Abstract
In mammals, very long chain fatty acids (VLCFAs) perform pleiotropic roles in a wide range of biological processes, such as cell membrane formation, cell signal transduction, and endocrine regulation. Beef and milk are abundant of palmitic acid which can be further elongated into stearic acid for synthesizing VLCFAs. Elongase of very long chain fatty acid 6 (ELOVL6) is a rate-limiting enzyme for converting palmitic acid to stearic acid. Consequently, investigating the tissue expression patterns and transcriptional regulation of bovine ELOVL6 can provide new insights into improving the composition of beneficial fats in cattle and expanding the knowledge of transcriptional regulation mechanism among domestic animals. In the current study, we found that bovine ELOVL6 expressed ubiquitously. Dual-luciferase reporter assay identified that the core promoter region (-130/-41 bp) was located in the second CpG island. In addition, the deletion mutation of binding sites demonstrated that sterol regulatory element binding transcription factor 1 (SREBF1) and specific protein 1 (SP1) both were able to stimulate bovine ELOVL6 promoter activity independently, while resulting the similar effect. To confirm these findings, further RNA interference assays were executed in bovine mammary epithelial cells (BMECs). In summary, these data suggest that bovine ELOVL6 expressed ubiquitously and is activated by SREBF1 and SP1, via two binding sites present in the ELOVL6 promoter region between -130 bp to -41bp.
Introduction
In vertebrates, increasing evidence has shown that very long chain fatty acids (VLCFAs) which are the fatty acids of 20 carbon or more in length perform a vital role in maintaining global metabolic homeostasis and normal physiological function [1]. Among VLCFAs, the polyunsaturated fatty acids (PUFAs) arachidonic acid and docosahexaenoic acid regulate several processes within the brain, including neurotransmission, cell survival and neuroinflammation [2]. PUFAs also participate in lipid metabolism by directly interacting with the lipid-sensing transcription factors (TFs) [3]. People benefit greatly from livestock products, such as beef and milk, which provide nutrition containing high-quality protein, and low-level fat with a desirable VLCFAs profile. Oleic acid (18:1n-9, 35.70%) which belongs to VLCFAs is the most abundant fatty acid in beef, followed by palmitic acid (16:0, 31.07%) [4]. With the help of mid-infrared predictions tools, milk was detected to possess more palmitic acid (33.44%) than oleic acid (17.31%) [5]. Lately researches showed that diets high in oleic acid improved the health condition for individuals through the effect on reducing central obesity and cardiovascular disease risk [6, 7].
In mammals, fatty acids with a chain length of up to 16 carbons are synthesized by fatty acid synthase(FASN), as well as are gained from diet. These short chain fatty acids (SCFAs) are further elongated and desaturated into VLCFAs [8]. The elongation of SCFAs is proceeded by a four-step biochemical cycle. In the elongation cycle, the first rate-limiting (condensation) step was catalyzed by a group of endoplasmic reticulum (ER) membrane-bound enzymes, termed ELOVL (Elongase of very long chain fatty acids) [9]. To date, seven distinct isoforms of ELONGASE family have been identified, which were designated from ELOVL1 to ELOVL7, reside in murine and human [10]. Each ELOVL protein exhibits a characteristic substrate specificity [11].
C18 fatty acids has been proved to be the precursor for the synthesis of VLCFAs [12]. ELOVL6 (LCE/FACE) is essential for synthesizing C18 fatty acids, owning to its specific activity to convert C16 saturated and monounsaturated fatty acids into C18 fatty acids [13]. Previous study indicated that ELOVL6 was ubiquitously expressed, especially in tissues with high lipid content such as brown/white adipose tissue, liver and brain in mouse [14]. The function researches of ELOVL6 demonstrated that the deficiency of ELOVL6 in mouse protected against metabolic diseases such as insulin resistance [15], nonalcoholic steatohepatitis [16]. Meanwhile, the overexpress of mouse ELOVL6 induced cancer diseases, included breast cancer [17], cystic fibrosis [18], pulmonary fibrosis [19] and lung squamous cell carcinoma [20]. ELOVL6 also regulates thermogenic capacity in brown adipose tissue [21]. Consequently, many researches poured attentions into the transcriptional regulation of ELOVL6.
Recent attempts utilizing advanced molecular biological techniques have provided some novel insights of the transcriptional regulation of ELOVL6. In general, sequence-specific transcription factors have been grouped into two categories: proximal promoter factors, and enhancer binding factors. Direct evidence indicated that hepatic expression of ELOVL6 in mouse was regulated by SREBF1 via SREBF1 binding sites (SRE) present in the ELOVL6 promoter. Further ChIP assay showed that the proximal SRE-1 binding site on the promoter of ELOVL6 had higher affinity to SREBF1 than the distal one [22]. Meanwhile, a recent study suggested that human carbohydrate response element binding protein (ChREBP) and SREBF1 synergistically stimulated ELOVL6 promoter activity in HepG2 cell lines [23]. It has been demonstrated that a portion of transcriptional regulation which was activated by SREBPs requires cooperation with other DNA binding transcription factors such as SP1, NF-Y, and CREB as well as with coactivators [24].The SREBF family is essential to the regulation of milk lipogenic genes expression, including acetyl-CoA carboxylase (ACC), fatty acid synthetase (FAS), stearoyl-CoA desaturase (SCD), mechanistic target of rapamycin (mTOR), desaturation fatty acid binding protein 3 (FABP3) and peroxisome proliferator activated receptor γ (PPARγ) [25]. Additionally, among the transcription factors involved in lipid metabolism of beef cattle, PPARs and SREBFs stand out [26].
