Abstract
Context:
Hypoparathyroidism is a disorder characterized by hypocalcemia due to insufficient secretion of PTH. Pseudohypoparathyroidism is a less common disorder due to target organ resistance to PTH. This report summarizes the results of the findings and recommendations of the Working Group on Epidemiology and Diagnosis of Hypoparathyroidism.
Evidence Acquisition:
Each contributing author reviewed the recent published literature regarding epidemiology and diagnosis of hypoparathyroidism using PubMed and other medical literature search engines.
Evidence Synthesis:
The prevalence of hypoparathyroidism is an estimated 37 per 100 000 person-years in the United States and 22 per 100 000 person-years in Denmark. The incidence in Denmark is approximately 0.8 per 100 000 person-years. Estimates of prevalence and incidence of hypoparathyroidism are currently lacking in most other countries. Hypoparathyroidism increases the risk of renal insufficiency, kidney stones, posterior subcapsular cataracts, and intracerebral calcifications, but it does not appear to increase overall mortality, cardiovascular disease, fractures, or malignancy. The diagnosis depends upon accurate measurement of PTH by second- and third-generation assays. The most common etiology is postsurgical hypoparathyroidism, followed by autoimmune disorders and rarely genetic disorders. Even more rare are etiologies including parathyroid gland infiltration, external radiation treatment, and radioactive iodine therapy for thyroid disease. Differentiation between these different etiologies is aided by the clinical presentation, serum biochemistries, and in some cases, genetic testing.
Conclusions:
Hypoparathyroidism is often associated with complications and comorbidities. It is important for endocrinologists and other physicians who care for these patients to be aware of recent advances in the epidemiology, diagnosis, and genetics of this disorder.
This summary statement reports findings of an expert panel that reviewed the evidence for management of hypoparathyroidism in connection with the first international meeting focused on this disease.
Hypoparathyroidism is an uncommon disorder characterized by low serum calcium, increased serum phosphorus, and deficient production of PTH (1, 2). The epidemiology of hypoparathyroidism has become better understood, with a number of recent studies quantitating aspects of the disease not previously appreciated. Pseudohypoparathyroidism (PHP), a disorder of PTH resistance, is an even less common disease characterized by similarly abnormal mineral biochemical abnormalities, but with increased circulating levels of PTH (1).
Operationally, hypoparathyroidism can be divided into primary hypoparathyroidism due to intrinsic defects within the parathyroid glands primarily due to genetic causes, and the much more common secondary or acquired forms due to etiologies that ablate, impair, or destroy parathyroid gland function. Secondary causes of hypoparathyroidism are by far the most common etiologies (2). Although the diagnosis of hypoparathyroidism is usually straightforward once serum calcium, phosphorus, and PTH levels are known, determining the cause of nonsurgical hypoparathyroidism may be challenging.
Epidemiology of Hypoparathyroidism
Anterior neck surgery is the most common cause of acquired hypoparathyroidism and is responsible for about 75% of cases (1). The next most common acquired cause in adults is thought to be autoimmune disease, affecting either the parathyroid glands alone or multiple other endocrine glands (2). Remaining cases of acquired hypoparathyroidism are secondary due to a variety of rare infiltrative disorders in which the parathyroid glands are affected by metastatic disease or iron or copper overload or by ionizing radiation exposure. These latter causes of hypoparathyroidism are covered in more detail in the accompanying paper on presentation of hypoparathyroidism, including what little is known regarding the epidemiology of these disorders (3). Rare genetic disorders may also cause hypoparathyroidism.
Prevalence
The best prevalence estimate of hypoparathyroidism in the United States is based on analysis of a large health plan claims database, which resulted in estimation of 77 000 cases (4). This figure is an interpolation based upon a review of a large U.S. claims database with 77 million patients from 75 health plans in October 2007-September 2008 that resulted in an estimated 58 793 insured U.S. adult patients diagnosed with chronic hypoparathyroidism. This diagnostic prevalence estimate was based on the number of new diagnoses of hypoparathyroidism in the database over the interval studied. A surgical incidence estimate was based on calculation of the proportion of total neck operations resulting in transient (<6 months) or chronic (>6 months) hypoparathyroidism. A physician primary market research study was conducted to assess disease severity and to determine the percentage of new nonsurgical patients with hypoparathyroidism. Surgical incidence data were entered into an epidemiological model to derive a second prevalence estimate. The surgical-based incidence approach gave an estimate of 117 342 relevant neck surgeries, resulting in 8901 cases of hypoparathyroidism over 12 months. Approximately 7.6% of surgeries resulted in hypoparathyroidism, with 75% of these cases being transient and 25% being chronic. The prevalence of insured patients with chronic hypoparathyroidism in the surgical database was 58 625, similar to that obtained in the diagnostic estimate. Assuming that 15.4% of the U.S. population was uninsured at the time this study was done, these findings were used to project an estimate of 77 000 total insured and uninsured individuals in the United States that had hypoparathyroidism.
Another estimate of the prevalence of hypoparathyroidism comes from the longitudinal population-based Rochester Epidemiology Project (Table 1) in which medical records linkage resources were used to identify all persons residing in Olmsted County, Minnesota, in 2009 with any diagnosis of hypoparathyroidism assigned by a health care provider since 1945 (5). Detailed medical records were reviewed to confirm the diagnosis of hypoparathyroidism and to assign an etiology. Subjects were assigned two age- and sex-matched controls per confirmed case. Fifty-four cases were confirmed, of mean age 58 ± 20 years, with 71% female, giving a prevalence estimate of 37 per 100 000 person-years. This prevalence estimate was projected to approximately 115 000 patients in the United States having hypoparathyroidism of any cause. Hypoparathyroidism was caused by neck surgery in 78% of cases, other secondary causes in 9%, familial disorders in 7%, and without an identified cause in 6%.
Table 1.
Epidemiology of Hypoparathyroidism
| Hypoparathyroidism | Postsurgical Hypoparathyroidism | Nonsurgical Hypoparathyroidism | |
|---|---|---|---|
| Prevalence | 37/100 000 person-years (5)a | 22/100 000 person-years (6)b | 2.3/100 000 person-years (8)b |
| 29/100 000 person-years (5)a | 8/100 000 person-years (5)a | ||
| Incidence | 0.8/100 000 person-years (6)b | ||
| Mortality | HR, 0.98; 95% CI, 0.76–1.26 (6)b | HR, 1.25; 95% CI, 0.90–1.73 (8)b | |
| Risk of hospitalization for complications and comorbiditiesb | |||
| Renal disease of all types | HR, 3.67; 95% CI, 2.41–5.59 (6)b | HR, 3.39; 95% CI, 1.67–6.88 (8)b | |
| Renal insufficiency | HR, 3.10; 95% CI, 1.73–5.55 (6)b | HR, 6.01; 95% CI, 2.45–14.75 (8)b | |
| Renal stones | HR, 4.02; 95% CI, 1.64–9.90 (6)b | ||
| Any cardiovascular disease | HR, 1.91; 95% CI, 1.29–2.81 (8)b | ||
| Ischemic cardiovascular disease | HR, 1.09; 95% CI, 0.83–1.45 (6)b | HR, 2.01; 95% CI, 1.31–3.09 (8)b | |
| Neuropsychiatric disease | HR, 1.26; 95% CI, 1.01–1.56 (7)b | HR, 2.45; 95% CI, 1.78–3.35 (8)b | |
| Depression and bipolar disease | HR, 2.01; 95% CI, 1.16–3.50 (7)b | ||
| Infection | HR, 1.42; 95% CI, 1.20–1.67 (7)b | HR, 1.94; 95% CI, 1.55–2.44 (8)b | |
| Seizures | HR, 3.82, 95% CI, 2.15–6.79 (7)b | HR, 10.05; 95% CI, 5.39–18.72 (8)b | |
| Cataracts | HR, 1.17; 95% CI, 0.66–2.09 (7)b | HR, 4.21; 95% CI, 2.13–8.34 (8)b | |
| Fractures | HR, 1.03; 95% CI, 0.83–1.29 (7)b | ||
| Upper extremity fractures | HR, 0.69; 95% CI, 0.49–0.97 (7)b | HR, 1.93; 95% CI, 1.31–2.85 (8)b | |
| Intracranial calcifications | 56% (21) | 69–74% (18–20) | |
| Malignancy | HR, 0.83; 95% CI, 0.61–1.13 (7)b | HR, 0.44; 95% CI, 0.24–0.82 (8)b |
United States;
Denmark.
Only a few data exist on the epidemiology of hypoparathyroidism in Europe. In a recent nationwide Danish historic cohort study, the prevalence of hypoparathyroidism was estimated using data from the Danish National Patient Registry (6–8). This study assessed mortality and comorbidities by comparing patients with age- and sex-matched population-based controls. A total of 1849 patients with postsurgical hypoparathyroidism and 180 patients with nonsurgical hypoparathyroidism were identified, among whom 1127 and 123 subjects, respectively, were alive at the time of follow-up. The estimated prevalence of postsurgical and nonsurgical hypoparathyroidism was 22/100 000 and 2.3/100 000, respectively (Table 1). Of the postsurgical cases, approximately 33% had acquired postsurgical hypoparathyroidism due to surgery for malignant diseases (mainly thyroid cancer), 33% due to surgery for nontoxic goiter, 25% due to surgery for toxic goiter, and 10% due to surgery for primary hyperparathyroidism (5).
In Hungary, a single state health insurance company insures practically everyone, with the population of 10 million maintained in a single database. Analysis of this database from 2004–2013 demonstrated a diagnosis-based prevalence estimate of approximately 1000 patients with chronic hypoparathyroidism in 2013. The yearly prevalence of hypoparathyroidism increased by more than 60% over the observational period, whereas the female to male ratio of 4:1 remained stable. Regional differences and trends in the incidence of hypoparathyroidism were relatively constant across the country, after adjusting for the population of each region. Postsurgical hypoparathyroidism is thought to be the most common cause of hypoparathyroidism in Hungary, as in other countries (9). There is an annual average of 5000 thyroid-related operations in Hungary. Transient postsurgical hypoparathyroidism is estimated to occur in 31% of these cases, whereas permanent parathyroid hypofunction is estimated to occur in 1.9% as an average. However, surgical centers with experienced endocrine surgeons report lower rates of post-thyroid surgical permanent hypoparathyroidism of as low as 0.1%, with some centers reporting up to 5.8%. A number of confounding factors are thought to influence these estimates. The incidence of hypoparathyroidism increases dramatically after a second neck operation in Hungary, and is thought to be around 15%.
