Abstract
Objective
To evaluate the cost-effectiveness of nurse-led multifactorial care to prevent or postpone new disabilities in community-living older people in comparison with usual care.
Methods
We conducted cost-effectiveness and cost-utility analyses alongside a cluster randomized trial with one-year follow-up. Participants were aged ≥ 70 years and at increased risk of functional decline. Participants in the intervention group (n = 1209) received a comprehensive geriatric assessment and individually tailored multifactorial interventions coordinated by a community-care registered nurse with multiple follow-up visits. The control group (n = 1074) received usual care. Costs were assessed from a healthcare perspective. Outcome measures included disability (modified Katz-Activities of Daily Living (ADL) index score), and quality-adjusted life-years (QALYs). Statistical uncertainty surrounding Incremental Cost-Effectiveness Ratios (ICERs) was estimated using bootstrapped bivariate regression models while adjusting for confounders.
Results
There were no statistically significant differences in Katz-ADL index score and QALYs between the two groups. Total mean costs were significantly higher in the intervention group (EUR 6518 (SE 472) compared with usual care (EUR 5214 (SE 338); adjusted mean difference €1457 (95% CI: 572; 2537). Cost-effectiveness acceptability curves showed that the maximum probability of the intervention being cost-effective was 0.14 at a willingness to pay (WTP) of EUR 50,000 per one point improvement on the Katz-ADL index score and 0.04 at a WTP of EUR 50,000 per QALY gained.
Conclusion
The current intervention was not cost-effective compared to usual care to prevent or postpone new disabilities over a one-year period. Based on these findings, implementation of the evaluated multifactorial nurse-led care model is not to be recommended.
Introduction
With the aging of the population and the associated increase in multimorbidity, the prevention of (new) disability in community-living older persons has received considerable attention.[1] Disability is defined as difficulty or dependence in performing (instrumental) daily activities essential for independent living [2], while the occurrence of new physical disabilities is often referred to as functional decline.[3] Functional decline in older people is associated with loss of quality of life,[4] loss of independence,[5] and strains on social and economic resources.[6] It has been suggested that proactive, integrated care provision for community-living older people with complex care needs may postpone new disabilities, support independent living, and curtail health and social costs by preventing, delaying, or reducing hospitalizations and nursing home admissions.[2, 7–12]
Although some recent studies concluded that multifactorial interventions were not cost-effective compared to usual care,[13–16] other studies described that they were cost-effective at high willingness-to-pay ratios.[17–19] Despite conflicting evidence on the (cost-) effectiveness of multifactorial interventions to prevent or postpone new disabilities as shown in a number of reviews, [20–27] different proactive strategies are already part of national policy of elderly care in several Western countries.[28]
Over the last two decades, in the Netherlands, there has been an increasing task delegation towards registered nurses (RN) working in general practice, especially in the care for patients with chronic conditions.[29] This has become fertile soil to implement multifactorial nurse-led interventions to prevent new disabilities in older people. In 2008, the Dutch government launched the National Care for the Elderly Programme (NCEP) stimulating innovative healthcare projects focused on older people with multifactorial care needs to promote physical, mental and social health and well-being.[30] We designed an intervention to prevent or postpone new disabilities targeting a community-living primary care population, at increased risk of functional decline, and offered them geriatric interventions based on current evidence or guidelines, patient-centered care, and nurse-led care coordination.[20, 23, 25, 31, 32] All these components were designed based on reviews and meta-analyses of interventions with a beneficial effect on overall functioning. The aim of this study was to investigate the cost-effectiveness of a multifactorial nurse-led care to prevent or postpone new disabilities in community-living older people at increased risk of functional decline compared to usual care from a healthcare perspective alongside a cluster randomized trial.
Methods
Design
We conducted a cost-effectiveness analysis (CEA) and a cost-utility analysis (CUA) from a healthcare perspective alongside a cluster randomized trial with a one-year follow-up in the Netherlands. The trial was registered at Trial Registration NTR2653 http://www.trialregister.nl). The study protocol was approved by the Medical Ethics Committee of the Academic Medical Center, University of Amsterdam, The Netherlands (protocol ID MEC10/182) and all eligible participants signed a written informed postponed consent before inclusion. We provide a summary of the materials and methods in the current article. A more detailed description of the materials and methods is published in the study protocol.[31] The results of the trial are published elsewhere.[32]
Setting and participants
The cluster randomized trial was conducted between December 2010 and May 2014, in the north-west of the Netherlands.[32] Twenty-four general practices who had not implemented nurse-led integrated care for community-living older people, participated. All participating general practitioners (GP) invited their patients aged 70 years and over to fill in a self-report questionnaire after first excluding patients who had a life expectancy less than three months, suffered from dementia, did not understand Dutch, planned to move or spend a long time abroad, or lived in a nursing home.[32] Participants who were at increased risk of functional decline based on a score of two or more on the Identification of Seniors At Risk—Primary Care (ISAR-PC) were eligible for study participation.[33] The ISAR-PC was developed to identify community-living older persons at increased risk of functional decline and is validated in Dutch. It comprises three dichotomous risk factors for functional decline, has a moderate predictive value, and is easy to use.[33]
Randomization
We performed a computerized stratified cluster randomization procedure, in which practices were the clusters. We stratified on socio-economic status, number of enlisted patients, and practitioners in both study groups.[31]
Intervention
The participants from practices randomized to the intervention group received nurse-led multifactorial care provided by the community-care registered nurses (CCRN) in addition to the usual care provided by the GP. The intervention was designed to identify and treat geriatric problems in an early stage, and to improve care coordination between healthcare professionals.[31, 32] The program included a comprehensive geriatric assessment (CGA), an individually tailored care and treatment plan (CTP) consisting of multifactorial interventions, and nurse-led care coordination with multiple follow-up home visits. The CGA focused on somatic, psychological, functional and social domains, including a physical examination and performance tests to identify conditions such as urinary incontinence, memory problems, increased risk of falling, and loneliness.[31, 32] Possible interventions were referral to a GP, referral to a paramedic, giving advice, or follow-up visit by the CCRN.[34]
Care as usual
The participants from general practices randomized to the usual care group received no extra care or information besides usual care. In the Dutch healthcare system, the general practitioner (GP) plays a central role as the gatekeeper of the healthcare system, (s)he is the first and only freely accessible medical professional, and people are used to visiting their GP first if they have a health problem.[35]
Outcome measurements
All data were collected using self-report questionnaires at baseline, six and twelve months. The primary outcome was participants’ change in (I)ADL measured with the modified Katz-ADL index score at one year of follow-up.[36] The modified Katz-ADL index score ranges from zero to 15 points with higher scores indicating more dependence in (I)ADL. The secondary outcome included Quality-Adjusted Life-Years (QALYs) based on the EuroQol (EQ-5D-3L) over one year.[37] The EQ-5D-3L is a five-dimension scale to measure health related quality of life. Resulting health states were converted to utilities using the Dutch EQ-5D-3L tariff which was based on a sample of the Dutch general population.[38] The utilities reflect the relative desirability of a particular health state where 0 indicates death and 1 indicates perfect health. We calculated QALYs by multiplying the utilities by the amount of time spent in a particular health state. Changes in health states between measurements were considered to be linear.
