Abstract
Background
Rehabilitation can improve physical activity after stroke. However, patients may be more prone to falls and fractures because of balance and gait deficits. Few reports have studied the relationship between rehabilitation and subsequent fractures after ischemic stroke.
Objective
To investigate whether post-stroke rehabilitation affects fracture risk.
Methods
We conducted a population-based retrospective cohort study based on the Taiwan National Health Insurance Research Database. Patients with a newly diagnosed ischemic stroke between 2000 and 2012 were included. After propensity score matching, a total of 8,384 patients were enrolled. Half of the patients (4,192) received post-stroke rehabilitation within 1 month; the other half did not receive any post-stroke rehabilitation. Cox proportional hazards regression model was used to calculate hazard ratios (HRs) for fractures among patients with and without rehabilitation within 1 year after ischemic stroke. Patients were further stratified by sex and age (20–64 and ≥65 years).
Results
Patients receiving post-stroke rehabilitation had a higher incidence of fracture (6.2 per 100 person-years) than those who did not (4.1 per 100 person-years) after adjustment for sociodemographic and coexisting medical conditions [HR = 1.53, 95% confidence interval (CI) = 1.25–1.87, p < 0.001]. The analyses performed after stratifying for sex and age showed that only older women undergoing rehabilitation had a significantly higher risk of fracture (HR = 1.62, 95% CI = 1.21–2.17, p = 0.001).
Conclusion
Rehabilitation after ischemic stroke is associated with an increased fracture risk in older women.
Introduction
Ischemic stroke is a devastating disease, resulting in disability in nearly half of the patients at hospital discharge [1]. It is also a major risk factor for hip fracture regardless of age or sex. It has been reported that stroke is associated with a two-fold increase in the risk of hip or femur fracture [2]. Compared with men, women with stroke have a higher fracture risk [3]. Ramnemark et al. found that more than 80% of all fractures after stroke were caused by falls and most frequently involved the femoral neck [4, 5]. Impaired balance may make it difficult to maintain erect upright posture while walking. Inability to maintain balance impairs functional performance and results in a higher frequency of falls [6]. Short- and long-term morbidity of fractures include pain, limitation of function, decreased health-related quality of life, and increased mortality [7]. Many studies have reported that hip [7–12] and spine fractures [7, 13–16] increase mortality in the general population.
Although rehabilitation enhances physical activity after stroke, it may be accompanied by falls due to balance and gait deficits [17]. Falls are common complications experienced during post-stroke rehabilitation [18, 19]. A prospective study estimated an incidence of 159 falls per 10,000 patient-days in an inpatient stroke rehabilitation setting, potentially subjecting patients to the serious complications of inactivity mentioned above. Acute fractures accounts for 4% of patients’ falling accidents during their rehabilitation stay [20]. To the best of our knowledge, there is no report on the long-term relationship between rehabilitation and fractures after ischemic stroke. We conducted a population-based cohort study to investigate the association between rehabilitation and fracture after ischemic stroke in Taiwan.
Materials and methods
Study design and data sources
The cohort data set was obtained from Taiwan’s National Health Insurance Research Database. In Taiwan, up to 99% of the population is covered by National Health Insurance (NHI), and 97% of hospital and clinics are contracted with NHI [21, 22]. For research purposes, the National Health Research Institute (NHRI) established the Longitudinal Health Insurance Database by randomly sampling a representative cohort of one million patients from the registry of all NHI beneficiaries from the year 2000 [23, 24]. There are no statistically significant differences in age, sex or health care costs between the sample cohort and all beneficiaries. We used this database to retrieve information about patient characteristics and medical records by linking ambulatory and inpatient care claims and the registry of beneficiaries. Because the data set used in this study comprised de-identified secondary data released to the public for research purposes, this study was granted exempt from review by the Institutional Review Board of the Tzu Chi Medical Center (REC No:IRB104-131C).
Study population
Because ischemic stroke accounts for more than 80% of total strokes [25] and the claims-based stroke severity index (described below) is only applicable to ischemic stroke patients, our study population was defined as patients with ischemic stroke. To identify patients in whom new-onset ischemic stroke was diagnosed between 2000 and 2012, we used the primary discharge diagnosis of ischemic stroke (International Classification of Diseases, 9th Revision, Clinical Modification [ICD-9-CM] codes 433 and 434). The date of ischemic stroke diagnosis was recorded as the index date and the concurrent hospitalization as the index hospitalization. Rehabilitation recipients were defined as patients receiving either in-patient or out-patient post-stroke rehabilitation, according to NHI medical records. The rehabilitation aimed at functional improvement of hemiplegic limbs, muscle strengthening, posture, ambulation, and balance training.
To evaluate an association between rehabilitation and fractures, patients were divided into two groups. The rehabilitation group included subjects who started post-stroke rehabilitation within 1 month after stroke, and the non-rehabilitation group comprised subjects who did not receive any post-stroke rehabilitation within 1 year after stroke. Exclusion criteria were: 1) age ≤20 years, 2) previous history of ischemic or hemorrhagic stroke, 3) fracture within 1 year before stroke, 4) diagnosis of fracture during index hospitalization, 5) death during index hospitalization, or 6) rehabilitation initiated 2 to 12 months after stroke (Fig 1).
