Abstract
Context:
Cushing disease, due to pituitary corticotroph tumor ACTH hypersecretion, drives excess adrenal cortisol production with adverse morbidity and mortality. Loss of glucocorticoid negative feedback on the hypothalamic-pituitary-adrenal axis leads to autonomous transcription of the corticotroph precursor hormone proopiomelanocortin (POMC), consequent ACTH overproduction, and adrenal hypercortisolism. We previously reported that R-roscovitine (CYC202, seliciclib), a 2,6,9-trisubstituted purine analog, suppresses cyclin-dependent-kinase 2/cyclin E and inhibits ACTH in mice and zebrafish. We hypothesized that intrapituitary cyclin E signaling regulates corticotroph tumor POMC transcription independently of cell cycle progression. The aim was to investigate whether R-roscovitine inhibits human ACTH in corticotroph tumors by targeting the cyclin-dependent kinase 2/cyclin E signaling pathway.
Methods:
Primary cell cultures of surgically resected human corticotroph tumors were treated with or without R-roscovitine, ACTH measured by RIA and quantitative PCR, and/or Western blot analysis performed to investigate ACTH and lineage-specific transcription factors. Cyclin E and E2F transcription factor 1 (E2F1) small interfering RNA (siRNA) transfection was performed in murine corticotroph tumor AtT20 cells to elucidate mechanisms for drug action. POMC gene promoter activity in response to R-roscovitine treatment was analyzed using luciferase reporter and chromatin immunoprecipitation assays.
Results:
R-roscovitine inhibits human corticotroph tumor POMC and Tpit/Tbx19 transcription with decreased ACTH expression. Cyclin E and E2F1 exhibit reciprocal positive regulation in corticotroph tumors. R-roscovitine disrupts E2F1 binding to the POMC gene promoter and suppresses Tpit/Tbx19 and other lineage-specific POMC transcription cofactors via E2F1-dependent and -independent pathways.
Conclusion:
R-roscovitine inhibits human pituitary corticotroph tumor ACTH by targeting the cyclin E/E2F1 pathway. Pituitary cyclin E/E2F1 signaling is a previously unappreciated molecular mechanism underlying neuroendocrine regulation of the hypothalamic-pituitary-adrenal axis, providing a subcellular therapeutic target for small molecule cyclin-dependent kinase 2 inhibitors of pituitary ACTH-dependent hypercortisolism, ie, Cushing disease.
The proopiomelanocortin (POMC) gene encodes the precursor protein for pituitary ACTH, which is constitutively expressed in corticotroph cells and regulated by positive and negative feedback loops of the hypothalamic-pituitary-adrenal (HPA) axis as the neuroendocrine stress response axis (1). Cushing disease (CD) is due to pituitary corticotroph tumors, in which glucocorticoid resistance in ACTH-producing tumor cells and persistent hypothalamic CRH stimulation contribute to autonomous ACTH hypersecretion and hypercortisolism (2, 3). CD morbidity and mortality, mostly due to cardiovascular complications, are determined by the duration of hypercortisolism exposure and ACTH levels (4, 5). Almost invariably benign and not highly proliferative, greater than 90% of corticotroph adenomas are less than a centimeter in size and often not visible on magnetic resonance imaging, challenging surgical tumor resection as primary therapy with recurrence rates reaching 20%–25% at 10 years after initial surgical resection by experienced neurosurgeons (6–8). Other therapeutic modalities, such as pituitary-directed radiation, adrenalectomy, and/or medical suppression of adrenal gland cortisol production, are limited by suboptimal efficacy and serious side effects (9, 10). Pituitary-targeted pharmacotherapy against CD is limited to pasireotide and cabergoline, only effective in approximately 20% of patients, mostly in those with mild CD, and accompanied by a high incidence of hyperglycemia in the case of pasireotide (11–13).
Molecular mechanisms for autonomous POMC activation in ACTH-secreting corticotroph adenomas are not fully understood (14). Constitutive POMC expression in corticotroph cells starts from early embryonic stages and is controlled by transcriptional complexes on the POMC gene promoter, including pituitary-specific and cell-specific transcription factors, paired-like homeodomain transcription factor 1 (Pitx1), pituitary-restricted transcription factor (Tpit)/T-box transcription factor 19 (Tbx-19), nerve growth factor 1B (NGF1B)/orphan nuclear receptor 77 (Nur77), Brahma-related gene 1 (Brg1), and testicular orphan nuclear receptor 4 (TR4) (15–19). Cofactors within the POMC promoter transcription complex are under positive control of hypothalamic CRH and negative inhibition by peripheral cortisol mediated by the nuclear glucocorticoid receptor (GR) (18, 20). Hypothalamic CRH stimulates the corticotroph cAMP/protein kinase A (PKA) pathway, leading to activation of POMC gene expression mediated by the NGFI-B receptor subfamily (21, 22). Binding of NGFI-B/Nur77 dimer on the Nur response element (NurRE) of POMC promoter is further synergized by CRH-induced Tpit/Tbx19/Pitx-RE interaction and relies in part on the chromatin remodeling protein Brg1 (18). CRH also activates pituitary POMC gene transcription by inhibiting pituitary nuclear factor-κB DNA binding (23). GR transrepression usually dominates over CRH stimulation as the complexes of Brg1, NGFI-B/Nur77, GR, and histone deacetylase-2 (HDAC2) remain on the promoter maintaining overall histone deacetylation (18).
Cyclin E, a regulatory subunit of cyclin-dependent kinase (CDK)-2, is cyclically expressed during the cell cycle (24). The active cyclin E-CDK2 complex leads to retinoblastoma (Rb) phosphorylation and release of E2F transcriptional activity, thereby promoting G1-S progression (25, 26), and inhibited by CDK inhibitors such as p27Kip1 (24). Tumors derived from diverse cell lineages overexpress cyclin E, altering cell proliferation, differentiation, survival, and senescence (27–29).