To address the question of whether the transcriptional pattern of ELOVL6 in bovine is conserved, we determined the tissue expression profile of bovine ELOVL6 in nine different tissues by quantitative real-time PCR (qPCR). In order to identify and narrow down the core promoter region of bovine ELOVL6, we constructed six dual-luciferase reporter plasmids harboring various 5’ flanking truncations and detected their promoter activities by dual-luciferase reporter assays. Seven transcription factor binding sites (TFBS) were predicated in the core promoter region of bovine ELOVL6. Subsequently, the dominant transcription factors were verified by site-directed deletion mutation and RNA interference assays. Our results suggest that bovine ELOVL6 expressed ubiquitously and is activated by SREBF1 and SP1, via two binding sites present in the ELOVL6 promoter region between -130 bp to -41bp.
Materials and methods
Ethics statement
All animal procedures were carried out in accordance with the Regulations for the Administration of Affairs Concerning Experimental Animals (Ministry of Science and Technology, China, 2004) and were approved by the Institutional Animal Care and Use Committee at the Northwest A&F University (Protocol NWAFAC1117). Cattles were raised under free food intake and humanely slaughtered in the Shannxi Kingbull Animal Husbandry Company, Ltd (Baoji, Shaanxi, China).
Quantitative real-time PCR (qPCR)
The tissues were collected from three 2 year-old male Qinchuan cattles, including heart, liver, spleen, lung, kidney, intestine, stomach, skeletal muscle and abdominal fat. The relative quantification was carried out against the quantification cycle (Cq) value of ELOVL6 in spleen tissue. Total RNA was extracted using Trizol reagent (Invitrogen, USA) and quantified by the Nanodrop 2000 spectrophotometry (Thermo Fisher Scientific, USA). cDNA was subsequently synthesized by using the All-in-one RT MasterMix (ABM, USA). The quantitative real-time PCR (qPCR) reactions were carried out in a CFX96 Real-Time PCR Detection System (Bio-Rad, USA) employing the SYBR® Premix Ex Taq II (Takara, China). The Cq values were normalized to reference gene (GAPDH) run on the same plate. The primers which amplified the transcripts were listed in S1 Table. All the experiments were performed in triplicates.
Dual-luciferase reporter assays
The 5’-flanking region of bovine ELOVL6 promoter was amplified and was identical to the GenBank database (Accession no. AC_000163). In order to determine the core region of bovine ELOVL6 promoter, multiple dual-luciferase reporter plasmids containing unidirectional truncations (from 5’ to 3’) were constructed. The primers were listed in S2 Table. After double digestions with MluI and XhoI, the fragments were cloned into pGL3-basic vector, respectively. The resulting constructs were designated as pGL3-F2, pGL3-F3, pGL3-F4, pGL3-F5, pGL3-F6 and pGL3-F7. Deletion mutation constructs were subsequently generated by overlapping extension methods using pGL3-F5 as template. All constructs were sequenced in both directions (Invitrogen, USA). The primers were listed in S3 Table. Constructed plasmids were then co-transfected with pRL-TK plasmid into 3T3-L1 and 293A cell lines for the dual-luciferase reporter assay.
RNA interference
All siRNAs were designed and synthesized according to the online prediction program at http://rnaidesigner.thermofisher.com/rnaiexpress/. SREBF1-siRNA sequences were as following, sence: 5'- UCUUCCAUCAAUGACAAGATT-3', anti-sence: 5'- UCUUGUCAUUGAUGGAAGATT-3'. SP1-siRNA sequences were as following, sence: 5'-GCCAAUAGCUACUCAACAATT-3', anti-sence: 5'-UUGUUGAGUAGCUAUUGGCTT-3'. Control-siRNA sequences were as following, sence: 5'- UUCUCCGAACGUGUCACGUTT-3', anti-sence: 5'- ACGUGACACGUUCGGAGAATT-3'. siRNAs were then transfected into BMECs following the manual of Lipofectamine™ 2000 (Invitrogen, USA). The expressions were measured by qPCR described above.
Cell culture and transfection
3T3-L1 (CL-173, ATCC) and 293A (R70507, Invitrogen) cell lines were maintained in Dulbecco’s modified eagle’s medium (DMEM) (Hyclone, GE, USA) which was supplemented with 10% fetal bovine serum (FBS) (Gibco, Invitrogen, USA) in 5% CO2 and 100% humidity at 37℃ and passaged using standard cell culture techniques. Before transfection, cells were plated at a density of 1.4×105 cells per well in 48-well plates and incubated for 12 hours until they reached 80–90% confluent. Plasmids described above were transfected using Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s instructions. After 48 hours transfection, cells were washed with 1× PBS and lysed with 1× passive lysis buffer for 15 min. Dual-luciferase reporter assay was carried out by using Varioskan Flash instrument (Thermo Fisher Scientific, USA). The level of firefly luciferase activity was normalized to renilla luciferase activity. In RNA interference assays, BMECs were cultured in 1640 medium (Hyclone, GE, USA) which was supplemented with 10% fetal bovine serum (FBS) (Gibco, Invitrogen, USA) in 5% CO2 and 100% humidity at 37℃ and passaged using standard cell culture techniques. Before transfection, cells were plated at a density of 7.5×105 cells per well in 48-well plates and incubated for 12 hours until they reached 70–80% confluent. siRNAs (20 umol/L) were transfected as 5 pmol mixed with 0.25 ul Lipofectamine 2000 per well. After 24 hours and 48 hours, samples of siRNA treatment were collected.
