Abstract
Context:
Resistance to thyroid hormone (RTH) β is due to mutations in the β-isoform of the thyroid hormone receptor (TR). TSH-secreting adenomas (TSHomas) are presumed to represent clonal expansion and have been reported to contain TRβ gene mutations. Mice with a knock-in mutation in the TRβ gene spontaneously develop TSHomas, although as yet no patient has been reported to have both a TSHoma and RTHβ.
Objective:
We investigated a 12-year-old girl with elevated serum T4 concentration, inappropriately high TSH levels, and a pituitary adenoma.
Design and Intervention:
Clinical, biochemical, and radiological assessments were performed at baseline and after a transsphenoidal pituitary adenomectomy.
Results:
The patient's laboratory results included: TSH, 21.12 mIU/L (0.35–4.94 mIU/L); free T3, 14.25 pmol/L (2.63–5.7 pmol/L); free T4, 28.79 pmol/L (9.01–19.05 pmol/L); serum glycoprotein hormone alpha-subunit (α-GSU), 0.32 ng/ml (0.22–0.39 ng/ml); and α-GSU/TSH, 0.15. Thyroid radioiodine uptake was increased by 94.4% at 24 hours. A T3 suppression test showed incomplete suppression of the serum TSH concentration and blunted response of the peripheral thyroid hormone markers. The sequence of TRβ exons confirmed a P453T mutation in the TRβ gene. Pituitary magnetic resonance imaging revealed a microadenoma in the left side of the pituitary. The patient underwent transsphenoidal pituitary adenomectomy. Histologically, the tumor stained positively for TSH-β, human Chorionic Gonadotropin alpha (HCG-α), GH, prolactin, and ACTH. After removal of the tumor, the patient's thyroid function improved significantly, and she experienced the onset of menarche and an increase in linear growth as well.
Conclusions:
This patient with RTHβ had a TSHoma consistent with previous findings linking somatic TRβ mutations to TSHomas.
Inappropriate secretion of TSH, in the presence of elevated serum T4 concentration, is due to either a TSH-secreting adenoma (TSHoma) of the pituitary or resistance to thyroid hormone (RTH) β. Both of these conditions are characterized by high levels of free T4 (FT4) and free T3 (FT3) in the presence of nonsuppressed TSH concentrations (1–3). RTHβ is due to the mutations in the β-isoform of the thyroid hormone receptor (TR) (2, 3). TSHomas are thought to represent clonal expansion of an abnormal cell. In this situation, TSH secretion is autonomous and refractory to the negative feedback of thyroid hormone (1). The molecular mechanism leading to a TSHoma is still unknown. It is speculated that down-regulation of TRs may be a mechanism for the defective negative regulation of TSH by thyroid hormone (1). It was recently demonstrated that knock-in mutant mice, a mutant TRβ (TRβPV/PV mouse), spontaneously develop TSHomas, suggesting that the unliganded TRβ may contribute to pituitary tumorigenesis (4). TSHomas have previously been shown to contain RTH-related TRβ mutations (5) as well as a deletion in the TRβ2 alternative mRNA splice product (6). However, no patient has been reported to have both a TSHoma and RTHβ.
Case Report
In June 2011, a 12-year-old Chinese girl was admitted to the local hospital for the evaluation of a goiter, sinus tachycardia, and tremors. Laboratory investigation revealed an elevated serum FT3 and FT4 and low TSH. She was diagnosed as hyperthyroid and treated with an antithyroid drug (Thyrozol, 15 mg/d orally). Four months later, her thyroid function was re-examined and showed elevated serum FT3, FT4, and TSH. Antithyroid drug treatment was continued.
One and a half years later, the patient was admitted to our hospital after Thyrozol therapy had been discontinued for at least 1 month. On admission, her height was 147 cm, and her weight was 41 kg. Her pulse rate was between 90 and 110 beats/min. Blood pressure ranged from 110/70 to 120/80 mm Hg. Basal metabolic rate (BMR) was about 25% (normal range, −15% to +5%). Her thyroid gland was moderately enlarged and firm, without nodules. There was no vascular bruit heard or thrill on palpation. There were no signs of galactorrhea, acromegaly, or ophthalmopathy. Laboratory investigation revealed elevated serum FT3 and FT4 levels and inappropriate TSH secretion (Supplemental Table 1). Thyroglobulin antibodies and thyroperoxidase antibodies were not detected. Thyroid 123I uptake was 52.5% at 2 hours and 94.4% at 24 hours (normal range, 10–32% at 2 h; 25–62% at 24 h). Her thyroid ultrasound showed diffuse thyroid enlargement (Table 1).