Healthcare utilization and costs
Healthcare utilization and associated costs were measured from a healthcare perspective. During one year, healthcare utilization rates were collected using self-report questionnaires at baseline, six and twelve months to measure the number of GP consultations, the number of GP visits after hours, the hours of personal care and home nursing received, the number of days receiving day care and residential care, the number of nursing home admission days, the number of emergency room visits, and the number of hospital admissions. The EMR of participants admitted to one hospital (n = 1421) were used to determine the mean length of stay per hospital admission at six and twelve months per participant. This data was subsequently used to calculate the total number of hospital admission days. Dutch standard costs were used to value healthcare utilization according to Dutch guidelines for costing research.[39] All prices were adjusted for the year 2016 using consumer price index figures.[40] Cost were calculated by multiplying the volumes of healthcare utilization with cost prices of that unit. S1 Table lists the cost categories and prices used in the CEA and the CUA.
To calculate the costs of the intervention, time to identify older persons at increased risk and time to train nurses for the intervention was valued using salary costs (S2 Table). In addition, the CCRNs used diaries to fill in the number of visits, and time per visit for each participant including consultation time with the GP. A top down approach was used by calculating the total costs of intervention (including the people screened) and dividing this by the total number of participants in the intervention group.
Statistical analysis
All analyses were performed according to the intention-to-treat principle. Baseline characteristics of participants were described for the two study groups. Missing values for cost and effect data were imputed using multiple imputation by chained equations (MICE) with predictive mean matching (PMM).[41, 42] Individual sub costs per category were imputed instead of total costs to maximize the accuracy of the imputation.[43] We created 50 imputed datasets that were analyzed separately. The analysis results from the imputed datasets were pooled using Rubin’s rules.[44]
Seemingly unrelated regression analyses were performed to estimate adjusted cost and effect differences. Cost and effect differences were adjusted for confounding variables, which were selected on the basis of causal diagrams. Confounders included baseline values of (i) the outcome variable, (ii) age, (iii) sex, (iv) education (three categories), and (v) socio-economic status (three categories).[45] Incremental Cost-Effectiveness Ratios (ICERs) were calculated by dividing the pooled cost difference by the pooled effect difference. Bias-corrected accelerated bootstrapping techniques (5000 replications) were used to estimate 95% confidence intervals around cost and effect differences, and the statistical uncertainty around the ICER. The uncertainty surrounding the ICER was visually presented in the cost-effectiveness (CE) planes.
Cost-effectiveness acceptability curves (CEAC) were estimated to quantify the uncertainty due to sampling and measurement errors. The CEAC is a plot of the probability that the intervention is cost-effective in comparison with care as usual (y-axis) as a function of the money society might be willing to pay for one additional unit of outcome (x-axis). The pooled coefficients and variance parameters from the regression models were used to estimate the CEACs.[46]
We used IBM SPSS, Version 20.0 (IBM Corp. 2011) and STATA 13 (StataCorp. 2013. College Station, TX) for data analysis.
Results
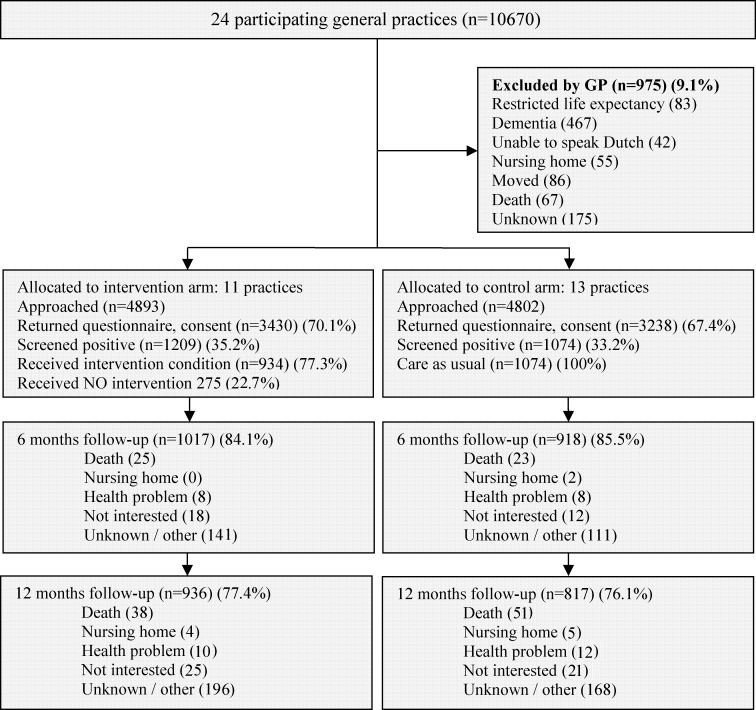
Eleven practices were randomized to the intervention group and 13 practices were randomized to the usual care group. In total, 35.2% (1209/3430) of the potential participants in the intervention group and 33.2% (1074/3238) of the potential participants in the usual care group (Fig 1) were at increased risk of functional decline based on a score ≥ 2 points on the ISAR-PC. The participants’ baseline characteristics were balanced between both study groups, except that there was a higher percentage of people with a high SES level in the intervention group (Table 1). The median age of the participants was almost 83 years in both groups. The median modified Katz-ADL index score was 2 (IQR 1–5) points in the intervention group and 3 (IQR 1–5) points in the usual care group. The follow-up rates after one year were 77.4% (936/1209) in the intervention group and 76.1% (817/1074) in the usual care group (Fig 1). At one year of follow-up, 23% of the cost data were missing, 24% of the modified Katz-ADL index data were missing and 23% of the QALY data were missing. The participants who declined the comprehensive geriatric assessment (n = 275) were older, had more (I)ADL disabilities, and more often lived in a home for the elderly (data not shown).