Bioinformatics analyses
The bovine ELOVL6 promoter was analyzed using a promoter prediction program at http://www.cbs.dtu.dk/services/Promoter/. The transcription factor binding sites were predicted by using an online prediction server at http://www.biobase-international.com/product/transcription-factor-binding-sites. The methylation CpG island of bovine ELOVL6 promoter was analyzed at http://www.urogene.org/cgi-bin/methprimer/methprimer.cgi. For the phylogenetic analysis, other promoter sequences from different mammalian species were aligned with bovine ELOVL6 promoter by the Clustal X 2.1 program. A phylogenetic tree was constructed by using the MEGA program (version 6.0) with the neighbor-joining method. Bootstrap values were obtained from 1000 repetitions and listed as percentages at the nodes. The core regions of promoter sequences from different mammalian species were aligned and visualized by DNAman software (version 7.0).
Statistical analyses
SPSS 19.0 (IBM, Armonk, NY, USA) software performed all statistical analyses. The relative expression quantification was evaluated by the algorithm of 2-ΔΔCT method. The results of each independent samples were normalized to the reference gene (GAPDH) run on the same plate. A one-way ANOVA test was conducted to determine the significant level. Mean values were compared by the LSD post-test. The results were expressed as mean ± SD, and the p value less than 0.05 was considered statistically significant.
Results
Tissue-specific expression patterns of bovine ELOVL6 transcripts
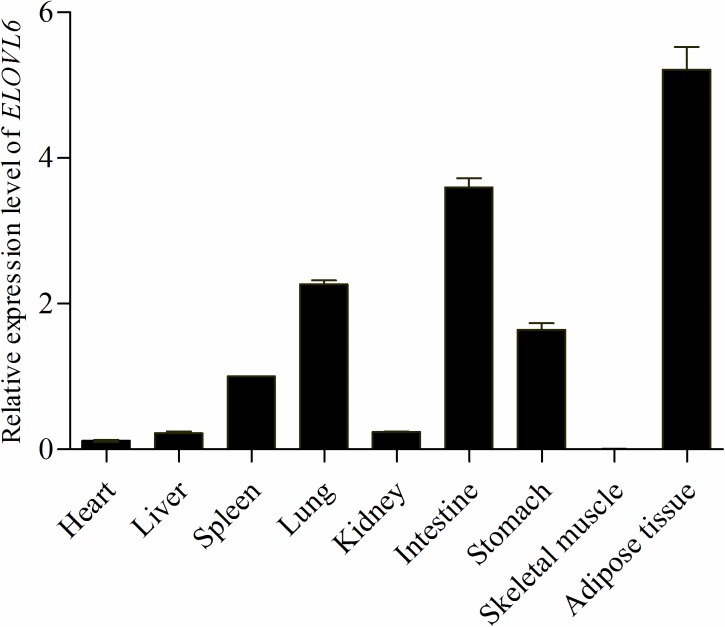
To enhance the understanding of the transcriptional regulation mechanism of bovine ELOVL6 in various tissues of Qin Chuan cattle, we investigated the expression profiles of bovine ELOVL6 in nine different tissues by qPCR. ELOVL6 was expressed ubiquitously on bovine (Fig 1). Significantly high transcript levels were observed in adipose tissue and intestine. The expression of bovine ELOVL6 in lung, stomach and spleen displayed moderate transcript levels, followed by kidney, liver, heart. Skeletal muscle had the lowest transcript level among the tissues investigated.
Fig 1. Tissue-specific expression patterns of bovine ELOVL6 mRNAs.
Each column represented the mean ± SD of three independent experiments which were performed in triplicate.
Bioinformatics analyses of bovine ELOVL6 promoter
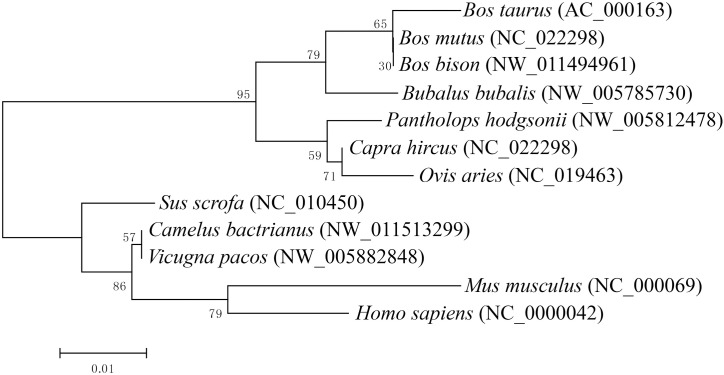
The 5’-flanking region of bovine ELOVL6 promoter was amplified and was identical to the GenBank database (accession no. AC_000163). To understand potential evolutionary processes of bovine ELOVL6 promoter among mammal species, a neighbor-joining phylogenetic tree was constructed by MEGA program (version 6.0). Bos Taurus has the close relationship with Bos mutus and Bos bison among Bovidae family. Mus musculus and homo sapiens display the greatest distance from Bos taurus (Fig 2).
Fig 2. The phylogenetic analyses of bovine ELOVL6 promoter.
The phylogenetic relationship was analyzed by Neighbor Joining method (Mega program version 6.0) utilizing bovine ELOVL6 promoter and homologous sequences from other mammal species. Bootstrap values were obtained from 1000 repetitions and illustrated as percentages at the nodes. The evolutionary distance of 0.01 nucleic acid substitutions per position was represented at the scale bars.
