts
| Rx-Naive |
Prior ALN-Rx |
|||
|---|---|---|---|---|
| Daily TPTD | Cyclic TPTD | Daily TPTD | Cyclic TPTD | |
| n | 43 | 43 | 30 | 34 |
| Age, y | 64.0 ± 8.8 | 62.8 ± 7.9 | 66.3 ± 10.2 | 68.4 ± 7.4 |
| Height, in | 62.6 ± 2.6 | 63.5 ± 2.8 | 63.3 ± 2.5 | 63.1 ± 2.4 |
| Weight, kg | 61.7 ± 13.2 | 65.1 ± 13.2 | 61.3 ± 9.0 | 61.6 ± 9.9 |
| BMI, kg/m2 | 24.4 ± 5.3 | 25.1 ± 5.0 | 23.6 ± 2.8 | 24.0 ± 4.1 |
| Time from menopause, y | 17.4 ± 11.6 | 14.3 ± 10.2 | 19.8 ± 13.0 | 18.7 ± 10.2 |
| Time on ALN, y | — | — | 6.0 ± 3.1 | 5.8 ± 2.8 |
| History of clinical fracture > age 50, n (%) | 14 (33) | 14 (33) | 13 (43) | 13 (38) |
| Morphometric vertebral fracture = 1, n (%) | 4 (10) | 6 (14) | 5 (17) | 9 (26) |
| Morphometric vertebral fracture > 1, n (%) | 4 (10) | 2 (5) | 7 (23) | 5 (15) |
| Spine BMD, g/cm2 | 0.804 ± 0.14 | 0.832 ± 0.08 | 0.849 ± 0.09 | 0.850 ± 0.13 |
| Spine T-score | −3.1 ± 1.1 | −2.9 ± 0.6 | −2.9 ± 0.7 | −2.7 ± 1.0 |
| TH BMD, g/cm2 | 0.734 ± 0.09 | 0.762 ± 0.11 | 0.759 ± 0.08 | 0.741 ± 0.10 |
| TH T-score | −2.2 ± 0.9 | −1.9 ± 0.86 | −2.0 ± 0.6 | −2.2 ± 0.8 |
| Serum OC, ng/mL | 23.9 ± 9.7 | 24.2 + 8.9 | 16.0 ± 5.8 | 16.0 ± 5.1 |
| Serum PINP, ng/mL | 49.7 ± 19.8 | 50.3 ± 20.2 | 23.4 ± 10.1 | 25.5 ± 9.8 |
| Serum CTX, pg/mL | 434 ± 206 | 459 ± 202 | 169 ± 100 | 177 ± 91 |
Data are expressed as mean ± SD, unless stated otherwise. —, not applicable in treatment naive cohort.
Within cohorts, daily and cyclic groups were well-matched for age, height, weight, BMI, BMD, prior fracture history, prevalent morphometric vertebral fractures, and serum BTM levels. Three women in the Rx-naive group had taken osteoporosis medication previously, as follows: raloxifene, stopped 6 months before the study; ALN for 3 years, but stopped 5 years before the study; and ALN for 9 months, but stopped 1.5 years before the study.
Overall characteristics in Rx-naive and ALN-Rx cohorts were similar, although ALN-Rx subjects were slightly older (mean age, 67.4 vs 63.4 y for Rx-naive; P = .005). The prevalence of prior clinical fracture was similar in ALN-Rx (41%) and Rx-naive (33%) cohorts. The average duration of ALN treatment (ALN-Rx cohort) was 5.9 years. Mean LS and TH T-scores were similar between cohorts. Mean BTM levels were significantly lower in ALN-Rx subjects.