Fig 1. Flow of practices and participants through the trial.
Eleven practices were randomized to the intervention group and 13 practices were randomized to the usual care group. In both groups around 35% of the invited persons were at increased risk of functional decline and participated in the study. In both groups the follow-up rates were around 77% after one year respectively.
Table 1. Distribution of baseline variables of participants with an ISAR-PC score ≥ 2, by study group (N = 2283).
| Characteristics | Intervention group | Usual care group | |
|---|---|---|---|
| N = 1209 | N = 1074 | ||
| n(%) | n(%) | ||
| Age, in years, median (IQR) | 82.6 (76.8–86.8) | 82.9 (77.3–87.3) | |
| Female sex | 789 (65.2) | 671 (62.7) | |
| Caucasian | 1141 (95.4) | 1022 (96.5) | |
| Level of education | |||
| primary school or less | 255 (21.3) | 281 (26.6) | |
| secondary education | 760 (63.7) | 648 (61.4) | |
| college or university | 179 (15.0) | 127 (12.0) | |
| Socio-economic status | |||
| low (≤1SD) | 57 (4.7) | 78 (7.3) | |
| intermediate | 931 (76.9) | 890 (83.2) | |
| high (≥1SD) | 223 (18.4) | 102 (9.5) | |
| Married/living together | 561 (46.7) | 489 (46.0) | |
| Living situation | |||
| independent | 530 (44.0) | 467 (43.9) | |
| aloneindependent | 535 (44.5) | 442 (41.6) | |
| togetherhome for elderly | 138 (11.5) | 154 (14.5) | |
| Multimorbidity (≥2) | 997 (83.2) | 856 (80.6) | |
| Polypharmacy (≥3) | 830 (69.3) | 748 (70.7) | |
| Modified Katz-ADL index (range 0–15), (median (IQR)) | 2 (1–5) | 3 (1–5) | |
| Katz-ADL (range 0–6), median (IQR) | 1 (0–1) | 1 (0–1) | |
| IADL scale (range 0–7), median (IQR) | 1 (0–3) | 2 (0–3) | |
| EuroQol-5D (range -0.33–1.0), mean (SD) | 0.75 (0.21) | 0.72 (0.22) | |
| Emotional wellbeing (Rand-36) (range 4–100), mean (SD) | 71.4 (17.4) | 70.3 (17.6) | |
| Self-perceived quality of Life (scale range 0–10), mean (SD) | 7.2 (1.3) | 7.2 (1.2) | |
| Healthcare utilization in past 12 months | |||
| hospital admission (≥1) | 306 (26.1) | 264 (25.6) | |
| GP after hours (≥1) | 232 (20.1) | 175 (17.2) | |
| home nursing | 193 (17.0) | 149 (14.7) | |
| personal care | 654 (56.3) | 523 (51.9) | |
| day care | 26 (2.2) | 36 (3.5) | |
| Falls (≥1) in past 12 months | 418 (34.9) | 344 (32.7) | |
| Identification of seniors at risk-primary care (range 0–7.5), median (IQR) | 4 (3–5) | 4 (3–5) | |
Values are numbers (percentages) unless stated otherwise. IQR = interquartile range; SD = standard deviation
Healthcare use and costs
The mean total costs after one year were EUR 6518 (SE 472) per participant in the intervention group and EUR 5214 (SE 338) in the usual care group (Table 2). The costs for the intervention were EUR 156 per participant. The mean total costs for GP care per participant were significantly lower in the intervention group (adjusted mean difference EUR -13 (95% CI: -21; -4). Mean cost for personal care and nursing home admission per participant were significantly higher in the intervention group as compared to the usual care group (adjusted mean difference personal care: EUR 247 (95% CI: 60; 453); nursing home: EUR 520 (95% CI: 189; 968)). Mean total home care costs and long-term care contributed most to the adjusted mean differences in total cost between both groups. The adjusted mean difference in total costs between the intervention and the usual care group was €1457 (95% CI: 572; 2537) indicating significantly higher cost in the intervention group.
Table 2. Mean costs in intervention and usual care group after one year.
| Intervention group | Usual caregroup | unadjusted mean difference in costs | ||
|---|---|---|---|---|
| N = 1209 | N = 1074 | EUR | ||
| mean costs (Standard Error)ae | mean costs (Standard Error)ae | (95% Confidence Interval)b | ||
| GP care | ||||
| GP consult | 71 (2) | 79 (3) | -8 (-15; -1) | |
| GP after hours | 15 (2) | 22 (2) | -5 (-11; 0) | |
| Total GP care | 86 (3) | 100 (4) | -14 (-23; -4) | |
| Home care | ||||
| Home nursing | 2239 (316) | 1873 (231) | 366 (-280; 1169) | |
| Personal care | 2383 (85) | 2118 (77) | 266 (65; 487) | |
| Total Home care | 4621 (356) | 3991 (261) | 630 (-105; 1535) | |
| Long-term care | ||||
| Daycare | 23 (4) | 23 (4) | 1 (-9; 10) | |
| Elderly home | 428 (100) | 552 (116) | -124 (-352; 111) | |
| Nursing home | 909 (242) | 350 (106) | 559 (203; 1041) | |
| Total long-term care | 1361 (312) | 924 (180) | 437 (-70; 1071) | |
| Secondary care | ||||
| Emergency room | 37 (3) | 44 (4) | -6 (-17; 2) | |
| Hospital | 676 (98) | 552 (81) | 124 (-53; 306) | |
| Total secondary care | 712 (99) | 595 (83) | 126 (-65; 302) | |
| Intervention (nurse patient, nurse GP, screening) | 168 | 0 | ||
| Total costs | 7012 (508) | 5609 (364) | 1338 (332; 2514) | |
a Mean cost per participant were unadjusted
b 95% confidence intervals were estimated using bootstrapped bivariate regression models b Bold = significant difference
e Dutch standard costs were used to value healthcare utilization. All prices were adjusted for the year 2016 using consumer price index figures
Outcomes of disability and QALYs
The adjusted effect of the intervention after one year was -0.07 (95% CI -0.22 to 0.07) on the modified Katz-ADL index and 0.004 (95% CI -0.009–0.017) on the QALY indicating no significant differences in clinical outcomes between groups.