Fig 1. Flow diagram of selection of study subjects.

Primary outcome
ICD-9-CM codes from 800.X to 829.X were used to identify subjects with a new diagnosis of fracture within 1 year after ischemic stroke. All study participants were followed from the index date until a new diagnosis of fracture, withdrawal from the Longitudinal Health Insurance Database, or until 1 year after ischemic stroke. The ICD-9-CM codes for fractures were validated in previous studies and the accuracy could be acceptable [26, 27].
Covariates
We identified comorbidities according to ICD-9-CM and procedure codes. A preexisting comorbidity was any disease diagnosed during at least one hospital admission or two outpatient visits in the 1 year preceding the index admission. Based on preexisting conditions identified from each patient’s medical records, Charlson comorbidity index scores were calculated [28]. Subjects’ demographic and clinical characteristics including age, sex, and comorbidities during index hospital admission were retrieved. Proxy indicators of stroke severity during the index hospitalization included Stroke Severity Index (SSI) score, diagnosis codes of hemiplegia, paraplegia, or aphasia; operation and procedure codes; mechanical ventilation; and intensive care unit admission [29]. The SSI score developed by Sung et al. was calculated to assess neurologic deficit severity of ischemic stroke [30]. The following seven features were used to calculate the SSI score: airway suctioning, bacterial sensitivity test, general ward stay, ICU stay, nasogastric intubation, osmotherapy, and urinary catheterization. Multiple linear regression model was used to calculate the SSI score based on the coefficients of these seven features reported by Sung et al. Previous studies revealed that the claims-based SSI correlated well with the National Institutes of Health Stroke Scale and the consequent functional outcomes after stroke [30, 31].
Socioeconomic data included the premium paid for NHI, which is set nationally according to income and is therefore a proxy for income. The premium cost was stratified in 4 levels: New Taiwan Dollars ≥40,000; 20,000–39,999; 1–19,999; and fixed. The fixed category included those designated by NHI as dependents, such as the unemployed, students, children, and elderly persons with no salary [32]. Subjects were also stratified by where they lived based on National Health Insurance Research Database information, identifying 7 levels of urbanization [33, 34], with level 1 indicating the most urbanized area. To simplify analysis, levels 5 through 7 were combined in a single group and counted as level 5 [32].
Statistical analysis
To minimize selection bias, propensity score matching was performed to balance covariates and baseline differences, including age, sex, baseline comorbidities, socioeconomic factors, and stroke severity proxies (Table 1). We matched (without replacement) patients who had rehabilitation with those who did not. The nearest-neighbor algorithm was applied to construct matched pairs.
Table 1. Baseline characteristics and comorbidity of patients with and without post-stroke rehabilitation after propensity score matching.
| Rehabilitation (n = 4192) | Non-rehabilitation (n = 4192) | p value | |
|---|---|---|---|
| Demographic factors | |||
| Age (y), mean ± SD | 68.0 ± 12.4 | 67.9 ± 12.4 | 0.774 |
| Male, n (%) | 2,533 (60.4%) | 2,521 (60.1%) | 0.789 |
| Comorbidities | |||
| Charlson Comorbidity Index | 12.7 ± 1.85 | 2.72 ± 1.82 | 0.938 |
| Hypertension | 3,311 (79.0%) | 3,310 (79.0%) | 0.979 |
| Diabetes mellitus | 1,768 (42.2%) | 1,744 (41.6%) | 0.595 |
| Osteoporosis | 169 (4.0%) | 171 (4.1%) | 0.912 |
| COPD | 772 (18.4%) | 736 (17.6%) | 0.306 |
| Congestive heart failure | 386 (9.2%) | 379 (9.0%) | 0.791 |
| Chronic kidney disease | 371 (8.9%) | 361 (8.6%) | 0.699 |
| Malignancy | 256 (6.1%) | 245 (5.8%) | 0.612 |
| Parkinsonism | 128 (3.1%) | 123 (2.9%) | 0.749 |
| Epilepsy | 60 (1.4%) | 64 (1.5%) | 0.717 |
| Dementia | 208 (5.0%) | 204 (4.9%) | 0.840 |
| Depression | 179 (4.3%) | 174 (4.2%) | 0.786 |
| Socioeconomic factors | |||
| Insurance premium | 0.186 | ||
| Fixed | 1,204 (28.7%) | 1,249 (29.8%) | |
| 1–19,999 | 1,658 (39.6%) | 1,604 (38.3%) | |
| 20,000–39,999 | 1,046 (25.0%) | 1,015 (24.2%) | |
| ≥40,000 | 284 (6.8%) | 324 (7.7%) | |
| Urbanization level | 0.029 | ||
| 1 (Most urbanized) | 1,067 (25.5%) | 1,073 (25.6%) | |
| 2 | 1,119 (26.7%) | 1,172 (28.0%) | |
| 3 | 795 (19.0%) | 722 (17.2%) | |
| 4 | 709 (16.9%) | 657 (15.7%) | |
| 5 (Least urbanized) | 502 (12.0%) | 568 (13.6%) | |
| Stroke severity proxies | |||
| SSI score | 6.07 ± 3.88 | 6.11 ± 4.36 | 0.599 |
| ICU | 534 (12.7%) | 544 (13.0%) | 0.744 |
| Mechanical ventilation | 157 (3.8%) | 156 (3.7%) | 0.954 |
| Aphasia | 52 (1.2%) | 62 (1.5%) | 0.346 |
| Hemiplegia or paraplegia | 500 (11.9%) | 551 (13.1%) | 0.093 |
| Neurosurgery | 22 (0.5%) | 21 (0.5%) | 0.878 |
Continuous data expressed as mean ± standard deviation and categorical data expressed as number (%)
Abbreviations: COPD = chronic obstructive pulmonary disease, SSI = Stroke severity index, ICU = Intensive Care Unit
A χ2 test for categorical data and a t test for continuous variables were conducted to analyze differences between the rehabilitation and non-rehabilitation groups. Fracture-free rates were estimated with Kaplan–Meier curves, and the difference between survival curves was compared with the log-rank test.
