Abstract
Bacterial cell division ends with the separation of the daughter cells, a process that requires peptidoglycan hydrolases (PGHs). Bacteria lacking cell separating PGHs are impaired in cell separation with the formation of long chains or clusters. We identified a gene in Streptococcus gordonii encoding for a putative glucosaminidase (lytB). The lytB isogenic mutant grew in long bacterial chains and resulted in impaired biofilm formation. Purified recombinant LytB showed a murolytic activity on Micrococcus lysodeikticus cell suspension and was able to disperse the long chains of the mutant, restoring the wild type diplococci/short chain phenotype. LytB protein was localized only in culture supernatant cell fraction of S. gordonii, and co-cultures of wild type and lytB mutant showed a significant reduction of bacterial chain length, indicating that LytB is a secreted enzyme. Our results demonstrate that LytB is a secreted peptidoglycan hydrolase required for S. gordonii cell separation.
Introduction
Bacteria produce enzymes able to cleave their own protective peptidoglycan cell wall. The number of peptidoglycan hydrolases (PGHs) encoded by a bacterial genome varies among species. Gram positive bacteria from Streptococcus and Lactobacillus genus possess a relative small number of PGHs [1], while 35 peptidoglycan hydrolases have been identified in Bacillus subtilis [2]. PGHs are involved in many bacterial physiological processes as autolysis, competence development, biofilm formation, sporulation, cell wall elongation and turnover, cell division and cell separation, and their classification is based on the catalytic activity on the different peptidoglycan bonds: N-acetylmuramyl-L-Alanine amidases, endopeptidases, carboxypeptidases, N-acetyl-β-D-muramidases, N-acetyl-β-D-glucosaminidases, and lytic transglycosylases [3, 4]. PGHs have a modular structure composed by one or two catalytic domains, and a single or multiple binding domains specific for the peptidoglycan or peptidoglycan anchored surface polymers, which localize the catalytic domain of the enzyme on the substrate [1].
Several PGHs involved in daughter cells separation have been identified. In the human pathogen Streptococcus pneumoniae, the deletion of lytB gene causes the formation of long chains of non-separated cells that can be dispersed by the addition of purified recombinant LytB enzyme [5]. The same defects in cell separation were observed in Streptococcus thermophilus cse deletion mutant and in rod-shaped Bacillus anthracis bslO deletion mutant [6, 7]. Defects in cell separation following cell division were also reported in several autolysin deficient mutants of Lactococcus lactis (acmA) [8], Listeria monocytogenes (murA) [9], Clostridium perfringens (acp) [10], Lactobacillus plantarum (Acm2) [11], and L. lactis AcmD [12], indicating that cell separation requires the involvement of PGHs.
Streptococci are elongated ellipsoids cocci that divide in successive parallel planes perpendicular to their long axis. The result of this type of cell division is the formation of single cells, diplococci or small chains [13]. During streptococcal cell division a complete septum is synthesized before cell separation and then cleaved by dedicated PGHs to allow the separation of daughter cells [14]. Streptococcus gordonii is an oral commensal gram positve, α-hemolytic, aerotolerant coccus that grows in short chains [15]. It is a member of the Mitis group, which comprises other oral streptococci as S. mitis, S. oralis, S. sanguis, S. parasanguis and the human pathogen S. pneumoniae [16]. S. gordonii is involved in the primary colonization of the saliva coated tooth surfaces where, by co-aggregating with other bacterial species, it contributes to the formation of the multispecies biofilm on healthy dental surfaces [17, 18]. S. gordonii has been used as mucosal vaccine vector for heterologous gene expression of surface recombinant antigens [19–21] and, despite belonging to the GRAS (generally regarded as safe) organisms, it is also an important etiologic agent of infective endocarditis [22, 23].
Here we report the identification of LytB as a PGH required for the cell separation of S. gordonii.
Materials and methods
Bacterial strains and growth conditions
S. gordonii V288 (Challis) [24] and the isogenic lytB mutant GP1485 were grown statically in Tryptic Soy Broth (TSB, Beckton Dickinson, Italy) at 37°C in sealed tubes. Growth was monitored spectrophotometrically at 590 nm with a Spectronic 20D+ spectrophotometer (Thermo Scientific, Waltham, MA, USA). Bacterial stocks were prepared from logarithmic growth phase cultures and stored frozen at -80°C in TSB containing 10% of glycerol. Escherichia coli DH5α and BL21-Codon Plus (DE3) strains (Stratagene, La Jolla, CA) were cultured in Luria-Bertani medium (LB) at 37°C in agitation or in Luria-Bertani agar (LBA).
Amino acid sequence analysis
LytB amino acid sequence was analyzed with Pfam tool available at the Wellcome Trust Sanger Institute (http://pfam.sanger.ac.uk/). Presence of signal peptides was predicted by using SignalP 4.0 Server [25], and Blast software (http://blast.ncbi.nlm.nih.gov/Blast.cgi) was used to conduct homology searches.
Construction of an isogenic mutant
To construct the S. gordonii isogenic mutant, the SGO_1138 gene (Gene ID: 25052579), which we named lytB, was inactivated by gene SOEing, essentially as previously described [26]. A 697 bp deletion from nucleotide 1,185,670 to 1,186,367 was generated using a spectinomycin resistance cassette (894bp, GenBank acc. no. AY334020) flanked by segments of lytB upstream and downstream genes, SGO_1137 (698 bp, Gene ID: 25052608) and engB (486 bp, Gene ID: 25052554), respectively. The DNA fragment containing the antibiotic-resistance cassette flanked by complementary regions upstream and downstream the lytB gene was obtained by subsequent PCR reactions using the following primers: IF622 (5’-TTTGCTGAGTGCGGCTAATA-3’), IF623 (5’-AATTGCTGCTCCAACTAGAT-3’), IF100 (5’-GCTCTAGAACTAGTGGATC-3’), IF101 (5’-TTCCCTTCAAGAGCGATAC-3’) IF624 (5’-GATCCACTAGTTCTAGAGCAGTCATAACTCCTAAAAAATACTAC-3’) and IF625 (5’-GTATCGCTCTTGAAGGGAAAGTAAGAAAAGAGTTAGGAAAAGA-3’). The obtained PCR product was used to transform S. gordonii V288 competent cells. Transformants were selected for acquisition of antibiotic resistance-marker by multilayer plating procedure [26]. The resulting mutant GP1485 was confirmed by PCR and DNA sequence analysis.