Table 1.
Follow-up of the Thyroid Parameters Before and After Transsphenoidal Pituitary Adenomectomy
| Item | Preoperative | Postoperative |
Reference Range | |||
|---|---|---|---|---|---|---|
| 4 mo Later | 6 mo Later | 10 mo Later | 14 mo Later | |||
| FT3, pmol/L | 14.25 | 13.92 | 10.60 | 8.32 | 9.05 | 2.63–5.7 |
| FT4, pmol/L | 28.79 | 27.16 | 27.66 | 25.22 | 30.27 | 9.01–19.05 |
| TSH, mIU/L | 21.11 | 17.53 | 5.5 | 2.60 | 1.54 | 0.35–4.94 |
| BMR | 25% | 9% | 19% | −15% to + 5% | ||
| Pulse rate, beats/min | 96 | 80 | 90 | 60–100 | ||
| Thyroid ultrasound (width*depth*length), mm | ||||||
| Left lobe | 39*24*55 | 35*26*55 | 37*27*55 | 33*26*55 | ||
| Right lobe | 36*27*55 | 36*27*55 | 34*26*55 | 37*28*55 | ||
| Isthmus | 12.9 | 11.2 | 8.9 | 9.1 | ||
Chemiluminescent microparticle immunoassay (Architect System; Abbott Ireland Diagnostics Division, Co) was used for analyzing serum FT4, FT3, and TSH concentrations.
Genomic DNA was isolated from peripheral blood using a DNA Kit (QIAGEN) and was amplified by PCR. The sequence of exon 1–10 of TRβ was performed, and a single nucleotide mutation (cytosine → adenosine) at codon 453 was identified in the peripheral blood in the index patient, but not in her parents or sister (Supplemental Figure 1).
Her pituitary magnetic resonance imaging revealed a 6 mm × 5 mm adenoma in the left lateral lobe, which did not enhance after gadolinium injection (Supplemental Figure 2). The considerations for the adenoma were a TSHoma or an incidental microadenoma. After treatment options were discussed with the patient's parents, they agreed to an exploratory transsphenoidal pituitary adenomectomy. An expert neurosurgeon performed this operation (surgery video for this patient is provided in Supplemental Data) and reported that it was an adenoma with an intact capsule and softer density than the surrounding tissues. Considering the patient's age and the risk of hypopituitarism, the surgeon removed the tumor but did not disrupt the surrounding tissues. We analyzed the distribution of pituitary hormone cell type by immunocytochemistry: 80–90% of the cells in the adenoma were immunoreactive with anti-β-TSH antibodies and anti-α-human Chorionic Gonadotropin (HCG) antibodies (staining intensity, + ∼ ++). Forty to 50% of the cells were GH-positive or prolactin-positive (staining intensity, ++ ∼ +++), but only a scattered distribution (10–15%) of ACTH-positive cells was observed in the adenomas (staining intensity, + ∼ ++). LH and FSH were negative (Figure 1). Some adenomatous cells were positive with two or more pituitary hormones, but others were only positive for one pituitary hormone.
Figure 1. Immunohistochemical staining of pituitary tumor.
