Abstract
Context:
Pregnancy-associated plasma protein-A2 (PAPP-A2) is a metalloproteinase that specifically cleaves IGFBP-3 and IGFBP-5. Mutations in the PAPP-A2 gene have recently been shown to cause postnatal growth failure in humans, with specific skeletal features, due to the resulting decrease in IGF-1 bioavailability. However, a pharmacological treatment of this entity is yet to be established.
Case Description:
A 10.5-year-old girl and a 6-year-old boy, siblings from a Spanish family, with short stature due to a homozygous loss-of-function mutation in the PAPP-A2 gene (p.D643fs25*) and undetectable PAPP-A2 activity, were treated with progressive doses (40, 80, 100, and 120 μg/kg) of recombinant human IGF-1 (rhIGF-1) twice daily for 1 year. There was a clear increase in growth velocity and height in both siblings. Bioactive IGF-1 was increased, and spontaneous GH secretion was diminished after acute administration of rhIGF-1, whereas serum total IGF-1 and IGFBP-3 levels remained elevated. No episodes of hypoglycemia or any other secondary effects were observed during treatment.
Conclusion:
Short-term treatment with rhIGF-1 improves growth in patients with PAPP-A2 deficiency.
We treated 2 children with PAPP-A2 deficiency with rhIGF-I, increasing circulating bioactive IGF-I. After 1 year, their growth velocity improved with no adverse side- effects observed.
We recently identified a novel autosomal recessive syndrome consisting of short stature, skeletal abnormalities, and high circulating IGF-1, IGFBP-3, IGFBP-5, and acid labile subunit (ALS) levels due to loss-of-function mutations in the pregnancy-associated plasma protein-A2 (PAPP-A2, pappalysin 2) gene (1). PAPP-A2 is a metalloproteinase that specifically cleaves IGFBP-3 and -5, resulting in dissociation of IGFs carried within ternary complexes with ALS and IGFBP-3 or -5, enabling unbound IGF to enter the extravascular space to stimulate the IGF-1 receptor (IGF-1R) in target tissues (2).
The objectives of the current study were to determine auxological and metabolic parameters after administration of rhIGF-1 to two patients lacking PAPP-A2 activity and to assess the safety of this treatment. We employed rhIGF-1 rather than rhGH because these patients are not GH deficient, whereas they are deficient in bioactive IGF-1. Moreover, we reasoned that increasing GH levels would further elevate not only total IGF-1, but also IGFBP-3 and ALS, allowing for the formation of more ternary complexes, which might not result in an increase in bioactive IGF-1 because these patients lack the proteolytic activity of PAPP-A2.
Subjects and Methods
The study was approved by the Institutional Ethical Review Board at the University Hospital Niño Jesús. Written informed consent was obtained from the legal guardians.
Clinical characteristics (Table 1)
Table 1.
Anthropometric Data at Baseline and After 6 Months and 12 Months of rhIGF-1 Therapy
| Subject ID | Age, y | BA (Greulich Pyle) | Height, cm (SDS) | BMI, kg/m2 (SDS) | HC, cm (SDS) | GV/y (SDS) | ΔTH (SDS)-H (SDS) | rhIGF-1 Therapy, μg/kg/dose |
|---|---|---|---|---|---|---|---|---|
| Patient 1 (female) | ||||||||
| Start | 10.54 | 10.75 | 132 (−1.25) | 14.1 (−1.51) | 49 (−2.06) | 3.7 (−1.5) | 1.94 | 40–80 |
| 6 mo | 11.03 | 11 | 136.9 (−0.87) | 14.4 (−1.44) | 49 (−2.06) | 9.8 (+4) | 1.56 | 80–120 |
| 12 mo | 11.54 | 11.25 | 139.6 (−0.86) | 14.8 (−1.44) | 49 (−2.08) | 7.6 (+1.6) | 1.55 | 120 |
| Patient 2 (male) | ||||||||
| Start | 6 | 4.5 | 111.5 (−0.74) | 13.38 (−1.81) | 47.5 (−2.76) | 5.8 (−1.6) | 1.17 | 40–80 |
| 6 mo | 6.5 | 5.0 | 115.5 (−0.47) | 13.38 (−1.9) | 48 (−2.92) | 8 (+1.91) | 0.90 | 80–120 |
| 12 mo | 7 | 5.5 | 118.5 (−0.34) | 13.64 (−1.69) | 48.5 (−2.94) | 7 (+1.06) | 0.77 | 120 |
Abbreviations: BA, bone age; BMI, body mass index; HC, head circumference; GV, growth velocity; ΔTH-H, Δtarget height − height.