Three different Cox proportional hazard regression models, including univariate, multivariate, and stratified Cox proportional hazard regressions, were conducted to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for fracture risk associated with post-stroke rehabilitation. We corrected for immortal time for all calculated incidence rates and HRs [35].
We used the same method of propensity score matching to perform analysis after stratifying for sex and age (20–64 and ≥65 years) on a total of 11,806 enrolled patients for assessing the association between rehabilitation and fracture risk. Database management and statistical analyses were performed using Stata version 13 (Stata Corporation, College Station, Texas, USA). A two-sided probability value of <0.05 was considered significant.
Results
Demographic characteristics of subjects
A total of 13945 adult patients with new-onset ischemic stroke were identified from the national cohort. After excluding patients not meeting study criteria and matching by propensity score, 8384 patients with ischemic stroke without recent or comorbid fracture were evaluated, of whom half (4192 subjects) received post-stroke rehabilitation (Fig 1).
Aside from a slight difference in the level of urbanization, there were no significant differences between the groups in terms of baseline characteristics, comorbidities, or stroke severity after propensity score matching (Table 1).
Risk of fractures after stroke
The Kaplan–Meier model showed a higher cumulative incidence of fracture in the rehabilitation than in the non-rehabilitation group (6.2 vs 4.1 per 100 person-years; log-rank test, p = 0.0001) (Fig 2), with the difference appearing at about 2 months after stroke. Rehabilitation was associated with a higher risk of fracture during follow-up (crude HR = 1.53, 95% CI = 1.25–1.88, p < 0.001) (Table 2). This effect remained on multivariate Cox regression even after adjusting for all baseline characteristics listed in Table 1 (adjusted HR [aHR] = 1.53, 95% CI = 1.25–1.87, p < 0.001) and on stratified Cox regression by stratifying propensity-score matched groups (aHR = 1.52, 95% CI = 1.23–1.88, p < 0.001).
Fig 2. Kaplan–Meier curves showing estimated fracture-free proportions of patients with and without post-stroke rehabilitation.
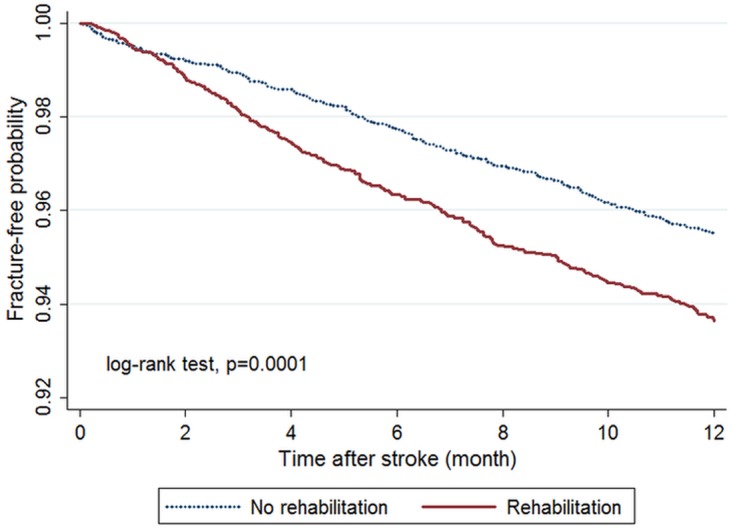
Table 2. Risk of fracture with or without post-stroke rehabilitation adjusted for covariates.
| Rehabilitation (n = 4192) | Non-rehabilitation (n = 4192) | |
|---|---|---|
| Subjects with fracture | 241 | 154 |
| Person-years | 3840.6 | 3806.5 |
| Incidence rate* | 6.2 | 4.1 |
| Univariate model | ||
| Crude HR (95% CI) | 1.53 (1.25–1.88) | 1 (ref.) |
| p value | <0.001 | |
| Model 1† | ||
| Adjusted HR (95% CI) | 1.53 (1.25–1.87) | 1 (ref.) |
| p value | <0.001 | |
| Model 2‡ | ||
| Adjusted HR (95% CI) | 1.52 (1.23–1.88) | 1 (ref.) |
| p value | <0.001 |
* Per 100 person-years, calculated by correcting immortal time.