Cloning, expression and purification of recombinant LytB
The nucleotide sequence of lytB was amplified by PCR using S. gordonii V288 genomic DNA as template [24] using primers IF687 (5´-GGATCCAGCTAACGAAGAACAAACA-3´) and IF675 (5´-AAGCTTACGGTTCGCCCAAGCGACG-3´) (underlined sequence indicates respectively BamHI and HindIII restriction sites). PCR products were cloned into the pGEM T-Easy vector (Promega, Madison, WI, USA), and transformed in E. coli DH5α. Transformants were selected on LBA plates containing 100 μg ampicillin ml-1. Recombinant plasmids were purified and digested with BamHI and HindIII restriction enzymes. Purified DNA fragments were inserted in the expression vector pET-21b (Novagen, Madison, WI, USA). The recombinant expression plasmid pSMB630::lytB was transformed in E. coli BL21-CodonPlus (DE3)-RIL strain (Stratagene La Jolla, CA, USA). The resulting strain GP1905 was grown in 500 mL of LB broth containing 50 μg ampicillin ml-1 and 10 μg chloramphenicol ml-1 at 37°C with agitation at 140 rpm to an OD600 of 0.6, and then induced with 1mM isopropyl β-D-1-thiogalactopyranoside (IPTG) (Sigma-Aldrich, St Louis, MO, USA) for 5 h. Cells were collected by centrifugation, resuspended in Lysis Buffer (50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole, pH 8), and incubated with 0.3 mg ml-1 lysozyme (Sigma-Aldrich, St Louis, MO, USA) and 0.003 μg ml-1 DNase I (Sigma-Aldrich, St Louis, MO, USA) at 4°C for 1 h. Bacteria were lysed by three cycle of sonication, and centrifuged at 13.000 × g at 4°C for 30 min. The supernatant was filtered with a 0.22 μm filter (Millipore, Billerica, MA, USA), and loaded onto a Ni-NTA column (Qiagen, Hilden, Germany), according to manufacturer’s instructions. The eluate was desalted and concentrated in Tris-HCl 50 mM pH 7.5 with 10% (v/v) glycerol using a 200 ml stirred cell ultrafiltration device having a 10 KDa Amicon membrane (Millipore, Billerica, MA, USA). Proteins were quantitated with Bradford reagent (Sigma-Aldrich, St Louis, MO, USA) following manufacturer’s protocol, and stored at -20°C. The purified recombinant LytB (recLytB) was analyzed in a mini cell electrophoresis apparatus in MES buffer using a NuPAGE 4–12% Bis-Tris acrylamide gel (Invitrogen, Carlsbad, CA, USA). Protein bands were visualized by Coomassie Brillant Blue R-250 staining (Bio-Rad, Bio-Rad, Hercules, CA, USA). For Western blot analysis, proteins were transferred on a nitrocellulose membrane with the I-Blot apparatus (Invitrogen, Carlsbad, CA, USA) according to manufacturer instructions. Membrane was blocked for 1 h with 3% (w/v) BSA in TBS buffer (100 mM Tris-HCl pH 7.5, 150 mM NaCl) and incubated with Anti-Penta His primary antibody (Qiagen, Hilden, Germany) diluted 1:1,000 in TBS containing 3% (w/v) BSA for 1.5 h. Membrane was washed three times in TBS-Tween 0.05% (v/v), incubated with anti-mouse secondary antibody conjugated with alkaline phosphatase (AP) diluted 1:10,000 in TBS 3% (w/v) skim milk powder for 1.5 h, washed, and incubated with NBT-BCIP solution (Sigma-Aldrich, St Louis, MO, USA). The reaction was stopped by using dH2O when protein bands were clearly visible.
Ethics statement
Animal experimentation in Italy is regulated by Decreto Legislativo 116/92 and Directive 210/63/EU. The animal protocol was approved by the “Comitato Etico Locale” of the Azienda Universitaria Ospedaliera Senese and received thereafter the relative project licence issued by the Italian Ministry of Health (193/2008-B).
Anti-LytB antibody production
Three six-week-old female BALB/c mice (Charles River, Italy) were immunized subcutaneously with 50 μg of recLytB adsorbed to aluminum hydroxide. To obtain the aluminum-adsorbed antigen, recLytB (500 μg) was resuspended in 1 ml of 0.15 M NaCl and added to a solution of 2 mg ml-1 Aluminum hydroxide gel (Sigma-Aldrich, St Louis, MO, USA) in NaCl 0.15 M. The suspension was incubated in agitation at room temperature for 1 hour, and then centrifuged at 3,000 × g, at room temperature, for 5 min. Supernatant was collected and analyzed for unabsorbed protein with Bradford reagent. The pellet containing the adsorbed protein to aluminum gel (Al-LytB) was resuspendend in NaCl 0.15 M, and prepared for subcutaneous inoculation. An Al-LytB dose of 50 μg/mouse was delivered at day 0 and day 21. Blood samples were collected from temporal plexus at day 0 and day 35, and at day 37 by cardiac puncture. Total anti-LytB IgG serum was analyzed by enzyme-linked immunosorbent assay (ELISA) as previously described [20]. Sera were stored at -35°C.
Lytic assay
The lytic activity of recLytB was tested on a M. lysodeikticus cell suspension. Autoclaved M. lysodeikticus ATCC 4697 cells (Sigma-Aldrich, St Louis, MO, USA) were resuspended in 50 mM Tris-HCl (pH 7.0) to an OD450 of 0.5 in 16-mm glass tubes. Ten-fold dilutions of the enzyme, ranging from 0.1 to 0.0001 mg ml-1, were added to the tubes, and mixtures were incubated at 37°C for 2 h. A cell suspension without enzyme was used as a negative control. The OD450 values were recorded by using a Spectronic 20D+ spectrophotometer (Thermo Scientific, Waltham, MA, USA) during the incubation. The lysis rate (OD min-1) was defined as the absolute value of the slope of the linear regression calculated on consecutive points on the steepest portion of the lysis curve.
De-chaining assay
S. gordonii V288 and GP1485 were grown in TSB at 37°C. For each strain, 2-ml culture aliquots were collected at the same OD590 value multiple times during growth. The OD590 values selected were: 0.2, 0.8, 1.9, and ~1.7 (ON). At each collection point, culture aliquots were frozen at -80°C in 10% (v/v) glycerol. At the time of the assay, bacterial cells were defrosted, centrifuged at 3,500 × g at 4°C for 5 min, washed in 1× PBS, and resuspended in Tris 50 mM pH 7.0 at a cell concentration of ~ 2 × 108 cells ml-1. The de-chaining activity of recLytB was tested by adding the enzyme to the GP1485 cell suspension at a final concentration of 0.2 mg ml-1. GP1485 and V288 cell suspensions without enzyme were used as controls. Samples were incubated at 37°C for 6 h. After incubation, a 10 μl aliquot of each bacterial suspension was spotted on a glass slide, stained with crystal violet, and observed by light microscopy with a Leica DM1000 microscope at 100× magnification. For each sample, at least three pictures of a representative field were captured with a Leica DFC 490 digital camera, and used to count the chain length. The chain length was expressed as the mean ± SEM of the number of bacterial cells forming a chain.