The study included two prepubertal siblings, a 10.5-year-old female (patient 1) and a 6-year-old boy (patient 2), born to nonconsanguineous Spanish parents. Birth weight and length were normal. Postnatally they exhibited a gradual decline in growth velocity causing a progressive deviation from their target height (TH). Other phenotypic features included a small chin, mild microcephaly, and long, thin digits on hands and feet. Skeletal maturation was consistent with chronological age in patient 1 (Δ chronological age–bone age = −0.21) and was delayed in patient 2 (Δ chronological age–bone age = 1.5). Both have a homozygous frameshift mutation in exon 3 of the PAPP-A2 gene (p.D643fs25*) resulting in a premature stop codon and absent PAPP-A2 activity (1). At treatment onset, the height of patient 1 was in the normal range (−1.25 SDS), but was 1.9 SDS below her TH. The height of patient 2 was also normal (−0.74 SDS), but 1.2 SDS below his TH. After 6 months of rhIGF-1 treatment, patient 1 spontaneously entered puberty (Tanner II; chronological and bone age, both 11 years). In an attempt to improve her final height, she received GnRH analog treatment (Triptorelin, 3.75 mg/28 days) during the remaining study period.
Study procedures
Initially, 40–80 μg/kg of rhIGF-1 (Mecasermin, Increlex; Ipsen) was administered sc twice daily for 6 months. The dose was progressively increased to 120 μg/kg twice daily after 9 months of treatment. During the first 3 days of rhIGF-1 administration, glucose levels were measured every 30 minutes, and thereafter at least six times daily (ACC-CHEK; Roche Diagnostics Corp). Patients underwent an anthropometric evaluation and physical examination every 3 months. At 0, 6, and 12 months, hematology and biochemical analyses, thyroid function, and serum insulin, GH, total and bioactive IGF-1 and IGFBP-3 were measured, and radiography of the left hand and wrist was performed. At 0 and 12 months of treatment, abdominal ultrasound (to evaluate spleen and kidney size), echocardiogram, and whole-body dual-energy x-ray absorptiometry for body composition were performed.
Spontaneous GH secretion over 8 hours (7 am to 3 pm), with extractions every 30 minutes, was evaluated at 0 and 6 months of treatment. Serum levels of total and bioactive IGF and IGFBP-3 were measured every 30 minutes during 8 hours after rhIGF-1 administration at 6 months of treatment.
Laboratory methods
Serum levels of total IGF-1 and IGFBP-3 were measured by ELISA (Ansh Labs), insulin by an immunoradiometric assay (DIAsource ImmunoAssays), and GH by chemiluminescence (IDS). IGF-1 bioactivity was estimated as the ability of serum to activate the IGF-1 receptor in vitro, using an IGF-1 kinase receptor activation assay. The assays were performed as previously reported (1).
Results
Clinical and auxological data (Table 1)
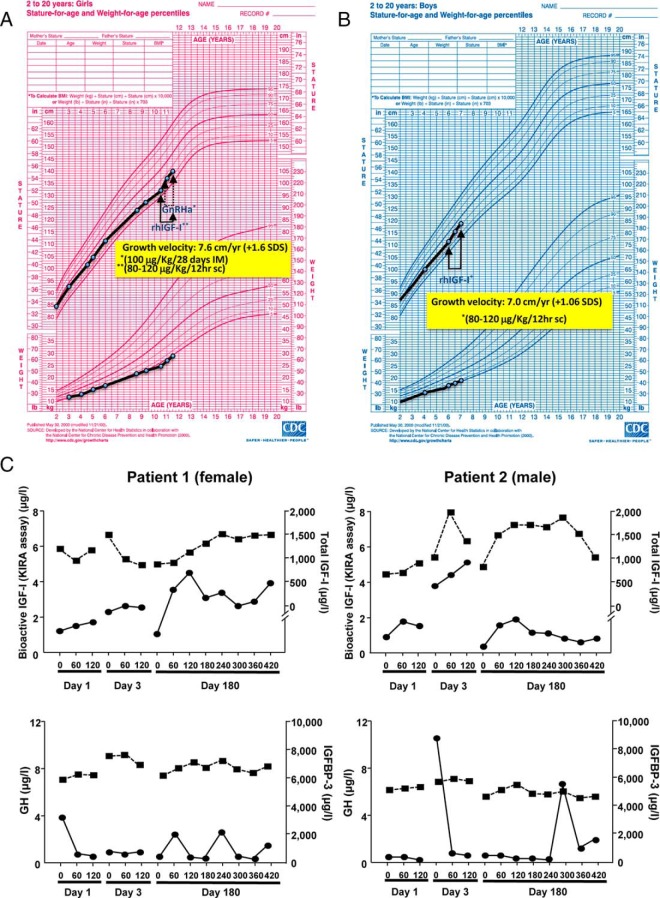
Treatment with rhIGF-1 accelerated growth velocity, clearly improving height SDS according to age and sex in both patients (Figure 1, A and B, and Table 1). No significant changes in weight, BMI, head circumference, or skeletal maturation were observed. Kidney and spleen size and auditory measures remained in the normal range.