Postsurgical hypoparathyroidism is thought to be the cause of 95% of cases in Russia, somewhat higher than in other countries (10). Estimates of rates of postsurgical hypoparathyroidism vary between 1 and 40% (11).
Incidence
Acquired hypoparathyroidism typically occurs after removal of, irreversible damage to, or vascular compromise of the parathyroid glands. The incidence of postsurgical hypoparathyroidism depends on the center, type of intervention, and surgical expertise. Transient postsurgical hypoparathyroidism lasting <6 months is estimated to occur in 25.4–83% of patients worldwide after neck surgery (12), whereas permanent postsurgical hypoparathyroidism, defined as lasting more than 6 months, has been estimated to occur in approximately 0.12–4.6% of cases (13). Incidence estimates of nonsurgical causes of hypoparathyroidism are generally not available in the United States due to the rarity of these causes.
In the Danish historic cohort study, the incidence of postsurgical hypoparathyroidism was reported to be 0.8/100 000/y (6) (Table 1). No other European or international studies have reported the incidence of postsurgical hypoparathyroidism. Estimates of nonsurgical causes of hypoparathyroidism are generally not available in most other European countries, but the incidence of autoimmune hypoparathyroidism due to autoimmune polyendocrinopathy syndrome type 1 in Hungary is estimated at 1 per million (14).
Cost and hospitalization
The population-based study by Leibson et al (15) quantitated the overall cost of medical care for patients with hypoparathyroidism in Olmsted County, Minnesota. The yearly cost of medical care for patients with hypoparathyroidism in 2007–2009 was estimated to be about three times that for healthy patients. This study did not quantify the costs related to, or the frequency of utilization of, outpatient clinics, hospitals, emergency departments, or pharmacies. No other studies have yet addressed the frequency of hospitalization of patients with hypoparathyroidism relative to normal controls, but it is probable that hospitalization for complications of hypoparathyroidism, such as tetany, bronchospasm, laryngospasm, seizures, or cardiac dysrhythmias, is increased. If one were to factor in these items in cost estimates for care of patients with hypoparathyroidism, they are likely to be much higher than the Olmsted County experience.
Morbidities
Various morbidities associated with hypoparathyroidism are related directly to hypocalcemia and/or hyperphosphatemia or indirectly to treatment, the latter due to excessive or insufficient amounts of calcium and active vitamin D. When patients are not adequately treated, symptoms and signs of neuromuscular excitability (tetany) due to hypocalcemia are common. When patients receive excessive amounts of calcium and vitamin D, hypercalcemia and/or hypercalciuria can result. Alterations in quality of life such as sense of well-being and mood can be related either to the disease itself or to its treatment. This is also true for ectopic calcification that can occur in the basal ganglia and the gray-white matter interface in the brain and in the kidney. Other complications of hypoparathyroidism can include posterior subcapsular cataracts and reduced skeletal remodeling. Clarke et al (5) demonstrated that in Olmsted County, patients with hypoparathyroidism were significantly more likely than healthy age- and sex-matched controls to have at least one diagnosis within seven of 17 major categories of disease, and 16 of 113 subcategories of disease within the major categories, as defined in the International Classification of Diseases, Version 9, Clinical Modification (ICD-9-CM) system. Mitchell et al (16) evaluated the prevalence of various morbidities associated with chronic hypoparathyroidism in a large Boston health system from 1988–2009. A total of 120 patients aged 52 ± 19 years were identified, with 73% female. Of the 54 patients who had renal imaging during follow-up, 31% had renal calcifications. Of 31 patients with head imaging, 52% had basal ganglia calcifications. Stage 3–5 chronic kidney disease was 2- to 17-fold greater than age-appropriate normal values.
In the Danish national cohort study (6), renal disease risk of all types compared to the general population was more than 3-fold higher in patients with postsurgical (hazard ratio [HR], 3.67; 95% confidence interval [CI], 2.41–5.59) and nonsurgical hypoparathyroidism (HR, 3.39; 95% CI, 1.67–6.88) (Table 1). Risk of renal insufficiency was 3-fold higher in postsurgical (HR, 3.10; 95% CI, 1.73–5.55) and 6-fold higher in nonsurgical hypoparathyroidism (HR, 6.01; 95% CI, 2.45–14.75). Patients with postsurgical hypoparathyroidism had a 4-fold increased risk of being hospitalized due to renal stone disease (HR, 4.02; 95% CI, 1.64–9.90). Cardiovascular disease was not increased in postsurgical hypoparathyroidism, but patients with nonsurgical hypoparathyroidism had a significantly increased risk of ischemic heart disease (HR, 2.01; 95% CI, 1.31–3.09) and any cardiovascular disease (HR, 1.91; 95% CI, 1.29–2.81). Compared with the general population, a higher proportion of patients with nonsurgical hypoparathyroidism had been hospitalized due to stroke (HR, 1.84; 95% CI, 0.95–3.94; P = .03) or arrhythmia (HR, 1.78, 95% CI, 0.96–3.30; P = .03).
Hospitalization for neuropsychiatric disease in the Danish national cohort study (7) was significantly increased by a factor of 2.45 in patients with postsurgical as well as nonsurgical hypoparathyroidism (HR, 2.45, 95% CI, 1.78–3.35) (Table 1). Among patients with surgical hypoparathyroidism, the risk of depression and bipolar disorders was significantly increased (HR, 2.01; 95% CI, 1.16–3.50). The risk of being hospitalized due to an infection was significantly increased among patients with postsurgical (HR, 1.42; 95% CI, 1.20–1.67) and nonsurgical hypoparathyroidism (HR, 1.94; 95% CI, 1.55–2.44). Risk of urinary tract infections was borderline significantly increased in postsurgical (HR, 1.36; 95% CI, 0.97–1.91) and significantly increased in nonsurgical hypoparathyroidism (HR, 3.84; 95% CI, 2.24–6.60). Risk of hospitalization due to infection remained significantly increased after exclusion of hospitalizations due to urinary tract infections. Calcium is known to serve many physiological functions, so it is not surprising that hypocalcemia might influence the immune response, and perhaps lead to increased risk of infections. Calcium acts as a second messenger in neutrophils, which in part depends on extracellular calcium (17).
Hospitalization for seizures was significantly increased in postsurgical (HR, 3.82, 95% CI, 2.15–6.79) as well as nonsurgical hypoparathyroidism (HR, 10.05; 95% CI, 5.39–18.72). Cataracts were significantly increased in nonsurgical hypoparathyroidism (HR, 4.21; 95% CI, 2.13–8.34), but not in postsurgical hypoparathyroidism (HR, 1.17; 95% CI, 0.66–2.09). Overall, risk of any fracture, as well as risk of fracture at specific skeletal sites, did not differ between patients and controls. However, in postsurgical hypoparathyroidism, the risk of upper extremity fracture was significantly decreased (HR, 0.69; 95% CI, 0.49–0.97). In nonsurgical hypoparathyroidism, risk of fracture of the upper extremities was significantly increased compared to the general population (HR, 1.93; 95% CI, 1.31–2.85), including risk of fractures at the forearm (HR, 2.83; 95% CI, 1.43–5.63) and proximal humerus (HR, 2.81; 95% CI, 1.34–5.85). No clear reasons were identified for the discrepancy in fracture risk seen between postsurgical and nonsurgical hypoparathyroidism.
In a small case series from Denmark (18), the presence of intracranial calcifications was systematically investigated by computed tomography imaging in 16 patients with nonsurgical hypoparathyroidism and eight patients with PHP. Calcifications were present in 69% of the patients with nonsurgical hypoparathyroidism and in all patients with PHP. In all 19 patients with intracerebral calcifications, the globus pallidus was affected. In five patients, calcifications were found only in this region, whereas the remaining 14 patients also had calcifications in the caudate nucleus. The putamen was affected in 11 cases, thalamus in 10, and cerebral cortex in nine. Calcification in the cerebellum and brainstem was found in four and three cases, respectively. Similar findings were reported in a study from India (19) including 145 patients with nonsurgical hypoparathyroidism, among whom 74% had intracranial calcifications. In this study, independent predictors of progression of calcification were a history of seizures at presentation and the calcium/phosphorus ratio during follow-up. Intracranial calcifications have also been reported in long-standing postsurgical hypoparathyroidism and may be associated with Parkinson-like symptoms (20), but these symptoms resolved with control of blood calcium. In a small case series of nine patients with postsurgical hypoparathyroidism, calcifications were detected by computed tomography imaging in five of the patients (21). These studies suggest that hypoparathyroidism is commonly associated with intracerebral calcifications, but the association between these calcifications and Parkinson-like symptoms is not yet established.
In the Danish cohort study (7), the risk of gastrointestinal cancer was significantly decreased in postsurgical hypoparathyroidism (HR, 0.63; 95% CI, 0.44–0.93), with a tendency toward lower risk of any malignant disease (HR, 0.83; 95% CI, 0.61–1.13). Risk of malignant disease was also significantly decreased in nonsurgical hypoparathyroidism (HR, 0.44; 95% CI, 0.24–0.82).
A number of the comorbidities seen in hypoparathyroidism are related to extraskeletal calcifications, such as cataracts, intracerebral calcifications, and renal stones or nephrocalcinosis. The increased risk of cardiovascular disease in nonsurgical hypoparathyroidism may be related to an increased tendency to precipitate calcium salts in vascular tissues. In the Danish cohort study, patients with postsurgical hypoparathyroidism (6) had a median duration of disease of only 8 years. Patients in the Danish cohort study with nonsurgical hypoparathyroidism (8) were of mean age 49.7 years, and most had had nonsurgical hypoparathyroidism since birth. More studies are needed to assess whether long-standing postsurgical hypoparathyroidism increases the risk of cardiovascular disease similar to nonsurgical hypoparathyroidism.
Conventional treatment of hypoparathyroidism with calcium and active vitamin D analogs causes an increase in serum calcium and relief of classical symptoms of hypocalcemia. However, although serum calcium levels improve to the low-normal range, they typically do not completely normalize, and calcium and phosphorus homeostasis does not normalize in a physiological manner in response to conventional treatment.