Cost-effectiveness analysis
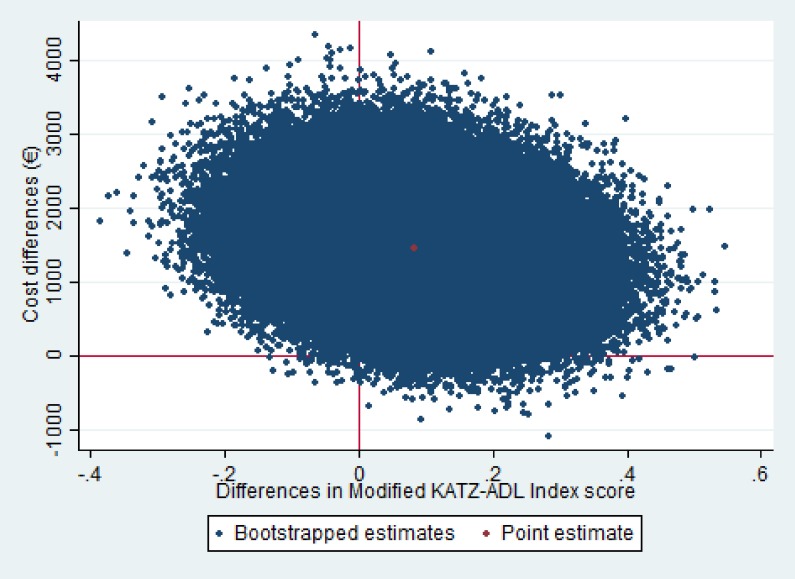
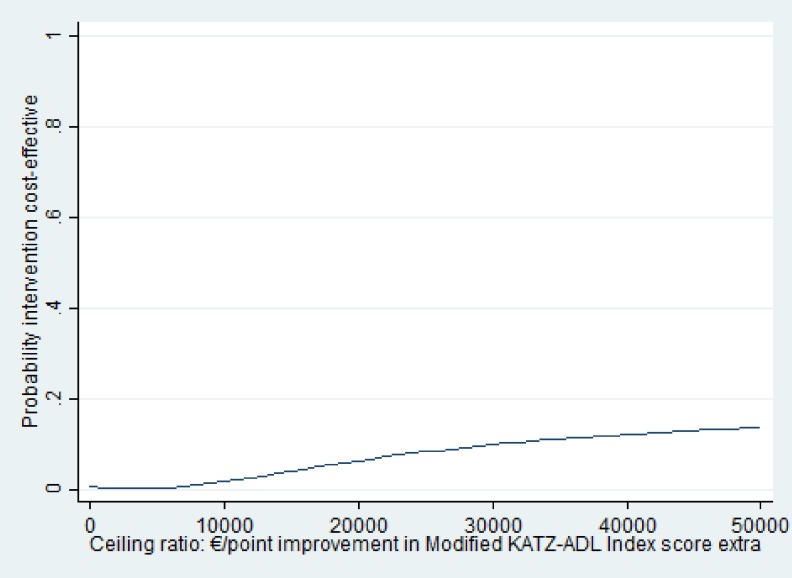
The ICER for the modified Katz-ADL index was 21,884 meaning that to gain one point of improvement in modified Katz-ADL index EUR 21,884 needs to be invested in the intervention group as compared to usual care. Fig 2 presents the cost-effectiveness plane for the modified Katz-ADL index. The majority (79%) of the cost-effect pairs for the modified Katz-ADL index were in the northeast quadrant indicating that the intervention was on average more effective and more expensive than usual care. The cost-effectiveness acceptability curves (CEAC) for improvement in modified Katz-ADL index score is shown in Fig 3. The CEAC for the modified Katz-ADL index shows that the probability that the intervention group was cost-effective in comparison with the usual care group was less than .01 at a willingness to pay (WTP) of 0 EUR/point improvement in modified Katz-ADL index score and this increased to 0.14 at a WTP of 50,000 EUR/point improvement in modified Katz-ADL index score.
Fig 2. Cost-Effectiveness (CE) Planes for disability.
The cost-effectiveness (CE) planes visualize the uncertainty surrounding the ICER estimated using bootstrapped bivariate regression models while adjusting for confounders. Modified Katz-Activities of daily living index score: (range 0–15). The effect difference was multiplied by -1 to keep the CE plane understandable (northeast: more effective, more expensive; southeast: more effective, less expensive; southwest: less effective, less expensive; northwest: less effective, more expensive).
Fig 3. Cost-effectiveness acceptability curves (CEAC) for disability.