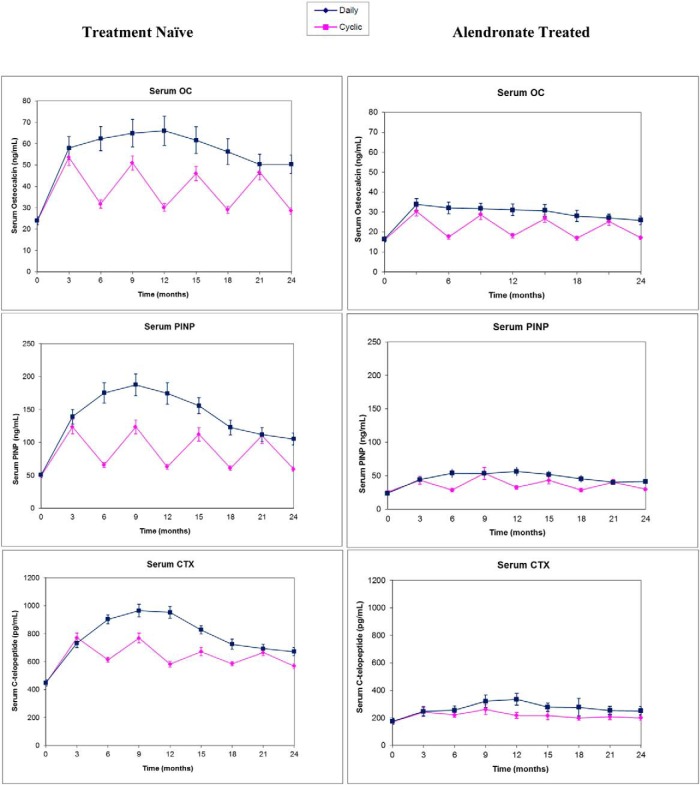
Bone turnover markers (Figure 2)
Figure 2. Serum biochemical BTM levels (OC, PINP, CTX) during TPTD treatment given daily or cyclically over 2 years in Rx-naive women (left column) and ALN-Rx women (right column).
Data are expressed as mean ± SEM.
Rx-naive cohort
Serum OC increased over 100% within 3 months of TPTD and, in the daily group, it plateaued 180% above baseline at 6–15 months and then declined. Mean OC was still 100% above baseline at 24 months (P < .0001). In the cyclic group, serum OC increased with each TPTD cycle and declined during each off-cycle. Nadir levels were still 30% above baseline, even at 24 months. Peak OC levels after the second and third TPTD cycles (9 and 15 mo) were lower than levels in the daily group at these times (group differences, P = .06 and 0.03, respectively). Serum OC in the cyclic group was also lower than the daily group at 24 months (P < .001).
Similar trends were seen with serum PINP. In the daily group, levels increased to 310% above baseline within 6 months, plateaued from 6–12 months, and then declined. Levels remained elevated by 130% at 24 months. With cyclic treatment, peak PINP levels were similar after each TPTD cycle, but again, peak levels after the second and third TPTD cycles at 9 and 15 months were lower than daily group levels at corresponding times (P < .01). Nadir levels after each off-cycle were still elevated 40% above baseline. At 24 months, serum PINP remained higher in the daily vs cyclic group (P < .001).
Mean CTX level increased by 90% at 3 months and continued to rise in the daily group, with a plateau at 6–12 months at 160% above baseline and a decline to 80% above baseline at 24 months. Similar to that seen for the formation markers, in the cyclic group, mean CTX increased with each TPTD cycle and declined during each off-cycle, with nadir levels remaining elevated by about 40%. The magnitude of the CTX changes was blunted during the third and fourth cycles. Mean CTX did not differ in the cyclic group compared with the daily group at 24 months (P = .09).
ALN-Rx cohort
Although the overall patterns were similar in ALN-Rx subjects, increments for all BTMs were dampened compared to those in Rx-naive subjects. In the daily group, mean OC level peaked at 3 months (110% above baseline) and declined thereafter, remaining 60% above baseline at 24 months. In the cyclic group, peak levels at 9, 15, and 21 months were similar to levels in the daily group at the same times (no group differences). In the cyclic group, with each off-cycle, mean OC declined but remained approximately 30% above baseline. There was a small but significant difference between mean OC in the cyclic and daily groups at 24 months (P < .005).
In the daily group, serum PINP peaked a bit later than did serum OC (12 months, 150% elevated) and remained 90% elevated above baseline at 24 months. In the cyclic group, serum PINP followed the same pattern as OC, with significant elevations during each on-cycle, and peaks similar to daily group peaks at corresponding times (9, 15, and 21 mo). Serum PINP levels declined with each off-cycle but remained approximately 25% above baseline at the nadir. At 24 months, mean PINP in the daily group was slightly greater than in the cyclic group (P < .05).