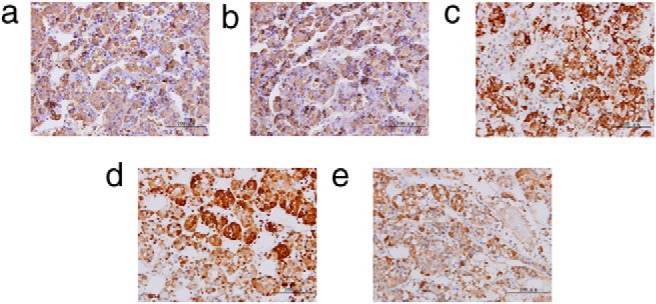
a, TSH-b; b, HCG-a; c, PRL; d, GH; e, ACTH. The pituitary tumor specimen was embedded in paraffin, and sections were cut at a thickness of 5 μm. After inactivation of endogenous peroxidase in PBS containing 3% H2O2 for 10 minutes, the slides were rinsed three times in PBS-Tween. Proteinase K treatment (1:50, 9 min; Dako) was used as antigen retrieval to detect the expression of TSH-β and HCG-α immunoreactivity. Samples were blocked in 5% normal goat serum for 20 minutes at room temperature. The sections were incubated with the primary antibody at 4°C overnight and then incubated with the second antibody (biotinylated antimouse IgG or antirabbit IgG; Maixin-Bio) for 10 minutes at room temperature. After washing out the secondary antibody, the sections were subjected to an streptavidin-peroxidase reaction using a SP Kit (Maixin-Bio). Primary antibodies: mouse monoclonal to TSH-β (catalog no. MA5–12159; Thermo Fisher Scientific), mouse monoclonal to GH (catalog no. RAB-0084; Maixin-Bio), mouse monoclonal to prolactin (catalog no. MAB-0148; Maixin-Bio), mouse monoclonal to LH (catalog no. MAB-0114; Maixin-Bio), mouse monoclonal to FSH (catalog no. MAB-0204; Maixin-Bio), mouse monoclonal to ACTH (catalog no. sc-57021; Santa Cruz Biotechnology), and rabbit polyclonal to human Chorionic Gonadotropin alpha (HCG-α) (catalog no. 25014–1-AP; Proteintech Group Inc).
The patient was followed for 14 months after surgery (Table 1). Within 3 months after the removal of her pituitary adenoma, menarche occurred. Within 4 months after the surgery (the first time of follow-up), her height had increased 5 cm. We analyzed her GH and LH levels at the 4-month follow-up time point and found that her GH increased from 0.47 to 13.70 mIU/L, with LH increasing from 1.35 to 3.46 mIU/mL. The L-T3 inhibition test was performed at the 4-month follow-up time point by a protocol that has been described previously (2). A T3 suppression test showed incomplete suppression of the serum TSH concentration and blunted response of the peripheral thyroid hormone markers, consistent with RTHβ (Supplemental Figure 3).
Discussion
We report the case of a girl with goiter, tachycardia, and elevated serum levels of FT4, FT3, and TSH. The sequence of exon 10 of TRβ confirmed P453T mutation in the TRβ gene. Interestingly, the patient also had a pituitary microadenoma demonstrated by magnetic resonance imaging, which raised the question of whether she had RTHβ and a TSH-secreting microadenoma or RTHβ and pituitary incidental microadenoma. Previous studies have identified TRβ mutations in TSHomas (5, 6). The common mechanistic link is disruption of T3-mediated TSH gene suppression with dominant-negative TR mutations. Such TR mutations, however, have been found in a minority of TSHomas.
TSHomas with positive immunoreactivity for multiple pituitary hormones not necessarily correlated with hypersecretion of the hormone have been reported (7, 8). Although we recognize that the staining for multiple hormones raises questions about the nature of the lesion, we believe the adenoma is distinct on imaging and in the surgery video.
The most significant evidence that this patient with RTHβ had a TSHoma is the 14-month follow-up results after the transsphenoidal pituitary adenomectomy. Her serum FT3 and TSH levels gradually decreased, but her FT4 level was unaltered during the 14-month period, suggesting that the TSH level was selectively reduced after surgery. Her thyroid gland decreased in size postoperatively without any medications, and the serum TSH fell into the normal reference range but was still “inappropriately normal” for the elevated serum FT3 and FT4 levels. Her BMR and pulse rate returned to the normal range. Previous studies have shown that the mean T4 level was higher and T3 level was lower in patients with RTHβ compared to those with TSHomas. The ratio of T3/T4 is significantly higher in patients with TSHomas than that in patients with RTHβ (2). We speculate that the elevated T4 is due to RTH, and high T3 relates to TSHomas, consistent with the progressive decrease of both TSH and FT3 levels during the 14-month period after surgery.
