Abstract
Background
We evaluated the changes in the levels of soluble biomarkers of inflammation and coagulation and T cell activation among participants of ACTG A5217 who were started on antiretroviral therapy (ART) within the first 6 months of HIV infection.
Methods
Cryopreserved specimens were obtained pre-ART (Week 0), at time of virologic suppression (Week 36), and at 36 weeks after treatment interruption (Week 72). Levels of D-dimer, C-reactive protein (CRP), and soluble CD14 (sCD14) were measured in plasma, while T cell activation levels defined as the frequencies of CD4+ and CD8+ T cells co-expressing HLA-DR and CD38, were measured in peripheral blood mononuclear cells.
Results
D-dimer levels were significantly lower at viral suppression (p=0.031) whereas CRP and sCD14 levels remained similar to pre-ART levels. At viral suppression, levels of the soluble markers did not correlate with each other. CD4+ T cell counts pre-ART tended to modestly correlate with levels of D-dimer (r=0.35, p=0.058) and CRP (r=0.33, p=0.078). At 36 weeks following treatment interruption (Week 72), D-dimer levels returned back to pre-ART levels. However, CD8+ T cell activation was significantly lower than pre-ART levels (35.8% at Week 0 vs 28.9% at Week 72; p=0.004).
Conclusion
Among the A5217 participants who started ART within the first 6 months of HIV infection, high levels of sCD14 and CRP remain similar to pre-ART levels suggesting that immune damage occurring in the initial stages of infection persists despite short-term virologic suppression.
Keywords: early ART, HIV, biomarkers, Setpoint Study
INTRODUCTION
Chronic, treated HIV-1 infection has been associated with an increased risk of chronic diseases such as cardiovascular disease and HIV-associated malignancies.1 This increased risk is hypothesized to be due to persistent immune activation and increased systemic inflammation. Although virologic suppression on antiretroviral therapy (ART) leads to a decrease in several biomarkers of immune activation and inflammation to levels observed in HIV-seronegative controls, levels of other inflammatory biomarkers remain elevated.2 Indeed, these increased levels of soluble inflammatory biomarkers during effective ART have been shown to predict non-AIDS-defining adverse outcomes.3 Initiation of ART during early HIV-1 infection has been proposed as a possible strategy to prevent persistent immune dysfunction despite virologic suppression. However, studies evaluating the effect of initiating ART in early infection on biomarkers of immune activation and inflammation have shown varying results.4 We therefore evaluated changes in the levels of soluble biomarkers of inflammation, monocyte activation, and coagulation among individuals who started ART within six months of HIV acquisition in the AIDS Clinical Trials Group (ACTG) Study A5217 (The Setpoint Study).5 In addition, we compared levels of these biomarkers as well as levels of T cell activation among these individuals at study entry, prior to ART, and during a period of treatment interruption.
METHODS
ACTG A5217 is a 96-week, open-label study in which recently HIV-1-infected adults adults (defined as a non-reactive detuned HIV-1 antibody test or documented seroconversion) were randomized to either receive 36 weeks of ART followed by treatment discontinuation (immediate treatment with boosted lopinavir, tenofovir, and emtricitabine; IT), or followed off-treatment (delayed treatment; DT) until study-defined criteria were reached and ART was restarted.5 The study sought to compare virologic setpoint 36 weeks after treatment discontinuation in the IT group with the virologic setpoint of the DT group 72 weeks into the study. Of the 66 A5217 study participants in the IT group, 32 participants with available plasma and peripheral blood mononuclear cell (PBMC) samples and the following clinical data were included in this immunologic analysis: HIV-1 RNA and CD4+ T cell counts available at Weeks 0, 36 (obtained prior to treatment interruption), and 72 (36 weeks after starting treatment interruption). Samples from the timepoints were thawed and analyzed in batches. Plasma concentrations of D-dimer (coagulation biomarker) and soluble CD14 (sCD14; monocyte activation marker) were quantified using the D-dimer ELISA kit (American Diagnostica, Lexington, MA) and sCD14 ELISA Quantikine kit (R&D systems, Minneapolis, MN), respectively per manufacturer’s instructions. Duplicates of 20% of the samples were included in each ELISA plate, and results were analyzed using Dynex Plate Reader (Dynex Technologies). Plasma concentrations of C-reactive protein (CRP; inflammatory biomarker) were quantified using the Single-plex CRP Luminex kit (Invitrogen, Grand Island NY), and analyzed using the Bio-Plex Multiple system platform (Bio-Rad, Hercules, CA). For assessment of T cell activation, thawed peripheral blood mononuclear cells (PBMC) were stained with the following monoclonal antibodies: anti-CD3 (APC-H7), anti-CD4 (V450), anti-CD8 (PE-Cy7), anti-HLA-DR (PE), and anti-CD38 (APC). Specimens were analyzed within 24 hours of staining using an LSR Fortessa flow cytometer (BD Biosciences). Changes in the levels of soluble markers and T cell activation were analyzed using Wilcoxon signed rank test whereas differences in demographic data were analyzed using Wilcoxon rank sum test. Spearman correlations were computed to explore associations between the immunologic parameters. P values <0.05 were considered significant.