† Model 1 used a multivariate Cox proportional hazard regression model to adjust for all baseline characteristics listed in Table 1.
‡ Model 2 used a stratified Cox proportional hazard regression model by stratifying propensity-score matched groups.
Anatomic distribution of fractures
The most common fracture sites were vertebra and femoral neck in both groups, accounting for more than 40% of total fractures (Table 3). The rehabilitation group exhibited significantly higher incidence of fractures of vertebral, pelvic, humeral, and femoral neck than non-rehabilitation groups.
Table 3. Comparison of fracture sites between post-stroke rehabilitation and non-rehabilitation groups.
| Anatomical sites | Rehabilitation (n = 4192) |
Non-rehabilitation (n = 4192) |
p value |
|---|---|---|---|
| Total fracture events* | 241 (5.7%) | 154 (3.7%) | <0.001 |
| Skull or facial | 5 (0.1%) | 7 (0.2%) | 0.563 |
| Vertebral | 60 (1.4%) | 40 (1.0%) | 0.044 |
| Rib or sternal | 16 (0.4%) | 19 (0.5%) | 0.611 |
| Pelvic | 10 (0.2%) | 0 (0%) | 0.002 |
| Clavicular or scapular | 8 (0.2%) | 4 (0.1%) | 0.248 |
| Humeral | 26 (0.6%) | 7 (0.2%) | 0.001 |
| Radial or ulnar | 14 (0.3%) | 20 (0.5%) | 0.303 |
| Carpal, metacarpal, or phalanges | 14 (0.3%) | 9 (0.2%) | 0.296 |
| Femoral (neck) | 59 (1.4%) | 28 (0.7%) | 0.001 |
| Femoral (other than neck) | 12 (0.3%) | 5 (0.1%) | 0.089 |
| Patella | 4 (0.1%) | 3 (0.1%) | 0.705 |
| Tibial or fibular | 9 (0.2%) | 7 (0.2%) | 0.617 |
| Ankle | 5 (0.1%) | 3 (0.1%) | 0.479 |
| Tarsal, metatarsal, or phalanges | 7 (0.2%) | 6 (0.1%) | 0.781 |
A χ2 test was used to compare the differences of fracture rate between the two groups.
*Because there may be more than one fracture site in the same patient, the sum of events of individual fracture sites is not equal to the total fracture events.
Risk of fractures by sex and age
We further analyzed the association between rehabilitation and fractures stratified for sex and age (Table 4). Among women aged ≥65 years, there was a significantly increased risk of fracture in those undergoing rehabilitation as showed by both multivariate analysis (aHR = 1.62, 95% CI = 1.21–2.17, p = 0.001) and stratified Cox regression model (aHR = 1.82, 95% CI = 1.33–2.50, p < 0.001). No such significantly increased risk was found among the other three subgroups.
Table 4. Risk of fractures for patients with and without post-stroke rehabilitation stratified by sex and age.
| Rehabilitation | Non-rehabilitation | Model 1† | Model 2‡ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n§ | Fracture cases | Person-years | Incidence rate* | n§ | Fracture cases | Person-years | Incidence rate* | aHR (95% CI) |
p value | aHR (95% CI) |
p value | |
| Male | ||||||||||||
| Age 20–64 | 1039 | 25 | 993.2 | 2.5 | 1039 | 30 | 991.8 | 3.0 | 0.85 (0.50–1.45) |
0.549 | 0.87 (0.51–1.49) |
0.618 |
| Age ≥ 65 | 1440 | 64 | 1313.0 | 4.9 | 1440 | 56 | 1280.0 | 4.4 | 1.12 (0.78–1.61) |
0.541 | 1.24 (0.85–1.81) |
0.274 |
| Female | ||||||||||||
| Age 20–64 | 476 | 17 | 451.7 | 3.8 | 476 | 11 | 454.9 | 2.4 | 1.60 (0.75–3.43) |
0.228 | 1.70 (0.78–3.71) |
0.183 |
| Age ≥ 65 | 1172 | 119 | 1026.3 | 11.6 | 1172 | 73 | 1012.6 | 7.2 | 1.62 (1.21–2.17) |
0.001 | 1.82 (1.33–2.50) |
<0.001 |
The propensity score matching procedure was performed after initial stratification for sex and age of the total of “11,806 patients”§ included in this study.
* Per 100 person-years, calculated by correcting immortal time.
† Model 1 used a multivariate Cox proportional hazard regression model to adjust for all baseline characteristics listed in Table 1.
‡ Model 2 used a stratified Cox proportional hazard regression model stratified for propensity-score matched groups.
§ After propensity score matching, a total of 8,384 patients were enrolled. However, the values in the table do not add up to 8,384 patients. This is because in order to keep all paired relationships when stratifying sex and age, we stratified sex and age from the initial total 11,806 patients. After stratification, we separately performed propensity score matching in each stratum. Therefore, there are 2078, 2880, 952 and 2344 patients included in each stratum.
Discussion
In the present study, we investigated the association of rehabilitation and the risk of fracture following ischemic stroke using a nationwide population database. We observed that patients receiving rehabilitation had a higher incidence of fracture compared with those who did not. This is the first large-scale study to demonstrate an increased fracture risk with rehabilitation after ischemic stroke.