In the daily group, mean CTX increased modestly within 3 months of TPTD (50% above baseline), with a peak at 12 months (150% over baseline), and elevation continued at 24 months (90% above baseline; P < 0. 02). In the cyclic group, cyclicity was greatly blunted during the on/off cycles in the first year, and there was almost no apparent change during the second year. Mean CTX level was not significantly above baseline in the cyclic group at 24 months (200 pg/mL vs baseline 177 pg/mL; P = .064).
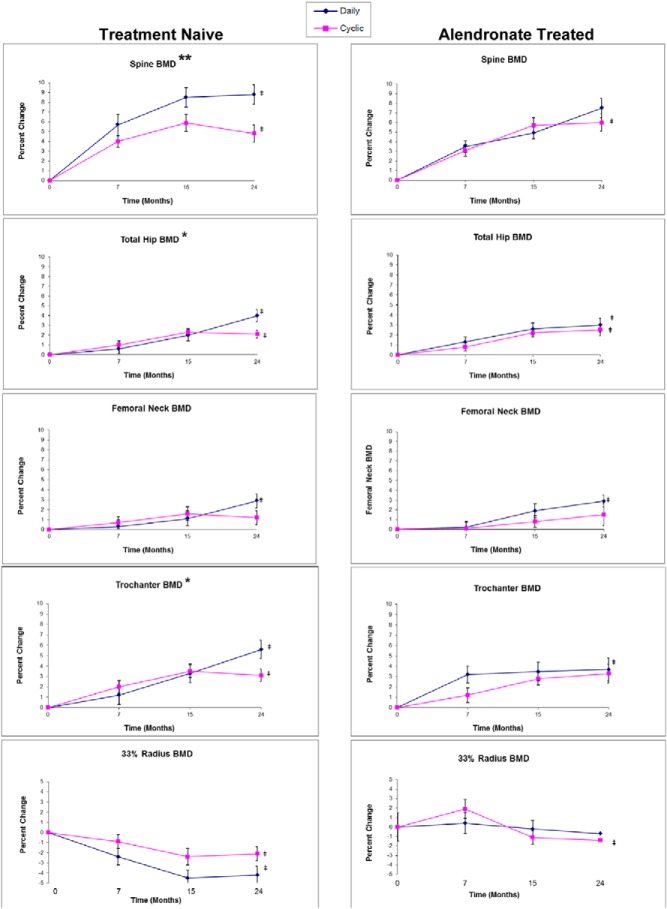
Bone density (Figure 3)
Figure 3. Bone density (by dual-energy x-ray absorptiometry [DXA]) percentage changes during TPTD treatment given daily or cyclically over 2 years in Rx-naive women (left column) and ALN-Rx women (right column) for different skeletal sites (from top to bottom: LS, TH, FN, Troch, radius).
*, P < .05; **, P < .005, difference between percentage BMD change between cyclic and daily groups; ‡, P < .005, difference from baseline within group.
Rx-naive cohort
In the Rx-naive cohort, BMD increased in LS, TH, Troch, and FN in both daily and cyclic groups (increments within groups, all P < .0002 vs baseline, except cyclic FN, P = .13), but increases were about 2-fold greater in the daily group (for LS, 8.8% daily and 4.8% cyclic; group difference, P = .004). In the hip, changes were similar between daily and cyclic groups up to 15 months, but thereafter, the daily group increased to reach a cumulative 4% TH gain, 2.9% FN gain, and 5.6% Troch gain (all P < .001 vs baseline). In contrast, there were no further increments in hip BMD in the cyclic group during the 15- to 24-month time period, and mean final gains were: TH, 2.1% (P < .001); FN, 1.2% (P = .13); and Troch, 3.1% (P < .001). Group differences in hip BMD accrual were significant at TH and Troch (both P < .05), but not at FN (P = .07).
Differences between BMD increments in cyclic and daily groups for LS, TH, and Troch remained after controlling for age, years from menopause, BMI, baseline BMD, and baseline BTMs. Radius BMD declined more in the daily group over the first 15 months, with no subsequent change in either group from 15–24 months (4.2% daily vs 2.1% cyclic; both P < .01 vs respective baselines; group difference, P = .08).
TBM increased 1.5% in the cyclic group (P = .06) and 1.4% in the daily group (P = .18) (group difference, not significant).