RESULTS
Demographics
Table 1 shows the baseline demographics of the 66 participants randomized to the IT arm and the 32 participants included in this analysis. Of these 32 study participants, 84% were male and 81% were white, and were older than the other participants in the IT group (median age of 39 years vs 27; p=0.028; Wilcoxon rank sum test) The median CD4+ T cell count was 494 cells/mm3 at Week 0, 663.5 cells/mm3 at Week 36 (vs. Week 0, p< 0.001), and 539 cells/mm3 at Week 72 (vs. Week 0, p= 0.092). The median HIV-1 RNA level at Week 0 was 4.37 log10 copies/mL while the median at Week 72 was 4.17 log10 copies/mL (p=0.073). The median duration of viral suppression (<50 cps/mL) is 26 weeks.
Table 1.
Demographics of A5217 participants who were randomized to the immediate treatment (IT) arm, including those with available samples and were included in the immunologic analyses and those who were not.
| Included in Immunology Analysis? | |||||
|---|---|---|---|---|---|
| Not included (N=34) |
Included (N=32) |
Total (N=66) |
P-Value* | ||
| Sex | Male | 31 (91%) | 27 (84%) | 58 (88%) | |
| Female | 3 (9%) | 5 (16%) | 8 (12%) | ||
| Age (yrs) | N | 34 | 32 | 66 | 0.028 |
| Median (Q1, Q3) | 27 (25, 36) | 38.5 (25.5, 44.0) | 34 (25, 40) | ||
| Race | White | 23 (68%) | 26 (81%) | 49 (74%) | |
| Black/African American | 4 (12%) | 6 (19%) | 10 (15%) | ||
| Other/Unknown | 7 (21%) | 0 (0%) | 7 (11%) | ||
| CD4+ T-cell count (cells/mm3) | N | 34 | 32 | 66 | 0.323 |
| Median (Q1, Q3) | 559 (421, 688) | 493.5 (406.0, 653.0) | 513.5 (415.0, 671.0) | ||
| 101–200 | 2 (6%) | 2 (6%) | 4 (6%) | ||
| 201–350 | 11 (32%) | 15 (47%) | 26 (39%) | ||
| 351–500 | 21 (62%) | 15 (47%) | 36 (55%) | ||
| HIV-1 RNA (log10copies/mL) | N | 34 | 32 | 66 | 0.822 |
| Median (Q1, Q3) | 4.4 (4.2, 4.7) | 4.4 (3.9, 4.8) | 4.4 (3.9, 4.8) | ||
| <10,000 | 8 (24%) | 9 (28%) | 17 (26%) | ||
| >=10,000 | 26 (76%) | 23 (72%) | 49 (74%) | ||
| Transmission risk | MSM | 27 (79%) | 25 (78%) | 52 (79%) | |
| Heterosexual | 3 (9%) | 7 (22%) | 10 (15%) | ||
| IVDU | 1 (3%) | 0 (0%) | 1 (2%) | ||
| MSM and IVDU | 3 (9%) | 0 (0%) | 3 (5%) | ||
Wilcoxon Rank Sum Test
Note: Baseline CD4 cell count (cells/mm3) and baseline HIV-1 RNA (log10(cp/mL)) were the values at study entry
Changes in Soluble Markers and T cell activation
We evaluated changes in the levels of CRP, sCD14, and D-dimer 36 weeks after starting ART, and after 36 weeks of treatment interruption (Figure 1A–C). At Week 36, only the plasma levels of D-dimer were significantly lower compared to pre-ART levels (from a median of 2.28 log10 ng/mL to 2.19; p=0.031). Despite virologic suppression on ART, levels of CRP and sCD14 remained similar to baseline levels. At Week 72 (36 weeks following ART-interruption), plasma levels of D-dimer and sCD14 biomarkers were similar to baseline, whereas there was a trend towards higher levels of CRP. We evaluated whether the plasma levels of biomarkers correlated with each other and with the CD4+ T cell counts at the time of virologic suppression (Week 36). There was a trend towards a positive correlation between CRP and D-dimer levels (r=0.34; p=0.066; data not shown). However, there were no observed correlations between CD4+ T cell counts and levels of the three soluble markers at Week 36.