Figure 1. A, Growth chart of the female patient with PAPP-A2 deficiency, representing the pattern of growth before and after rhIGF-1 therapy with a dose between 40 and 120 μg/kg every 12 hours. B, Growth chart of the male patient with PAPP-A2 deficiency, representing the pattern of growth before and after rhIGF-1 therapy with a dose between 40 and 120 μg/kg every 12 hours. C, The top graphs represent changes in bioactive IGF-1 as measured by kinase receptor activation (dark circles and continuous line) and total IGF-1 (dark squares and dashed lines) in response to treatment. The bottom graphs represent changes in GH secretion (dark circles and continuous line) and IGFBP-3 (dark squares and dashed line) in response to treatment.
GH secretion and the peripheral GH-IGF axis during treatment
Mean GH secretion was elevated before rhIGF-1 treatment (patient 1, 4.8 ng/mL; patient 2, 5.3 ng/mL), with integrated secretion over 8 hours diminishing markedly after 6 months of treatment (patient 1, 0.80 ng/mL; patient 2, 0.65 ng/mL). Treatment had no significant effect on basal levels of total IGF-1, bioactive IGF-1, or IGFBP-3 at 6 or 12 months (data not shown). Acutely, rhIGF-1 administration increased bioactive IGF-1 60–120 minutes later (Figure 1C), and this was associated with a decrease in the GH secretory peaks occurring at this time. Levels of IGFBP-3 remained constant during the treatment period.
Metabolic measures
Both patients exhibited moderate fasting hyperinsulinemia (15 and 27 μU/mL, respectively; normal range, 4–13 μU/mL), with normal glycemia and glycosylated hemoglobin before treatment. At 1 year of treatment, serum insulin was normalized (patient 1, 11 μU/mL; patient 2, 8 μU/mL). Treatment with rhIGF-1 produced no significant change in bone mineral density but increased the percentage of lean body mass (4.8 and 5%) and decreased the percentage of total body fat mass (4.5%) in both patients.
Safety and adverse events
Neither patient experienced episodes of clinically relevant hypoglycemia or hyperglycemia or any other previously described secondary effect of rhIGF-1.
Discussion
We show that treatment with rhIGF-1 is effective in promoting short-term growth in patients with PAPP-A2 deficiency, who exhibit increased total IGF-1, yet decreased IGF-1 bioactivity (1). The response was in the higher range of that observed in patients with GH insensitivity syndrome treated with rhIGF-1 (3). Treatment of PAPP-A2-deficient patients with rhIGF-1 induced modifications in the GH axis that could affect the long-term response. The decrease in spontaneous GH secretion resulting from rhIGF-1 administration could reduce the non-IGF-dependent effects of GH on growth. This decline in GH secretion most likely results from an increased negative feedback exerted by the rise in bioactive IGF-1 after rhIGF-1 injection (4). The chronic lack of PAPP-A2 activity in these patients may be responsible for maintaining high levels of IGFBPs bound to both endogenous IGFs and exogenous rhIGF-1, even if there is a decrease in GH-dependent de novo synthesis. However, bioactive IGF-1 was significantly increased up to at least 7 hours after administration, suggesting that it underlies the observed increase in growth velocity.
The mild basal hyperinsulinemia observed before treatment suggests some degree of insulin resistance, which is most likely secondary to the increased GH secretion and decreased insulin-like effects due to low bioactive IGF-1 levels (5). This view is supported by the observation that treatment with rhIGF-1 improved both phenomena, as reported by others (6). Levels of total IGF-1 and IGFBP-3, both GH dependent, did not change with treatment.