Cell fractionation
Bacterial cell fractions of S. gordonii V288 were prepared as described [27]. Briefly, four different fractions were obtained: (i) cell wall, which contains cell wall fragments released following lysozyme and mutanolysin digestion; (ii) envelope, which represents membranes and cell wall-membrane fragments; (iii) clarified cytoplasm, (iv) culture supernatant. S. gordonii cultures were grown to early stationary phase and sedimented by centrifugation. Bacteria were washed once, resuspended in 0.1 ml of protoplasting buffer (100 mM Tris, pH 7.2; 30% (w/v) sucrose; 5 mM EDTA; 5 mM dithiothreitol) containing 1 mM PMSF, 1 mg ml-1 lysozyme (Sigma-Aldrich, St Louis, MO, USA), and 100 U ml-1 mutanolysin (Sigma-Aldrich, St Louis, MO, USA), and incubated at 37°C with gentle shaking for 1h. Protoplasts were centrifuged at 16,000 × g for 2 min. The recovered supernatant represented the cell wall fraction. Protoplasts were lysed by performing five washes in sterile distilled water. The supernatant collected in each step was pooled and ultra-centrifuged at 100,000 × g at 10°C for 30 min in a Beckmann Coulter Optima LE-80K ultracentrifuge. The pellet, representing the envelope fraction, was resuspended in 0.1 ml of 0.25 M Tris HCl pH 6.8,. The supernatant was precipitated with 20% (w/v) TCA by centrifugation at 13,000 × g, at 4°C, for 30 min. The recovered pellet was resuspended in 0.1 ml of 0.25 M Tris pH 6.8 to obtain the clarified cytoplasmic fraction. Culture supernatant was filtered (0.22 μm), loaded in an Amicon® Ultracel device (Millipore, Billerica, MA, USA) with 10 KDa membrane cut off and concentrated (53 ×) according to manufacturer instructions. Cell fractions (~1 × 1010 cell equivalents) were separated on a NuPAGE 4–12% Bis-Tris acrylamide gels and transferred on nitrocellulose membranes. The presence of LytB was detected with an anti-LytB mouse serum (1:1,000 dilution), and with an anti-mouse IgG serum (1:10,000 dilution) conjugated with alkaline phosphatase (Sigma-Aldrich, St Louis, MO, USA).
Co-culture
S. gordonii V288 and GP1485 were inoculated from frozen stocks in 10 mL of TSB in sealed 16mm-diameter tubes and incubated at 37°C. Growth was monitored by a Spectronic 20D+ spectrophotometer. When bacterial cultures reached an OD590 value of 0.020, a volume of 5 mL of each culture was sampled, transferred in a new tube and co-cultured to early stationary phase (OD590 1.080). Chain length was determined by directly spotting 10 μl of the co-culture on a glass slide. Equal volumes of V288 and GP1485 cultures were mixed and spotted to the glass slide, as controls. Bacteria were heat-fixed, stained with crystal violet and observed by using a light microscope at 100× magnification. For each sample, at least three pictures of a representative field were captured with a Leica DFC 490 digital camera and used to count the chain length. The chain length was expressed as the mean ± SEM of the number of bacterial cells forming a chain.
In-vitro biofilm assay
Biofilm formation was analyzed in 6 well flat bottom polystyrene tissue culture plates (Sarstedt, Newton, NC, USA). Bacteria from frozen stocks (~ 4 × 108 CFU ml-1) were inoculated in the wells containing 5ml of TSB. Plates were incubated at 37°C in a 5% CO2 enriched atmosphere. At 2, 4, 6, 8, 10 and 24 h. plates were analyzed for biofilm formation. Planktonic cells were removed and each well was washed 3 times with 5 ml of PBS in order to remove loosely attached bacteria. Plates were dried at 30°C for 15 min and stained with 0.5 ml of crystal violet for 15 min. Wells were washed with distilled water and dried for a second time. Biofilm was removed with 1 ml of absolute ethanol and quantified by measuring the absorbance at OD590.
Statistical analysis
Two tailed Student’s t-test was used to analyze the data; statistical significance was defined as P < 0.05.
Results
Identification of LytB
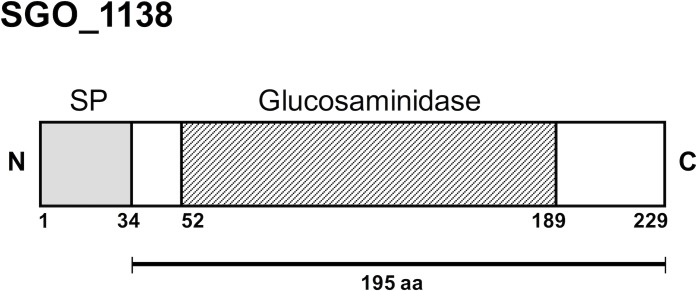
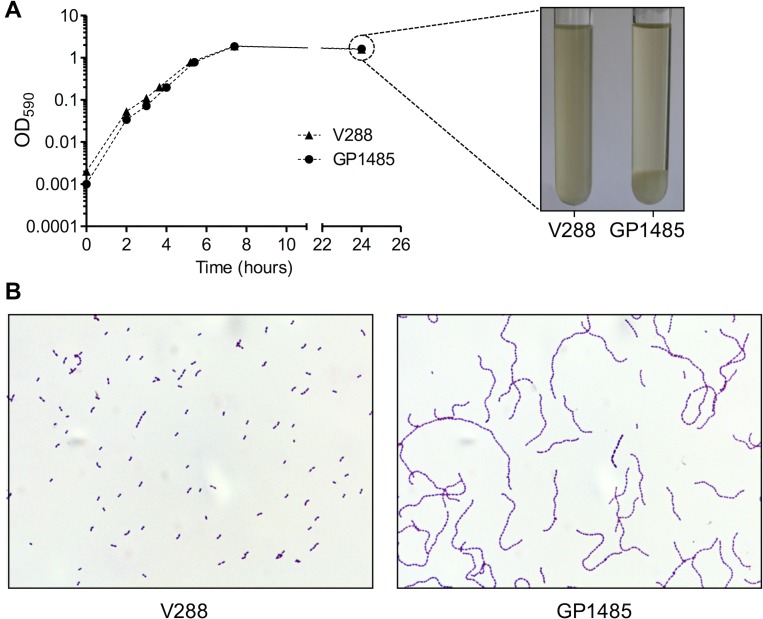
The lytB gene of S. pneumoniae has been previously shown to encode a putative endo-beta-N-acetylglucosaminidase that functions as a chain-dispersing enzyme [5]. The genome of S. gordonii does not encode a homolog of S. pneumoniae lytB. However, a search for glucosaminidase catalytic domains (Pfam PF01832) yielded SGO_1138 (gene ID: 25052579) in the chromosome of S. gordonii Challis strain (NCBI RefSeq: NC_009785). SGO_1138 is a 627 bp ORF that encodes for a 229-amino acids polypeptide characterized by a putative 34-amino acids signal peptide and a 137-amino acid glucosaminidase catalytic domain (Pfam PF01832) (Fig 1). To investigate whether SGO_1138 was involved in daughter cell separation, we constructed a SGO_1138 deletion mutant (GP1485) and characterized its growth phenotype. GP1485 showed a growth curve similar to wild type S. gordonii strain Challis V288 in liquid medium (Fig 2A). When the early stationary phase bacterial cultures were observed under a light microscope, we observed that the wild type strain grew in short chains and diplococci, whereas GP1485 formed long bacterial chains and aggregates that were dispersed by vortexing (Fig 2B). The GP1485 mutant phenotype resembles that observed with the lytB mutation of S. pneumoniae. Thus, SGO_1138 was renamed lytB.