Figure 1. Percent changes from pre-ART baseline in the levels of soluble biomarkers and T cell activation.
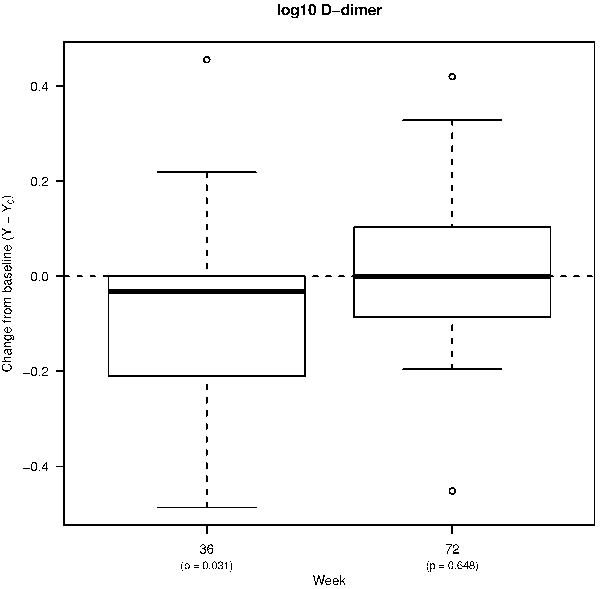
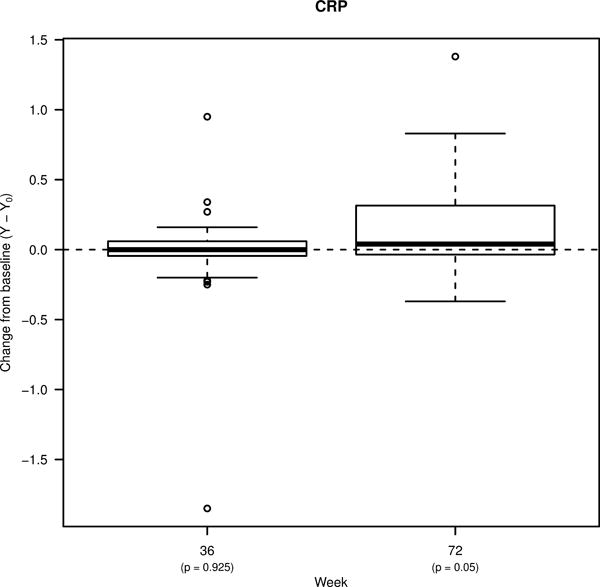

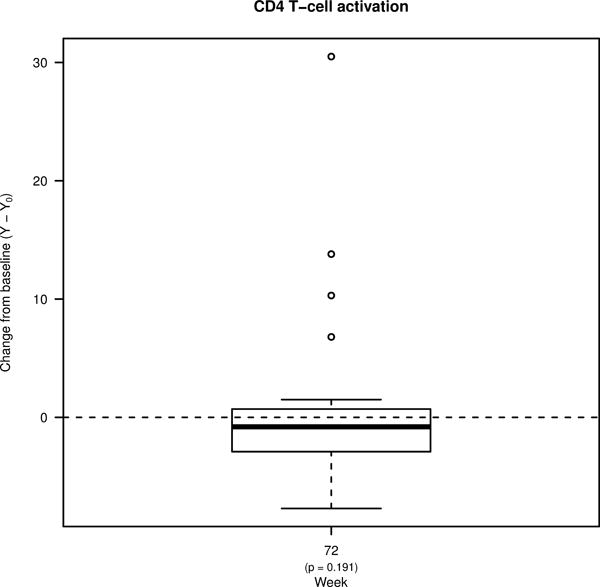
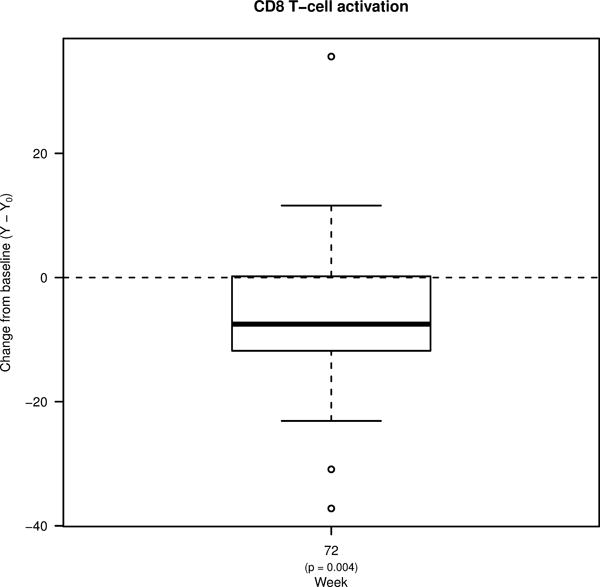
Box plots showing changes in the plasma levels of A) D-dimer, B) C-reactive protein (CRP), and C) soluble CD14 (sCD14) from pre-ART baseline to week 36 of ART, and to week 72 (36 weeks following treatment interruption). Box plots also show changes in the frequencies of D) CD4+ and E) CD8+ T cells co-expressing HLA-DR and CD38 from pre-ART baseline to week 72. Bold lines in the box plots represent the median change for each immunologic biomarker.
Given the availability of samples, we were only able to also compare levels of T cell activation (defined as the frequency of CD4 and CD8+ T cells that co-express HLA-DR and CD38) at the Week 0 timepoint and at Week 72 (Figure 1D–E). Although levels of CD4+ T cell activation were similar between the two timepoints, levels of CD8+ T cell activation were significantly lower at Week 72 (from a median of 35.3% at Week 0 to 28.9% at Week 72; p=0.004).
Association of baseline CD4+ T cell counts and HIV-1 RNA with soluble biomarker levels
We then assessed whether baseline levels of CD4+ T cell count and plasma HIV-1 RNA were predictive of the levels of soluble biomarkers during virologic suppression (Week 36). Baseline CD4+ T cell count tended to modestly correlate with the levels of D-dimer during viral suppression (r=0.35, p=0.058), but did not correlate with levels of sCD14 and CRP (Supplementary Figure 1). On the other hand, baseline HIV-1 RNA was not predictive of the levels in any of the soluble biomarkers at Week 36.