Patients may have fractures after stroke because of fragile bones and falling [36]. Other risk factors besides stroke include older age, female gender, osteoporosis, dementia, depression, immobilization, and inactivity [3, 36, 37]. To account for these factors, we used propensity scores to match differences in baseline characteristics and stroke severity proxies between the two groups. Furthermore, all confounding factors were adjusted for in the multivariate Cox proportional hazard regression models.
Several guidelines suggest that early mobilization and rehabilitation after stroke, including sitting, standing, and walking, yield beneficial effects [38, 39]. Musculoskeletal, cardiovascular, respiratory, and immune systems are negatively affected by bed rest in the acute phase of stroke [40]. Inactive patients are subject to immobility-related complications, including pneumonia, deep vein thrombosis, and pressure sores [19, 41, 42], which may be reduced by early mobilization after acute ischemic stroke [43]. After a stroke, the window of opportunity for brain plasticity and repair may be narrow, making early neurologic rehabilitation optimal [44, 45]. Agility exercise training prevents falls after stroke in older adults by improving postural reflexes, functional balance, and mobility [46]. Muscle and bone strength benefit from rehabilitation, which can help maintain fitness and bone mass, prevent osteoporosis, improve balance, and potentially reduce the risk of falls and fractures [47, 48]. However, although stroke patients benefit from rehabilitation, some do not receive post-stroke rehabilitation. Previous studies have revealed that the rate of rehabilitation usage was approximately 50% in ischemic stroke patients in Taiwan [49, 50]. Chen et al. reported on the possible determinants of rehabilitation for in-patient stroke care [51]. The positive factors for receiving rehabilitation include treatment in a teaching hospital, older age, intracranial hemorrhage, prior surgery, and hemiplegia or hemiparesis; the negative factors include male gender, higher income, higher comorbidity, use of a mechanical ventilator, and participation of a neurologist in patient care.
Even though rehabilitation benefits patients by improving functional status and mobility, we found that it did not protect patients from fractures. Similarly, a meta-analysis study indicated that exercise did not prevent falls after stroke [52]. Simpson et al. found that increasing mobility made patients more vulnerable to falls after stroke. Patients recently discharged from stroke rehabilitation are at a greater risk of falling in their home [53]. Once ambulatory, higher mobility may be associated with incremental odds of falling [54, 55].
A nationwide retrospective cohort study in Sweden reported that 9% of stroke patients had subsequent fractures during a mean follow-up of 2.54 years. The highest fracture risk was during the first year after hospital discharge [56]. A population-based cohort study in Ontario, Canada reported that the 2-year post-stroke fracture rate was 5.7% [57] and another in North Dublin, Ireland reported that 5.4% of patients had fractures in the 2 years after stroke [58]. In this study, the overall fracture rate in the first year after stroke was 4.7%, which is comparable to previous studies.
Patients with rehabilitation had higher incidences of vertebral, pelvic, femoral neck, and humeral fractures than those without rehabilitation; vertebral, femoral neck, and humeral fractures were the most frequent. Vertebral compression fractures are characteristic of osteoporosis and result from low-energy trauma [59]. Femoral neck fractures tend to result from falling to the side or straight down [60], and proximal humeral fractures result from falling obliquely or to the side [61]. Patients receiving post-stroke rehabilitation may have experienced fractures while engaging in mobile activities.
As both age and female gender increase fracture risk [36], we stratified the study groups based on both sex and age. In the rehabilitation group, only older women had a significantly increased fracture risk. Younger women with rehabilitation had a trend toward increased fracture risk but HR was not statistically significant. We did not determine the reason for the difference in fracture rate but suggest that it may have resulted from the high prevalence of osteoporosis in postmenopausal women, which increased vulnerability to low-energy fractures [62]. Apart from osteoporosis, falls and muscle weakness have been associated with fractures in woman [63]. Women may also experience more frequent falls than men. A previous study reported that women had an increased risk of falling because of variation in gait pattern during dual-task activities, which in turn contributed to a greater fracture risk in women compared with men [64]. Fear of falling appeared to be higher in women, and fear of falling in patients after stroke may indicate a person who is at a high risk of falling [65, 66]. According to the Taiwan Ministry of Health and Welfare, the population of single elderly women increased from 19,893 to 25,598 between 2000 and 2012. Single and disabled elderly women may be prone to fractures because of the mobility required for most daily activities. Additional precautions must be taken to prevent fractures in older women receiving post-stroke rehabilitation.
Although we could not determine whether fractures after stroke directly resulted from fall using the administrative database, previous studies have confirmed this hypothesis [5, 36]. Thus, effective interventions for preventing fall should be established to prevent fractures after stroke. Several studies have evaluated strategies to prevent fall, such as physical activity intervention (including balance training, exercise, and strength training) [67–69], environment modification or use of assistive technology [70, 71] and use of different models for stroke care [72]. However, a systemic review concluded that the above mentioned strategies were no more effective than usual care [73]. Moreover, a randomized controlled trial showed that multifactorial fall prevention programs were not effective in reducing this risk in stroke patients [74]. Various drugs increase the risk of falling in the elderly, including sedatives and hypnotics, neuroleptics and antipsychotics, antidepressants, benzodiazepines, and nonsteroidal anti-inflammatory drugs [75]. Medication use was not evaluated in this study, but unnecessary prescription of such drugs should be avoided to reduce the risk of falling after stroke. Therefore, further research is required to identify effective interventions to prevent fall and consequent fractures in stroke patients, especially in those undergoing rehabilitation owing to their higher susceptibility to fractures.