Fig 1. Domain organization of SGO_1138 of S. gordonii Challis (NCBI RefSeq: WP_012000547.1).
Domains and amino acid positions were assigned with Pfam (http://pfam.sanger.ac.uk/) and drawn to scale. Numbers indicate the amino acid positions. SGO_1138 (229 amino acids) contains a 34 amino acids signal peptide (SP) and, a 137 amino acids glucosaminidase domain (Pfam PF01832). The 195 amino acid segment expressed in E. coli is indicated.
Fig 2. Growth curve and microscopic analysis of wild type and lytB mutant S. gordonii.
(A) S. gordonii V288 and GP1485 growth curves. Strains were inoculated from frozen stocks in tubes containing 10 ml of TSB, and growth was monitored spectrophotometrically for 24 h. Parallel tubes were incubated statically and photographed after 24 h of incubation. (B) Long chain phenotype of S. gordonii lytB mutant GP1485. Early stationary phase S. gordonii cultures (OD590 1.1) were spotted onto a glass slide, heat-fixed and stained with crystal violet. Samples were observed with a light microscope at 100× magnification. Pictures are representative of the whole bacterial population. Data from one representative experiment (of three independent experiments) are shown.
LytB muralytic activity on M. lysodeikticus cells
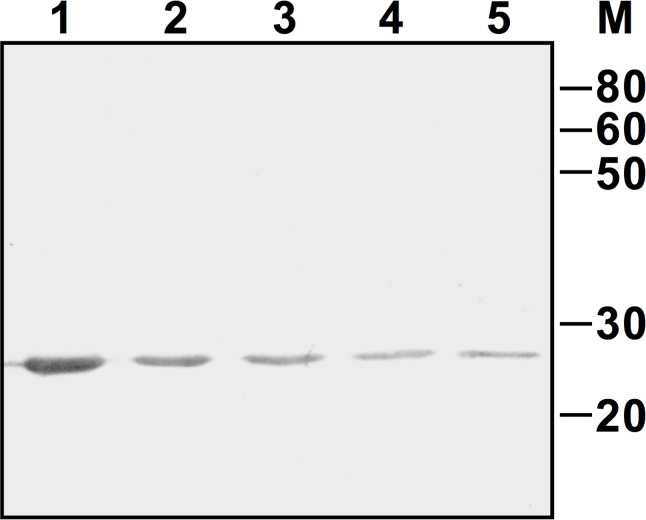
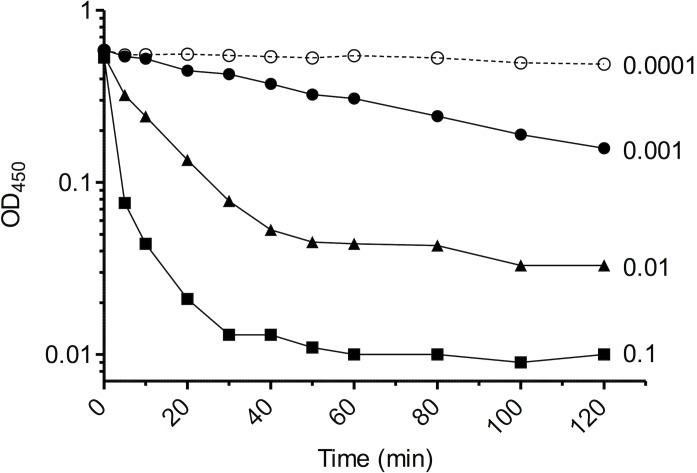
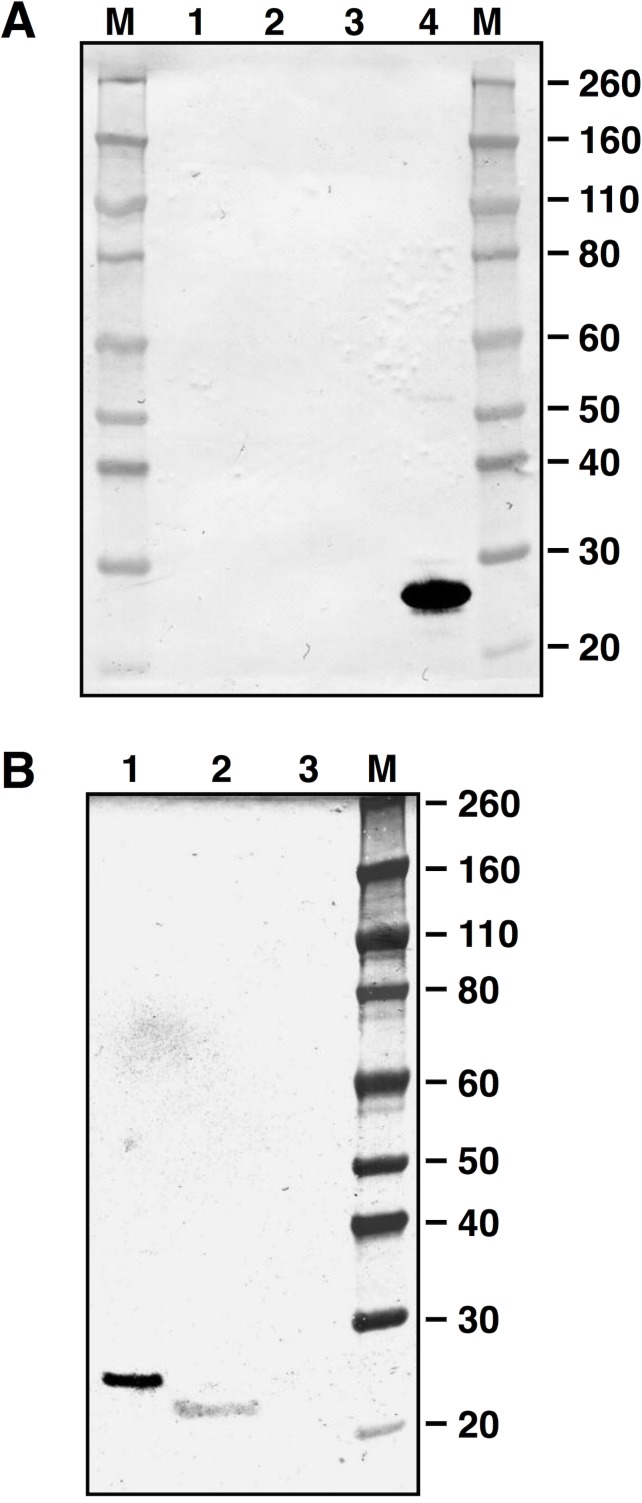
The mature form of LytB was produced in E. coli as a fusion with a C-terminal His tag, and purified by affinity chromatography under native conditions. The expected molecular mass (25.1 KDa) of recLytB was confirmed by Western blot analysis (Fig 3). The lytic activity of recLytB was tested on a whole-cell suspension of M. lysodeikticus using ten-fold serial dilutions of the recombinant enzyme, ranging from 0.1 to 0.0001 mg ml-1 (Fig 4). When recLytB was added at a concentration of 0.1 mg ml-1, the lysis rate was 260-fold higher than that of the untreated control, and 98.1% of the bacterial population was lysed after 2 hours of incubation. The lytic activity was clearly dose-dependent and was still present at a concentration of 0.001 mg ml-1 (Table 1).