Mortality
The effects of chronic hypocalcemia, intermittent hypercalcemia, hypercalciuria, and multiple comorbidities on mortality in patients with hypoparathyroidism are not yet certain. No studies have yet quantified overall or cause-specific mortality due to hypoparathyroidism in the United States. Analyses of mortality and comorbidities among patients with postsurgical hypoparathyroidism in the Danish historical cohort study (6) were limited to patients who developed hypoparathyroidism after neck surgery for nonmalignant diseases (toxic or nontoxic goiter, or primary hyperparathyroidism), and also excluded patients with postsurgical hypoparathyroidism after parathyroidectomy due to severe renal insufficiency. Analyses were adjusted for history of the disease in question before the diagnosis of postsurgical hypoparathyroidism. Mortality was not increased among patients with postsurgical (HR, 0.98; 95% CI, 0.76–1.26) or nonsurgical hypoparathyroidism (HR, 1.25; 95% CI, 0.90–1.73), so the available evidence does not support an association between mortality and hypoparathyroidism.
Diagnosis
Hypoparathyroidism is characterized by hypocalcemia, defined as a total serum calcium below the lower limit of normal and hyperphosphatemia, both of which result from deficiency in circulating PTH (22, 23). By contrast, PHP is characterized by hypocalcemia and elevated levels of PTH (23–27).
Hypoparathyroidism
In hypoparathyroidism, serum concentrations of PTH are typically low or undetectable, but in very rare cases, elevated levels of a mutant form of PTH can be measured with certain assays (22, 23, 28). The concentrations of 1,25-dihydroxyvitamin D [1,25(OH)2 vitamin D] and bone turnover markers including alkaline phosphatase activity are usually in the low normal to low range (Figure 1 and Table 2) (22, 23, 29, 30). The daily urinary excretion of calcium is reduced when patients are hypocalcemic, although the fractional excretion of calcium is increased (22, 23). Nephrogenous cAMP excretion is low, and renal tubular reabsorption of phosphate is elevated. Urinary cAMP, plasma cAMP, and urinary phosphate excretion increase markedly after administration of exogenous bioactive PTH (modified Ellsworth-Howard test based on Chase-Aurbach test) (Table 2) (22, 23, 26, 29). Such measurements of urinary cAMP and plasma cAMP are rarely performed now and are usually only undertaken by specialist centers, if required, to distinguish between the different forms of hypoparathyroidism and PHP (Table 2). Hypocalcemia may result from agenesis (eg, GCM2 mutation) or destruction of the parathyroid glands (eg, after neck surgery, in autoimmune diseases), from reduced secretion of PTH (eg, neonatal hypocalcemia or hypomagnesemia), resistance to PTH (which may occur secondary to hypomagnesemia or as a primary disorder, eg, a variant of PHP), or due to mutations in the PTH gene that impair synthesis or bioactivity of PTH (22, 31). In addition, hypoparathyroidism may occur as a component of a complex inherited syndromic disorder (Table 3) that may be either a complex congenital defect (eg, DiGeorge syndrome) or an autoimmune disorder (Figure 2) (22). Hypoparathyroidism may also occur as a nonsyndromic solitary endocrinopathy, which has been referred to as isolated or idiopathic hypoparathyroidism. Familial occurrences of isolated hypoparathyroidism with autosomal dominant, autosomal recessive, and X-linked recessive inheritances have been established (22).
Figure 1. Clinical approach to investigation of causes of hypocalcemia.
CKD, chronic kidney disease; Cr, creatinine; eGFR, estimated glomerular filtration rate; 25(OH)D, 25-hydroxyvitamin D; CK, creatine kinase. [Adapted from R. V. Thakker: The parathyroid glands, hypercalcemia, and hypocalcemia. In: Goldman L, Shafer AI, eds. Goldman-Cecil Medicine. 25th ed. Atlanta, GA: Elsevier Ltd; 2016:1649–1661 (67), with permission.]
Table 2.
Clinical, Biochemical, and Genetic Features of Hypoparathyroid and Pseudohypoparathyroid Disorders
| Hypoparathyroidism | PHP |
|||||
|---|---|---|---|---|---|---|
| PHP1A | PPHP | PHP1B | PHP1C | PHP2 | ||
| AHO manifestations | No | Yes | Yes | No/rarely | Yes | No |
| Serum calcium | ↓ | ↓ | N | ↓ | ↓ | ↓ |
| Serum PO4 | ↑ | ↑ | N | ↑ | ↑ | ↑ |
| Serum PTH | ↓ | ↑ | N | ↑ | ↑ | ↑ |
| Response to PTH | ||||||
| Urinary cAMPa (Chase-Aurbach test) | ↑ | ↓ | ↑ | ↓ | ↓ | ↑ |
| Urinary PO4 (Ellsworth-Howard test) | ↑ | ↓ | ↑ | ↓ | ↓ | ↓ |
| Gsα activity | N | ↓ | ↓ | N | N | N |
| Inheritance | AD/AR/X | AD | AD | AD/sporadic | AD | Sporadic |
| Molecular defect | PTH/CaSR/GATA3/GCM2/others | GNAS | GNAS | STX16/GNASb | GNAS | ? cAMP targets |
| Other hormonal resistance | No | Yes | No | In some patients | Yes | No |
Abbreviations: ↓, decreased; ↑, increased; N, normal; AD, autosomal dominant; AR, autosomal recessive; X, X-linked, AHO, Albright's hereditary osteodystrophy presumed, but not proven.
Plasma cAMP responses are similar to those of urinary cAMP.
Involves deletions that are located upstream of GNAS.
[Adapted from R. V. Thakker: The parathyroid glands, hypercalcemia, and hypocalcemia. In: Goldman L, Shafer AI, eds. Goldman-Cecil Medicine. 25th ed. Atlanta, GA: Elsevier Ltd; 2016:1649–1661 (67), with permission.]
Table 3.
Genetic Disorders Associated With Hypoparathyroidism
| Disease | Inheritance | Gene/Protein | Chromosomal Location |
|---|---|---|---|
| Syndromic forms | |||
| Hypoparathyroidism associated with polyglandular autoimmune syndrome (APECED) | Autosomal recessive | AIRE-1 | 21q22.3 |
| DiGeorge type 1 | Autosomal dominant | TBX1 | 22q11.2/10p |
| DiGeorge type 2 | Autosomal dominant | NEBL | 10p13-p12 |
| CHARGE | Autosomal dominant | CHD7, SEMA3E | 8q12.1-q12.2, 7q21.11 |
| HDR syndrome | Autosomal dominant | GATA3 | 10p14 |
| Kenney-Caffey type 1, Sanjad-Sakati | Autosomal dominant/recessive | TBCE | 1q42.3 |
| Kenney-Caffey type 2 | Autosomal recessive | FAM111A | 11q12.1 |
| Barakat | Autosomal recessivec | Unknown | ? |
| Dubowitz | Autosomal recessivec | Unknown | ? |
| Bartter type 5 | Autosomal dominant | CaSR | 3q21.1 |
| Lymphedema | Autosomal recessive | Unknown | ? |
| Nephropathy, nerve deafness | Autosomal dominantc | Unknown | ? |
| Nerve deafness without renal dysplasia | Autosomal dominant | Unknown | ? |
| Hypoparathyroidism associated with KSS, MELAS and MTPDS | Maternal | Mitochondrial genome | |
| Nonsyndromic forms | |||
| Isolated hypoparathyroidism | Autosomal dominant | PTH, GCMB | 11p15,a 6p24.2 |
| Autosomal recessive | PTH, GCMB | 11p15,a 6p24.2 | |
| X linked recessive | SOX3b | Xq26–27 | |
| ADH1 | Autosomal dominant | CaSR | 3q21.1 |
| ADH2 | Autosomal dominant | Gα11 | 19p13 |
Claudin 16 (CLDN16), Claudin 19 (CLDN19), and transient receptor potential cation channel, subfamily M, member 6 (TRPM6) whose mutations are associated with hypomagnesemia and thereby impairment of PTH secretion, are not included. ?, Not defined.
Mutations of PTH gene identified only in some families.
Deletion-insertion in possibly regulatory region.
Most likely inheritance shown, chromosomal location of the mutant gene not known.
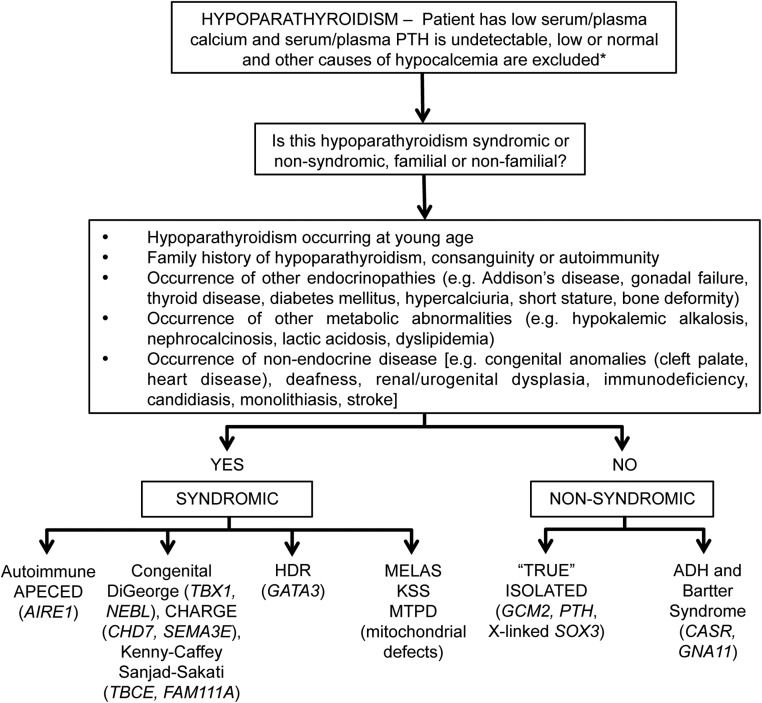
Figure 2. Clinical approach to establishing the genetic etiology of hypoparathyroidism.
The genes for each disorder are indicated in italics, and additional details are provided in Table 1. *, In PHP the plasma PTH is high. [Adapted from Thakker RV, Bringhurst FR, Juppner H. Regulation of calcium homeostasis and genetic disorders that affect calcium metabolism. In: De Groot LJ, Jameson JL, eds. Endocrinology. 7th ed. Philadelphia, PA: Elsevier; 2016:1063–1089 (67) with permission.]