Cyclin E levels are uniquely increased in corticotroph tumors but not in tumors arising from other pituitary lineages, and cyclin E expression is undetectable in normal pituitary, the mechanisms for which remain to be defined (30). Cyclin E expression in corticotroph adenomas correlates with deficient p27Kip1 and Brg1 expression (18, 31). We previously showed that E2F transcription factor 1 (E2F1) induces pituitary tumor-transforming gene (PTTG) expression in human pituitary tumors, and PTTG overexpression in corticotroph tumor cells is associated with lineage-specific cyclin E up-regulation and G1/S disruption (32, 33). Using a zebrafish CD model, we identified R-roscovitine (CYC202, seliciclib), a 2,6,9-trisubstituted purine analog with high selectivity for CDK1/2/5/7/9, which down-regulates CDK2/cyclin E activity and dephosphorylates pRb, as leading to murine corticotroph tumor cell cycle arrest and apoptosis (32).
Here we report that activation of cyclin E/E2F1 signaling in corticotroph tumors contributes to autonomous ACTH overproduction. R-roscovitine inhibits human POMC gene expression by targeting the lineage-specific cyclinE/E2F1 pathway. We therefore propose small-molecule CDK inhibitors as pharmacotherapy against CD with dual effects of suppressing ACTH overproduction to alleviate hypercortisolemia and metabolic complications while also achieving control or shrinkage of pituitary corticotroph tumor growth.
Materials and Methods
Human subjects and tumor sample collection
This study was approved by the Institutional Review Board at Cedars-Sinai Medical Center (Los Angeles, California). Male and female patients older than 18 years with a clinical diagnosis of CD were enrolled through the Pituitary Center of Cedars-Sinai Medical Center. Written informed consent was obtained from the subjects. Surgical pituitary tumor specimens remaining after pathology analysis for clinical diagnosis were obtained and processed for primary culture. Deidentified patient clinical information included history, duration of disease, clinical features, mean preoperative serum ACTH and 24-hour urinary cortisol levels, and treatment history (surgery, medical therapy, and recurrence).
Cell culture, trypan blue assay, and small interfering RNA (siRNA) transfection
Human pituitary corticotroph tumor specimens were processed using tissue dissociate kits (Miltenyi Biotec) and cultured in low-glucose DMEM 10% fetal bovine serum (FBS) with or without R-roscovitine (Cayman Chemical) for 48 hours. Cell viability was monitored by trypan blue staining. After 48 hours of cell culture, cells were stained by trypan blue and live cells counted manually by hemocytometer in four corner squares and averaged. AtT20 cells (mouse corticotroph tumor) were cultured in low-glucose DMEM with 10% fetal bovine serum. Cyclin E1 siRNA (M-044240-01; Thermo Scientific Dharmacon) and E2f1 siRNA (AM16708; Ambion) transfections were performed in 70%–80% confluent cells using Lipofectamine 2000 (Invitrogen).
Hormone assay
Primary cell culture medium of human corticotroph tumors were collected and stored at −20°C until further use. ACTH levels were measured by RIAs (MP Biomedicals).
Quantitative RT-PCR analysis
Total RNA was purified using the RNeasy minikit (QIAGEN) and cDNA prepared using the QuantiTect reverse transcription kit (Applied Biosystems). Real-time PCR was performed with Taqman gene expression assays (human POMC probe; Hs01596743_m1, human glyceraldehyde-3-phosphate dehydrogenase (hGAPDH) probe; Hs02758991, hTbx19 probe; Hs0193027, Taqman gene expression master mix; 439016).
Western blot analysis
Twenty micrograms of protein aliquots from total cell lysates or nuclear extracts were separated by 15% SDS-PAGE and transferred to nitrocellulose membranes. The membranes were probed with antibodies to ACTH (ab20358; Abcam), E2F1 (sc-251; Santa Cruz Biotechnology), cyclin E (sc-481; Santa Cruz Biotechnology), Tpit/Tbx-19 (ab139165; Abcam), NGFI-B/Nur77 (ab109180), Ptix1 (sc-18924; Santa Cruz Biotechnology), lamin A/C (sc-20681; Santa Cruz Biotechnology), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (sc-25778; Santa Cruz Biotechnology).
Construction of promoters and luciferase assays
Rat POMC promoter constructs (−379/+63 and −480/+63) were generated as described (34). Luciferase reporter assays were performed using 8 × 104 AtT20 cells, 0.5 μg luciferase reporter plasmids, and 50 ng of pRL-TK as an internal control plasmid. Mutations in promoter sequence were introduced into rPomc promoter fragment by PCR assemble procedure, and the mutated fragments were integrated into the NheI (5′) and HindIII (3′) in the rPomc (−480/+63) promoter reporter plasmid (16). DNA sequences of all the inserted fragments were determined to remove defective fragments generated by PCR errors. Cells were transfected using Lipofectamine 2000 (Invitrogen) and cultured in 24-well plates. As required, R-roscovitine (5–20 μM) was added. Forty-eight hours after transtransfection, cells were harvested and luciferase activity analyzed by a dual-luciferase reporter assay system (Promega). Luciferase assays were repeated more than three times and luciferase activities were normalized by internal renilla activities.
Chromatin immunoprecipitation assay (ChIP)
AtT20 cells were fixed in 1% formaldehyde, 4.5 mM HEPES (pH 8.0), 9 mM NaCl, 0.09 mM EDTA, and 0.045 mM EGTA for 10 minutes at room temperature, and sonicated (Bioruptor) in lysis buffer (1% sodium dodecyl sulfate; 10 mM EDTA; and 50 mM Tris-HCl, pH 8.0) with proteinase inhibitor (Sigma-Aldrich; P8340). Cell lysates were incubated overnight at 4°C with polyclonal antibodies to E2F1 (sc-251X; Santa Cruz Biotechnology) or acetyl histone H4 (06-866; Millipore) or with normal rabbit IgG (sc-2027; Santa Cruz Biotechnology) polyclonal antibodies. DNA fragments were isolated from the immunoprecipitated chromatin and analyzed by real-time PCR analysis with SYBR Green PCR master mix (Applied Biosystems). Results were normalized to input in each case. Three sets of PCR primers were designed to represent different regions of the POMC promoter: enhancer region (ChIP1) (35), distal (ChIP2), and basal promoter region (ChIP3). Primer sequences are as follows: mouse POMC (mPOMC), 5′-CCATGCAGGTCACAAGACTC, 5′-CTGTCCTTGCTTGCTGCATA, 5′-ATGGGGAGTAACCTCACTGG, 5′-AGCTCCCAAACAAGAGCAAG, 5′-CCCAACCCTGCAAGTATAA, and 5′-TCCGCTACAAACCAGAAC.