Fig 3. Western blot analysis of recLytB.
Membrane was incubated with anti-His tag antibodies. Two-fold dilutions of purified recLytB ranging from 1 μg to 0.0625 μg are displayed (Lane 1–5). Numbers on the side indicate the molecular mass marker expressed in KDa.
Fig 4. Muralytic activity of recLytB.
Ten-fold serial dilutions of recLytB were added to a whole-cell suspension of M. lysodeikticus. Lytic activity of recLytB on M. lysodeikticus cells was monitored over time by measuring OD450 using a spectrophotometer. Data from one representative experiment (of three independent experiments) are shown.
Table 1. Muralytic activity of recLytB.
| LytB concentration (mg ml-1) | Lysis Rate | Lysis Outcome | ||
|---|---|---|---|---|
| OD min-1a | Fold increaseb | Final ODc | % of lysisd | |
| 0.1 | 0.091 | 260 | 0.010 | 98.1 |
| 0.01 | 0.030 | 85.7 | 0.033 | 93,9 |
| 0.001 | 0.0039 | 11.1 | 0.158 | 73,2 |
| 0.0001 | 0.00058 | 1.7 | 0.486 | 17.1 |
| None | 0.00035 | 1 | 0.478 | 4.0 |
aLytic rate was expressed as the absolute value of the slope of the linear regression calculated on consecutive points in the steepest portion of the lysis curve (Fig 4).
bFold increase of lysis rate relative to the control with no enzyme.
cOD450 value measured after 2 h of incubation.
dPercentage of OD decreases after 2 h of incubation.
Functional complementation of the ΔlytB mutant by recLytB
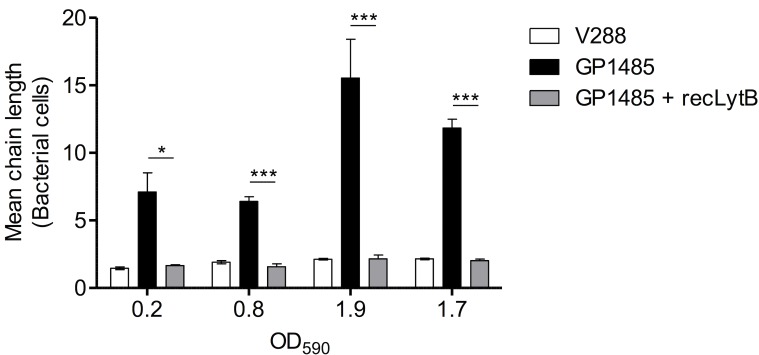
The capacity of recLytB to complement the chain-dispersing function, which is lacking in the lytB knock-out mutant GP1485, was tested by adding purified LytB to the mutant. Aliquots from cultures of wild type and mutant strains were collected at the same OD590 values during growth. At each OD590 tested, addition of LytB caused a significant reduction (3- to 7- fold) of the chain length of GP1485 (P < 0.05) (Fig 5). The resulting phenotype, which was characterized by short chains, diplococci and single bacterial cells, was indistinguishable from that of wild type cells (Fig 5).
Fig 5. De-chaining activity of recLytB.
Aliquots from cultures of S. gordonii V288 and ΔlytB mutant GP1485 were collected at the following OD590 values: 0.2, 0.8, 1.9 and ~1.7 (ON) (since the growth curves of the two strains were essentially indistinguishable (see Fig 1), same OD590 values corresponded to the same time points). Cells were washed, resuspended in buffer Tris 50 mM pH 7.0, and incubated with or without recLytB at 37°C. V288 without recLytB (white bar), GP1485 without recLytB (black bar), GP1485 with 0.2 μg μl-1 of recLytB (gray bar). After 6 hours of incubation, bacteria were observed by light microscopy to count the number of chain-forming bacteria. Data are expressed as the mean ± SEM of the number of cells forming a chain (mean chain length) in at least three microscopy fields. The asterisks indicate *P < 0.05 and ***P < 0.001. Data from one representative experiment (of two independent experiments) are shown.
Sub-cellular localization of LytB and complementation of the ΔlytB mutant phenotype in co-culture experiments
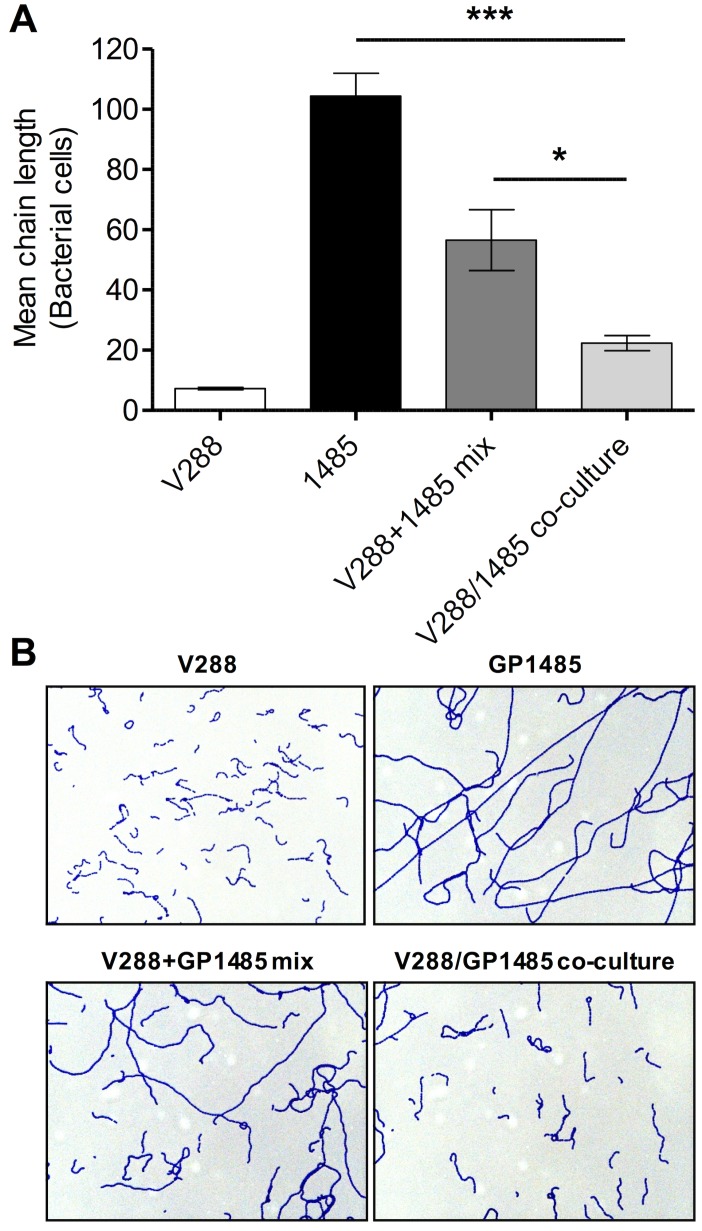
We next determined the sub-cellular localization of LytB by assaying bacterial cell fractions of S. gordonii V288 and ΔlytB mutant GP1485 for the presence of LytB by Western blot analysis. LytB was detected only in wild type culture supernatants (Fig 6A and 6B). Since LytB was found in culture supernatants, we reasoned that co-culturing the wild type strain with ΔlytB mutant strain might complement the absence of LytB in GP1485. After 8 hours of co-culture, the mean chain length of mutant GP1485 was significantly decreased (~ 5-fold) relative to GP1485 culture alone (Fig 7A and 7B). Mixing the two cultures immediately prior to microscopy analysis only resulted in halving the chain length, as expected (Fig 7A and 7B), indicating that the effect of co-culturing on chain length results from enzymatic activity rather than the mere presence of both wild type and mutant cells in the mix.