The CEAC is a plot of the probability that the intervention is cost-effective in comparison with usual care (y-axis) as a function of the money society might be willing to pay for one additional unit of outcome (x-axis) Modified Katz-Activities of daily living index score
Cost-utility analysis
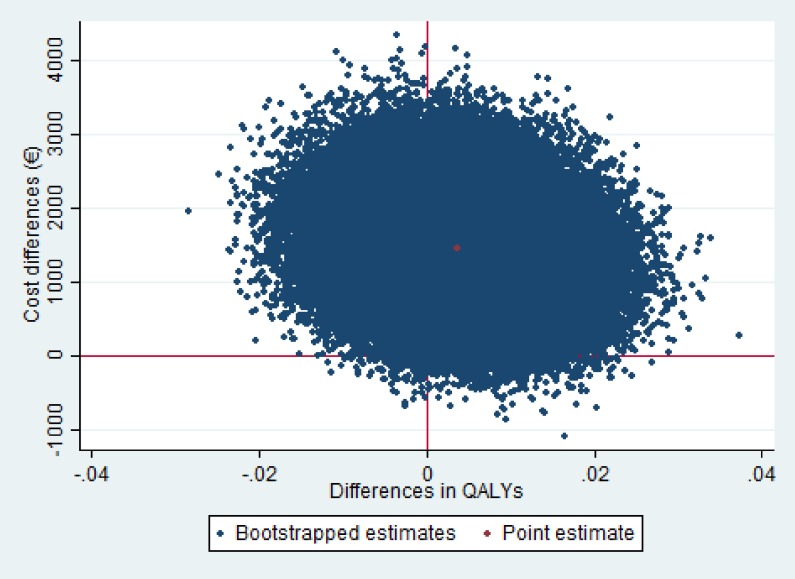
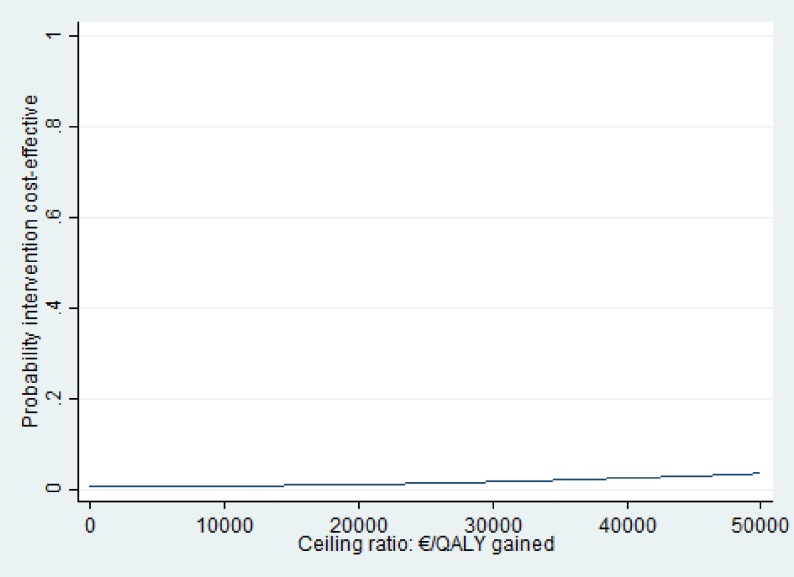
The ICER for QALYs was 287,879 indicating that to gain one QALY an additional EUR 287,879 needs to be invested in the intervention group as compared with usual care. For QALYs, the majority of the cost-effect pairs was also situated in the northeast quadrant of the CE plane (Fig 4). The probability of cost-effectiveness at a WTP of 0 EUR/ QALY gained was less than .01 as well, and this increased to 0.04 at a WTP of 50,000 EUR/QALY gained (Fig 5).
Fig 4. Cost-Effectiveness (CE) Planes for QALY.
Qaly = Quality-adjusted-life-year: (range -0.33–1.0) positive results indicate improvement in QALY
Fig 5. Cost-effectiveness acceptability curves (CEAC) for disability.
Qaly = Quality-adjusted-life-year
Discussion
The results of our study show that a one-year nurse-led multifactorial care program is not cost-effective compared with care as usual in community-living older people at increased risk of functional decline in the Netherlands. There were no statistically significant differences in Katz-ADL index score and QALYs between the two groups. Costs in the intervention group were significantly higher than in the usual care group. Costs for personal care and nursing home nursing were significantly higher in the intervention group as compared to the usual care group.
Strengths and limitations
Our study has several strengths. We conducted a large cluster randomized trial in a population at increased risk of functional decline. Other strengths of the study include the patient-centered approach, the high participation rate, the high adherence rate to the structured study protocol, and the evidence-based toolkit. Some limitations should also be taken in consideration. First, the cost-effectiveness analysis was conducted from a healthcare perspective instead of societal perspective which is recommended in to Dutch guidelines for costing research.[39] Lost productivity costs were not included because these costs are not considered relevant at this age. Informal care data were collected, but could not be used because of a considerable amount of missing data due to non-response. It is possible that when more appropriate care is arranged after discharge the amount of informal care needed is reduced resulting in lower informal care costs in the intervention group as compared to the usual care group. For this study, we focused on healthcare services that contribute most to total costs (home care, long-term care, and secondary care). However, since the intervention did not specifically target hospital outpatient care, medication use, or consultations with allied healthcare professions (physiotherapy, occupational therapy), these services were not included. In total, 14% (254/1856) of all initiated interventions consisted of referrals to allied health care professionals and 7% (136/1856) of all interventions concerned medication use. Not including allied healthcare professions and medication use may have resulted in an underestimation of the intervention cost and total societal costs in the intervention group. However, since these costs only represent a small proportion of the total costs, we expect that it is unlikely that including these costs would have changed the overall conclusion of this study. Second, healthcare utilization was measured using self-report questionnaires. As people become older and more functionally impaired, collecting healthcare utilization from the EMR should be considered to avoid measurement bias.[47] However, no uniform source is available to collect all healthcare utilization. To increase the validity of the largest cost driver, hospital admissions, we used data from the hospital EMR to calculate these costs. Third, in the intervention group at one year of follow-up around 23% of total cost and 24% of the effect data were missing. To prevent bias, we imputed missing values for cost and effect data using multiple imputation with predictive mean matching (PMM). Multiple imputation is highly preferred above naïve imputation methods such as Last Value Carried Forward (LVCF) which has been shown to bias parameter estimates.[41].
Comparison with other studies
Before the start of this study, cost-effectiveness analyses on multifactorial care to prevent or postpone new disabilities were scarce.[19] Recent studies in the Netherlands and Finland which were performed from a societal perspective, found no evidence for the cost-effectiveness of multifactorial preventive interventions in preventing or postponing new disabilities as compared with usual care.[13–16, 48] This is consistent with the results of our study. Two other recent studies which were performed from a societal perspective, found that their intervention was cost-effective at high willingness to pay ratios (EUR 20,000 and 50,000 Australian dollars (= EUR 32,327) respectively).[17, 18] These positive results can largely be explained by fewer hospital admissions and nursing home admissions, and less informal care in the intervention groups as compared to the control groups resulting in cost savings.[17] However, considering the heterogeneity in the study populations and interventions in these different studies, it is difficult to adequately explain these contradicting results. A large variety consists between the total cost per participant over a one-year period between different studies in the intervention group (the estimated total amounts vary between EUR 13,251 and EUR 19,353).[15, 48] The total cost per participant described in our study are substantially lower than the total costs described in these other studies. This may have resulted from the exclusion of informal care cost in our study. However, home care and nursing home admission were the main cost drivers in other studies as well.[15, 18, 48]
Explanation of findings and implications for future research
There are several possible explanations why we found no evidence of cost-effectiveness in our study. First, the cost-effectiveness analyses were conducted after one year, which may have been too short to see preventive effects emerge. When targeting a pre-frail population, and aiming to prevent future incidents, longer intervention and follow-up periods may be necessary to be able to show beneficial effects on new disabilities, hospital admission and costs.[11, 48–50] Higher costs for personal care and home nursing admission in our study may have been the direct result of the initiated interventions. This ‘intervention effect’ has been described before.[15, 16, 48, 51, 52] Second, the effect estimate of the intervention on disability (-0.07 on the modified Katz-ADL index) and on health-related quality of life (0.004 on the EQ-5D) was very small and not clinically relevant. The outcome measure, the modified Katz-ADL index may not be sensitive enough to detect clinically relevant change in disability over time and may poorly discriminate between people with relatively few disabilities in a community-dwelling setting.[53, 54] Besides, the EQ-5D-3L that was used to measure health-related quality of life strongly focusses on health, whereas in older adults aspects of quality of life beyond health, may be more important contributors to quality of life in older people. The Adult Social Care Outcomes Toolkit (ASCOT) is an example of an outcome measure that assesses quality of life from a broader perspective than health alone, and that can be used in economic evaluations of care interventions for older people.[55]
Conclusions and implications for further research
In this cost-effectiveness analysis, we found that one-year multifactorial nurse-led care was not cost-effective compared to usual care in community-living older people at increased risk of functional decline in The Netherlands. This adds to the accumulating evidence that proactive integrated care provision over a one-year period is not (cost-)effective in preventing new disabilities in countries with a well-developed primary care system. Based on these findings, implementation of the evaluated multifactorial nurse-led care model is not to be recommended. However, the implementation of multifactorial nurse-led care in general practice is ongoing in many healthcare systems throughout the Western world. In view of the ageing of Western societies, and the burden the care for older people incurs for GPs, increasing task delegation from GPs to nurses warrant further evaluation with regard to both quality of life and costs. Such studies should indicate whether programs like these may result in valuable service provision at acceptable costs and quality.