Pseudohypoparathyroidism
The hallmark of PHP is resistance to PTH, which may occur due to a variety of defects (24–26). Currently defined postreceptor defects that lead to PTH resistance and the biochemical hallmarks of hypocalcemia, hyperphosphatemia, and elevated serum levels of PTH include PHP type 1a (PHP1A; GNAS mutations affecting exons 1–13), PHP type 1b (PHP1B) (GNAS methylation abnormalities), PHP type 2 (PHP2), acrodysostosis type I and type II (mutations in the regulatory subunit of protein kinase A and the phosphodiesterase PDE4D, respectively, with the type 1 form resembling PHP2); and PHP type 1c (PHP1C; GNAS mutations affecting exon 13), which is a variant of PHP1A (32–34). The most extreme example of PTH resistance is observed when both alleles encoding the type 1 PTH receptor are mutated, as in Blomstrand lethal chondrodysplasia (22, 25). This is not a form of PHP, however.
Patients with PHP1 exhibit resistance to PTH in the proximal renal tubule and show an impaired increase in serum and urinary cAMP and urinary phosphate after administration of PTH; patients with PHP1A (and less frequently with PHP1B) also manifest the features of Albright's hereditary osteodystrophy (AHO), which includes short stature, subcutaneous ossifications, variable degrees of reduced mental acuity, round faces, dental hypoplasia, and brachydactyly (ie, shortening of the metacarpals and metatarsals, particularly of the third, fourth, and fifth metacarpals and metatarsals) that is not present at birth, but usually becomes evident by the second decade of life (24–26, 35). In addition to brachydactyly, other skeletal abnormalities of the long bones also occur. The absence of a normal rise in urinary excretion of nephrogenous cAMP after an infusion of PTH in PHP1 indicates a defect at some site of the PTH receptor-adenylyl cyclase system (24–26, 35). Responsiveness to PTH is regulated by at least two heterotrimeric G proteins: the stimulatory G protein (Gs) couples the PTH receptor to adenylyl cyclase and thus stimulates formation of cAMP, while Gq/11 couples this receptors to phospholipase C with subsequent formation of inositol trisphosphate (24–26). There is, however, decreased expression or activity of the α-subunit of Gs (Gsα) encoded by GNAS that causes patients with PHP1A to be resistant to PTH and frequently other hormones, for example TSH, gonadotropins (FSH and LH), and hypothalamic neurotransmitters that lead to neurocognitive defects and obesity, because these hormones require Gsα to couple their receptors to adenylyl cyclase (24–26, 35, 36). Patients with PHP1B typically exhibit only PTH resistance, and only a few have the somatic features of AHO (24, 26). Patients with PHP1C have genetic Gsα mutations that are not detected by conventional assays assessing Gsα activity but are otherwise indistinguishable from PHP1A patients, ie, impaired urinary excretion of nephrogenous cAMP and phosphate after PTH administration, and typically AHO features (24, 26, 35). Patients with pseudoseudohypoparathyroidism (PPHP) have most of the somatic features of AHO that are observed in PHP1A, but without hormonal resistance (Table 2) (24–26, 31–35). In contrast, PHP2 patients have no somatic features of AHO and show a conserved cAMP response to PTH but no phosphaturic response, consistent with a molecular defect distal to cAMP generation in the PTH-mediated signal transduction pathway (24, 26, 35). The defective renal phosphaturic response to PTH in patients with PHP2 may be the consequence of unrecognized vitamin D deficiency, which leads to a reversible form of PTH resistance, or of acrodysostosis (Table 4) (24, 28, 35).
Table 4.
Classification of PHP and Related Disorders
| Molecular Defect | Parental Origin of GNAS Mutation | Endocrine Defects | Clinical Features | Other Features | |
|---|---|---|---|---|---|
| PHP1A | Heterozygous mutations in GNAS gene that reduce expression or function of Gαs | Maternal | Multihormone resistancea | 1. AHOb; 2. early onset obesity | Cognitive disability, short stature |
| PPHP | Heterozygous mutations in GNAS gene | Paternal | None | AHO | Small for gestational age at birth, short stature |
| PHP1C | Heterozygous mutations in GNAS that reduce expression or function of Gs | Maternal | Multihormone resistance | 1. AHO; 2. early onset obesity | Cognitive disability, short stature |
| Familial PHP1B | Heterozygous deletions in STX16, NESP55, and/or AS exons | Maternal | PTH resistance, partial resistance to TSH or other hormones in some | Mild brachydactyly in some | Loss of methylation at GNAS exon A/B |
| Sporadic PHP1B | Paternal UPD of chromosome 20q in some, but most cases are unresolved | N/A | PTH resistance, partial resistance to TSH or other hormones in some | Mild brachydactyly in some | Methylation defect affecting all four GNAS DMRs |
| Progressive osseous heteroplasia | Heterozygous mutations in GNAS gene that reduce expression or function of Gαs | Paternal | None | Progressive heterotopic ossification extending to deep connective tissues | |
| Osteoma cutis | Heterozygous mutations in GNAS gene that reduce expression or function of Gαs | Paternal | None | Heterotopic ossification that is limited to dermis and subcutaneous tissues | |
| PHP type 2 | None known | N/A | PTH resistance | Severe hypocalcemia | Vitamin D deficiency |
| Acrodysostosis type 1 | PRKAR1A | N/A | TSH and PTH resistance | Brachydactyly and facial dysostosis | Obesity |
| Acrodysostosis type 2 | PDE4D | N/A | None or mild PTH resistance | Brachydactyly and facial dysostosis | No obesity |
Abbreviation: N/A, not available; UPD, uniparental disomy.
Multiple hormone resistance, resistance to PTH, TSH, and GHRH, and often to gonadotropins as well.
AHO comprising round face, short stature, brachydactyly/brachymetacarpia, and heterotopic ossification.
In patients affected by PHP, hypocalcemia can be treated more aggressively with calcium supplements and active vitamin D analogs than in individuals affected by hypoparathyroidism because there is less risk of developing hypercalciuria (24, 26). Thus, calcium and 1,25(OH)2 vitamin D therapy may be given at doses that result in normal blood calcium levels as long as PTH levels remain at the upper end of normal or are only mildly elevated. Failure to reduce PTH levels close to the normal range results in persistently elevated bone turnover and may even lead to tertiary hyperparathyroidism (24, 26, 27). Patients with PHP1A/C or PHP1B generally do not develop hypercalciuria when circulating PTH levels remain near the upper end of the normal range because the distal renal tubule retains the ability to respond to PTH, thus reabsorbing calcium from the glomerular filtrate at this site. Hence, it is important to avoid treatment with amounts of calcium and 1,25(OH)2 vitamin D that suppress PTH levels below the normal range because this can result in the loss of PTH action in the distal tubules and in hypercalciuria and increased risk of developing nephrocalcinosis and nephrolithiasis (24, 26).
The diagnosis of these hypocalcemic disorders has been greatly facilitated by the advent of improved PTH assays (37, 38) and the identification of genes that are responsible for many of these disorders (22). This section will therefore focus on the advances in PTH assays and molecular genetics for these disorders.
PTH assays
Low, or inappropriately normal, concentration of serum PTH in association with hypocalcemia is the hallmark of hypoparathyroidism and helps to differentiate this disease from other disorders associated with hypocalcemia (eg, vitamin D deficiency) (Figure 1) (22, 23). Hence, a reliable assay for measuring serum PTH is critical for making the diagnosis.
PTH is synthesized by the parathyroid glands as preproPTH, a 115-amino acid precursor peptide that is subsequently processed and stored in secretory granules as the biologically active PTH(1–84) (22, 37). PTH is released from secretory granules that fuse with the parathyroid cell membranes in response to a decrease in extracellular ionized calcium. Circulating PTH consists of the full-length PTH(1–84) peptide as well as several carboxyl-terminal fragments, most of which being PTH(34–84) and PTH(37–84) (22, 37). These fragments cannot bind to and activate the classic PTH/PTHrP receptor. Although plasma half-life of intact PTH(1–84) is several minutes, renal clearance of PTH fragments is slower (22). Therefore, under normocalcemic conditions, up to 80% of circulating PTH is inactive fragments, and only about 20% is intact, biologically active PTH (38). This abundance of inactive PTH fragments, which arises from proteolytic cleavage of intact PTH either within the parathyroid glands or peripherally (eg, in hepatic Kupffer cells), has made it a challenge to establish reliable assays for measurement of the intact, biologically active form of PTH (34). The first-generation PTH assays were RIAs, which used antibodies that were developed using parathyroid extracts from various species (37, 38). Epitopes were later found to recognize mid- and C-terminal parts of the PTH molecule. Although this assay for the first time allowed for the measurement of PTH, it detected not only intact PTH but also the bioinactive C-terminal PTH fragments that represented the preponderance of PTH immunoactivity, thereby limiting its utility (37). To improve clinical utility, a two-site immunoradiometric assay was introduced (the intact PTH assay) (37). This sandwich assay uses an antibody directed against the carboxyl-terminal PTH portion that is linked to a solid phase for capture and an antibody directed against an epitope within the PTH(1–34) portion for detection, thereby facilitating the measurement of PTH(1–84) without interference by carboxyl-terminal fragments. Several variants of this “second-generation” PTH assay are commercially available, and these usually use an enzyme-linked, rather than a radiolabeled detection antibody, some of which are directed against the (15–34) portion of PTH (28, 37). “Third-generation” PTH assays, also referred to as “whole PTH” or “biointact PTH” assays, use similar antibodies directed against the carboxyl-terminal portion of PTH for capture, but an antibody directed against the extreme amino-terminal amino acid residues 1–3 or 1–6 for detection (37). Such PTH assays therefore detect the full-length PTH(1–84), but not large, amino-terminally truncated fragments, such as PTH(7–84), which lack bioactivity. Interestingly, the third-generation assays, whereas theoretically better, have not proved to be superior to second-generation assays in clinical practice. This has been disappointing for the evaluation of patients with chronic kidney disease (38–40) in which such large inactive fragments accumulate. Furthermore, third-generation assays detect an insufficiently characterized form of PTH, which is thought to be phosphorylated at serine 17 (41). For the diagnosis of hypoparathyroidism and other conditions associated with hypocalcemia (22, 38), second-generation “intact PTH” assays are usually satisfactory.