Results
R-roscovitine inhibits ACTH expression in human pituitary corticotroph tumors
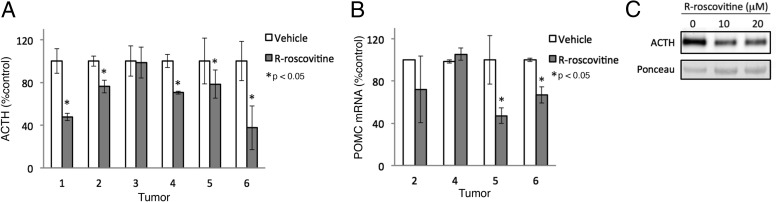
To test whether R-roscovitine directly targets human corticotroph tumor ACTH production, we treated primary cell cultures derived from six consecutive, surgically resected human pituitary corticotroph tumors with R-roscovitine (Table 1). Although R-roscovitine treatment minimally affected cell viability as determined by trypan blue assay (84% ± 2.7% vs 80% ± 1.9%), the drug attenuated ACTH levels in the culture medium by up to 62% in five of six tumors (Table 1 and Figure 1A). In three (tumor numbers 2, 5, and 6) of the four tumors with sufficient mRNA yielded from primary cultured human cells, RT-PCR analysis showed 30%–50% suppressed POMC mRNA abundance by R-roscovitine treatment, statistically not significant for tumor number 2 due to a wide SD (Figure 1B). Tumor number 5 is a 3.7-cm-diameter macroadenoma (Table 1) and was the only one that yielded sufficient cells from primary culture for Western blot analysis of protein extracts, which demonstrated ACTH inhibition by R-roscovitine treatment compared with vehicle-treated controls (Figure 1C). The other macroadenoma, tumor number 4 (Table 1), was the only tumor derived from a patient exposed to prior irradiation and exhibited suppressed ACTH by R-roscovitine treatment, although not at the mRNA level (Figure 1, A and B). These results indicate that R-roscovitine inhibits lineage-specific POMC expression and/or ACTH production in human corticotroph tumors.
Table 1.
Clinical Features of Human Pituitary Corticotroph Tumors Treated With R-Roscovitine in Primary Cell Culture
| Patient Number | ACTH Reduction With R-Roscovitine Treatment, % | Clinical Features |
||||||
|---|---|---|---|---|---|---|---|---|
| Age and Gender | ACTH, pg/mLa | 24 Hours UFC, μg/da | Tumor Diameter, mm | Duration, yb | Prior Rx | Comorbidities | ||
| 1 | 56 ± 2.4 | 16, M | 98 | 459 | 5.7 | 3 | None | DM, HTN, obesity, mood changes, myopathy, hypogonadism |
| 2 | 24 ± 5.9 | 22, F | 30 | 820 | 9 | 3 | TSS, K, C | DM, obesity, osteoporosis |
| 3 | 1.3 ± 14.5c | 29, F | 54 | 281 | 8 | 4 | None | HTN, obesity |
| 4 | 30 ± 1.3 | 73, M | 296 | 59 | 28 | 15 | TSS × 3, R | DM, HTN, obesity, CAD, hypogonadism |
| 5 | 22 ± 13.1 | 16, F | 139 | 661 | 37 | 5 | K | DM, HTN, obesity, amenorrhea, hirsutism, acne, myopathy, anxiety |
| 6 | 62 ± 20.5 | 33, F | 44 | 239 | 4 | 2 | None | HTN, obesity, myopathy, rash, irregular menses |
Abbreviations: C, cabergoline; CAD, coronary artery disease; DM, diabetes mellitus; F, female; HTN, hypertension; K, ketoconazole; M, male; R, radiotherapy; Rx, treatment; TSS, transsphenoidal surgery; UFC, urinary free cortisol. Medium ACTH concentrations in primary cultures were measured by RIA (normalized for viable cell numbers).
Preoperative mean value.
CD duration before surgery.
Nonsignificant (P > .05).
Figure 1. Inhibition of ACTH expression by R-roscovitine in human pituitary corticotroph tumors.
A, Primary cultures of human corticotroph tumor (numbers 1–6) cells treated with vehicle or R-roscovitine for 48 hours. Medium ACTH concentrations in primary cultures were measured by RIA (normalized for viable cell numbers; n = 6 tumors, mean ± SE). *, P < .05. B, POMC mRNA measured by RT-PCR in extracts derived from human corticotroph tumor (numbers 2, 4, 5, and 6) cells treated with vehicle or R-roscovitine. C, Western blot analysis of ACTH expression in primary cultures of human corticotroph tumor (from patient 5 in Table 1) treated with vehicle or R-roscovitine. Tumor numbers correspond to patient numbers in Table 1. RT-PCR was performed in triplicates.