Our study has several advantages. First, it was based on a nationwide population database that includes a representative sample of one million individuals, allowing selection of a sufficient sample size for our research. Second, in contrast to case-control or cross-sectional designs, the retrospective cohort study design generates a higher level of epidemiologic evidence. To eliminate confounding by sociodemographic factors and coexisting medical conditions, we used propensity score matching to balance the groups with and without post-stroke rehabilitation. The use of multivariable as well as stratified Cox proportional hazard models helped control for residual confounding effects. Finally, we calculated the fracture risk in person-years with correction of immortal time to reduce bias [35].
This study has several limitations. First, we could not retrieve lifestyle information; physical, psychiatric, or laboratory data; or marriage and family support status. We also could not determine the actual mechanism of fractures in the absence of additional information to differentiate between intrinsic or extrinsic causes of falls. The actual cause-effect relation between rehabilitation and post-stroke fracture may require further clinical trials. Second, the use of several proxies of stroke severity is not the best way to determine stroke severity. However, we calculated the claims-based SSI score to represent neurologic deficit severity, and this was well matched between the two groups by propensity score matching. A previous study suggested that SSI was an acceptable indicator of severity of ischemic stroke when conducting outcomes research using administrative data [30]. The SSI correlates well with clinical stroke severity measured by the National Institutes of Health Stroke Scale and is significantly associated with the modified Rankin Scale up to 1 year after stroke [31]. Therefore, the difference in stroke severity between the two groups was diminished with adequate matching. However, bias related to confounders might persist in a cohort study. Even if we adequately controlled for known confounders, there might still be bias related to unmeasured or unknown confounders. Finally, the diagnoses in NHI claim records are used for administrative rather than scientific purposes. The anonymized patient data does not allow independent confirmation of diagnoses [76]. Nonetheless, previous studies have reported the high accuracy and validity of ICD-9-CM coding for diagnoses in the database, including stroke and fracture [27, 77]. Hospitals or doctors in Taiwan would be penalized and heavily fined for wrong diagnoses or coding, further establishing the reliability of the diagnoses in the database.
Conclusions
In summary, we found that patients with ischemic stroke who received post-stroke rehabilitation had a higher risk of fractures compared with patients without rehabilitation, although this risk was increased only in older women. Further investigation is necessary to establish whether preventive measures, such as safety education, environmental modification, and adjustments in rehabilitation programs, would reduce the risk of fractures in patients receiving post-stroke rehabilitation.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Mittmann N, Seung SJ, Hill MD, Phillips SJ, Hachinski V, Cote R, et al. Impact of disability status on ischemic stroke costs in Canada in the first year. Can J Neurol Sci. 2012;39(6):793–800. [DOI] [PubMed] [Google Scholar]
- 2.Pouwels S, Lalmohamed A, Leufkens B, de Boer A, Cooper C, van Staa T, et al. Risk of hip/femur fracture after stroke: a population-based case-control study. Stroke. 2009;40(10):3281–5. Epub 2009/08/08. 10.1161/STROKEAHA.109.554055 [DOI] [PubMed] [Google Scholar]
- 3.Wu CH, Liou TH, Hsiao PL, Lin YC, Chang KH. Contribution of ischemic stroke to hip fracture risk and the influence of gender difference. Arch Phys Med Rehabil. 2011;92(12):1987–91. Epub 2011/12/03. 10.1016/j.apmr.2011.06.028 [DOI] [PubMed] [Google Scholar]
- 4.Ramnemark A, Nilsson M, Borssen B, Gustafson Y. Stroke, a major and increasing risk factor for femoral neck fracture. Stroke. 2000;31(7):1572–7. Epub 2000/07/08. [DOI] [PubMed] [Google Scholar]
- 5.Ramnemark A, Nyberg L, Borssen B, Olsson T, Gustafson Y. Fractures after stroke. Osteoporos Int. 1998;8(1):92–5. Epub 1998/08/06. 10.1007/s001980050053 [DOI] [PubMed] [Google Scholar]