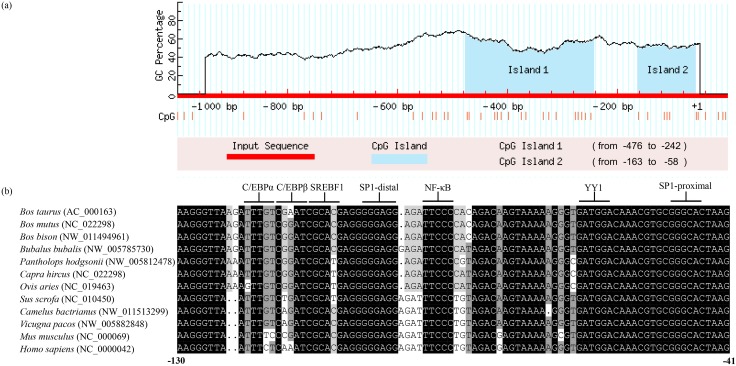
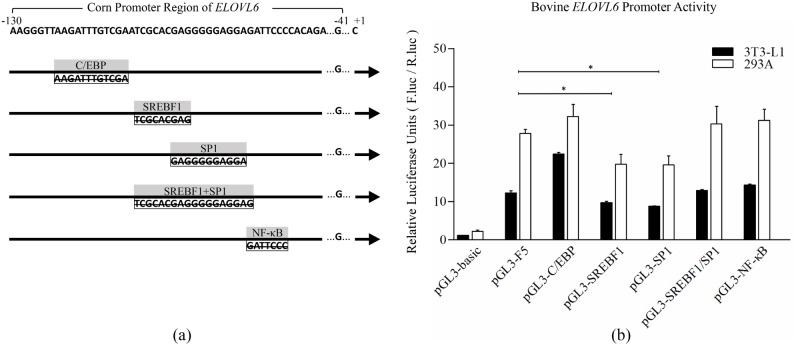
Utilizing the MatInspector program (Genomatix, USA), we analyzed the cloned promoter fragment consisting 980 bp upstream of the transcription start site (TSS). Interestingly, neither a TATA box nor a CAAT box was identified at the upstream of TSS. However, this region was found to be GC-rich. The online program MethPrimer revealed two predicted CpG islands within the promoter region of the bovine ELOVL6 (Fig 3A). Conserved nucleic acids were identified in the proximal CpG island by multiple sequence alignment (Fig 3B). Subsequently, we analyzed the sequence between -130 bp to -41 bp, a total of seven transcription factor binding sites were predicted on both strands of the promoter core sequence.
Fig 3. The prediction and analyses of CpG islands in bovine ELOVL6 promoter.
(a) The predicted CpG islands in the bovine ELOVL6 promoter (+1 to -1000 bp). The red vertical illustrated the GC-rich regions. The blue shading regions indicated the predicated CpG islands; (b) Multiple sequence alignment of the second CpG island. Seven predicted transcription factors binding sites were underlined. Black shaded sequences indicated that the base pair was identical in all sequences of the alignment. Dark grey shadow indicated conserved substitutions and light grey shadow illustrated semi-conserved substitutions.
Identification of the core promoter region
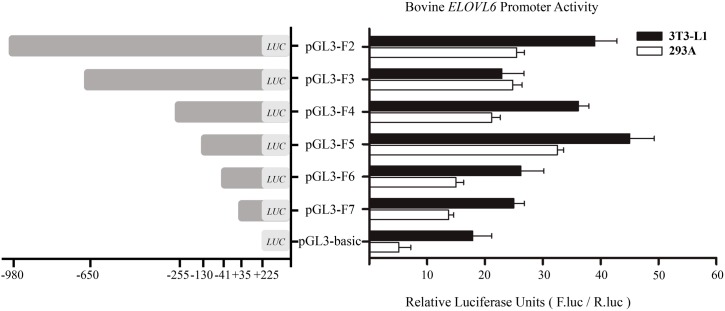
In order to narrow down the core region of bovine ELOVL6 promoter, we constructed six pGL3 reporter plasmids (designated as F2 to F7), which contained unidirectional truncations (from 5’ to 3’) of bovine ELOVL6 promoter, and then co-transfected with pRL-TK plasmid into 3T3-L1 and 293A cell lines for dual-luciferase reporter assay. In 3T3-L1 cell line, the dual-luciferase reporter assay showed that bovine ELOVL6 promoter activity gave a “down-up-down” pattern along with the piecewise truncation (Fig 4). Significantly higher promoter activity was observed in 3T3-L1 cell line transfected with the constructs containing the region between -130 bp and -41 bp, which indicated that this fragment contained the core region of bovine ELOVL6 promoter and probably harbored important transcriptional factor binding sites. Meanwhile, similar results were obtained in 293A cell line.
Fig 4. The structures and promoter activities of various truncation constructs of bovine ELOVL6 promoter region.
The promoter activities of 3T3-L1 cells (black bars) and 293A cells (white bars) were shown as the mean ± SD of the independent experiment performed in triplicate.
Deletion of potential transcription factor binding sites
The significant decrease of promoter activity in pGL3-F6 construct (-41/+225 bp) which was compared with pGL3-F5 construct (-131/+225 bp) may be attributed to the loss of cis elements contained within the deleted DNA region. To confirm the hypothesis, we chose four predicted transcription factor binding sites involved in fatty acids synthesis pathway to investigate their effects on bovine ELOVL6 promoter activity. We performed the deletion mutation of potential transcription factor binding sites to identify the key regulatory elements, which was schematically represented in Fig 5A. After measured by dual-luciferase reporter assay, no significant change in promoter activity was found in the deletions of C/EBP and NF-κB binding sites, suggesting that C/EBP and NF-κB may not be dominant regulators for bovine ELOVL6 promoter. In contrast, the respective deletion mutation of SREBF1 and SP1 binding sites dramatically decreased the promoter activities, which indicated SREBF1 and SP1 may be positive and dominant transcriptional regulators for bovine ELOVL6 promoter (Fig 5B).
Fig 5. Luciferase activities of different deletion constructs.
(a) Schematic represented various deletion constructs in the core region of bovine ELOVL6 promoter. The transcription initiation site was designated as +1.; (b) Luciferase activities of different deletion constructs were indicated as mean ± SD of the independent experiment performed in triplicate.