R-roscovitine targets POMC promoter and corticotroph transcription factors
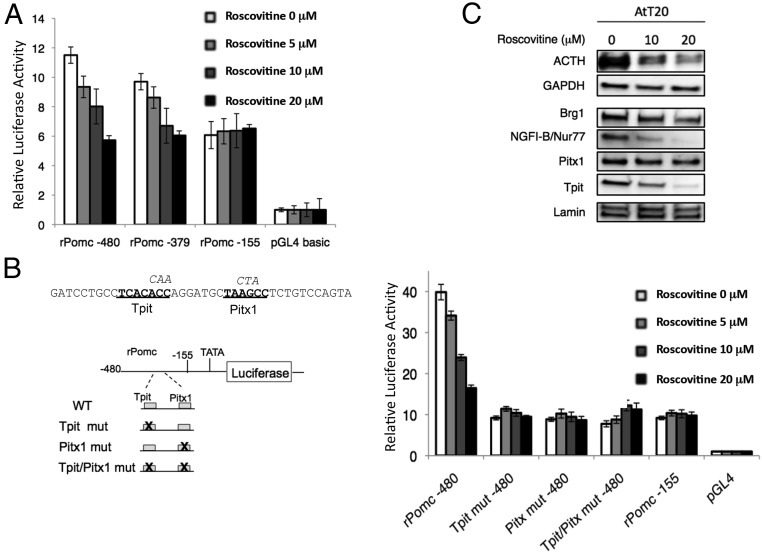
No human pituitary corticotroph cell line is available. However, pituitary-specific and hormonally regulated POMC expression exhibits functionally conserved cis-trans transcription control despite divergence of promoter sequences (36). To investigate transcriptional mechanisms underlying R-roscovitine-mediated suppression of POMC mRNA, we first performed luciferase reporter assays using rat POMC proximal promoter constructs that are 379 and 480 bp upstream of the transcription initiation site (−379/+63 and −480/+63) and contain cis-acting elements activated by transcription factors Pitx1, Tpit/Tbx-19, NGFI-B/Nur77, and Brg1 in the mouse ortholog (18, 36). We observed a dose-dependent inhibition of rat POMC (rPOMC)-379 and rPOMC-480 proximal promoter activity by R-roscovitine treatment in the AtT20 cells (Figure 2A).
Figure 2. R-roscovitine inhibition of rPomc promotor activity and corticotroph transcriptional factors.
A, Luciferase assay was performed using rat POMC gene proximal promoter constructs in AtT20 cells treated with or without R-roscovitine under indicated concentrations. B, Luciferase assay of rat Pomc gene promoter construct with Tpit and Pitx1 binding sites mutation in AtT20 cells treated with or without R-roscovitine under the indicated concentrations. Left panel, DNA sequence of the Tpit/Pitx1 binding site is shown. Tpit and Pitx1 sites are indicated in bold. Mutations are indicated by italic capital letters for Tpit and Pitx1 binding sites. C, Western blot of protein extract derived from AtT20 cells treated with vehicle or R-roscovitine. Data shown are representative results from at least two independent experiments.
The T-box pituitary-restricted transcription factor, Tpit/Tbx-19, is essential for both adequate cell-specific POMC transcription and terminal differentiation of corticotrophs (16, 17). Tpit/Tbx19 activates POMC gene transcription in cooperation with the homeoprotein Pitx1, and both factors bind DNA as monomers at contiguous sites on the POMC promoter. Tpit/Tbx19 null mutation leads to failure of precursor cells to reach terminal differentiation as defined by POMC expression (37, 38). Human TPIT mutations account for 60% of neonatal-onset congenital isolated ACTH deficiency (39). We therefore performed luciferase reporter assays on the rPOMC-480 proximal promoter with mutated Pitx1 and/or Tpit/Tbx-19 binding sites. Dose-dependent R-roscovitine inhibition on the proximal POMC promoter was diminished without the presence of intact Pitx1 and/or Tpit/Tbx-19 binding sites (Figure 2B). Western blot analysis of protein extracts derived from R-roscovitine-treated pituitary corticotroph tumor cells showed that R-roscovitine inhibits the expression of the lineage-specific transcription factors Tpit/Tbx-19, NGFI-B/Nur77, and Brg1 without altering the levels of the pan-pituitary factor, Pitx1 (Figure 2C), indicating that R-roscovitine inhibits POMC proximal promoter activity by also targeting Tpit/Tbx-19 and transcription cofactors (Figure 2B).
R-roscovitine suppresses corticotroph transcription factors mediated by the cyclin E/E2F1 pathway
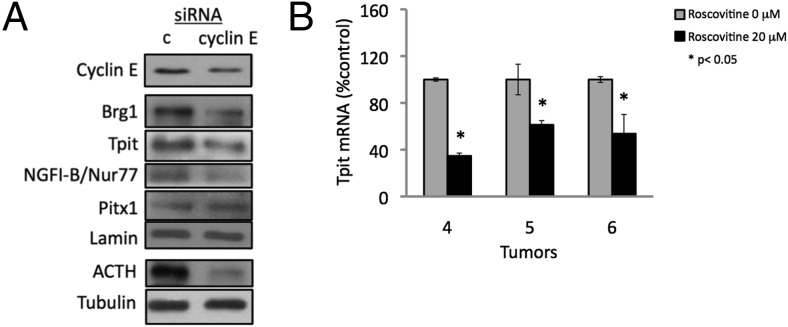
R-roscovitine suppresses cyclin E expression and dephosphorylates pRb, inhibiting xenografted murine corticotroph tumor growth (32). To test whether cyclin E down-regulation leads to inhibition of corticotroph lineage-specific transcription factors, we performed Western blot analysis of protein extracts derived from AtT20 corticotroph tumor cells transfected with cyclin E siRNA (Figure 3A). Cyclin E down-regulation in AtT20 cells leads to decreased levels of lineage-specific transcription factors Tpit/Tbx-19, NGFI-B/Nur77, and Brg1without altering Pitx1 levels. Furthermore, corticotroph tumors derived from patients 4, 5, and 6 (Figure 1A) yielded sufficient cells in primary culture to enable further RT-PCR analysis, which showed suppressed Tpit expression in response to R-roscovitine (Figure 3B). These results indicate that pituitary corticotroph tumor cyclin E signaling targets POMC lineage-specific transcription factors, which are suppressed by R-roscovitine.
Figure 3. R-roscovitine inhibition of cyclin E-mediated regulation of human POMC gene.