Fig 6. LytB sub-cellular localization.
Membranes were incubated with an anti-LytB specific serum raised in mice. Numbers on the sides indicate the molecular mass marker (M) expressed in KDa. (A) Lane 1, cell wall; lane 2 envelope; lane 3 cytoplasm; lane 4, 1 μg of recLytB. Fractions loaded in each well corresponds to 1 × 1010 cell equivalents. (B) Lane 1, 0.2 μg of recLytB; lane 2, culture supernatant of V288; lane 3, culture supernatant of ΔlytB mutant GP1485.
Fig 7. V288 and ΔlytB mutant co-culture.
(A) Co-culture assay. S. gordonii wild type strain V288 (white bar) and lytB mutant GP1485 (black bar) were grown separately or in co-culture (light grey bar) and spotted directly on a glass slide for chain enumeration. An equal culture volume of V288 and GP1485 was mixed and spotted on a glass slide as control (dark grey bar). Data were expressed as the mean ± SEM of the number of cells forming a chain (mean chain length) considering at least three microscopy fields. The asterisks indicate *P < 0.05 and ***P < 0.001. Data from one representative experiment (of two independent experiments) are shown. (B) Representative microscopy pictures of the conditions described in panel A.
The ΔlytB mutant is impaired in biofilm formation
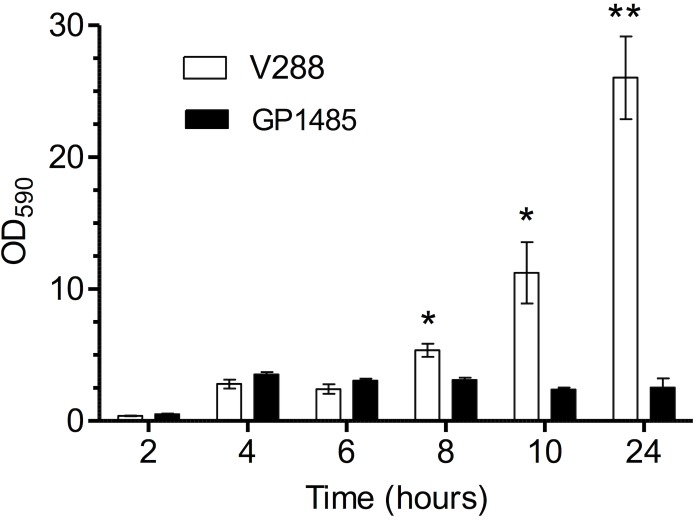
To investigate whether the ΔlytB mutant was affected in biofilm formation, the isogenic pair (mutant GP1485 and wild type V288) was tested in an in-vitro kinetic assay of biofilm formation, with bacteria growing in liquid medium in six-well tissue culture plates. During the exponential phase of growth (2 to 6 hours after the inoculum), wild type and mutant produced the same amount of biofilm, while in late exponential phase (8 hours), early stationary phase (10 hours), and late stationary phase (24 hours), wild type V288 produced from 2- to 10-fold higher amount of biofilm relative to the ΔlytB mutant (Fig 8).
Fig 8. In-vitro biofilm kinetic assay.
Biofilm formation was assayed in 6 well polystyrene tissue culture plates. The wild type V288 and ΔlytB mutant GP1485 strains were inoculated from frozen stocks and incubated at 37°C in a CO2 enriched atmosphere. At 2, 4, 6, 8, 10 and 24 hours of incubation, plates were washed and stained with crystal violet. Biofilm was removed with ethanol and quantified at 590 nm wavelength. Data represent the mean ± SEM of two experiments performed in triplicate. The asterisks indicate *P < 0.05.
Discussion
Numerous observations in the literature have clearly established the essential role of PGHs during bacterial cell separation. Mutants deficient in cell-separating PGHs can replicate but grow in long chains of cells [5–11]. Here we report that the genome of the oral commensal S. gordonii carries lytB, a gene coding for a putative glucosaminidase. Even though lytB mutant grew at the same rate of the wild type strain, it was impaired in cell separation and showed a long-chain phenotype. Addition of recombinant LytB to a lytB deficient mutant functionally complemented the mutant phenotype, demonstrating that S. gordonii LytB functions as a cell-separating enzyme. Since the de-chaining activity of S. gordonii LytB does not lead to cell lysis (not shown), future experimentation will test the putative polar localization of this enzyme.
Our work shows that LytB of S. gordonii has unique properties among PGH. LytB is presumably a secreted protein, since it is found only in the bacterial culture supernatant and carries a putative secretion signal peptide. Moreover, LytB carries no recognizable conserved peptidoglycan binding domains. The reduction of the mean chain length in the lytB mutant observed when wild type and mutant where co-cultured is consistent with the proposed secreted nature of this protein. In contrast, PGHs involved in cell separation are typically localized on the peptidoglycan by single or multiple binding domains. For example, a single LysM domain targets Cse of S. thermophilus [6] and AcmA of L. lactis [28] directly to the peptidoglycan, while S. pneumoniae LytB binds to choline residues on teichoic acids by repeated choline binding domains (ChBD) [29]. Among the PGHs lacking specific cell-wall-binding domains, L. plantarum Acm1 does not alter chain length [11], while S. thermophilus Mur1, L. lactis AcmC, and Leuconostoc citreum Mur have not been demonstrated to be cell-separating enzyme due to the lack of mutational or gene inactivation studies [30–32]. Based on these considerations, LytB of S. gordonii constitutes a novel example of an extracellular cell-separating enzyme.