- 6.Schmid AA, Van Puymbroeck M, Altenburger PA, Dierks TA, Miller KK, Damush TM, et al. Balance and balance self-efficacy are associated with activity and participation after stroke: a cross-sectional study in people with chronic stroke. Arch Phys Med Rehabil. 2012;93(6):1101–7. Epub 2012/04/17. 10.1016/j.apmr.2012.01.020 [DOI] [PubMed] [Google Scholar]
- 7.Teng GG, Curtis JR, Saag KG. Mortality and osteoporotic fractures: is the link causal, and is it modifiable? Clin Exp Rheumatol. 2008;26(5 Suppl 51):S125–37. Epub 2008/12/17. [PMC free article] [PubMed] [Google Scholar]
- 8.Alegre-Lopez J, Cordero-Guevara J, Alonso-Valdivielso JL, Fernandez-Melon J. Factors associated with mortality and functional disability after hip fracture: an inception cohort study. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2005;16(7):729–36. Epub 2004/11/05. [DOI] [PubMed] [Google Scholar]
- 9.Panula J, Pihlajamaki H, Mattila VM, Jaatinen P, Vahlberg T, Aarnio P, et al. Mortality and cause of death in hip fracture patients aged 65 or older: a population-based study. BMC Musculoskelet Disord. 2011;12:105 Epub 2011/05/24. 10.1186/1471-2474-12-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Empana JP, Dargent-Molina P, Breart G. Effect of hip fracture on mortality in elderly women: the EPIDOS prospective study. J Am Geriatr Soc. 2004;52(5):685–90. Epub 2004/04/17. 10.1111/j.1532-5415.2004.52203.x [DOI] [PubMed] [Google Scholar]
- 11.Franzo A, Francescutti C, Simon G. Risk factors correlated with post-operative mortality for hip fracture surgery in the elderly: a population-based approach. Eur J Epidemiol. 2005;20(12):985–91. Epub 2005/12/07. 10.1007/s10654-005-4280-9 [DOI] [PubMed] [Google Scholar]
- 12.Lee AY, Chua BS, Howe TS. One-year outcome of hip fracture patients admitted to a Singapore hospital: quality of life post-treatment. Singapore Med J. 2007;48(11):996–9. Epub 2007/11/03. [PubMed] [Google Scholar]
- 13.Bouza C, Lopez T, Palma M, Amate JM. Hospitalised osteoporotic vertebral fractures in Spain: analysis of the national hospital discharge registry. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2007;18(5):649–57. Epub 2007/01/16. [DOI] [PubMed] [Google Scholar]
- 14.Cauley JA, Thompson DE, Ensrud KC, Scott JC, Black D. Risk of mortality following clinical fractures. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2000;11(7):556–61. Epub 2000/11/09. [DOI] [PubMed] [Google Scholar]
- 15.Kanis JA, Oden A, Johnell O, De Laet C, Jonsson B. Excess mortality after hospitalisation for vertebral fracture. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2004;15(2):108–12. Epub 2003/11/05. [DOI] [PubMed] [Google Scholar]
- 16.Naves M, Diaz-Lopez JB, Gomez C, Rodriguez-Rebollar A, Rodriguez-Garcia M, Cannata-Andia JB. The effect of vertebral fracture as a risk factor for osteoporotic fracture and mortality in a Spanish population. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2003;14(6):520–4. Epub 2003/05/06. [DOI] [PubMed] [Google Scholar]
- 17.Weerdesteyn V, de Niet M, van Duijnhoven HJ, Geurts AC. Falls in individuals with stroke. J Rehabil Res Dev. 2008;45(8):1195–213. [PubMed] [Google Scholar]
- 18.McLean DE. Medical complications experienced by a cohort of stroke survivors during inpatient, tertiary-level stroke rehabilitation. Arch Phys Med Rehabil. 2004;85(3):466–9. Epub 2004/03/20. [DOI] [PubMed] [Google Scholar]
- 19.Langhorne P, Stott DJ, Robertson L, MacDonald J, Jones L, McAlpine C, et al. Medical complications after stroke: a multicenter study. Stroke. 2000;31(6):1223–9. Epub 2000/06/03. [DOI] [PubMed] [Google Scholar]
- 20.Nyberg L, Gustafson Y. Patient falls in stroke rehabilitation. A challenge to rehabilitation strategies. Stroke. 1995;26(5):838–42. [DOI] [PubMed] [Google Scholar]
- 21.Chen HF, Lee SP, Li CY. Sex differences in the incidence of hemorrhagic and ischemic stroke among diabetics in Taiwan. J Womens Health (Larchmt). 2009;18(5):647–54. Epub 2009/05/02. [DOI] [PubMed] [Google Scholar]
- 22.Chou YC, Liao CC, Su LT, Yang PY, Sung FC. Stroke rehabilitation is associated with a reduction in dementia risk: a population-based retrospective cohort study. J Rehabil Med. 2012;44(4):319–24. Epub 2012/03/01. 10.2340/16501977-0935 [DOI] [PubMed] [Google Scholar]
- 23.Tsan YT, Lee CH, Wang JD, Chen PC. Statins and the risk of hepatocellular carcinoma in patients with hepatitis B virus infection. J Clin Oncol. 2012;30(6):623–30. Epub 2012/01/25. 10.1200/JCO.2011.36.0917 [DOI] [PubMed] [Google Scholar]