Treatment with rhIGF-1 can induce adverse effects, mainly hypoglycemia and disproportionate growth of specific organs (spleen and kidneys) (3, 7), that are most likely caused by increased IGF-1 actions, as well as the marked insulin-like effect of high concentrations of IGF-1 after treatment (8). Neither of our patients experienced any of these complications. This could be due to the short duration of treatment or to the specific abnormalities in their GH-IGF-1 axes. They have an excess of antagonistic binding proteins that is not observed in other patients treated with IGF-1. This may protect them, at least in part, from adverse rhIGF-1-related effects.
In summary, rhIGF-1 treatment of children with PAPP-A2 deficiency improves short-term growth with no apparent adverse effects. Thus, we suggest that the indication for rhIGF-1 treatment could be extended to include patients with growth retardation caused by PAPP-A2 mutations, although further and long-term studies are necessary.
Acknowledgments
We thank Dr. Santiago Vila-Dupla for GH measurements.
The authors express their gratitude for the financial support received from the Spanish Ministry of Economy and Competitiveness (Grant CTQ2014–55279-R), and by Fondos de Investigación Sanitaria and Fondos FEDER (Grant PI1302195; to J.A.), Ministerio de Ciencia e Innovación (Grant BFU2014-51836-C2-2-R; to J.A.C.), Centro de Investigación Biomédica en Red Fisiopatología de Obesidad y Nutrición (CIBEROBN), Instituto de Salud Carlos III (to J.A.), and Fundación Endocrinología y Nutrición.
Disclosure Summary: The authors declare no conflict of interest.
Funding Statement
The authors express their gratitude for the financial support received from the Spanish Ministry of Economy and Competitiveness (Grant CTQ2014–55279-R), and by Fondos de Investigación Sanitaria and Fondos FEDER (Grant PI1302195; to J.A.), Ministerio de Ciencia e Innovación (Grant BFU2014-51836-C2-2-R; to J.A.C.), Centro de Investigación Biomédica en Red Fisiopatología de Obesidad y Nutrición (CIBEROBN), Instituto de Salud Carlos III (to J.A.), and Fundación Endocrinología y Nutrición.
Footnotes
- ALS
- acid labile subunit
- PAPP-A2
- pregnancy-associated plasma protein-A2
- TH
- target height.
References
- 1. Dauber A, Muñoz-Calvo MT, Barrios V, et al. . Mutations in pregnancy-associated plasma protein A2 cause short stature due to low IGF-I availability. EMBO Mol Med. 2016;8:363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Overgaard MT, Boldt HB, Laursen LS, Sottrup-Jensen L, Conover CA, Oxvig C. Pregnancy-associated plasma protein-A2 (PAPP-A2), a novel insulin-like growth factor-binding protein-5 proteinase. J Biol Chem. 2001;276:21849–21853. [DOI] [PubMed] [Google Scholar]
- 3. Chernausek SD, Backeljauw PF, Frane J, Kuntze J, Underwood LE. Long-term treatment with recombinant insulin-like growth factor (IGF)-I in children with severe IGF-I deficiency due to growth hormone insensitivity. J Clin Endocrinol Metab. 2007;92:902–910. [DOI] [PubMed] [Google Scholar]
- 4. Chen JW, Ledet T, Orskov H, et al. . A highly sensitive and specific assay for determination of IGF-I bioactivity in human serum. Am J Physiol Endocrinol Metab. 2003;284:E1149–E1155. [DOI] [PubMed] [Google Scholar]
- 5. Dominici FP, Turyn D. Growth hormone-induced alterations in the insulin-signaling system. Exp Biol Med (Maywood). 2002;227:149–157. [DOI] [PubMed] [Google Scholar]
- 6. El Kholy M, Amr NH, Elsedfy H. Further observations on the effects of long-term treatment with recombinant human insulin-like growth factor 1 in growth hormone insensitivity syndrome. Horm Res Paediatr. 2014;81:258–265. [DOI] [PubMed] [Google Scholar]
- 7. Guevara-Aguirre J, Vasconez O, Martinez V, et al. . A randomized, double blind, placebo-controlled trial on safety and efficacy of recombinant human insulin-like growth factor-I in children with growth hormone receptor deficiency. J Clin Endocrinol Metab. 1995;80:1393–1398. [DOI] [PubMed] [Google Scholar]
- 8. Savage MO. Phenotypes, investigation and treatment of primary IGF-1 deficiency. Endocr Dev. 2013;24:138–149. [DOI] [PubMed] [Google Scholar]