Silence of SREBF1 and SP1 in bovine mammary epithelial cells (BMECs)
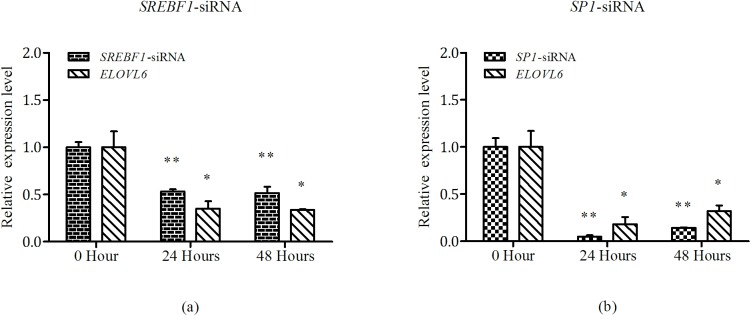
Based on estimates of cell volume, maximum intracellular concentration of siRNA is on the order of 5 pmol. After treatment with siRNA of SREBF1 and SP1 for 24 hours and 48 hours, respectively, both mRNA expression of ELOVL6 were significantly decreased. Relative to the experission in 0 hour, silencing of SREBF1 in 24 hours markedly decreased 65% expression of ELOVL6 (Fig 6A). Meanwhile, the expression of ELOVL6 in SP1 silencing group were induced a 82% reduction compare with 0 hour group (Fig 6B). Similar results were obtained in 48 hours group. These results demonstrated that both SREBF1 and SP1 regulated the transcription of bovine ELOVL6.
Fig 6. siRNA decreased the mRNA expression of ELOVL6.
(a) The silencing of SREBF1 affected the mRNA expression of ELOVL6; (b) The silencing of SP1 affected the mRNA expression of ELOVL6. GAPDH was measured as the reference gene. Each column represented the mean ± SD of the independent experiment performed in triplicate. * p < 0.05; ** p < 0.01.
Discussion
VLCFAs are fatty acids with greater than 20 carbon atoms, which can be characteristically divided into saturated, monunsaturated and polyunsaturated fatty acids [10]. VLCFAs regulate a variety of cellular functions and improve the resistance of numerous diseases, for instance cardiovascular disease and metabolic syndrome [1]. The enzymes involved in the process of VLCFAs synthesis were regulated by dietary [27] and transcription factors [28]. A recent study has indicated that PUFAs synthesis is regulated more by substrate competition for existing enzymes than by an increase in their mRNA expression [29]. In this research, we attempted to improve the fatty acids profiles of beef and milk, and raise the content of VLCFA through increasing its precursor stearic acid. Numerous studies have demonstrated that stearic acid which is the precursor of VLCFA was specifically elongated from palmitic acid by ELOVL6 [8, 11, 13]. Understanding the transcriptional regulation of bovine ELOVL6 resulted in ways towards guiding the production of high-quality beef and increasing the commercial value of cattle. Data derived from mouse has provided that the core region of mouse ELOVL6 promoter contained an E-box and a SREBF1 binding site (SRE) [22]. Further works demonstrated that SREBF1 were the dominant transcription factors for mouse ELOVL6 [13, 14]. The findings on human compared with murine revealed another transcriptional regulation pattern that ChREBP and SREBF1 synergistically activated human ELOVL6 promoter [23]. ELOVL1, as a member of ELONGASE family, was regulated by mTOR through the activating the transcription factors SREBF1 and PPARγ in Cashmere goat [30]. The transcription factor type and its regulatory pattern were strongly determined by species and cell type [31]. We hypothesized that the transcriptional regulation of bovine ELOVL6 would be different within patterns on mouse, human or goat.
In general, ELOVL6 mRNAs are ubiquitously expressed but their ratios vary across different tissues. In the current study, we demonstrated that the expression profile of bovine ELOVL6 slightly differ from that in human. In bovine, the transcript levels of ELOVL6 in intestine was higher than that in lung, whereas opposite observation was found in human as previously described by Ohno [11]. However, similar expression patterns were observed in other tissues. The different expression patterns in intestine were probably owning to the high fat diets for fattening beef cattle, which may increase the expression of bovine ELOVL6. The small intestine is commonly thought of as a lipid storage organ, however, a recent research clarified a novel function of intestine that enterocytes stored the dietary fat in cytoplasmic lipid droplets (CLDs), when meals and diets containing large amounts of fat were consumed [32]. In order to alleviate the lipotoxicity to enterocytes induced by high concentrations of free fatty acids absorbed from high fat diets, enterocytes required high expression level of ELOVL6 to elongate the palmitic acid into stearic acid for the storage in CLDs [33]. Since the limitation of samples collection, we were fail to investigate the tissue expression of bovine ELOVL6 on cow.
We have identified that the core region (-131/-41 bp) of bovine ELOVL6 promoter located at the second putative CpG island, harboring seven predicted TFBSs. The findings consisted with previous report that the DNA methylation of CpG island influenced the binding between transcription factor and its target DNA [34]. Among the seven predicted TFBSs, we chose four sites to verify the key transcription factors. C/EBP and SREBF1 play pivotal roles in regulating genes associated with fatty acid synthesis [35]. A recent study has been demonstrated that SP1 which belongs to Sp/Kruppel super family regulated gene related to fatty acids metabolism in goat [36]. NF-κB is recruited to many of the enhancer elements associated with the set of repressed-induced genes for inhibiting the reprogramming of fatty acid metabolism [37]. The site-directed deletion mutation assay in bovine ELOVL6 promoter proved our hypothesis that both SREBF1 and SP1 can activate the transcription of bovine ELOVL6 individually and exhibited equal effects on the promoter activities. For verification of results, we designed RNA interference of SREBF1 and SP1 in BMECs which was in line with previous findings.