A recent report illustrates the importance of carefully defining the specificity of currently available PTH assays. Genetic analysis of three siblings, who had presented with severe hypocalcemia, revealed a homozygous arginine-to-cysteine mutation at position 25 (R25C) of the mature PTH(1–84) polypeptide that reduces the hormone's biological activity (28). Measurement with the second-generation “intact” PTH assay revealed low hormone levels for the two untreated, asymptomatic patients, whereas considerably elevated PTH levels were measured with a third-generation assay. This discrepancy in circulating concentrations of immunoreactive PTH is caused by the substitution of cysteine for arginine at position 25, which is part of the epitope recognized by the second-generation detection antibody and thus interferes with antibody binding to the captured peptide. By contrast, the R25C mutation does not interfere with binding of the detection antibody used in third-generation PTH assays. Therefore, depending on the assay that is used for evaluating these patients, two very different diagnoses, namely hypoparathyroidism or PHP, could have been made.
Genetics of hypoparathyroidism and pseudohypoparathyroidism
The genetics of syndromic and nonsyndromic forms of hypoparathyroidism and PHP, the value of genetic testing in clinical practice, indications for mutational analysis, and the clinical approach to gene testing in a patient are reviewed below.
Hypoparathyroidism
Genetic forms of hypoparathyroidism may occur as part of syndromic disorders or as a nonsyndromic solitary endocrinopathy, which is called isolated or idiopathic hypoparathyroidism (Table 3).
Syndromic forms of hypoparathyroidism include: autoimmune polyglandular syndrome type 1 in which hypoparathyroidism occurs with candidiasis and Addison's disease; DiGeorge syndrome (DGS), in which hypoparathyroidism occurs with immunodeficiency due to thymic aplasia, congenital heart defects, and deformities of the ear, nose, and mouth; coloboma-heart anomaly-choanal atresia-retardation-genital-ear anomalies (CHARGE) syndrome; hypoparathyroidism-deafness-renal dysplasia (HDR) anomaly in which hypoparathyroidism occurs with sensorineural deafness and renal cysts and impairment; Kenny-Caffey syndrome, in which hypoparathyroidism occurs with short stature, osteosclerosis, cortical thickening of long bones, delayed fontanel closure, basal ganglia calcification, nanophthalmos, and hyperopia; Barakat syndrome, in which hypoparathyroidism occurs with nerve deafness, and a steroid-resistant nephrosis leading to chronic kidney disease; Dubowitz syndrome, in which hypoparathyroidism occurs with intrauterine growth retardation, short stature, microcephaly, mental retardation, eczema, blepharophimosis, ptosis, and micrognathia; Bartter syndrome type 5, in which hypocalcemia is accompanied by hypokalemic acidosis, renal salt-wasting that may lead to hypotension, hyperreninemic hyperaldosteronism, increased urinary excretion of prostaglandin and its metabolites, hypercalciuria, and nephrocalcinosis; Kearns-Sayre syndrome (KSS), in which hypoparathyroidism may occur with progressive external ophthalmoplegia, pigmentary retinopathy, cardiomyopathy, heart block, and sensorineural deafness; the mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes (MELAS) syndrome, in which hypoparathyroidism may occur with diabetes mellitus; and the mitochondrial trifunctional protein deficiency syndrome (MTPDS), a disorder of fatty-acid oxidation in which hypoparathyroidism may occur in association with peripheral sensorimotor polyneuropathy and a dilated cardiomyopathy (22, 42–45). These syndromic forms of hypoparathyroidism are due to mutations as follows: autoimmune polyglandular syndrome type 1 is caused by mutations of the autoimmune regulator 1 (AIRE1) gene, encoding a 545-amino acid protein that mediates E3 ubiquitin ligase activity and eliminates organ-specific T cells in the thymus; DGS type 1 (DGS1) is due to abnormalities of TBX1, which is a DNA-binding transcription factor of the T-Box family, whereas DGS2 is likely caused by mutations of the nebulette (NEBL) gene; CHARGE syndrome in most patients is due to mutations of chromodomain helicase DNA-binding protein 7 (CHD7), a transcriptional regulator that binds to enhancer elements in the nucleoplasm, and a minority of patients may have abnormalities involving sempahorin 3E (SEMA3E), that controls cell positioning during embryonic development; HDR is due to mutations of GATA3, a zinc-finger transcription factor; Kenny-Caffey types 1 and 2 are due to mutations of the tubulin-specific chaperone (TBCE) and in FAM111A, a member of the 111 family of proteins, respectively; Bartter syndrome type 5 is due to mutations of the calcium-sensing receptor (CaSR); and KSS, MELAS, and MTPDS are due to mitochondrial mutations and deletions (22, 42–46). The genetic abnormalities causing the Barakat and Dubowitz syndromes and other familial forms of syndromic hypoparathyroidism, which may be associated with lymphedema, nerve deafness, developmental delay, nephropathy, mitral valve prolapse, or brachytelephalangy, remain to be identified (22).
Nonsyndromic (ie, isolated) forms of hypoparathyroidism may be inherited as autosomal dominant, autosomal recessive, and X-linked recessive disorders and involve abnormalities of the following gene products: glial cells missing 2 (GCM2), a parathyroid-specific transcription factor; CaSR; the α-subunit of the G11 signaling protein (GNA11); PTH; and SOX3, a high-mobility group box transcription factor (22, 42, 46–50). Gain-of-function CaSR mutations result in autosomal dominant hypocalcaemia type 1 (ADH1), and patients affected by this disorder generally have normal serum PTH concentrations and hypomagnesemia. Treatment with vitamin D or its active metabolites to correct the hypocalcemia may result in marked hypercalciuria, nephrocalcinosis, nephrolithiasis, and renal impairment. Heterozygous gain-of-function GNA11 mutations result in ADH2, which resembles ADH1.
Pseudohypoparathyroidism
PHP1 variants and PPHP are caused by heterozygous mutations in GNAS, which is a complex transcriptional unit that has several alternative first exons that are alternatively spliced to the same downstream exons, antisense transcripts, and reciprocal, parent-specific methylation imprints (22, 24–26). The GNAS complex and the specific abnormalities resulting in PHP1 variants and PPHP will be reviewed (51).
GNAS is located on chromosome 20q13.3, and exons 1–13 encode Gsα that is synthesized as a 52- or 45-kDa protein based on the inclusion or exclusion of exon 3, respectively (22, 25). Upstream of exon 1 are three alternative first exons that each splice onto exons 2–13 to generate novel transcripts. These include XL, which is expressed only from the nonmethylated paternal allele and which generates a transcript with overlapping open reading frames that encodes XLαs and ALEX (25, 36). XLαs, which is a much larger signaling protein than Gαs (≈78 kDa vs 45–52 kDa), differs from Gαs only in its amino-terminal portion encoded by exon XL. It can interact with receptors for PTH and a variety of other hormones in vitro, but the native receptors that interact with XLαs in vivo are presently unknown. A second alternative promoter encodes the secretory protein NESP55, which is expressed only from the maternal allele and shares no protein homology with Gαs (22, 25, 52). An antisense transcript (AS or Nespas in mice), derived from five exons flanking NESP55, is expressed only from the paternal GNAS allele. A third alternative first exon A/B (also termed exon 1A in mice) is transcribed only from the paternal allele and encodes a protein that either is nontranslated or gives rise to an amino-terminally truncated form of Gαs that may behave as a competitive inhibitor of Gαs action (42). The alternative first exons and AS are associated with promoters that contain differentially methylated regions (DMRs), each of which is methylated on the nonexpressed allele (22, 25, 32, 53). By contrast, Gαs is expressed from both parental alleles in most cells, but in some cells (eg, pituitary somatotrophs, proximal renal tubular cells, thyroid epithelial cells, gonadal cells) Gαs is primarily expressed from the maternal allele (25, 51, 53, 54). There is no DMR that regulates expression of the Gαs transcript through parent-specific methylation, but cis-acting elements that control tissue-specific paternal silencing of Gαs appear to be located within the primary imprint region in exon A/B (53). It is important to note that PTH-dependent bone resorption is maintained in patients affected by PHP1A and PHP1B, and in conjunction with 1,25(OH)2 vitamin D, this allows calcium mobilization and maintenance of relative normocalcemia. However, dissolution of bone leads to the possibility of hyperparathyroid bone disease as well as the release of phosphate and thus, typically more profound hyperphosphatemia than in patients affected by hypoparathyroidism.
PHP1 variants and PPHP are associated with specific genetic abnormalities in the 13 exons of GNAS that encode Gαs, such that mutational analysis of GNAS allows distinction between different variants of PHP1 and related conditions (Table 4) (22, 24, 35, 55). Thus, patients with heterozygous mutations involving exons 1–13 of the maternally-derived GNAS gene have a widespread deficiency of Gαs that results in PHP1A (exons 1–12) or PHP1C (exon 13) (24, 26). Moreover, PHP1A and PHP1C patients have a complex clinical phenotype that includes resistance to multiple hormones (eg, PTH, TSH, gonadotropins, calcitonin, ACTH, and GHRH), mild to moderate intellectual disability, and early-onset morbid obesity due to decreased resting energy expenditure, features that are consistent with the role of Gαs in normal transmembrane signal transduction of many hormones and neurotransmitters (24, 26, 54–58). These diverse features reflect the effect of mutations within the maternal GNAS allele in tissues in which little or no Gαs is produced from the paternal allele (26, 54). By contrast, responsiveness to other hormones (eg, vasopressin) is normal in tissues in which Gαs is transcribed from both parental GNAS alleles. Patients with PHP1A and PHP1C also have the developmental defects of AHO, which include formation of heterotopic membranous bone, short stature, brachydactyly, and various degrees of intellectual disabilities (24, 26, 35, 59–61). Haploinsufficiency of Gαs is likely the basis for brachydactyly, which becomes apparent during the first decade of life and appears to be due in part to premature fusion of epiphyses in tubular and long bones. This implies a requirement of two functional copies of GNAS for normal differentiation and maturation of the growth plate. PPHP, which is characterized by some, but not all, AHO features, without hormonal resistance, is due to paternally inherited GNAS mutations (24, 26). PPHP patients with mutations in exons 2–13 furthermore show severe intrauterine growth retardation, which likely reflects the effect of reduced Gαs and/or absence of XLαs (62). Some patients with GNAS mutations on the paternally derived allele manifest only ectopic ossification, which can be limited to the dermis as osteoma cutis or be more invasive and debilitating as progressive osseous heteroplasia (59, 60, 63–66) (Table 4). Although patients with osteoma cutis and progressive osseous heteroplasia have paternally inherited GNAS defects, the basis for the striking difference in heterotopic ossification, as well as the absence of other features of AHO, remains uncertain (66). Patients with PHP1A, PHP1C, or PPHP may also have clinical similarities to those with acrodysostosis, an equally uncommon skeletal dysplasia characterized by very early and severe brachydactyly that is associated with facial dysostosis, nasal hypoplasia, short stature, and often resistance to multiple hormones (Table 4) (31, 33, 34). Patients with acrodysostosis type 1 have germline mutations in the gene encoding PRKAR1A, the cAMP-dependent regulatory subunit of protein kinase A, that reduce responsiveness of protein kinase A to cAMP; these patients thus have a post-cAMP defect in hormone responsiveness (31, 33). By contrast, patients with acrodysostosis type 2 have mutations in PDE4D, which encodes a class IV cAMP-specific phosphodiesterase that hydrolyzes cAMP; these patients develop hormone resistance due to accelerated degradation of cAMP (33). Although reduced PDE4D activity would be predicted to enhance cAMP signaling, there is a compensatory increase in expression levels of PDE4A and PDE4B isoforms, which accounts for a paradoxical decrease in cAMP levels in patient cells (34).