Watanabe et al (9) previously reported a young Japanese girl with suspected TSHoma and RTHβ, but the TRβ gene sequence was not reported. Our patient had a higher TSH than those in a report of eight unrelated families with RTH and the P453T mutation (10). Her TSH returned to the normal range, similar to these other RTH patients, after the surgery. In a study of 20 TSHomas, the microadenomas did not have stromal fibrosis and calcification (7). We speculate that the soft density of our patient's pituitary adenoma was the result of its having been diagnosed at an early stage.
In conclusion, our patient had a TSHoma and RTHβ. This case, along with previous reports and animals models, suggests that RTHβ may predispose to the development of TSH-secreting pituitary adenomas.
Acknowledgments
This work was supported by the National Science Foundation of China (30800918) and National Clinical Key College Fund.
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported by the National Science Foundation of China (30800918) and National Clinical Key College Fund.
Footnotes
- BMR
- basal metabolic rate
- FT3
- free T3
- FT4
- free T4
- RTH
- resistance to thyroid hormone
- TR
- thyroid hormone receptor
- TSHoma
- TSH-secreting adenoma.
References
- 1. Beck-Peccoz P, Brucker-Davis F, Persani L, Smallridge RC, Weintraub BD. Thyrotropin-secreting pituitary tumors. Endocr Rev. 1996;17:610–638. [DOI] [PubMed] [Google Scholar]
- 2. Refetoff S, Weiss RE, Usala SJ. The syndromes of resistance to thyroid hormone. Endocr Rev. 1993;14:348–399. [DOI] [PubMed] [Google Scholar]
- 3. Refetoff S, Bassett JH, Beck-Peccoz P, et al. . Classification and proposed nomenclature for inherited defects of thyroid hormone action, cell transport, and metabolism. J Clin Endocrinol Metab. 2014;99:768–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Furumoto H, Ying H, Chandramouli GV, et al. . An unliganded thyroid hormone β receptor activates the cyclin D1/cyclin-dependent kinase/retinoblastoma/E2F pathway and induces pituitary tumorigenesis. Mol Cell Biol. 2005;25:124–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ando S, Sarlis NJ, Oldfield EH, Yen PM. Somatic mutation of TRβ can cause a defect in negative regulation of TSH in a TSH-secreting pituitary tumor. J Clin Endocrinol Metab. 2001;86:5572–5576. [DOI] [PubMed] [Google Scholar]
- 6. Ando S, Sarlis NJ, Krishnan J, et al. . Aberrant alternative splicing of thyroid hormone receptor in a TSH-secreting pituitary tumor is a mechanism for hormone resistance. Mol Endocrinol. 2001;15:1529–1538. [DOI] [PubMed] [Google Scholar]
- 7. Wang EL, Qian ZR, Yamada S, et al. . Clinicopathological characterization of TSH-producing adenomas: special reference to TSH-immunoreactive but clinically non-functioning adenomas. Endocr Pathol. 2009;20:209–220. [DOI] [PubMed] [Google Scholar]
- 8. Brucker-Davis F, Oldfield EH, Skarulis MC, Doppman JL, Weintraub BD. Thyrotropin-secreting pituitary tumors: diagnostic criteria, thyroid hormone sensitivity, and treatment outcome in 25 patients followed at the National Institutes of Health. J Clin Endocrinol Metab. 1999;84:476–486. [DOI] [PubMed] [Google Scholar]
- 9. Watanabe K, Kameya T, Yamauchi A, et al. . Thyrotropin-producing microadenoma associated with pituitary resistance to thyroid hormone. J Clin Endocrinol Metab. 1993;76:1025–1030. [DOI] [PubMed] [Google Scholar]
- 10. Wu SY, Sadow PM, Refetoff S, Weiss RE. Tissue responses to thyroid hormone in a kindred with resistance to thyroid hormone harboring a commonly occurring mutation in the thyroid hormone receptor β gene (P453T). J Lab Clin Med. 2005;146:85–94. [DOI] [PubMed] [Google Scholar]