Study on murine demonstrated that SREBFs including three isoforms (SREBF-1a, SREBF-1c and SREBF-2), are established as global lipid synthetic regulators [38]. However, only two isoforms, SREBF1 and SREBF2, have been identified on bovine. The research using RNA interference in bovine mammary epithelial cells revealed that SREBF1 plays an important role in integrated regulation of lipid synthesis through regulation of key enzymes, including ACC, FAS, FABP3, and SCD [39]. For providing the optimal transcriptional activation, SREBFs require additional coregulatory factors that, are limited to SP1, NF-Y or CREB to date [40]. Recent studies have shown that SP1 regulated the transcriptional activity of FAS and acyl-CoA synthetase long-chain family member 1 (ACSL1) which involved in fatty acids synthesis [41, 42]. SP1 regulates the transcription via two different mechanisms that SP1 can directly bind to the promoter and activate the transcription of adipogenesis genes, as well as which can indirectly enhance the expression of these genes by up-regulating SREBF1 [43, 44]. Taken together, these findings indicated transcription factors not work individually instead of cooperatively functioning as a comprehensive regulation network, integrating multiple signaling pathways at specific genomic regions. However, in our current study, we found simultaneous deletion of SREBF1 and SP1 binding sites reverted the promoter activity to a higher level than that of two single deletions but still lower than that of wild type, leaving a curious question for the regulation pattern in bovine ELOVL6 promoter. We speculated that SREBF1 and SP1 synergistically regulated bovine ELOVL6 promoter activity, which should be verified by our further works.
Supporting information
(DOC)
(DOC)
(DOC)
Acknowledgments
Si Chen and Xiaolin Liu conceived the experiments. Si Chen conducted the experiments and analyzed the results. Xiaolin Liu and Hua He contributed reagents and materials. The manuscript was written by Si Chen. All authors reviewed the manuscript. In addition, the financial disclosure was amended as follows: This work was financially supported by the Sci-Tech Integrated Innovation Engineering Projects of Shaanxi Province (2015KTCL02-11); the First-Class General Financial Grant from the China Postdoctoral Science Foundation (2015M570856); the First-Class General Financial Grant from the Shaanxi Province Postdoctoral Science Foundation (2016BSHYDZZ44); the National 12th“Five-Year”National Science and Technology Key Project (2011AA100307); the Science and Technology funds from Northwest A&F University (A2990215123). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was financially supported by the First-Class General Financial Grant from the China Postdoctoral Science Foundation (2015M570856); the First-Class General Financial Grant from the Shaanxi Province Postdoctoral Science Foundation (2016BSHYDZZ44); the National 12th"Five-Year"National Science and Technology Key Project (2011AA100307); the Sci-Tech Integrated Innovation Engineering Projects of Shaanxi Province (2015KTCL02-11); the Science and Technology funds from Northwest A&F University(A2990215123). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hammad S, Pu S, Jones PJ. Current Evidence Supporting the Link Between Dietary Fatty Acids and Cardiovascular Disease. Lipids. 2016;51(5):507–17. Epub 2016/01/01. doi: 10.1007/s11745-015-4113-x [DOI] [PubMed] [Google Scholar]
- 2.Bazinet RP, Laye S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nature reviews Neuroscience. 2014;15(12):771–85. Epub 2014/11/13. doi: 10.1038/nrn3820 [DOI] [PubMed] [Google Scholar]
- 3.Desvergne B, Michalik L, Wahli W. Transcriptional regulation of metabolism. Physiological reviews. 2006;86(2):465–514. Epub 2006/04/08. doi: 10.1152/physrev.00025.2005 [DOI] [PubMed] [Google Scholar]
- 4.Ahmad Nizar NN, Nazrim Marikkar JM, Hashim DM. Differentiation of lard, chicken fat, beef fat and mutton fat by GCMS and EA-IRMS techniques. Journal of oleo science. 2013;62(7):459–64. Epub 2013/07/05. [DOI] [PubMed] [Google Scholar]
- 5.Bonfatti V, Degano L, Menegoz A, Carnier P. Short communication: Mid-infrared spectroscopy prediction of fine milk composition and technological properties in Italian Simmental. Journal of dairy science. 2016;99(10):8216–21. Epub 2016/08/09. doi: 10.3168/jds.2016-10953 [DOI] [PubMed] [Google Scholar]
- 6.Liu X, Kris-Etherton PM, West SG, Lamarche B, Jenkins DJ, Fleming JA, et al. Effects of canola and high-oleic-acid canola oils on abdominal fat mass in individuals with central obesity. Obesity (Silver Spring, Md). 2016;24(11):2261–8. Epub 2016/11/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mennella I, Savarese M, Ferracane R, Sacchi R, Vitaglione P. Oleic acid content of a meal promotes oleoylethanolamide response and reduces subsequent energy intake in humans. Food & function. 2015;6(1):204–10. Epub 2014/10/28. [DOI] [PubMed] [Google Scholar]
- 8.Jakobsson A, Westerberg R, Jacobsson A. Fatty acid elongases in mammals: their regulation and roles in metabolism. Progress in lipid research. 2006;45(3):237–49. Epub 2006/03/28. doi: 10.1016/j.plipres.2006.01.004 [DOI] [PubMed] [Google Scholar]