Supporting information
(DOCX)
(DOCX)
Acknowledgments
We are grateful to the following parties: all participants and participating GPs and CCRNs who were involved in the study; the study-monitors; the toolkit expert panels; Jose de Koning; Ameen Abu-Hanna and Rob de Haan for their advice on the study design; Marjan Hoogerheide and Petra Wempe; The Amsterdam GP Organization (HKA); Huisartsen Zorg Noord-Kennemerland; Paul Witteman; Frans van de Vijver; Melinda van Moorst; Daniella Konopase; and Jeroen van der Aa for their support.
Data Availability
The minimal dataset underlying the results of your study in a public repository: https://figshare.com/articles/FIT_dataset_dta/4558012.
Funding Statement
This work was supported by ZonMW ‘The Netherlands Organization for Health Research and Development’ (ZonMw no. 313020201) and was part of the Dutch National Care for the Elderly Programme. http://www.zonmw.nl/en/.
References
- 1.Cesari M, Vellas B, Hsu FC, Newman AB, Doss H, King AC, et al. A Physical Activity Intervention to Treat the Frailty Syndrome in Older Persons-Results From the LIFE-P Study. The journals of gerontology Series A, Biological sciences and medical sciences. 2014. Epub 2014/11/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. The journals of gerontology Series A, Biological sciences and medical sciences. 2004;59(3):255–63. Epub 2004/03/20. [DOI] [PubMed] [Google Scholar]
- 3.Beaton K, McEvoy C, Grimmer K. Identifying indicators of early functional decline in community-dwelling older people: A review. Geriatrics & gerontology international. 2014. Epub 2014/10/11. [DOI] [PubMed] [Google Scholar]
- 4.Groessl EJ, Kaplan RM, Rejeski WJ, Katula JA, King AC, Frierson G, et al. Health-related quality of life in older adults at risk for disability. American journal of preventive medicine. 2007;33(3):214–8. PubMed Central PMCID: PMC1995005. doi: 10.1016/j.amepre.2007.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hardy SE, Allore HG, Guo Z, Dubin JA, Gill TM. The effect of prior disability history on subsequent functional transitions. JGerontolA BiolSciMedSci. 2006;61(3):272–7. [DOI] [PubMed] [Google Scholar]
- 6.Rechel BD, Grundy Y., Mckee E., M. How can health systems respond to population aging?,: World Health Organization; 2009. Available from: http://www.euro.who.int/__data/assets/pdf_file/0004/64966/E92560.pdf. [Google Scholar]
- 7.Health Counsil of the Netherlands. Health care for the elderly with multimorbidity. 2008. Available from: https://www.gezondheidsraad.nl/sites/default/files/200801.pdf.
- 8.Rechel B, Grundy E, Robine JM, Cylus J, Mackenbach JP, Knai C, et al. Ageing in the European Union. Lancet. 2013;381(9874):1312–22. doi: 10.1016/S0140-6736(12)62087-X [DOI] [PubMed] [Google Scholar]
- 9.Fairhall N, Sherrington C, Kurrle SE, Lord SR, Lockwood K, Cameron ID. Effect of a multifactorial interdisciplinary intervention on mobility-related disability in frail older people: randomised controlled trial. BMCMed. 2012;10:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernabei R, Landi F, Gambassi G, Sgadari A, Zuccala G, Mor V, et al. Randomised trial of impact of model of integrated care and case management for older people living in the community. Bmj. 1998;316(7141):1348–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stuck AE, Minder CE, Peter-Wuest I, Gillmann G, Egli C, Kesselring A, et al. A randomized trial of in-home visits for disability prevention in community-dwelling older people at low and high risk for nursing home admission. Archives of internal medicine. 2000;160(7):977–86. Epub 2000/04/13. [DOI] [PubMed] [Google Scholar]
- 12.Hebert R, Raiche M, Dubois MF, Gueye NR, Dubuc N, Tousignant M. Impact of PRISMA, a coordination-type integrated service delivery system for frail older people in Quebec (Canada): A quasi-experimental study. The journals of gerontology Series B, Psychological sciences and social sciences. 2010;65b(1):107–18. Epub 2009/05/06. doi: 10.1093/geronb/gbp027 [DOI] [PubMed] [Google Scholar]
- 13.Blom J, den Elzen W, van Houwelingen AH, Heijmans M, Stijnen T, Van den Hout W, et al. Effectiveness and cost-effectiveness of a proactive, goal-oriented, integrated care model in general practice for older people. A cluster randomised controlled trial: Integrated Systematic Care for older People-the ISCOPE study. Age Ageing. 2016;45(1):30–41. PubMed Central PMCID: PMCPMC4711660. doi: 10.1093/ageing/afv174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kehusmaa S, Autti-Ramo I, Valaste M, Hinkka K, Rissanen P. Economic evaluation of a geriatric rehabilitation programme: a randomized controlled trial. Journal of rehabilitation medicine. 2010;42(10):949–55. Epub 2010/10/30. doi: 10.2340/16501977-0623 [DOI] [PubMed] [Google Scholar]
- 15.Looman WM, Huijsman R, Bouwmans-Frijters CA, Stolk EA, Fabbricotti IN. Cost-effectiveness of the 'Walcheren Integrated Care Model' intervention for community-dwelling frail elderly. Family practice. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Metzelthin SF, van Rossum E, Hendriks MR, De Witte LP, Hobma SO, Sipers W, et al. Reducing disability in community-dwelling frail older people: cost-effectiveness study alongside a cluster randomised controlled trial. Age Ageing. 2015;44(3):390–6. doi: 10.1093/ageing/afu200 [DOI] [PubMed] [Google Scholar]
- 17.Drubbel I NR, Bleijenberg N, Schuurmans MJ, ten Dam VH, Numans ME, de Wit G, de Wit NJ,. Frailty screening in older patients in primary care using routine care data. Utrecht: University Utrecht; 2013. [Google Scholar]
- 18.Fairhall N, Sherrington C, Kurrle SE, Lord SR, Lockwood K, Howard K, et al. Economic evaluation of a multifactorial, interdisciplinary intervention versus usual care to reduce frailty in frail older people. Journal of the American Medical Directors Association. 2015;16(1):41–8. doi: 10.1016/j.jamda.2014.07.006 [DOI] [PubMed] [Google Scholar]