DISCUSSION
Early ART is hypothesized to be an important strategy in preventing persistent HIV-associated inflammation elevated despite virologic suppression on ART. Indeed, initiation of ART during early infection has been associated with normalization of CD4+ T cell counts within the first year, lower levels of immune activation, and a smaller HIV DNA and RNA reservoir size.6,7 Given that soluble markers of inflammation and coagulation have been associated with non-AIDS adverse outcomes during virologic suppression3, we evaluated changes in the levels of soluble biomarkers in A5217 participants who were randomized to immediate ART, that is, within the first 6 months of HIV infection. D-dimer and CRP have both been associated with mortality in HIV(+) individuals.8 D-dimer has been independently associated with endothelial dysfunction9 while sCD14 has been associated with cardiovascular disease in treated infection10 among treated HIV(+) individuals. Levels of D-dimer significantly decreased with ART which is consistent with findings from the Strategies for Management of Antiretroviral Therapy (SMART) Trial11. This is also consistent with findings from another early treatment study, the SPARTAC trial12, and supports the importance of early ART especially since D-dimer levels are associated with serious non-AIDS conditions or death in those with suppressed viremia.13 However, even with early ART and with a median virologic suppression of 26 weeks, the levels of sCD14 and CRP did not significantly change at Week 36. These findings are consistent with results from two other studies evaluating inflammatory biomarkers in the setting of ART-induced viral suppression,2,14 but in contrast to another study which showed a decline in these markers following ART initiation.15 Our findings suggest that viremia alone is not responsible for persistent monocyte activation and inflammation during treatment. It is likely that damage to the immune system during the acute stages of HIV infection may be responsible for the persistent elevation of these markers despite viral suppression. Given the limitations with sample availability, we were unable to evaluate levels of T cell activation during viral suppression. Results from other studies have shown that these levels (measured by %co-expression of HLA-DR and CD38 on T cells or by levels of soluble CD27) decrease upon start ART.2,15 Although the study population in these two studies include individuals who started ART during chronic HIV infection, we believe that the dynamics of T cell activation levels in our population will be the same. It is important to note that A5217 did not include participants who were treated during acute infection, and studies evaluating changes in inflammatory biomarker levels in this population will be important in fully evaluating the impact of early treatment on the levels of chronic inflammation during viral suppression.
With treatment interruption, levels of all the soluble markers returned to pre-ART levels showing that the initiation of ART in the IT group did not impact any of the soluble markers after ART was interrupted. Although we expected levels of T cell activation to return to pre-ART baseline upon treatment interruption, we observed significantly lower levels of Week 72 CD8+ T cell activation compared to pre-ART levels which suggests a benefit of early treatment, especially given the association of T cell activation with disease progression.16 However, this was counteracted by the lack of change in the levels of the soluble biomarkers, specifically sCD14, which has been associated with higher hazards of long-term mortality and serious non-AIDS events.17,18
Our study has several limitations. Since we did not include individuals diagnosed during acute infection, we cannot fully evaluate the impact of early ART in levels of inflammation and immune activation. Also, participants in the study were virally suppressed for only a median of 26 weeks. Thus, it is possible that further reductions in the levels of CRP and sCD14 may be evident with prolonged viral suppression. It is also possible that the older age in our study population may have affected some of our results, although we do not believe that the difference could have significantly altered the results of the study. Given that the study was conducted in 2005, it is important to note that the ART regimen used in this study, specifically the protease inhibitor (PI), is no longer recommended as the initial line of therapy. It is possible that levels of these soluble markers may be influenced by the difference in PI choice given in vitro findings showing differences in the effect of different PIs on endothelial cells, including their secretion of inflammatory markers.19 In summary, in our study population of individuals who were diagnosed within the first 6 months of infection, but not during acute infection, initiation of ART has some beneficial effects, however, immune damage occurring during the initial weeks of HIV infection likely leads to residual immune dysfunction that does not appear to be reversed with short-term treatment.
Supplementary Material
Acknowledgments
Sources of Support: Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number UM1 AI068634, UM1 AI68636, and UM1 AI106701. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health. This study was also supported by NIH Grants AI43638 and AI69432. We also like to thank AbbVie Pharmaceuticals and Gilead Sciences for providing study medications. The authors would like to thank Chanelle Houston, Rob Camp, Eric Daar, Karen Tashima, Carlos Del Rio, Susan Fiscus, C. Bradley Hare, Martin Markowitz, and Elizabeth Connick of the A5217 Study Team, Luann Borowski, Dr. Jillian Roper, and Aarika Yates from the University of Pittsburgh Immunology Specialty Laboratory of the AIDS Clinical Trials Group.