Patients with autosomal dominant PHP1B have deletions associated with methylation changes affecting either GNAS exon A/B alone (different STX16 deletions or a deletion comprising only GNAS exon NESP) or all four differentially methylated GNAS regions (ie, GNAS deletions comprising exon NESP and/or antisense exons 3 and 4) (26, 51–53, 68, 69). In association with the lack of methylation at the maternal GNAS exon A/B, little or no Gαs is made from the maternal allele (26, 70). In those tissues where Gαs expression from the nonmethylated, paternal GNAS allele is normally silenced, particularly in the proximal renal tubules, virtually no Gαs is thought to be made when an inactivating mutation affects maternal GNAS exons 1–13 (as in PHP1A patients) or when a loss of methylation occurs at GNAS exon A/B (as in PHP1B patients) (26). Because of the Gαs deficiency resulting from these genetic or epigenetic abnormalities, PTH-stimulated synthesis of 1,25(OH)2 vitamin D in the renal proximal tubules is impaired or inappropriately normal, thus leading to reduced intestinal calcium absorption. Due to impaired cAMP generation, there is insufficient PTH-mediated down-regulation of the two sodium-dependent phosphate transporters, NPT2a and NPT2c, and consequently impaired urinary phosphate excretion (26). Taken together, these changes in the proximal renal tubules explain the PTH-resistant hypocalcemia and hyperphosphatemia that occur in PHP1A and PHP1B (26). The importance of GNAS methylation in maintaining Gαs expression was further illustrated by patients affected by the sporadic form of PHP1B, which is the most common form of PHP1B and has not yet been resolved at the molecular level, except for a few cases who have paternal uniparental isodisomy affecting chromosome 20q (patUPD20q) (71–74). Most of the patients with sporadic PHP1B show methylation abnormalities on both GNAS alleles, which can be incomplete. Thus, GNAS methylation changes alone are sufficient to cause hormonal resistance (26). However, although GNAS methylation abnormalities are present at birth, it is interesting to note that these epigenetic changes do not cause elevations in circulating PTH levels until the age of 2–3 years, and that symptomatic hypocalcemia usually does not develop until the second decade of life (26, 52, 69). Furthermore, some PHP1B patients never develop severe PTH resistance, and some seem to “outgrow” their resistance (26, 74). Moreover, some PHP1B patients present with hypothyroidism well before PTH resistance becomes apparent, indicating that silencing of Gαs expression from both nonmethylated parental alleles can be first encountered in the thyroid (36). Thus, the observed variability in disease severity among PHP1B patients is most likely related to temporal and tissue-specific differences in the silencing of paternal Gαs expression.
The genetic abnormalities causing PHP2 remain to be identified (Table 4).
Gene testing in clinical practice
Genetic testing can be performed using DNA obtained from leukocytes, salivary cells, skin cells, or hair follicles. It is important to emphasize that the best clinical practice for such genetic testing should include agreement (ie, informed consent) from the patient and access to genetic counselors. Genetic testing should be performed by accredited centers, some of which can be contacted using the following links: http://www.ncbi.nlm.nih.gov/sites/GeneTests/ (giving details of centers in Canada, Denmark, Greece, Israel, Japan, and the United States); http://www.orpha.net/consor/cgi-bin/index.php, or www.eddnal.com (giving details of centers in Austria, Belgium, Denmark, Finland, France, Germany, Holland, Ireland, Italy, Norway, Portugal, Spain, Sweden, Switzerland, and the United Kingdom). The utility of genetic testing in the hypoparathyroid and pseudohypoparathyroid disorders is reviewed.
Hypoparathyroidism
Genetic testing for mutations in patients with hypoparathyroidism is helpful in clinical practice in several ways that include: 1) confirmation of the clinical diagnosis so that appropriate screening for associated endocrinopathies or organ dysfunction can be undertaken; 2) implementation of appropriate treatment, eg, avoiding vitamin D treatment to restore normocalcemia in ADH1 patients because this will lead to renal complications, and commencement of appropriate hormone replacement such as in patients with Addison's disease; 3) identification of family members who may be asymptomatic but harbor the mutation and therefore require screening for development of endocrinopathies or other disorders and early/appropriate treatment; and 4) identification of the 50% of family members who do not harbor the familial germline mutation and can therefore be reassured and alleviated of the anxiety burden of developing future endocrine disease and other associated disorders (22). This latter aspect cannot be overemphasized because it helps to reduce the cost to the individuals and their children; it also helps health services in not having to undertake unnecessary investigations. One study has reported that overall 35% of 20 patients who had childhood-onset, permanent hypoparathyroidism, which was not due to a chromosome 22q11 deletion, had a mutation in one of three genes (GATA3, GCM2, or CASR) (75). Moreover, among the 13 patients with syndromic hypoparathyroidism, which was associated with deafness and/or renal dysplasia, six (ie, >45%) had a mutation involving GATA3, GCM2, or CASR, and among seven patients with nonsyndromic hypoparathyroidism, two (ie, >25%) had a mutation involving GCM2 (75). These studies indicate that the likelihood of a genetic etiology in hypoparathyroidism is likely to be high. Indications for testing for germline mutations in idiopathic hypoparathyroid patients include: 1) hypoparathyroidism occurring at a young age; 2) occurrence of other endocrinopathies, metabolic abnormalities, or nonendocrine congenital abnormalities (Figure 2); 3) family history of hypoparathyroidism, consanguinity, or autoimmunity; and 4) being a near relative of a known mutation carrier (22, 76). A clinical approach to genetic testing in a patient who has idiopathic hypoparathyroidism is as follows (Figure 2) (22). Patients with hypoparathyroidism in whom there is a high suspicion of a genetic etiology (eg, young age of onset, family history of autoimmunity or consanguinity) should be offered genetic counseling and germline mutation testing of an appropriate candidate gene (eg, AIRE1, TBX1, NEBL, CHD7, SEMA3E, TBCE, FAM111A, GATA3, CASR, GNA11, GCM2, PTH, and mitochondrial genes) (22). Such patients may have de novo mutations, which occur in about 10% of patients, or they may have an undetected family history for the disease. Near relatives of a hypoparathyroid patient with a germline mutation should be identified and offered genetic counseling and appropriate gene testing, and individuals who have inherited the mutation should be offered periodic biochemical screening, even if asymptomatic (22). Near relatives who have not inherited the causative mutation require no further follow-up and may be alleviated of the anxiety associated with the development of hypoparathyroidism and associated disorders (22).
Pseudohypoparathyroidism
Genetic and epigenetic analyses of GNAS as well as mutation analysis of PPKAR1A and PDE4D can help to distinguish between the different variants of PHP and its related disorders (24, 26, 35). Patients affected by PHP1B require testing for GNAS methylation changes; some laboratories will analyze exon A/B only as a screen because the loss of methylation of this DMR on the maternally derived GNAS allele is present in all reported cases of both inherited and sporadic forms of PHP1B (24, 26, 35). Patients with PHP1B can be further analyzed for paternal uniparental isodisomy of chromosome 20q or small deletions within STX16 and GNAS; these tests have furthermore been shown to identify deletions within GNAS as the cause of some PHP1A cases (Table 4) (24, 26, 35).
Conclusions
Recent advances described in this report have clarified certain aspects of the epidemiology and diagnosis of hypoparathyroidism. Postsurgical hypoparathyroidism causes about three-fourths of all cases of recognized hypoparathyroidism. In patients undergoing anterior neck surgery, less than 1–5% experience permanent hypoparathyroidism, although as many as 50% may develop transient hypoparathyroidism. Remaining cases of hypoparathyroidism are due to autoimmune disease, metastatic disease, iron or copper overload, radiation therapy, radioactive iodine treatment, or a variety of rare genetic disorders.
The prevalence of hypoparathyroidism has been quantified recently in the United States and Denmark, and the incidence in Denmark has been quantified. Most other countries lack these data currently. Mortality does not appear to be increased. Complications of hypoparathyroidism include chronic kidney disease, kidney stones or nephrocalcinosis, seizures, posterior subcapsular cataracts, and intracerebral calcifications. Infections may be increased in this disorder. Cardiovascular disease, fractures, and malignancy do not appear to be increased, except for increased upper extremity fractures in nonsurgical hypoparathyroidism. Gastrointestinal malignancies may be reduced in hypoparathyroidism. A large international registry would help to better define the prevalence, comorbidities, and best treatment of hypoparathyroidism.
Diagnosis of the genetic causes of hypoparathyroidism and PHP has been significantly clarified in recent years, with recognition of an increasing variety of different genes affecting parathyroid gland differentiation and function. Assessment for most of the recognized mutations can now be done by research or commercially available genetic testing. Research for new mutations causing hypoparathyroidism continues.