- 9.Guillou H, Zadravec D, Martin PG, Jacobsson A. The key roles of elongases and desaturases in mammalian fatty acid metabolism: Insights from transgenic mice. Progress in lipid research. 2010;49(2):186–99. Epub 2009/12/19. doi: 10.1016/j.plipres.2009.12.002 [DOI] [PubMed] [Google Scholar]
- 10.Leonard AE, Pereira SL, Sprecher H, Huang YS. Elongation of long-chain fatty acids. Progress in lipid research. 2004;43(1):36–54. Epub 2003/11/26. [DOI] [PubMed] [Google Scholar]
- 11.Ohno Y, Suto S, Yamanaka M, Mizutani Y, Mitsutake S, Igarashi Y, et al. ELOVL1 production of C24 acyl-CoAs is linked to C24 sphingolipid synthesis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(43):18439–44. Epub 2010/10/13. doi: 10.1073/pnas.1005572107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lomakin IB, Xiong Y, Steitz TA. The crystal structure of yeast fatty acid synthase, a cellular machine with eight active sites working together. Cell. 2007;129(2):319–32. Epub 2007/04/24. doi: 10.1016/j.cell.2007.03.013 [DOI] [PubMed] [Google Scholar]
- 13.Moon YA, Shah NA, Mohapatra S, Warrington JA, Horton JD. Identification of a mammalian long chain fatty acyl elongase regulated by sterol regulatory element-binding proteins. The Journal of biological chemistry. 2001;276(48):45358–66. Epub 2001/09/22. doi: 10.1074/jbc.M108413200 [DOI] [PubMed] [Google Scholar]
- 14.Matsuzaka T, Shimano H, Yahagi N, Yoshikawa T, Amemiya-Kudo M, Hasty AH, et al. Cloning and characterization of a mammalian fatty acyl-CoA elongase as a lipogenic enzyme regulated by SREBPs. Journal of lipid research. 2002;43(6):911–20. Epub 2002/05/29. [PubMed] [Google Scholar]
- 15.Matsuzaka T, Shimano H. Elovl6: a new player in fatty acid metabolism and insulin sensitivity. Journal of molecular medicine. 2009;87(4):379–84. Epub 2009/03/05. doi: 10.1007/s00109-009-0449-0 [DOI] [PubMed] [Google Scholar]
- 16.Laggai S, Kessler SM, Boettcher S, Lebrun V, Gemperlein K, Lederer E, et al. The IGF2 mRNA binding protein p62/IGF2BP2-2 induces fatty acid elongation as a critical feature of steatosis. Journal of lipid research. 2014;55(6):1087–97. Epub 2014/04/24. doi: 10.1194/jlr.M045500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng YH, Chen WY, Kuo YH, Tung CL, Tsao CJ, Shiau AL, et al. Elovl6 is a poor prognostic predictor in breast cancer. Oncology letters. 2016;12(1):207–12. Epub 2016/06/28. doi: 10.3892/ol.2016.4587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomsen KF, Laposata M, Njoroge SW, Umunakwe OC, Katrangi W, Seegmiller AC. Increased elongase 6 and Delta9-desaturase activity are associated with n-7 and n-9 fatty acid changes in cystic fibrosis. Lipids. 2011;46(8):669–77. Epub 2011/05/06. doi: 10.1007/s11745-011-3563-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sunaga H, Matsui H, Ueno M, Maeno T, Iso T, Syamsunarno MR, et al. Deranged fatty acid composition causes pulmonary fibrosis in Elovl6-deficient mice. Nature communications. 2013;4:2563 Epub 2013/10/12. doi: 10.1038/ncomms3563 [DOI] [PubMed] [Google Scholar]
- 20.Marien E, Meister M, Muley T, Gomez Del Pulgar T, Derua R, Spraggins JM, et al. Phospholipid profiling identifies acyl chain elongation as a ubiquitous trait and potential target for the treatment of lung squamous cell carcinoma. Oncotarget. 2016;7(11):12582–97. Epub 2016/02/11. doi: 10.18632/oncotarget.7179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan CY, Virtue S, Bidault G, Dale M, Hagen R, Griffin JL, et al. Brown Adipose Tissue Thermogenic Capacity Is Regulated by Elovl6. Cell reports. 2015;13(10):2039–47. Epub 2015/12/03. doi: 10.1016/j.celrep.2015.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumadaki S, Matsuzaka T, Kato T, Yahagi N, Yamamoto T, Okada S, et al. Mouse Elovl-6 promoter is an SREBP target. Biochemical and biophysical research communications. 2008;368(2):261–6. Epub 2008/01/30. doi: 10.1016/j.bbrc.2008.01.075 [DOI] [PubMed] [Google Scholar]
- 23.Bae JS, Oh AR, Lee HJ, Ahn YH, Cha JY. Hepatic Elovl6 gene expression is regulated by the synergistic action of ChREBP and SREBP-1c. Biochemical and biophysical research communications. 2016;478(3):1060–6. Epub 2016/08/16. doi: 10.1016/j.bbrc.2016.08.061 [DOI] [PubMed] [Google Scholar]
- 24.Edwards PA, Tabor D, Kast HR, Venkateswaran A. Regulation of gene expression by SREBP and SCAP. Biochimica et biophysica acta. 2000;1529(1–3):103–13. Epub 2000/12/09. [DOI] [PubMed] [Google Scholar]
- 25.Li N, Zhao F, Wei C, Liang M, Zhang N, Wang C, et al. Function of SREBP1 in the milk fat synthesis of dairy cow mammary epithelial cells. International journal of molecular sciences. 2014;15(9):16998–7013. Epub 2014/09/25. doi: 10.3390/ijms150916998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ladeira MM, Schoonmaker JP, Gionbelli MP, Dias JC, Gionbelli TR, Carvalho JR, et al. Nutrigenomics and Beef Quality: A Review about Lipogenesis. International journal of molecular sciences. 2016;17(6). Epub 2016/06/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valenzuela R, Barrera C, Espinosa A, Llanos P, Orellana P, Videla LA. Reduction in the desaturation capacity of the liver in mice subjected to high fat diet: Relation to LCPUFA depletion in liver and extrahepatic tissues. Prostaglandins, leukotrienes, and essential fatty acids. 2015;98:7–14. Epub 2015/04/26. doi: 10.1016/j.plefa.2015.04.002 [DOI] [PubMed] [Google Scholar]