- 19.Melis RJ, Adang E, Teerenstra S, van Eijken MI, Wimo A, van Achterberg T, et al. Cost-effectiveness of a multidisciplinary intervention model for community-dwelling frail older people. The journals of gerontology Series A, Biological sciences and medical sciences. 2008;63(3):275–82. [DOI] [PubMed] [Google Scholar]
- 20.Beswick AD, Rees K, Dieppe P, Ayis S, Gooberman-Hill R, Horwood J, et al. Complex interventions to improve physical function and maintain independent living in elderly people: a systematic review and meta-analysis. Lancet. 2008;371(9614):725–35. doi: 10.1016/S0140-6736(08)60342-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bouman A, van Rossum E, Nelemans P, Kempen GI, Knipschild P. Effects of intensive home visiting programs for older people with poor health status: a systematic review. BMC health services research. 2008;8:74 PubMed Central PMCID: PMC2364620. doi: 10.1186/1472-6963-8-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elkan R, Kendrick D, Dewey M, Hewitt M, Robinson J, Blair M, et al. Effectiveness of home based support for older people: systematic review and meta-analysis. Bmj. 2001;323(7315):719–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huss A, Stuck AE, Rubenstein LZ, Egger M, Clough-Gorr KM. Multidimensional preventive home visit programs for community-dwelling older adults: a systematic review and meta-analysis of randomized controlled trials. The journals of gerontology Series A, Biological sciences and medical sciences. 2008;63(3):298–307. [DOI] [PubMed] [Google Scholar]
- 24.Mayo-Wilson E, Grant S, Burton J, Parsons A, Underhill K, Montgomery P. Preventive home visits for mortality, morbidity, and institutionalization in older adults: a systematic review and meta-analysis. PloS one. 2014;9(3):e89257 PubMed Central PMCID: PMC3951196. doi: 10.1371/journal.pone.0089257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stuck AE, Egger M, Hammer A, Minder CE, Beck JC. Home visits to prevent nursing home admission and functional decline in elderly people: systematic review and meta-regression analysis. JAMA: the journal of the American Medical Association. 2002;287(8):1022–8. [DOI] [PubMed] [Google Scholar]
- 26.Markle-Reid M, Browne G, Weir R, Gafni A, Roberts J, Henderson SR. The effectiveness and efficiency of home-based nursing health promotion for older people: a review of the literature. Medical care research and review: MCRR. 2006;63(5):531–69. Epub 2006/09/07. doi: 10.1177/1077558706290941 [DOI] [PubMed] [Google Scholar]
- 27.Eklund K, Wilhelmson K. Outcomes of coordinated and integrated interventions targeting frail elderly people: a systematic review of randomised controlled trials. Health & social care in the community. 2009;17(5):447–58. [DOI] [PubMed] [Google Scholar]
- 28.Department of Health. National service framework for older people London2001. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/198033/National_Service_Framework_for_Older_People.pdf.
- 29.Dutch College of General Practitioners. Core values of General Practice Utrecht, The Netherlands2011. Available from: https://www.nhg.org/sites/default/files/content/nhg_org/uploads/position_paper_core_values_of_general_practice_family_medicine_sept_2011_0.pdf.
- 30.The National Care for the Elderly Programme Available from: http://www.nationaalprogrammaouderenzorg.nl/english/the-national-care-for-the-elderly-programme/.
- 31.Suijker JJ, Buurman BM, ter Riet G, van Rijn M, de Haan RJ, de Rooij SE, et al. Comprehensive geriatric assessment, multifactorial interventions and nurse-led care coordination to prevent functional decline in community-dwelling older persons: protocol of a cluster randomized trial. BMCHealth ServRes. 2012;12:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suijker JJ, van Rijn M, Buurman BM, Ter Riet G, Moll van Charante EP, de Rooij SE. Effects of Nurse-Led Multifactorial Care to Prevent Disability in Community-Living Older People: Cluster Randomized Trial. PloS one. 2016;11(7):e0158714 PubMed Central PMCID: PMCPMC4961429. doi: 10.1371/journal.pone.0158714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suijker JJ, Buurman BM, van Rijn M, van Dalen MT, ter Riet G, van Geloven N, et al. A simple validated questionnaire predicted functional decline in community-dwelling older persons: prospective cohort studies. Journal of clinical epidemiology. 2014;67(10):1121–30. doi: 10.1016/j.jclinepi.2014.05.014 [DOI] [PubMed] [Google Scholar]
- 34.van Rijn M, Suijker JJ, Bol W, Hoff E, Ter Riet G, de Rooij SE, et al. Comprehensive geriatric assessment: recognition of identified geriatric conditions by community-dwelling older persons. Age Ageing. 2016;45(6):894–9. doi: 10.1093/ageing/afw157 [DOI] [PubMed] [Google Scholar]
- 35.Jones R, Schellevis F, Westert G. The changing face of primary care: the second Dutch national survey. Family practice. 2004;21(6):597–8. Epub 2004/10/07. doi: 10.1093/fampra/cmh603 [DOI] [PubMed] [Google Scholar]
- 36.Weinberger M, Samsa GP, Schmader K, Greenberg SM, Carr DB, Wildman DS. Comparing proxy and patients' perceptions of patients' functional status: results from an outpatient geriatric clinic. JAmGeriatrSoc. 1992;40(6):585–8. [DOI] [PubMed] [Google Scholar]
- 37.Brooks R. EuroQol: the current state of play. Health policy. 1996;37(1):53–72. [DOI] [PubMed] [Google Scholar]
- 38.Lamers LM, Stalmeier PF, McDonnell J, Krabbe PF, van Busschbach JJ. [Measuring the quality of life in economic evaluations: the Dutch EQ-5D tariff]. Nederlands tijdschrift voor geneeskunde. 2005;149(28):1574–8. [PubMed] [Google Scholar]
- 39.Hakkart-van Roijen RT SS; Boumans CAM;. Handleiding voor kostenonderzoek: Methoden en standaard kostprijzen voor economische evaluaties in de gezondheidzorg Diemen: College voor Zorgverzekeringen; 2010. [Google Scholar]
- 40.Centraal Bureau voor de Statistiek. Prijsindexen 2010.