ALN-Rx cohort
In ALN-Rx subjects, mean increments at 24 months were similar between daily and cyclic groups: LS, 7.5 and 6.0%; TH, 3.0 and 2.5%; FN, 2.9 and 1.5%; and Troch, 3.7 and 3.3%, respectively (all P < .003 vs baseline, except cyclic FN, P = .2). Radius BMD declined 0.7% (not significant) in the daily group and 1.4% in the cyclic group (P < .05) at 24 months. TBM increased 2.3% in the daily group and 3.0% in the cyclic group (P < .001). There were no group differences for any skeletal site in the ALN-Rx cohort. No group differences were seen after controlling for age, years from menopause, BMI, baseline BMD, and baseline BTMs.
Observations across cohorts
Mean BMD increments in the Rx-naive daily group were similar to those in the ALN-Rx daily group in the LS (8.8 vs 7.5%), TH (4 vs 3%), Troch (5.6 vs 3.7%), and FN (2.9 vs 2.9%). TBM changes were also similar (1.4 vs 2.3%). Radius BMD declined more in the Rx-naive daily group (−4.2%) vs the ALN-Rx daily group (−0.7%; P < .005).
Fractures
Clinical fractures (pelvis, wrist, patella, rib, ankle, and ilium) occurred in eight of 150 subjects: two in Rx-naive daily, one in Rx-naive cyclic, three in ALN-Rx daily, and two in ALN-Rx cyclic. Incident morphometric vertebral fractures occurred in four subjects (one from each of the four groups).
Safety
Thirty-four serious AEs occurred in 24 subjects, were balanced across groups and cohorts, and appeared unrelated to TPTD. There was no hypercalcemia and/or excessive bone loss confirmed on repeat BMD.
Discussion
Over 24 months, in both Rx-naive and ALN-Rx women, both daily and cyclic TPTD administration increased BMD in the LS, TH, FN, Troch, and TBM but decreased radius BMD. In Rx-naive women given cyclic TPTD, BMD gains are consistent with the lower (50%) cumulative dose of medication. In these women, there may be no skeletal advantage to administering TPTD cyclically. In contrast, in the setting of ongoing ALN treatment, TPTD can be used cyclically over 24 months with similar BMD gains, despite the lower total dose of TPTD. This latter finding is consistent with our previous observations over a shorter time period (5).
It is important to consider why effects of cyclic TPTD differ in women on continued ALN compared with Rx-naive women. In Rx-naive women during the off-TPTD interval, it is possible that bone resorption exceeds formation, which could mitigate net BMD accrual during off-periods. This inference may be supported by the BTM observations. In Rx-naive women, the peak bone formation marker responses in cyclic-treated patients almost never achieve the same levels seen in daily-treated patients, whereas in ALN-Rx women, peak bone formation marker levels with each TPTD cycle were the same as in the daily group at corresponding times. Another possible explanation for the greater BMD accrual with cyclic treatment in ALN-Rx women is the time allotted for secondary mineralization during the off-cycles. This is likely to be more efficient in ALN-Rx than Rx-naive women because the lifespan of bone packets and duration of secondary mineralization is prolonged in the former group. Differences in cellular activity and matrix mineralization density might be determined by upcoming analyses of our histomorphometric and mineralization density data from biopsies obtained at various times during the study.
Hip BMD changes in the cyclic group for up to 15 months in Rx-naive women were very similar to those on daily therapy (despite using only 60% of the cumulative TPTD dose), but thereafter BMD continued to increase only in the daily group. We did not perform BMD measurements frequently enough to determine whether there were cyclic BMD changes that correspond to cyclic BTM increments (such as BMD at 21 mo). The hip BMD results are consistent, however, with some previous data, indicating that the rate of rise at this site might accelerate during the latter 6 months of a 2-year treatment course (12). A remaining question, also, is whether cyclical TPTD administration over 4 years (utilizing the same cumulative dose over twice the time) will provide a greater BMD effect compared with 2 years of daily therapy in Rx-naive women as well as ALN-Rx women in both the hip and spine.
It is well known that TPTD lowers radius BMD, perhaps due to increased cortical porosity. Despite this, nonvertebral fracture risk is reduced, and the number of wrist fractures is lower in TPTD-treated vs placebo-treated patients (1). In the present study, BMD decline in the radius is reduced by ongoing treatment with ALN; however, whether this could confer a superior benefit to risk of nonvertebral or wrist fracture is unknown.