A, Western blot of protein extract derived from AtT20 cells transfected with control siRNA (c) or cyclin E siRNA (cyclin E). Representative results from two independent experiments are shown. B, Tpit expression measured by RT-PCR of cDNA derived from human corticotroph tumors numbers 4–6 (Figure 1A) treated with vehicle or R-roscovitine. Each RT-PCR was performed in triplicate and also repeated for tumors 4 and 5. #, Levels are too low to be detected.
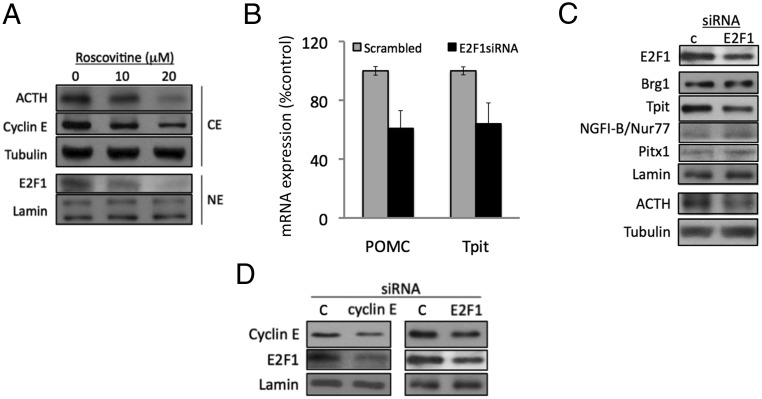
Activation of the cyclin E-CDK2 complex leads to Rb phosphorylation and release of E2F transcriptional activity. In AtT20 corticotroph tumor cells, R-roscovitine dose dependently suppressed cyclin E and E2F1 protein expression (Figure 4A). AtT20 cells were therefore transfected with E2F1 siRNA (Figure 4, B and C), which leads to decreased Tpit/Tbx-19 mRNA and protein levels as assessed by RT-PCR and Western blot analysis, respectively (Figure 4, B and C). However, NGFI-B/Nur77, Brg1, and Pitx1 expressions were not altered by E2F1 suppression (Figure 4C). These results also indicate that cyclin E and E2F1 exhibit a reciprocal positive feedback on their respective expression in pituitary corticotroph tumor cells (Figure 4D).
Figure 4. Reciprocal regulation of cyclin E/E2F1 expression mediates Tpit suppression by R-roscovitine.
A, Western blot of protein extracts derived from AtT20 cells treated with vehicle or R-roscovitine. B, POMC and Tpit expression measured by RT-PCR of cDNA derived from AtT20 cells transfected with control siRNA (scrambled) or E2F1 siRNA. C, Western blot of protein extract derived from AtT20 cells transfected with control siRNA (c) or E2F1 siRNA (E2F1). D, Western blot of protein extract derived from AtT20 cells transfected with control siRNA (c), cyclin E (cyclin E), or E2F1 siRNA (E2F1). For illustrative comparison, E2F1 and cyclin E expression from the same Western blot experiments in Figures 3A (cyclin E siRNA) and 4C (E2F1 siRNA) are depicted side by side. CE, cellular protein extract; NE, nuclear protein extract. Data shown are representative results from at least two independent experiments.
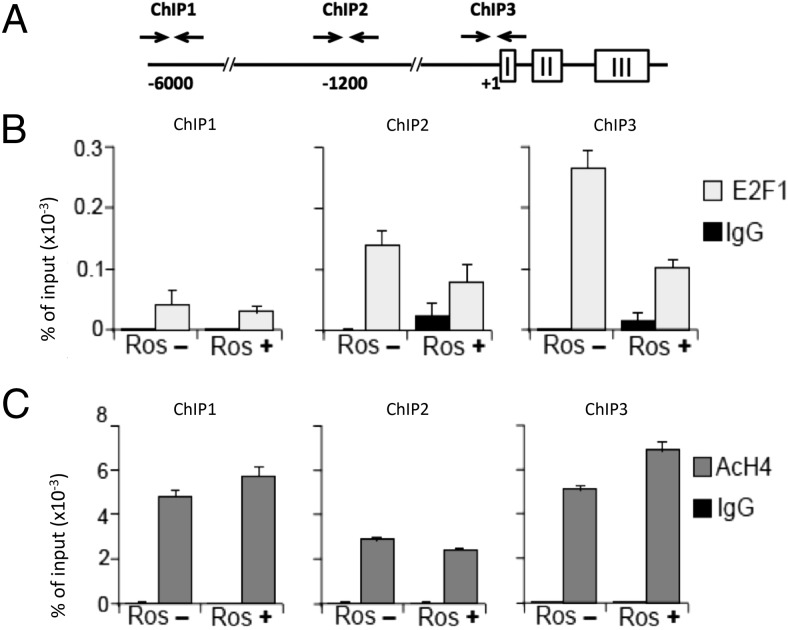
R-roscovitine inhibits E2F1 interaction with POMC regulatory sequences in corticotroph tumors
To test whether E2F1 mediates transcriptional activity of the POMC promoter, ChIP assays using an anti-E2F1 antibody were performed and demonstrated E2F1 binding to the proximal (ChIP3) and distal (ChIP2) promoter region, which was attenuated by R-roscovitine (Figure 5, A and B). Further distally, the previously reported enhancer region (35) exhibited only minimal E2F1 binding activity (Figure 5, A and B). R-roscovitine appears not to change POMC chromatin structure because it did not alter histone H4 acetylation (Figure 5C). These results further indicate that E2F1 mediates R-roscovitine inhibition of POMC gene expression in pituitary corticotroph tumors.
Figure 5. Inhibition of E2F1 interaction with POMC regulatory sequences by R-roscovitine in corticotroph tumors.
ChIP assays were performed in AtT20 cells treated with vehicle (Ros−) or R-roscovitine (Ros+) using PCR primer sets corresponding to the enhancer (ChIP1), the distal (ChIP2), and proximal (ChIP3) promoter region (A) with anti-E2F1 (E2F1) (B), antiacetyl histone H4 (AcH4) (C) antibodies, or negative control (IgG).