- 41.Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. Pharmacoeconomics. 2014;32(12):1157–70. PubMed Central PMCID: PMCPMC4244574. doi: 10.1007/s40273-014-0193-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. 2011;30(4):377–99. doi: 10.1002/sim.4067 [DOI] [PubMed] [Google Scholar]
- 43.Eekhout I, de Vet HC, Twisk JW, Brand JP, de Boer MR, Heymans MW. Missing data in a multi-item instrument were best handled by multiple imputation at the item score level. Journal of clinical epidemiology. 2014;67(3):335–42. doi: 10.1016/j.jclinepi.2013.09.009 [DOI] [PubMed] [Google Scholar]
- 44.Rubin DB. Inference and Missing Data. Biometrika. 1976;63(3):581–90. [Google Scholar]
- 45.Williamson EJ, Aitken Z, Lawrie J, Dharmage SC, Burgess JA, Forbes AB. Introduction to causal diagrams for confounder selection. Respirology (Carlton, Vic). 2014;19(3):303–11. Epub 2014/01/23. [DOI] [PubMed] [Google Scholar]
- 46.Fenwick E, Marshall DA, Levy AR, Nichol G. Using and interpreting cost-effectiveness acceptability curves: an example using data from a trial of management strategies for atrial fibrillation. BMC health services research. 2006;6:52 PubMed Central PMCID: PMCPMC1538588. doi: 10.1186/1472-6963-6-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Dalen MT, Suijker JJ, MacNeil-Vroomen J, van Rijn M, Moll van Charante EP, de Rooij SE, et al. Self-report of healthcare utilization among community-dwelling older persons: a prospective cohort study. PloS one. 2014;9(4):e93372 PubMed Central PMCID: PMCPMC3977826. doi: 10.1371/journal.pone.0093372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Leeuwen KM, Bosmans JE, Jansen AP, Hoogendijk EO, Muntinga ME, van Hout HP, et al. Cost-Effectiveness of a Chronic Care Model for Frail Older Adults in Primary Care: Economic Evaluation Alongside a Stepped-Wedge Cluster-Randomized Trial. Journal of the American Geriatrics Society. 2015;63(12):2494–504. doi: 10.1111/jgs.13834 [DOI] [PubMed] [Google Scholar]
- 49.Counsell SR, Callahan CM, Tu W, Stump TE, Arling GW. Cost analysis of the Geriatric Resources for Assessment and Care of Elders care management intervention. Journal of the American Geriatrics Society. 2009;57(8):1420–6. Epub 2009/08/20. PubMed Central PMCID: PMCPmc3874584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stuck AE, Moser A, Morf U, Wirz U, Wyser J, Gillmann G, et al. Effect of health risk assessment and counselling on health behaviour and survival in older people: a pragmatic randomised trial. PLoS medicine. 2015;12(10):e1001889 PubMed Central PMCID: PMCPMC4610679. doi: 10.1371/journal.pmed.1001889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Latour CH, Bosmans JE, van Tulder MW, de Vos R, Huyse FJ, de Jonge P, et al. Cost-effectiveness of a nurse-led case management intervention in general medical outpatients compared with usual care: an economic evaluation alongside a randomized controlled trial. Journal of psychosomatic research. 2007;62(3):363–70. Epub 2007/02/28. doi: 10.1016/j.jpsychores.2006.10.016 [DOI] [PubMed] [Google Scholar]
- 52.van Hout HP, Jansen AP, van Marwijk HW, Pronk M, Frijters DF, Nijpels G. Prevention of adverse health trajectories in a vulnerable elderly population through nurse home visits: a randomized controlled trial [ISRCTN05358495]. The journals of gerontology Series A, Biological sciences and medical sciences. 2010;65(7):734–42. doi: 10.1093/gerona/glq037 [DOI] [PubMed] [Google Scholar]
- 53.Lin JS, Whitlock EP, Eckstrom E, Fu R, Perdue LA, Beil TL, et al. Challenges in synthesizing and interpreting the evidence from a systematic review of multifactorial interventions to prevent functional decline in older adults. Journal of the American Geriatrics Society. 2012;60(11):2157–66. doi: 10.1111/j.1532-5415.2012.04214.x [DOI] [PubMed] [Google Scholar]
- 54.Lutomski JE, Krabbe PF, den Elzen WP, Olde-Rikkert MG, Steyerberg EW, Muntinga ME, et al. Rasch analysis reveals comparative analyses of activities of daily living/instrumental activities of daily living summary scores from different residential settings is inappropriate. Journal of clinical epidemiology. 2015. Epub 2015/11/26. [DOI] [PubMed] [Google Scholar]
- 55.van Leeuwen KM, Bosmans JE, Jansen AP, Hoogendijk EO, van Tulder MW, van der Horst HE, et al. Comparing measurement properties of the EQ-5D-3L, ICECAP-O, and ASCOT in frail older adults. Value in health: the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2015;18(1):35–43. Epub 2015/01/18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
The minimal dataset underlying the results of your study in a public repository: https://figshare.com/articles/FIT_dataset_dta/4558012.