Our BMD findings in the daily groups of the two cohorts are in fact quite similar. In contrast, in several previous studies comparing cohorts of Rx-naive and bisphosphonate-treated women, BMD accrual is reduced by about 30% in the latter group (3, 17). The difference is likely due to continuation of ALN in this trial compared with discontinuation in most other trials. We have shown this difference directly in a study where women on ALN were randomized to switch to TPTD or add TPTD to ongoing ALN (6, 18). Our data here are consistent with the previous findings when TPTD was added to ongoing ALN. Another novel cyclic regimen utilized two cycles, beginning with 3 months of PTH and followed by 9 months of ibandronate (19). The findings indicated that short courses of PTH in the setting of an intercurrent bisphosphonate could produce a BMD benefit greater than expected for the total cumulative PTH dose (19).
Limitations of this study include its insufficient size to analyze fractures. Furthermore, it is possible that our study was underpowered to find differences in BMD gain between cyclic and daily groups of small magnitude in the ALN-Rx cohort. However, our prestudy calculations suggested that with 25 completers per group, we could determine a 2.5% difference in spine BMD increment between cyclic and daily groups. Although treatment assignment was known to the investigative teams, all data review (BMD, radiographs) were performed in a blinded fashion. Because there were two separate cohorts identified for this study, limited comparisons across cohorts may be confounded by cohort differences. However, the main outcomes here, comparing daily vs cyclic administration of TPTD within each cohort, were based on randomized group assignments.
In conclusion, we are still learning how to optimize anabolic and antiresorptive therapies in sequence and/or in combination to achieve the best outcomes for patients with severe osteoporosis. The cyclic approach is valid for those on ongoing oral ALN and might apply to those on other bisphosphonates (19). However, there does not appear to be a BMD advantage (superior BMD gain for total cumulative TPTD dose) to cyclic administration in women on no antiresorptive therapy for at least up to 24 months.
Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number RO1 AR059204. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Teriparatide medication was supplied by Eli Lilly.
Disclosure Summary: F.C. received consulting/advisory board fees from Eli Lilly, Novartis, Merck, Amgen, Zosano, GSK, Unigene, Enteris, and Radius; lecture fees from Eli Lilly, Novartis, and Amgen; and grant support from Eli Lilly, Novartis, Amgen, and Merck. D.D. received consulting/advisory board fees from Eli Lilly, Merck, Amgen, and Radius; lecture fees from Eli Lilly and Amgen; and grant support from Eli Lilly. R.L. received consulting/advisory board/speaking fees from Eli Lilly and Amgen and grant support from Eli Lilly and Amgen. J.W.N., M.Z., P.G., and S.N. have no conflicts to report.
Funding Statement
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number RO1 AR059204. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
- AE
- adverse event
- ALN
- alendronate
- ALN-Rx
- ALN-treated
- BMD
- bone mineral density
- BMI
- body mass index
- BTM
- bone turnover marker
- CTX
- cross-linked C-telopeptide
- FN
- femoral neck
- LS
- lumbar spine
- OC
- osteocalcin
- 25(OH)D
- 25-hydroxyvitamin D
- PINP
- propeptide of type I procollagen
- Rx-naive
- minimal or no prior osteoporosis treatment
- TBM
- total bone mineral
- TH
- total hip
- TPTD
- teriparatide
- Troch
- trochanter.