Discussion
Neuroendocrine tumors are uniquely characterized by disrupted homeostasis resulting from uncontrolled hormone production. A pathognomonic feature of CD is autonomous ACTH production, resistant to glucocorticoid negative feedback and apparently cell cycle independent because the degree of ACTH hypersecretion is not necessarily associated with corticotroph tumor size. Molecular mechanisms for autonomous POMC activation in ACTH-dependent Cushing's syndrome are not fully understood.
We showed previously that R-roscovitine down-regulates CDK2/cyclin E in murine corticotroph tumor AtT20 cells, leading to cell cycle arrest with suppressed POMC expression (32). We now report lineage-specific amplification of cyclin E/E2F1 signals as a mechanism contributing to uncontrolled POMC transcription and autonomous ACTH production, both of which are suppressed by R-roscovitine treatment. Despite significant suppression of ACTH production, we observed only minimal changes of viable tumor cell numbers in primary cultures with R-roscovitine treatment at the same concentrations as used in AtT20 cells, suggesting that the inhibitory effect of R-roscovitine in human corticotroph tumors preferentially targets ACTH expression. R-roscovitine has been undergoing phase I and II clinical trials for non-small cell lung cancer and nasopharyngeal cancer and has shown a mild adverse effect profile (40, 41). CD tumors are often benign and small in size with low proliferation indices. Our current study supports a predominant effect of R-roscovitine suppressing human pituitary tumor ACTH expression, therefore elucidating a unique therapeutic target of small molecule CDK inhibitors for neuroendocrine CD tumors.
We demonstrate that cyclin E signaling induces human corticotroph POMC expression via the transcription regulatory complexes assembled on the POMC promoter, involving cofactors NGFI-B/Nur77, Tpit/Tbx19, and Brg1. Constitutive POMC transcription relies on multiple ubiquitous and cell-restricted factors such as NGFI-B/Nur77, Tpit/Tbx19, NeuroD1, and Brg1. Our results show that down-regulating cyclin E in corticotroph tumors leads to POMC and ACTH suppression with decreased expression of NGFI-B/Nur77, Tpit/Tbx19, and Brg1, suggesting that cyclin E derepression may enhance constitutive POMC expression by activating these cofactors. NGFI-B/Nur77 and Tpit/Tbx19 respond synergistically to CRH through activation of PKA and MAPK pathways that trigger their binding to the NurRE and Tpit/Pitx-RE sequence on the POMC promoter, respectively (22). Therefore, the cyclin E-mediated expression of NGFI-B/Nur77 and Tpit/Tbx19 resemble CRH activity on POMC transcription. Finally, cyclin E derepression leading to up-regulated NGFI-B/Nur77 and Brg1 may disrupt the stoichiometric relationships of these factors, leading to diminished GR repression of POMC transcription. Because cyclin E up-regulation is predominantly present in tumors but not in normal corticotroph cells, cyclin E activation therefore appears to alter the homeostatic balance between the positive and negative feedback loops of the HPA axis, exerting more dominant positive stimulation over negative inhibition on ACTH expression in corticotroph adenomas.
The pathological significance of corticotroph cyclin E/E2F1 signal amplification in CD is further supported by our observations that cyclin E knockdown in a corticotroph tumor leads to decreased E2F1 expression and vice versa, suggesting reciprocal positive feedback of cyclin E and E2F1 on their mutual expression, which further amplifies the role of cyclin E/E2F1 as activators of human POMC transcription and ACTH expression in pituitary corticotroph tumors. Less than 50% of human corticotroph adenomas have misexpressed Brg1 or HDAC2, both critical for formation of stable in vivo complexes of GR/NGFI-B/Nur77 and GR/HDAC2, as well as histone deacetylation, supporting their role in tumor glucocorticoid resistance in CD (18). In addition, rare GR mutations have been associated with loss of glucocorticoid-negative feedback in corticotroph tumors (42). Our current study defines a new mechanism for activation of human POMC and ACTH expression in CD, mediated by cyclin E/E2F1 signals.
Limitations of this study reflect multiple challenges of drug discovery for treating CD, including very sparse human pituitary tumor tissue availability due to the rarity of the disease [annual incidence of 0.7–2.4 per million population and a US prevalence of 11 000–15 000 (10, 43)] and the small adenoma size (usually < 1 cm in diameter). In addition, no human pituitary ACTH-secreting cells lines have been established, which limited our POMC gene promoter study to be performed in murine AtT20 cells. Although the small human sample number constrains our conclusions, our results show an overall association of R-roscovitine inhibition of human POMC mRNA expression and ACTH production, independent of viable cell numbers. When sufficient cells for a comprehensive study were yielded, R-roscovitine consistently inhibited of Tpit, POMC, and ACTH mRNA, protein, and hormone production levels. These results may reflect individual tumor variation in primary culture, and it is also possible that prior radiation-induced signaling alterations may override R-roscovitine effects on cyclin E- and Tpit-mediated POMC gene expression, and suppressed ACTH after irradiation may result from posttranscriptional mechanisms independent of cyclin E and Tpit. In addition, POMC gene expression appeared to be less sensitive to R-roscovitine in recurrent tumors (tumor numbers 2 and 4).
Our results show that cyclin E/E2F1 signals on neuroendocrine pituitary ACTH expression, indicating that cyclin E/E2F1, PKA/MAPK, and hormonal pathways appear to converge in regulating HPA axis differentiation and activity. R-roscovitine inhibits kinase activity with high selectivity for CDK1/2/5/7/9, and the drug differentially regulated transcriptional activity of the GR mediated by CDK5 in rat cortical neuronal cells (44). Our results demonstrate the potential therapeutic effect of small molecule CDK inhibitors on pituitary tumor ACTH production and tumor growth.
Acknowledgments
We thank Drs Song-Guang Ren for ACTH measurements, Adam Mamelak and Odelia Cooper for human pituitary corticotroph tumor and clinical information collections, and Cuiqi Zhou for helpful discussions.