References
- 1.Althoff KN, McGinnis KA, Wyatt CM, et al. Comparison of risk and age at diagnosis of myocardial infarction, end-stage renal disease, and non-AIDS-defining cancer in HIV-infected versus uninfected adults. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2015;60(4):627–638. doi: 10.1093/cid/ciu869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wada NI, Jacobson LP, Margolick JB, et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. Aids. 2015;29(4):463–471. doi: 10.1097/QAD.0000000000000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tenorio AR, Zheng Y, Bosch RJ, et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. The Journal of infectious diseases. 2014;210(8):1248–1259. doi: 10.1093/infdis/jiu254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rajasuriar R, Wright E, Lewin SR. Impact of antiretroviral therapy (ART) timing on chronic immune activation/inflammation and end-organ damage. Current opinion in HIV and AIDS. 2015;10(1):35–42. doi: 10.1097/COH.0000000000000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hogan CM, Degruttola V, Sun X, et al. The setpoint study (ACTG A5217): effect of immediate versus deferred antiretroviral therapy on virologic set point in recently HIV-1-infected individuals. The Journal of infectious diseases. 2012;205(1):87–96. doi: 10.1093/infdis/jir699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ananworanich J, Sacdalan CP, Pinyakorn S, et al. Virological and immunological characteristics of HIV-infected individuals at the earliest stage of infection. J Virus Erad. 2016;2:43–48. doi: 10.1016/S2055-6640(20)30688-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jain V, Hartogensis W, Bacchetti P, et al. Antiretroviral therapy initiated within 6 months of HIV infection is associated with lower T-cell activation and smaller HIV reservoir size. The Journal of infectious diseases. 2013;208(8):1202–1211. doi: 10.1093/infdis/jit311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS medicine. 2008;5(10):e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hileman CO, Longenecker CT, Carman TL, et al. Elevated D-dimer is independently associated with endothelial dysfunction: a cross-sectional study in HIV-infected adults on antiretroviral therapy. Antivir Ther. 2012;17(7):1345–1349. doi: 10.3851/IMP2297. [DOI] [PubMed] [Google Scholar]
- 10.Longenecker CT, Jiang Y, Orringer CE, et al. Soluble CD14 is independently associated with coronary calcification and extent of subclinical vascular disease in treated HIV infection. Aids. 2014;28(7):969–977. doi: 10.1097/QAD.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baker JV, Neuhaus J, Duprez D, et al. Changes in inflammatory and coagulation biomarkers: a randomized comparison of immediate versus deferred antiretroviral therapy in patients with HIV infection. Journal of acquired immune deficiency syndromes. 2011;56(1):36–43. doi: 10.1097/QAI.0b013e3181f7f61a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamlyn E, Fidler S, Stohr W, et al. Interleukin-6 and D-dimer levels at seroconversion as predictors of HIV-1 disease progression. Aids. 2014;28(6):869–874. doi: 10.1097/QAD.0000000000000155. [DOI] [PubMed] [Google Scholar]
- 13.Grund B, Baker JV, Deeks SG, et al. Relevance of Interleukin-6 and D-Dimer for Serious Non-AIDS Morbidity and Death among HIV-Positive Adults on Suppressive Antiretroviral Therapy. PLoS One. 2016;11(5):e0155100. doi: 10.1371/journal.pone.0155100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallien S, Charreau I, Fonsart J, et al. Early changes in coagulation but not inflammatory biomarkers under intermittent ART: the randomized ANRS 106 WINDOW trial. J Int AIDS Soc. 2014;17(4 Suppl 3):19551. doi: 10.7448/IAS.17.4.19551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Funderburg NT, Andrade A, Chan ES, et al. Dynamics of immune reconstitution and activation markers in HIV+ treatment-naive patients treated with raltegravir, tenofovir disoproxil fumarate and emtricitabine. PLoS One. 2013;8(12):e83514. doi: 10.1371/journal.pone.0083514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giorgi JV, Hultin LE, McKeating JA, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. The Journal of infectious diseases. 1999;179(4):859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 17.Wada NI, Bream JH, Martinez-Maza O, et al. Inflammatory Biomarkers and Mortality Risk Among HIV-Suppressed Men: A Multisite Prospective Cohort Study. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2016 doi: 10.1093/cid/ciw409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sunil M, Nigalye M, Somasunderam A, et al. Unchanged Levels of Soluble CD14 and IL-6 Over Time Predict Serious Non-AIDS Events in HIV-1-Infected People. AIDS Res Hum Retroviruses. 2016 doi: 10.1089/aid.2016.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Auclair M, Afonso P, Capel E, Caron-Debarle M, Capeau J. Impact of darunavir, atazanavir and lopinavir boosted with ritonavir on cultured human endothelial cells: beneficial effect of pravastatin. Antivir Ther. 2014;19(8):773–782. doi: 10.3851/IMP2752. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