References
- 1. Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344(19):1434–1441. [DOI] [PubMed] [Google Scholar]
- 2. McClung MR, San Martin J, Miller PD, et al. Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass. Arch Intern Med. 2005;165(15):1762–1768. [DOI] [PubMed] [Google Scholar]
- 3. Obermayer-Pietsch BM, Marin F, McCloskey EV, et al. Effects of two years of daily teriparatide treatment on BMD in postmenopausal women with severe osteoporosis with and without prior antiresorptive treatment. J Bone Miner Res. 2008;23(10):1591–1600. [DOI] [PubMed] [Google Scholar]
- 4. Cosman F, Nieves J, Woelfert L, et al. Parathyroid hormone added to established hormone therapy: effects on vertebral fracture and maintenance of bone mass after parathyroid hormone withdrawal. J Bone Miner Res. 2001;16(5):925–931. [DOI] [PubMed] [Google Scholar]
- 5. Cosman F, Nieves J, Zion M, Woelfert L, Luckey M, Lindsay R. Daily and cyclic parathyroid hormone in women receiving alendronate. N Engl J Med. 2005;353(6):566–575. [DOI] [PubMed] [Google Scholar]
- 6. Cosman F, Wermers RA, Recknor C, et al. Effects of teriparatide in postmenopausal women with osteoporosis on prior alendronate or raloxifene: differences between stopping and continuing the antiresorptive agent. J Clin Endocrinol Metab. 2009;94(10):3772–3780. [DOI] [PubMed] [Google Scholar]
- 7. Dempster DW, Cosman F, Kurland ES, et al. Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis: a paired biopsy study. J Bone Miner Res. 2001;16(10):1846–1853. [DOI] [PubMed] [Google Scholar]
- 8. Arlot M, Meunier PJ, Boivin G, et al. Differential effects of teriparatide and alendronate on bone remodeling in postmenopausal women assessed by histomorphometric parameters. J Bone Miner Res. 2005;20(7):1244–1253. [DOI] [PubMed] [Google Scholar]
- 9. Keaveny TM, Donley DW, Hoffmann PF, Mitlak BH, Glass EV, San Martin JA. Effects of teriparatide and alendronate on vertebral strength as assessed by finite element modeling of QCT scans in women with osteoporosis. J Bone Miner Res. 2007;22(1):149–157. [DOI] [PubMed] [Google Scholar]
- 10. Lindsay R, Nieves J, Formica C, et al. Randomised controlled study of effect of parathyroid hormone on vertebral-bone mass and fracture incidence among postmenopausal women on oestrogen with osteoporosis. Lancet. 1997;350(9077):550–555. [DOI] [PubMed] [Google Scholar]
- 11. Tsai JN, Uihlein AV, Lee H, et al. Teriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: the DATA study randomised trial. Lancet. 2013;382(9886):50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leder BZ, Tsai JN, Uihlein AV, et al. Two years of denosumab and teriparatide administration in postmenopausal women with osteoporosis (The DATA Extension Study): a randomized controlled trial. J Clin Endocrinol Metab. 2014;99(5):1694–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dempster DW, Zhou H, Recker RR, et al. Skeletal histomorphometry in subjects on teriparatide or zoledronic acid therapy (SHOTZ) study: a randomized controlled trial. J Clin Endocrinol Metab. 2012;97(8):2799–2808. [DOI] [PubMed] [Google Scholar]
- 14. Jiang Y, Zhao JJ, Mitlak BH, Wang O, Genant HK, Eriksen EF. Recombinant human parathyroid hormone (1–34) [teriparatide] improves both cortical and cancellous bone structure. J Bone Miner Res. 2003;18(11):1932–1941. [DOI] [PubMed] [Google Scholar]
- 15. Bogado C, Zanchetta JR, Zhou H, et al. Temporal effects of teriparatide on bone microarchitecture assessed by high resolution peripheral quantitative computerized tomography and paired bone biopsies in postmenopausal women with osteoporosis. In: Proceedings from the American Society for Bone and Mineral Research; September 11–15, 2009; Denver, CO, Abstract 1105. [Google Scholar]
- 16. Lindsay R, Cosman F, Zhou H, et al. A novel tetracycline labeling schedule for longitudinal evaluation of the short-term effects of anabolic therapy with a single iliac crest bone biopsy: early actions of teriparatide. J Bone Miner Res. 2006;21(3):366–373. [DOI] [PubMed] [Google Scholar]
- 17. Boonen S, Marin F, Obermayer-Pietsch B, et al. Effects of previous antiresorptive therapy on the bone mineral density response to two years of teriparatide treatment in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 2008;93(3):852–860. [DOI] [PubMed] [Google Scholar]
- 18. Cosman F, Keaveny TM, Kopperdahl D, et al. Hip and spine strength effects of adding versus switching to teriparatide in postmenopausal women with osteoporosis treated with prior alendronate or raloxifene. J Bone Miner Res. 2013;28(6):1328–1336. [DOI] [PubMed] [Google Scholar]
- 19. Schafer AL, Sellmeyer DE, Palermo L, et al. Six months of parathyroid hormone (1–84) administered concurrently versus sequentially with monthly ibandronate over two years: the PTH and ibandronate combination study (PICS) randomized trial. J Clin Endocrinol Metab. 2012;97(10):3522–3529. [DOI] [PMC free article] [PubMed] [Google Scholar]