This work was supported by National Institutes of Health Grants DK103198 and T32DK007770.
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported by National Institutes of Health Grants DK103198 and T32DK007770.
Footnotes
- Brg1
- Brahma-related gene 1
- CD
- Cushing disease
- CDK
- cyclin-dependent kinase
- ChIP
- chromatin immunoprecipitation assay
- E2F1
- E2F transcription factor 1
- GR
- glucocorticoid receptor
- HDAC2
- histone deacetylase-2
- HPA
- hypothalamic-pituitary-adrenal
- NGF1B
- nerve growth factor 1B
- Nur77
- orphan nuclear receptor 77
- Pitx1
- paired-like homeodomain transcription factor 1
- PKA
- protein kinase A
- POMC
- proopiomelanocortin
- PTTG
- pituitary tumor-transforming gene
- Rb
- retinoblastoma
- rPOMC
- rat POMC
- siRNA
- small interfering RNA
- Tbx-19
- T-box transcription factor 19
- Tpit
- pituitary-restricted transcription factor
- TR4
- testicular orphan nuclear receptor 4.
References
- 1. Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5:374–381. [DOI] [PubMed] [Google Scholar]
- 2. Stewart PM, Krone NP. Classification and pathophysiology of Cushing's syndrome. In: Melmed S, Polonsky KS, Larsen PR, Kronenberg HM, eds. Williams Textbook of Endocrinology. 12th ed Philadelphia: Elsevier; 2012:500–515. [Google Scholar]
- 3. Raverot G, Wierinckx A, Jouanneau E, et al. . Clinical, hormonal and molecular characterization of pituitary ACTH adenomas without (silent corticotroph adenomas) and with Cushing's disease. Eur J Endocrinol. 2010;163:35–43. [DOI] [PubMed] [Google Scholar]
- 4. Dekkers OM, Biermasz NR, Pereira AM, et al. . Mortality in patients treated for Cushing's disease is increased, compared with patients treated for nonfunctioning pituitary macroadenoma. J Clin Endocrinol Metab. 2007;92:976–981. [DOI] [PubMed] [Google Scholar]
- 5. Clayton RN, Raskauskiene D, Reulen RC, Jones PW. Mortality and morbidity in Cushing's disease over 50 years in Stoke-on-Trent, UK: audit and meta-analysis of literature. J Clin Endocrinol Metab. 2011;96:632–642. [DOI] [PubMed] [Google Scholar]
- 6. Hammer GD, Tyrrell JB, Lamborn KR, et al. . Transsphenoidal microsurgery for Cushing's disease: initial outcome and long-term results. J Clin Endocrinol Metab. 2004;89:6348–6357. [DOI] [PubMed] [Google Scholar]
- 7. Hassan-Smith ZK, Sherlock M, Reulen RC, et al. . Outcome of Cushing's disease following transsphenoidal surgery in a single center over 20 years. J Clin Endocrinol Metab. 2012;97:1194–1201. [DOI] [PubMed] [Google Scholar]
- 8. Tritos NA, Biller BM, Swearingen B. Management of Cushing disease. Nat Rev Endocrinol. 2011;7:279–289. [DOI] [PubMed] [Google Scholar]
- 9. Biller BM, Grossman AB, Stewart PM, et al. . Treatment of adrenocorticotropin-dependent Cushing's syndrome: a consensus statement. J Clin Endocrinol Metab. 2008;93:2454–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nieman LK, Biller BM, Findling JW, et al. . The diagnosis of Cushing's syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93:1526–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Colao A, Petersenn S, Newell-Price J, et al. . A 12-month phase 3 study of pasireotide in Cushing's disease. N Engl J Med. 2012;366:914–924. [DOI] [PubMed] [Google Scholar]
- 12. Pivonello R, De Martino MC, Cappabianca P, et al. . The medical treatment of Cushing's disease: effectiveness of chronic treatment with the dopamine agonist cabergoline in patients unsuccessfully treated by surgery. J Clin Endocrinol Metab. 2009;94:223–230. [DOI] [PubMed] [Google Scholar]
- 13. Pivonello R, Ferone D, Lamberts SW, Colao A. Cabergoline plus lanreotide for ectopic Cushing's syndrome. N Engl J Med. 2005;352:2457–2458. [DOI] [PubMed] [Google Scholar]
- 14. Melmed S. Pathogenesis of pituitary tumors. Nat Rev Endocrinol. 2011;7:257–266. [DOI] [PubMed] [Google Scholar]
- 15. Treier M, Rosenfeld MG. The hypothalamic-pituitary axis: co-development of two organs. Curr Opin Cell Biol. 1996;8:833–843. [DOI] [PubMed] [Google Scholar]
- 16. Lamolet B, Pulichino AM, Lamonerie T, et al. . A pituitary cell-restricted T box factor, Tpit, activates POMC transcription in cooperation with Pitx homeoproteins. Cell. 2001;104:849–859. [DOI] [PubMed] [Google Scholar]
- 17. Liu J, Lin C, Gleiberman A, et al. . Tbx19, a tissue-selective regulator of POMC gene expression. Proc Natl Acad Sci USA. 2001;98:8674–8679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bilodeau S, Vallette-Kasic S, Gauthier Y, et al. . Role of Brg1 and HDAC2 in GR trans-repression of the pituitary POMC gene and misexpression in Cushing disease. Genes Dev. 2006;20:2871–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Du L, Bergsneider M, Mirsadraei L, et al. . Evidence for orphan nuclear receptor TR4 in the etiology of Cushing disease. Proc Natl Acad Sci USA. 2013;110:8555–8560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lugo DI, Pintar JE. Ontogeny of basal and regulated secretion from POMC cells of the developing anterior lobe of the rat pituitary gland. Dev Biol. 1996;173:95–109. [DOI] [PubMed] [Google Scholar]
- 21. Labrie F, Veilleux R, Lefevre G, Coy DH, Sueiras-Diaz J, Schally AV. Corticotropin-releasing factor stimulates accumulation of adenosine 3′, 5′-monophosphate in rat pituitary corticotrophs. Science. 1982;216:1007–1008. [DOI] [PubMed] [Google Scholar]