S. gordonii is a close relative of the human pathogen S. pneumoniae [16]. A S. pneumoniae lytB mutant has reduced adhesion properties when tested in biofilm assays in vitro [33] and in vivo [34]. In our experimental biofilm model, S. gordonii lytB mutant resulted impaired in late stages of biofilm formation compared to the wild type. Since the maximum chain length of the mutant was measured during the late stages of growth, we hypothesize that the reduced adhesion of the mutant is a consequence of the shearing forces to which the long chains of lytB mutant are subjected. In the oral cavity, mouth fluid flow, tongue movements, and other physiologic movements of the mouth represent shear forces to which bacteria are exposed during the initial adhesion to the tooth [35, 36]. Thus, our results indicate that bacterial chain length can be a contributing factor to the success of S. gordonii adherence, pointing to the possibility that arrangement of streptococci in long chains can affect the initial colonization of tooth surfaces and the interaction with other bacterial species of dental biofilms.
Acknowledgments
We would like to thank Anna Maria Cuppone for technical assistance with mice immunization and Elisa Lazzeri for providing the protocol on biofilm experiments. We also thank Valentina Guerrini, Francesco Santoro and Maria Laura Gennaro for critical comments on the manuscript.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was founded by Ministero dell’Istruzione dell’Università e della Ricerca (MIUR) under Programs PRIN 2008 and PRIN 2012. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Layec S, Decaris B, Leblond-Bourget N. Diversity of Firmicutes peptidoglycan hydrolases and specificities of those involved in daughter cell separation. Research in microbiology. 2008;159(7–8):507–15. Epub 2008/07/29. doi: 10.1016/j.resmic.2008.06.008 [DOI] [PubMed] [Google Scholar]
- 2.Smith TJ, Blackman SA, Foster SJ. Autolysins of Bacillus subtilis: multiple enzymes with multiple functions. Microbiology (Reading, England). 2000;146 (Pt 2):249–62. Epub 2000/03/09. [DOI] [PubMed] [Google Scholar]
- 3.Vollmer W, Joris B, Charlier P, Foster S. Bacterial peptidoglycan (murein) hydrolases. FEMS microbiology reviews. 2008;32(2):259–86. Epub 2008/02/13. doi: 10.1111/j.1574-6976.2007.00099.x [DOI] [PubMed] [Google Scholar]
- 4.Chapot-Chartier M-P. Bacterial Autolysins In: König H, Claus H, Varma A, editors. Prokaryotic cell wall compounds, Structure and Biochemistry. 1 ed: Springer-Verlag Berlin Heidelberg; 2010. p. 383–406. [Google Scholar]
- 5.De Las Rivas B, Garcia JL, Lopez R, Garcia P. Purification and polar localization of pneumococcal LytB, a putative endo-beta-N-acetylglucosaminidase: the chain-dispersing murein hydrolase. Journal of bacteriology. 2002;184(18):4988–5000. Epub 2002/08/24. PubMed Central PMCID: PMCPMC135310. doi: 10.1128/JB.184.18.4988-5000.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Layec S, Gerard J, Legue V, Chapot-Chartier MP, Courtin P, Borges F, et al. The CHAP domain of Cse functions as an endopeptidase that acts at mature septa to promote Streptococcus thermophilus cell separation. Molecular microbiology. 2009;71(5):1205–17. Epub 2009/01/28. doi: 10.1111/j.1365-2958.2009.06595.x [DOI] [PubMed] [Google Scholar]
- 7.Anderson VJ, Kern JW, McCool JW, Schneewind O, Missiakas D. The SLH-domain protein BslO is a determinant of Bacillus anthracis chain length. Molecular microbiology. 2011;81(1):192–205. Epub 2011/05/19. PubMed Central PMCID: PMCPMC3124567. doi: 10.1111/j.1365-2958.2011.07688.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buist G, Kok J, Leenhouts KJ, Dabrowska M, Venema G, Haandrikman AJ. Molecular cloning and nucleotide sequence of the gene encoding the major peptidoglycan hydrolase of Lactococcus lactis, a muramidase needed for cell separation. Journal of bacteriology. 1995;177(6):1554–63. Epub 1995/03/01. PubMed Central PMCID: PMCPMC176772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carroll SA, Hain T, Technow U, Darji A, Pashalidis P, Joseph SW, et al. Identification and characterization of a peptidoglycan hydrolase, MurA, of Listeria monocytogenes, a muramidase needed for cell separation. Journal of bacteriology. 2003;185(23):6801–8. Epub 2003/11/18. PubMed Central PMCID: PMCPMC262698. doi: 10.1128/JB.185.23.6801-6808.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camiade E, Peltier J, Bourgeois I, Couture-Tosi E, Courtin P, Antunes A, et al. Characterization of Acp, a peptidoglycan hydrolase of Clostridium perfringens with N-acetylglucosaminidase activity that is implicated in cell separation and stress-induced autolysis. Journal of bacteriology. 2010;192(9):2373–84. Epub 2010/03/02. PubMed Central PMCID: PMCPMC2863477. doi: 10.1128/JB.01546-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rolain T, Bernard E, Courtin P, Bron PA, Kleerebezem M, Chapot-Chartier MP, et al. Identification of key peptidoglycan hydrolases for morphogenesis, autolysis, and peptidoglycan composition of Lactobacillus plantarum WCFS1. Microbial cell factories. 2012;11:137 Epub 2012/10/17. PubMed Central PMCID: PMCPMC3533731. doi: 10.1186/1475-2859-11-137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Visweswaran GR, Steen A, Leenhouts K, Szeliga M, Ruban B, Hesseling-Meinders A, et al. AcmD, a homolog of the major autolysin AcmA of Lactococcus lactis, binds to the cell wall and contributes to cell separation and autolysis. PloS one. 2013;8(8):e72167 Epub 2013/08/21. PubMed Central PMCID: PMCPMC3738550. doi: 10.1371/journal.pone.0072167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zapun A, Vernet T, Pinho MG. The different shapes of cocci. FEMS microbiology reviews. 2008;32(2):345–60. Epub 2008/02/13. doi: 10.1111/j.1574-6976.2007.00098.x [DOI] [PubMed] [Google Scholar]
- 14.Egan AJ, Vollmer W. The physiology of bacterial cell division. Annals of the New York Academy of Sciences. 2013;1277:8–28. Epub 2012/12/12. doi: 10.1111/j.1749-6632.2012.06818.x [DOI] [PubMed] [Google Scholar]