- 28.Yao D, Luo J, He Q, Shi H, Li J, Wang H, et al. SCD1 Alters Long-Chain Fatty Acid (LCFA) Composition and Its Expression Is Directly Regulated by SREBP-1 and PPARgamma 1 in Dairy Goat Mammary Cells. Journal of cellular physiology. 2017;232(3):635–49. Epub 2016/06/25. doi: 10.1002/jcp.25469 [DOI] [PubMed] [Google Scholar]
- 29.Tu WC, Cook-Johnson RJ, James MJ, Muhlhausler BS, Gibson RA. Omega-3 long chain fatty acid synthesis is regulated more by substrate levels than gene expression. Prostaglandins, leukotrienes, and essential fatty acids. 2010;83(2):61–8. Epub 2010/06/25. doi: 10.1016/j.plefa.2010.04.001 [DOI] [PubMed] [Google Scholar]
- 30.Wang W, He Q, Guo Z, Yang L, Bao L, Bao W, et al. Inhibition of Mammalian Target of Rapamycin Complex 1 (mTORC1) Downregulates ELOVL1 Gene Expression and Fatty Acid Synthesis in Goat Fetal Fibroblasts. International journal of molecular sciences. 2015;16(7):16440–53. Epub 2015/07/25. doi: 10.3390/ijms160716440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dowell RD. Transcription factor binding variation in the evolution of gene regulation. Trends in genetics. 2010;26(11):468–75. Epub 2010/09/25. doi: 10.1016/j.tig.2010.08.005 [DOI] [PubMed] [Google Scholar]
- 32.D'Aquila T, Hung Y-H, Carreiro A, Buhman KK. Recent discoveries on absorption of dietary fat: Presence, synthesis, and metabolism of cytoplasmic lipid droplets within enterocytes. Biochimica et Biophysica Acta (BBA)—Molecular and Cell Biology of Lipids. [DOI] [PMC free article] [PubMed]
- 33.Uchida A, Lee HJ, Cheng J-X, Buhman KK. Chapter 9—Imaging Cytoplasmic Lipid Droplets in Enterocytes and Assessing Dietary Fat Absorption Methods in Cell Biology. Volume 116: Academic Press; 2013. p. 151–66. doi: 10.1016/B978-0-12-408051-5.00014-0 [DOI] [PubMed] [Google Scholar]
- 34.Suzuki MM, Bird A. DNA methylation landscapes: provocative insights from epigenomics. Nature reviews Genetics. 2008;9(6):465–76. Epub 2008/05/09. doi: 10.1038/nrg2341 [DOI] [PubMed] [Google Scholar]
- 35.White UA, Stephens JM. Transcriptional factors that promote formation of white adipose tissue. Molecular and cellular endocrinology. 2010;318(1–2):10–4. Epub 2009/09/08. doi: 10.1016/j.mce.2009.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu J, Sun Y, Luo J, Wu M, Li J, Cao Y. Specificity protein 1 regulates gene expression related to fatty acid metabolism in goat mammary epithelial cells. International journal of molecular sciences. 2015;16(1):1806–20. Epub 2015/01/17. doi: 10.3390/ijms16011806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oishi Y, Spann NJ, Link VM, Muse ED, Strid T, Edillor C, et al. SREBP1 Contributes to Resolution of Pro-inflammatory TLR4 Signaling by Reprogramming Fatty Acid Metabolism. Cell metabolism. 2016. Epub 2017/01/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimano H. Sterol regulatory element-binding proteins (SREBPs): transcriptional regulators of lipid synthetic genes. Progress in lipid research. 2001;40(6):439–52. Epub 2001/10/10. [DOI] [PubMed] [Google Scholar]
- 39.Ma L, Corl BA. Transcriptional regulation of lipid synthesis in bovine mammary epithelial cells by sterol regulatory element binding protein-1. Journal of dairy science. 2012;95(7):3743–55. Epub 2012/06/23. doi: 10.3168/jds.2011-5083 [DOI] [PubMed] [Google Scholar]
- 40.Bennett MK, Osborne TF. Nutrient regulation of gene expression by the sterol regulatory element binding proteins: increased recruitment of gene-specific coregulatory factors and selective hyperacetylation of histone H3 in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(12):6340–4. Epub 2000/06/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ordovas L, Roy R, Pampin S, Zaragoza P, Osta R, Rodriguez-Rey JC, et al. The g.763G>C SNP of the bovine FASN gene affects its promoter activity via Sp-mediated regulation: implications for the bovine lactating mammary gland. Physiological genomics. 2008;34(2):144–8. Epub 2008/05/15. doi: 10.1152/physiolgenomics.00043.2008 [DOI] [PubMed] [Google Scholar]
- 42.Zhao ZD, Zan LS, Li AN, Cheng G, Li SJ, Zhang YR, et al. Characterization of the promoter region of the bovine long-chain acyl-CoA synthetase 1 gene: Roles of E2F1, Sp1, KLF15, and E2F4. Scientific reports. 2016;6:19661 Epub 2016/01/20. doi: 10.1038/srep19661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jeon BN, Kim YS, Choi WI, Koh DI, Kim MK, Yoon JH, et al. Kr-pok increases FASN expression by modulating the DNA binding of SREBP-1c and Sp1 at the proximal promoter. Journal of lipid research. 2012;53(4):755–66. Epub 2012/02/15. doi: 10.1194/jlr.M022178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu S, Archer MC. Sp1 coordinately regulates de novo lipogenesis and proliferation in cancer cells. International journal of cancer. 2010;126(2):416–25. Epub 2009/07/22. doi: 10.1002/ijc.24761 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.