- 22. Maira M, Martens C, Batsche E, Gauthier Y, Drouin J. Dimer-specific potentiation of NGFI-B (Nur77) transcriptional activity by the protein kinase A pathway and AF-1-dependent coactivator recruitment. Mol Cell Biol. 2003;23:763–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Karalis KP, Venihaki M, Zhao J, van Vlerken LE, Chandras C. NF-κB participates in the corticotropin-releasing, hormone-induced regulation of the pituitary proopiomelanocortin gene. J Biol Chem. 2004;279:10837–10840. [DOI] [PubMed] [Google Scholar]
- 24. Sherr CJ, Roberts JM. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 2004;18:2699–2711. [DOI] [PubMed] [Google Scholar]
- 25. Hinds PW, Mittnacht S, Dulic V, Arnold A, Reed SI, Weinberg RA. Regulation of retinoblastoma protein functions by ectopic expression of human cyclins. Cell. 1992;70:993–1006. [DOI] [PubMed] [Google Scholar]
- 26. Chellappan SP, Hiebert S, Mudryj M, Horowitz JM, Nevins JR. The E2F transcription factor is a cellular target for the RB protein. Cell. 1991;65:1053–1061. [DOI] [PubMed] [Google Scholar]
- 27. Porter PL, Malone KE, Heagerty PJ, et al. . Expression of cell-cycle regulators p27Kip1 and cyclin E, alone and in combination, correlate with survival in young breast cancer patients. Nat Med. 1997;3:222–225. [DOI] [PubMed] [Google Scholar]
- 28. Loeb KR, Kostner H, Firpo E, et al. . A mouse model for cyclin E-dependent genetic instability and tumorigenesis. Cancer Cell. 2005;8:35–47. [DOI] [PubMed] [Google Scholar]
- 29. Akli S, Van Pelt CS, Bui T, Meijer L, Keyomarsi K. Cdk2 is required for breast cancer mediated by the low-molecular-weight isoform of cyclin E. Cancer Res. 2011;71:3377–3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jordan S, Lidhar K, Korbonits M, Lowe DG, Grossman AB. Cyclin D and cyclin E expression in normal and adenomatous pituitary. Eur J Endocrinol. 2000;143:R1–R6. [DOI] [PubMed] [Google Scholar]
- 31. Roussel-Gervais A, Bilodeau S, Vallette S, et al. . Cooperation between Cyclin E and p27(Kip1) in pituitary tumorigenesis. Mol Endocrinol. 2010;24:1835–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu NA, Jiang H, Ben-Shlomo A, et al. . Targeting zebrafish and murine pituitary corticotroph tumors with a cyclin-dependent kinase (CDK) inhibitor. Proc Natl Acad Sci USA. 2011;108:8414–8419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhou C, Wawrowsky K, Bannykh S, Gutman S, Melmed S. E2F1 induces pituitary tumor transforming gene (PTTG1) expression in human pituitary tumors. Mol Endocrinol. 2009;23:2000–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fukuoka H, Cooper O, Ben-Shlomo A, et al. . EGFR as a therapeutic target for human, canine, and mouse ACTH-secreting pituitary adenomas. J Clin Invest. 2011;121:4712–4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Langlais D, Couture C, Sylvain-Drolet G, Drouin J. A pituitary-specific enhancer of the POMC gene with preferential activity in corticotrope cells. Mol Endocrinol. 2011;25:348–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bumaschny VF, de Souza FS, Lopez Leal RA, et al. . Transcriptional regulation of pituitary POMC is conserved at the vertebrate extremes despite great promoter sequence divergence. Mol Endocrinol. 2007;21:2738–2749. [DOI] [PubMed] [Google Scholar]
- 37. Pulichino AM, Vallette-Kasic S, Tsai JP, Couture C, Gauthier Y, Drouin J. Tpit determines alternate fates during pituitary cell differentiation. Genes Dev. 2003;17:738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pulichino AM, Vallette-Kasic S, Couture C, et al. . Human and mouse TPIT gene mutations cause early onset pituitary ACTH deficiency. Genes Dev. 2003;17:711–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Couture C, Saveanu A, Barlier A, et al. . Phenotypic homogeneity and genotypic variability in a large series of congenital isolated ACTH-deficiency patients with TPIT gene mutations. J Clin Endocrinol Metab. 2012;97:E486–E495. [DOI] [PubMed] [Google Scholar]
- 40. Benson C, White J, De Bono J, et al. . A phase I trial of the selective oral cyclin-dependent kinase inhibitor seliciclib (CYC202; R-Roscovitine), administered twice daily for 7 days every 21 days. Br J Cancer. 2007;96:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Le Tourneau C, Faivre S, Laurence V, et al. . Phase I evaluation of seliciclib (R-roscovitine), a novel oral cyclin-dependent kinase inhibitor, in patients with advanced malignancies. Eur J Cancer. 2010;46:3243–3250. [DOI] [PubMed] [Google Scholar]
- 42. Lamberts SW. Glucocorticoid receptors and Cushing's disease. Mol Cell Endocrinol. 2002;197:69–72. [DOI] [PubMed] [Google Scholar]
- 43. Lindholm J, Juul S, Jorgensen JO, et al. . Incidence and late prognosis of Cushing's syndrome: a population-based study. J Clin Endocrinol Metab. 2001;86:117–123. [DOI] [PubMed] [Google Scholar]
- 44. Kino T, Ichijo T, Amin ND, et al. . Cyclin-dependent kinase 5 differentially regulates the transcriptional activity of the glucocorticoid receptor through phosphorylation: clinical implications for the nervous system response to glucocorticoids and stress. Mol Endocrinol. 2007;21:1552–1568. [DOI] [PubMed] [Google Scholar]