- 15.Kilian M ML, Henrichsen J. Taxonomic study of viridans streptococci: description of Streptococcus gordonii sp. nov. and emended descriptions of Streptococcus sanguis (White and Niven 19461, Streptococcus oralis (Bridge and Sneath 1982), and Streptococcus mitis (Andrewes and Horder 1906). International journal of systematic bacteriology. 1989;39(4):471–84. [Google Scholar]
- 16.Kawamura Y, Hou XG, Sultana F, Miura H, Ezaki T. Determination of 16S rRNA sequences of Streptococcus mitis and Streptococcus gordonii and phylogenetic relationships among members of the genus Streptococcus. International journal of systematic bacteriology. 1995;45(2):406–8. Epub 1995/04/01. doi: 10.1099/00207713-45-2-406 [DOI] [PubMed] [Google Scholar]
- 17.Kolenbrander PE, Palmer RJ Jr., Periasamy S, Jakubovics NS. Oral multispecies biofilm development and the key role of cell-cell distance. Nature reviews Microbiology. 2010;8(7):471–80. Epub 2010/06/02. doi: 10.1038/nrmicro2381 [DOI] [PubMed] [Google Scholar]
- 18.Nyvad B, Kilian M. Comparison of the initial streptococcal microflora on dental enamel in caries-active and in caries-inactive individuals. Caries research. 1990;24(4):267–72. Epub 1990/01/01. [DOI] [PubMed] [Google Scholar]
- 19.Oggioni MR, Pozzi G. A host-vector system for heterologous gene expression in Streptococcus gordonii. Gene. 1996;169(1):85–90. Epub 1996/02/22. [DOI] [PubMed] [Google Scholar]
- 20.Medaglini D, Ciabattini A, Spinosa MR, Maggi T, Marcotte H, Oggioni MR, et al. Immunization with recombinant Streptococcus gordonii expressing tetanus toxin fragment C confers protection from lethal challenge in mice. Vaccine. 2001;19(15–16):1931–9. Epub 2001/03/03. [DOI] [PubMed] [Google Scholar]
- 21.Ciabattini A, Giomarelli B, Parigi R, Chiavolini D, Pettini E, Arico B, et al. Intranasal immunization of mice with recombinant Streptococcus gordonii expressing NadA of Neisseria meningitidis induces systemic bactericidal antibodies and local IgA. Vaccine. 2008;26(33):4244–50. Epub 2008/06/28. doi: 10.1016/j.vaccine.2008.05.049 [DOI] [PubMed] [Google Scholar]
- 22.Douglas CW, Heath J, Hampton KK, Preston FE. Identity of viridans streptococci isolated from cases of infective endocarditis. Journal of medical microbiology. 1993;39(3):179–82. Epub 1993/09/01. doi: 10.1099/00222615-39-3-179 [DOI] [PubMed] [Google Scholar]
- 23.Watanakunakorn C, Pantelakis J. Alpha-hemolytic streptococcal bacteremia: a review of 203 episodes during 1980–1991. Scandinavian journal of infectious diseases. 1993;25(4):403–8. Epub 1993/01/01. [DOI] [PubMed] [Google Scholar]
- 24.Pozzi G, Musmanno RA, Renzoni EA, Oggioni MR, Cusi MG. Host-vector system for integration of recombinant DNA into chromosomes of transformable and nontransformable streptococci. Journal of bacteriology. 1988;170(4):1969–72. Epub 1988/04/01. PubMed Central PMCID: PMCPMC211061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nature methods. 2011;8(10):785–6. Epub 2011/10/01. doi: 10.1038/nmeth.1701 [DOI] [PubMed] [Google Scholar]
- 26.Iannelli F, Pozzi G. Method for introducing specific and unmarked mutations into the chromosome of Streptococcus pneumoniae. Molecular biotechnology. 2004;26(1):81–6. Epub 2004/01/22. doi: 10.1385/MB:26:1:81 [DOI] [PubMed] [Google Scholar]
- 27.Pozzi G, Oggioni MR, Manganelli R, Medaglini D, Fischetti VA, Fenoglio D, et al. Human T-helper cell recognition of an immunodominant epitope of HIV-1 gp120 expressed on the surface of Streptococcus gordonii. Vaccine. 1994;12(12):1071–7. Epub 1994/09/01. [DOI] [PubMed] [Google Scholar]
- 28.Steen A, Buist G, Horsburgh GJ, Venema G, Kuipers OP, Foster SJ, et al. AcmA of Lactococcus lactis is an N-acetylglucosaminidase with an optimal number of LysM domains for proper functioning. The FEBS journal. 2005;272(11):2854–68. Epub 2005/06/10. doi: 10.1111/j.1742-4658.2005.04706.x [DOI] [PubMed] [Google Scholar]
- 29.Moscoso M, Obregon V, Lopez R, Garcia JL, Garcia E. Allelic variation of polymorphic locus lytB, encoding a choline-binding protein, from streptococci of the mitis group. Applied and environmental microbiology. 2005;71(12):8706–13. Epub 2005/12/08. PubMed Central PMCID: PMCPMC1317417. doi: 10.1128/AEM.71.12.8706-8713.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cibik R, Tailliez P, Langella P, Chapot-Chartier MP. Identification of Mur, an atypical peptidoglycan hydrolase derived from Leuconostoc citreum. Applied and environmental microbiology. 2001;67(2):858–64. Epub 2001/02/07. PubMed Central PMCID: PMCPMC92659. doi: 10.1128/AEM.67.2.858-864.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huard C, Miranda G, Redko Y, Wessner F, Foster SJ, Chapot-Chartier MP. Analysis of the peptidoglycan hydrolase complement of Lactococcus lactis: identification of a third N-acetylglucosaminidase, AcmC. Applied and environmental microbiology. 2004;70(6):3493–9. Epub 2004/06/09. PubMed Central PMCID: PMCPMC427759. doi: 10.1128/AEM.70.6.3493-3499.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Husson-Kao C, Mengaud J, Benbadis L, Chapot-Chartier MP. Mur1, a Streptococcus thermophilus peptidoglycan hydrolase devoid of a specific cell wall binding domain. FEMS microbiology letters. 2000;187(1):69–76. Epub 2000/06/01. [DOI] [PubMed] [Google Scholar]
- 33.Moscoso M, Garcia E, Lopez R. Biofilm formation by Streptococcus pneumoniae: role of choline, extracellular DNA, and capsular polysaccharide in microbial accretion. Journal of bacteriology. 2006;188(22):7785–95. Epub 2006/08/29. PubMed Central PMCID: PMCPMC1636320. doi: 10.1128/JB.00673-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramos-Sevillano E, Moscoso M, Garcia P, Garcia E, Yuste J. Nasopharyngeal colonization and invasive disease are enhanced by the cell wall hydrolases LytB and LytC of Streptococcus pneumoniae. PloS one. 2011;6(8):e23626 Epub 2011/09/03. PubMed Central PMCID: PMCPMC3160309. doi: 10.1371/journal.pone.0023626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scannapieco FA, Solomon L, Wadenya RO. Emergence in human dental plaque and host distribution of amylase-binding streptococci. Journal of dental research. 1994;73(10):1627–35. Epub 1994/10/01. doi: 10.1177/00220345940730100701 [DOI] [PubMed] [Google Scholar]
- 36.Richard J. Lamont HFJ. Oral Microbiology at a Glance: Wiley-Blackwell; 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.