Acknowledgments
Disclosure Summary: B.L.C. receives research support from Shire Pharmaceuticals and serves on two Data Monitoring Committees: Denosumab Oncology and Glucocorticoid-Induced Osteoporosis (Amgen). E.M.B. has a financial interest in the calcimimetic, Cinacalcet. M.A.L. is a member of the Scientific Advisory Board and receives research support from Shire Pharmaceuticals. M.M.M. is a member of the Scientific Advisory Board for Shire Pharmaceuticals. J.P.B. is a consultant for Amgen, Radius, Lilly, and Merck and receives research support from Shire Pharmaceuticals. R.V.T. serves in an advisory capacity for ENETS, Novartis, and Ipsen and is a consultant for AstraZeneca. The other authors have no disclosures to report.
- ADH1
- autosomal dominant hypocalcaemia type 1
- AHO
- Albright's hereditary osteodystrophy
- CaSR
- calcium-sensing receptor
- CHARGE
- coloboma-heart anomaly-choanal atresia-retardation-genital-ear
- CHD7
- chromodomain helicase DNA-binding protein 7
- CI
- confidence interval
- DGS
- DiGeorge syndrome
- DMR
- differentially methylated region
- GCM2
- glial cells missing 2
- HDR
- hypoparathyroidism-deafness-renal dysplasia
- HR
- hazard ratio
- KSS
- Kearns-Sayre syndrome
- MELAS
- mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes
- MTPDS
- mitochondrial trifunctional protein deficiency syndrome
- NEBL
- nebulette
- 1,25(OH)2 vitamin D
- 1,25-dihydroxyvitamin D
- PDE4D
- phosphodiesterase 4D
- PHP
- pseudohypoparathyroidism
- PPHP
- pseudopseudohypoparathyroidism
- SEMA3E
- sempahorin 3E
- TBCE
- tubulin-specific chaperone.
References
- 1. Bilezikian JP, Khan A, Potts JT Jr, et al. . Hypoparathyroidism in the adult: epidemiology, diagnosis, pathophysiology, target-organ involvement, treatment, and challenges for future research. J Bone Miner Res. 2011;26:2317–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shoback D. Clinical practice. Hypoparathyroidism. N Engl J Med. 2008;359:391–403. [DOI] [PubMed] [Google Scholar]
- 3. Shoback DM, Bilezikian JP, Costa AG, et al. . Presentation of hypoparathyroidism: etiologies and clinical features. J Clin Endocrinol Metab. 2016;101:2300–2312. [DOI] [PubMed] [Google Scholar]
- 4. Powers J, Joy K, Ruscio A, Lagast H. Prevalence and incidence of hypoparathyroidism in the United States using a large claims database. J Bone Miner Res. 2013;28:2570–2576. [DOI] [PubMed] [Google Scholar]
- 5. Clarke BL, Leibson C, Emerson J, Ransom JE, Lagast H. Co-morbid-medical conditions associated with prevalent hypoparathyroidism: a population-based study. J Bone Miner Res. 2011;26:S182 (Abstract SA1070). [Google Scholar]
- 6. Underbjerg L, Sikjaer T, Mosekilde L, Rejnmark L. Cardiovascular and renal complications to postsurgical hypoparathyroidism: a Danish nationwide controlled historic follow-up study. J Bone Miner Res. 2013;28:2277–2285. [DOI] [PubMed] [Google Scholar]
- 7. Underbjerg L, Sikjaer T, Mosekilde L, Rejnmark L. Postsurgical hypoparathyroidism–risk of fractures, psychiatric diseases, cancer, cataract, and infections. J Bone Miner Res. 2014;29:2504–2510. [DOI] [PubMed] [Google Scholar]
- 8. Underbjerg L, Sikjaer T, Mosekilde L, Rejnmark L. The epidemiology of nonsurgical hypoparathyroidism in Denmark: a nationwide case finding study. J Bone Miner Res. 2015;30:1738–1744. [DOI] [PubMed] [Google Scholar]
- 9. Ito Y, Kihara M, Kobayashi K, Miya A, Miyauchi A. Permanent hypoparathyroidism after completion total thyroidectomy as a second surgery: how do we avoid it? Endocr J. 2014;61:403–408. [DOI] [PubMed] [Google Scholar]
- 10. Romanchishen AF. The use of chromothyrolymphography for selection of surgical volume in patients with thyroid cancer. Voprosy Oncologii. 1989;35:1037–1040. [PubMed] [Google Scholar]
- 11. Romanchishen AF. Surgery of Thyroid and Parathyroid Glands. Saint Petersburg, Russia: Vesty; 2009. [Google Scholar]
- 12. Page C, Strunski V. Parathyroid risk in total thyroidectomy for bilateral, benign, multinodular goitre: report of 351 surgical cases. J Laryngol Otol. 2007;121:237–241. [DOI] [PubMed] [Google Scholar]
- 13. Asari R, Passler C, Kaczirek K, Scheuba C, Niederle B. Hypoparathyroidism after total thyroidectomy: a prospective study. Arch Surg. 2008;143:132–137; discussion 138. [DOI] [PubMed] [Google Scholar]
- 14. Betterle C, Garelli S, Presotto F. Diagnosis and classification of autoimmune parathyroid disease. Autoimmun Rev. 2014;13:417–422. [DOI] [PubMed] [Google Scholar]
- 15. Leibson C, Clarke BL, Ransom JE, Lagast H. Medical care costs for persons with and without prevalent hypoparathyroidism: a population-based study. J Bone Miner Res. 2011;26:S183 (Abstract SA1071). [Google Scholar]
- 16. Mitchell DM, Regan S, Cooley MR, et al. . Long-term follow-up of patients with hypoparathyroidism. J Clin Endocrinol Metab. 2012;97:4507–4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Krause KH, Campbell KP, Welsh MJ, Lew DP. The calcium signal and neutrophil activation. Clin Biochem. 1990;23:159–166. [DOI] [PubMed] [Google Scholar]
- 18. Illum F, Dupont E. Prevalences of CT-detected calcification in the basal ganglia in idiopathic hypoparathyroidism and pseudohypoparathyroidism. Neuroradiology. 1985;27:32–37. [DOI] [PubMed] [Google Scholar]
- 19. Goswami R, Sharma R, Sreenivas V, Gupta N, Ganapathy A, Das S. Prevalence and progression of basal ganglia calcification and its pathogenic mechanism in patients with idiopathic hypoparathyroidism. Clin Endocrinol (Oxf). 2012;77:200–206. [DOI] [PubMed] [Google Scholar]
- 20. Tambyah PA, Ong BK, Lee KO. Reversible parkinsonism and asymptomatic hypocalcemia with basal ganglia calcification from hypoparathyroidism 26 years after thyroid surgery. Am J Med. 1993;94:444–445. [DOI] [PubMed] [Google Scholar]
- 21. Forman MB, Sandler MP, Danziger A, Kalk WJ. Basal ganglia calcification in postoperative hypoparathyroidism. Clin Endocrinol (Oxf). 1980;12:385–390. [DOI] [PubMed] [Google Scholar]
- 22. Thakker RV, Bringhurst FR, Jüppner H. Regulation of calcium homeostasis and genetic disorders that affect calcium metabolism. In: De Groot LJ, Jameson JL, eds. Endocrinology. 7th ed Philadelphia, PA: Elsevier; 2016:1063–1089. [Google Scholar]
- 23. Hannan FM, Thakker RV. Investigating hypocalcaemia. BMJ. 2013;346:f2213. [DOI] [PubMed] [Google Scholar]
- 24. Levine MA. An update on the clinical and molecular characteristics of pseudohypoparathyroidism. Curr Opin Endocrinol Diabetes Obes. 2012;19:443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bastepe M. Genetics and epigenetics of parathyroid hormone resistance. Endocr Dev. 2013;24:11–24. [DOI] [PubMed] [Google Scholar]
- 26. Bastepe M, Jüppner H. Pseudohypoparathyroidism, Albright's hereditary osteodystrophy, and progressive osseous heteroplasia: disorders caused by inactivating GNAS mutations. In: Jameson JL, DeGroot LJ, eds. Endocrinology. 7th ed Philadelphia, PA: Elsevier; 2016:1147–1159. [Google Scholar]
- 27. Neary NM, El-Maouche D, Hopkins R, Libutti SK, Moses AM, Weinstein LS. Development and treatment of tertiary hyperparathyroidism in patients with pseudohypoparathyroidism type 1B. J Clin Endocrinol Metab. 2012;97:3025–3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee S, Mannstadt M, Guo J, et al. . A homozygous [Cys25]PTH(1–84) mutation that impairs PTH/PTHrP receptor activation defines a novel form of hypoparathyroidism. J Bone Miner Res. 2015;30:1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Akın L, Kurtoğlu S, Yıldız A, Akın MA, Kendirici M. Vitamin D deficiency rickets mimicking pseudohypoparathyroidism. J Clin Res Pediatr Endocrinol. 2010;2:173–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Winer KK, Zhang B, Shrader JA, et al. . Synthetic human parathyroid hormone 1–34 replacement therapy: a randomized crossover trial comparing pump versus injections in the treatment of chronic hypoparathyroidism. J Clin Endocrinol Metab. 2012;97:391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Linglart A, Menguy C, Couvineau A, et al. . Recurrent PRKAR1A mutation in acrodysostosis with hormone resistance. N Engl J Med. 2011;364:2218–2226. [DOI] [PubMed] [Google Scholar]
- 32. Thiele S, de Sanctis L, Werner R, et al. . Functional characterization of GNAS mutations found in patients with pseudohypoparathyroidism type Ic defines a new subgroup of pseudohypoparathyroidism affecting selectively Gsα-receptor interaction. Hum Mutat. 2011;32:653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Michot C, Le Goff C, Goldenberg A, et al. . Exome sequencing identifies PDE4D mutations as another cause of acrodysostosis. Am J Hum Genet. 2012;90:740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kaname T, Ki CS, Niikawa N, et al. . Heterozygous mutations in cyclic AMP phosphodiesterase-4D (PDE4D) and protein kinase A (PKA) provide new insights into the molecular pathology of acrodysostosis. Cell Signal. 2014;26:2446–2459. [DOI] [PubMed] [Google Scholar]
- 35. Mantovani G. Clinical review: pseudohypoparathyroidism: diagnosis and treatment. J Clin Endocrinol Metab. 2011;96:3020–3030. [DOI] [PubMed] [Google Scholar]
- 36. Molinaro A, Tiosano D, Takatani R, et al. . TSH elevations as the first laboratory evidence for pseudohypoparathyroidism type Ib (PHP-Ib). J Bone Miner Res. 2015;30:906–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Eastell R, Brandi ML, Costa AG, D'Amour P, Shoback DM, Thakker RV. Diagnosis of asymptomatic primary hyperparathyroidism: proceedings of the Fourth International Workshop. J Clin Endocrinol Metab. 2014;99:3570–3579. [DOI] [PubMed] [Google Scholar]
- 38. D'Amour P. Acute and chronic regulation of circulating PTH: significance in health and in disease. Clin Biochem. 2012;45:964–969. [DOI] [PubMed] [Google Scholar]
- 39. Zhang CX, Weber BV, Thammavong J, Grover TA, Wells DS. Identification of carboxyl-terminal peptide fragments of parathyroid hormone in human plasma at low-picomolar levels by mass spectrometry. Anal Chem. 2006;78:1636–1643. [DOI] [PubMed] [Google Scholar]
- 40. Inaba M, Nakatsuka K, Imanishi Y, et al. . Technical and clinical characterization of the Bio-PTH (1-84) immunochemiluminometric assay and comparison with a second-generation assay for parathyroid hormone. Clin Chem. 2004;50:385–390. [DOI] [PubMed] [Google Scholar]
- 41. D'Amour P, Brossard JH, Räkel A, Rousseau L, Albert C, Cantor T. Evidence that the amino-terminal composition of non-(1-84) parathyroid hormone fragments starts before position 19. Clin Chem. 2005;51:169–176. [DOI] [PubMed] [Google Scholar]
- 42. Grigorieva IV, Thakker RV. Transcription factors in parathyroid development: lessons from hypoparathyroid disorders. Ann NY Acad Sci. 2011;1237:24–38. [DOI] [PubMed] [Google Scholar]
- 43. Ogata T, Niihori T, Tanaka N, et al. . TBX1 mutation identified by exome sequencing in a Japanese family with 22q11.2 deletion syndrome-like craniofacial features and hypocalcemia. PLoS One. 2014;9:e91598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Naiki M, Ochi N, Kato YS, et al. . Mutations in HADHB, which encodes the β-subunit of mitochondrial trifunctional protein, cause infantile onset hypoparathyroidism and peripheral polyneuropathy. Am J Med Genet. 2014;164A:1180–1187. [DOI] [PubMed] [Google Scholar]
- 45. Jyonouchi S, McDonald-McGinn DM, Bale S, Zackai EH, Sullivan KE. CHARGE (coloboma, heart defect, atresia choanae, retarded growth and development, genital hypoplasia, ear anomalies/deafness) syndrome and chromosome 22q11.2 deletion syndrome: a comparison of immunologic and nonimmunologic phenotypic features. Pediatrics. 2009;123:e871–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hannan FM, Nesbit MA, Zhang C, et al. . Identification of 70 calcium-sensing receptor mutations in hyper- and hypo-calcaemic patients: evidence for clustering of extracellular domain mutations at calcium-binding sites. Hum Mol Genet. 2012;21:2768–2778. [DOI] [PubMed] [Google Scholar]
- 47. Nesbit MA, Hannan FM, Howles SA, et al. . Mutations affecting G-protein subunit α11 in hypercalcemia and hypocalcemia. N Engl J Med. 2013;368:2476–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mannstadt M, Harris M, Bravenboer B, et al. . Germline mutations affecting Gα11 in hypoparathyroidism. N Engl J Med. 2013;368:2532–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li D, Opas EE, Tuluc F, et al. . Autosomal dominant hypoparathyroidism caused by germline mutation in GNA11: phenotypic and molecular characterization. J Clin Endocrinol Metab. 2014;99:E1774–E1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Taylor JC, Martin HC, Lise S, et al. . Factors influencing success of clinical genome sequencing across a broad spectrum of disorders. Nat Genet. 2015;47:717–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chillambhi S, Turan S, Hwang DY, Chen HC, Jüppner H, Bastepe M. Deletion of the noncoding GNAS antisense transcript causes pseudohypoparathyroidism type Ib and biparental defects of GNAS methylation in cis. J Clin Endocrinol Metab. 2010;95:3993–4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Richard N, Abeguilé G, Coudray N, et al. . A new deletion ablating NESP55 causes loss of maternal imprint of A/B GNAS and autosomal dominant pseudohypoparathyroidism type Ib. J Clin Endocrinol Metab. 2012;97:E863–E867. [DOI] [PubMed] [Google Scholar]
- 53. Elli FM, de Sanctis L, Peverelli E, et al. . Autosomal dominant pseudohypoparathyroidism type Ib: a novel inherited deletion ablating STX16 causes loss of imprinting at the A/B DMR. J Clin Endocrinol Metab. 2014;99:E724–E728. [DOI] [PubMed] [Google Scholar]
- 54. Weinstein LS, Xie T, Qasem A, Wang J, Chen M. The role of GNAS and other imprinted genes in the development of obesity. Int J Obes (Lond). 2010;34:6–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lemos MC, Thakker RV. GNAS mutations in pseudohypoparathyroidism type 1a and related disorders. Hum Mut. 2015;36:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mouallem M, Shaharabany M, Weintrob N, et al. . Cognitive impairment is prevalent in pseudohypoparathyroidism type Ia, but not in pseudopseudohypoparathyroidism: possible cerebral imprinting of Gsα. Clin Endocrinol (Oxf). 2008;68:233–239. [DOI] [PubMed] [Google Scholar]
- 57. Al-Salameh A, Despert F, Kottler ML, Linglart A, Carel JC, Lecomte P. Resistance to epinephrine and hypersensitivity (hyperresponsiveness) to CB1 antagonists in a patient with pseudohypoparathyroidism type Ic. Eur J Endocrinol. 2010;162:819–824. [DOI] [PubMed] [Google Scholar]
- 58. Dekelbab BH, Aughton DJ, Levine MA. Pseudohypoparathyroidism type 1A and morbid obesity in infancy. Endocr Pract. 2009;15:249–253. [DOI] [PubMed] [Google Scholar]
- 59. Lebrun M, Richard N, Abeguilé G, et al. . Progressive osseous heteroplasia: a model for the imprinting effects of GNAS inactivating mutations in humans. J Clin Endocrinol Metab. 2010;95:3028–3038. [DOI] [PubMed] [Google Scholar]
- 60. Pignolo RJ, Ramaswamy G, Fong JT, Shore EM, Kaplan FS. Progressive osseous heteroplasia: diagnosis, treatment, and prognosis. Appl Clin Genet. 2015;8:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Regard JB, Malhotra D, Gvozdenovic-Jeremic J, et al. . Activation of hedgehog signaling by loss of GNAS causes heterotopic ossification. Nat Med. 2013;19:1505–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Richard N, Molin A, Coudray N, Rault-Guillaume P, Jüppner H, Kottler ML. Paternal GNAS mutations lead to severe intrauterine growth retardation (IUGR) and provide evidence for a role of XLαs in fetal development. J Clin Endocrinol Metab. 2013;98:E1549–E1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lau K, Willig RP, Hiort O, Hoeger PH. Linear skin atrophy preceding calcinosis cutis in pseudo-pseudohypoparathyroidism. Clin Exp Dermatol. 2012;37:646–648. [DOI] [PubMed] [Google Scholar]
- 64. Martin J, Tucker M, Browning JC. Infantile osteoma cutis as a presentation of a GNAS mutation. Pediatr Dermatol. 2012;29:483–484. [DOI] [PubMed] [Google Scholar]
- 65. Ward S, Sugo E, Verge CF, Wargon O. Three cases of osteoma cutis occurring in infancy. A brief overview of osteoma cutis and its association with pseudo-pseudohypoparathyroidism. Australas J Dermatol. 2011;52:127–131. [DOI] [PubMed] [Google Scholar]
- 66. Adegbite NS, Xu M, Kaplan FS, Shore EM, Pignolo RJ. Diagnostic and mutational spectrum of progressive osseous heteroplasia (POH) and other forms of GNAS-based heterotopic ossification. Am J Med Genet A. 2008;146A:1788–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Thakker RV. The parathyroid glands, hypercalcemia, and hypocalcemia. In: Goldman L, Shafer AI, eds. Goldman-Cecil Medicine. 25th ed Atlanta, GA: Elsevier Ltd; 2016:1649–1661. [Google Scholar]
- 68. Sanchez J, Perera E, Jan de Beur S, et al. . Madelung-like deformity in pseudohypoparathyroidism type 1b. J Clin Endocrinol Metab. 2011;96:E1507–E1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Turan S, Ignatius J, Moilanen JS, et al. . De novo STX16 deletions: an infrequent cause of pseudohypoparathyroidism type Ib that should be excluded in sporadic cases. J Clin Endocrinol Metab. 2012;97:E2314–E2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Xie T, Plagge A, Gavrilova O, et al. . The alternative stimulatory G protein α-subunit XLαs is a critical regulator of energy and glucose metabolism and sympathetic nerve activity in adult mice. J Biol Chem. 2006;281:18989–18999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bastepe M, Altug-Teber O, Agarwal C, Oberfield SE, Bonin M, Jüppner H. Paternal uniparental isodisomy of the entire chromosome 20 as a molecular cause of pseudohypoparathyroidism type Ib (PHP-Ib). Bone. 2011;48:659–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Fernández-Rebollo E, Lecumberri B, Garin I, et al. . New mechanisms involved in paternal 20q disomy associated with pseudohypoparathyroidism. Eur J Endocrinol. 2010;163:953–962. [DOI] [PubMed] [Google Scholar]
- 73. Takatani R, Minagawa M, Molinaro A, et al. . Similar frequency of paternal uniparental disomy involving chromosome 20q (patUPD20q) in Japanese and Caucasian patients affected by sporadic pseudohypoparathyroidism type Ib (sporPHP1B). Bone. 2015;79:15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Jin HY, Lee BH, Choi JH, et al. . Clinical characterization and identification of two novel mutations of the GNAS gene in patients with pseudohypoparathyroidism and pseudopseudohypoparathyroidism. Clin Endocrinol (Oxf). 2011;75:207–213. [DOI] [PubMed] [Google Scholar]
- 75. Mitsui T, Narumi S, Inokuchi M, et al. . Comprehensive next-generation sequencing analyses of hypoparathyroidism: identification of novel GCM2 mutations. J Clin Endocrinol Metab. 2014;99:E2421–E2428. [DOI] [PubMed] [Google Scholar]