- 24.Chiu HF, Ho SC, Chen CC, Yang CY. Statin use and the risk of liver cancer: a population-based case-control study. Am J Gastroenterol. 2011;106(5):894–8. Epub 2010/12/16. 10.1038/ajg.2010.475 [DOI] [PubMed] [Google Scholar]
- 25.Andersen KK, Olsen TS, Dehlendorff C, Kammersgaard LP. Hemorrhagic and ischemic strokes compared: stroke severity, mortality, and risk factors. Stroke. 2009;40(6):2068–72. Epub 2009/04/11. 10.1161/STROKEAHA.108.540112 [DOI] [PubMed] [Google Scholar]
- 26.Yang NP, Chan CL, Yu IL, Lee CY, Chou P. Estimated prevalence of orthopaedic fractures in Taiwan--A cross-sectional study based on nationwide insurance data. Injury. 2010;41(12):1266–72. Epub 2011/02/04. 10.1016/j.injury.2010.05.025 [DOI] [PubMed] [Google Scholar]
- 27.Wang WJ, Chao CT, Huang YC, Wang CY, Chang CH, Huang TM, et al. The impact of acute kidney injury with temporary dialysis on the risk of fracture. J Bone Miner Res. 2014;29(3):676–84. Epub 2013/08/10. 10.1002/jbmr.2061 [DOI] [PubMed] [Google Scholar]
- 28.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40(5):373–83. Epub 1987/01/01. [DOI] [PubMed] [Google Scholar]
- 29.Lee HC, Chang KC, Huang YC, Lan CF, Chen JJ, Wei SH. Inpatient rehabilitation utilization for acute stroke under a universal health insurance system. Am J Manag Care. 2010;16(3):e67–e74. Epub 2010/03/09. [PubMed] [Google Scholar]
- 30.Sung SF, Hsieh CY, Kao Yang YH, Lin HJ, Chen CH, Chen YW, et al. Developing a stroke severity index based on administrative data was feasible using data mining techniques. J Clin Epidemiol. 2015;68(11):1292–300. Epub 2015/02/24. 10.1016/j.jclinepi.2015.01.009 [DOI] [PubMed] [Google Scholar]
- 31.Sung SF, Hsieh CY, Lin HJ, Chen YW, Chen CH, Kao Yang YH, et al. Validity of a stroke severity index for administrative claims data research: a retrospective cohort study. BMC Health Serv Res. 2016;16(1):509 Epub 2016/09/24. 10.1186/s12913-016-1769-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu JC, Liu L, Wen-Cheng H, Chen YC, Ko CC, Wu CL, et al. The incidence of adjacent segment disease requiring surgery after anterior cervical diskectomy and fusion: estimation using an 11-year comprehensive nationwide database in Taiwan. Neurosurgery. 2012;70(3):594–601. Epub 2012/02/22. 10.1227/NEU.0b013e318232d4f2 [DOI] [PubMed] [Google Scholar]
- 33.Lin HC, Chao PZ, Lee HC. Sudden sensorineural hearing loss increases the risk of stroke: a 5-year follow-up study. Stroke. 2008;39(10):2744–8. Epub 2008/06/28. 10.1161/STROKEAHA.108.519090 [DOI] [PubMed] [Google Scholar]
- 34.Liu CY H Y, Chuang YL, Chen YJ, Weng WS, Liu JS Liang KY. Incorporating development stratification of Taiwan townships into sampling design of large scale health interview survey (in Chinese). J Health Manage. 2006;4(1):1–22. [Google Scholar]
- 35.Levesque LE, Hanley JA, Kezouh A, Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ. 2010;340:b5087 Epub 2010/03/17. 10.1136/bmj.b5087 [DOI] [PubMed] [Google Scholar]
- 36.Dennis MS, Lo KM, McDowall M, West T. Fractures after stroke: frequency, types, and associations. Stroke. 2002;33(3):728–34. Epub 2002/03/02. [DOI] [PubMed] [Google Scholar]
- 37.Pisani P, Renna MD, Conversano F, Casciaro E, Di Paola M, Quarta E, et al. Major osteoporotic fragility fractures: Risk factor updates and societal impact. World J Orthop. 2016;7(3):171–81. Epub 2016/03/24. 10.5312/wjo.v7.i3.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Govan L, Langhorne P, Weir CJ. Does the prevention of complications explain the survival benefit of organized inpatient (stroke unit) care?: further analysis of a systematic review. Stroke. 2007;38(9):2536–40. Epub 2007/08/11. 10.1161/STROKEAHA.106.478842 [DOI] [PubMed] [Google Scholar]
- 39.Indredavik B, Bakke F, Slordahl SA, Rokseth R, Haheim LL. Treatment in a combined acute and rehabilitation stroke unit: which aspects are most important? Stroke. 1999;30(5):917–23. Epub 1999/05/07. [DOI] [PubMed] [Google Scholar]
- 40.Allen C, Glasziou P, Del Mar C. Bed rest: a potentially harmful treatment needing more careful evaluation. Lancet. 1999;354(9186):1229–33. Epub 1999/10/16. [DOI] [PubMed] [Google Scholar]
- 41.Bernhardt J, Dewey H, Thrift A, Donnan G. Inactive and alone: physical activity within the first 14 days of acute stroke unit care. Stroke. 2004;35(4):1005–9. Epub 2004/02/28. 10.1161/01.STR.0000120727.40792.40 [DOI] [PubMed] [Google Scholar]
- 42.Langhorne P. Measures to improve recovery in the acute phase of stroke. Cerebrovasc Dis. 1999;9 Suppl 5:2–5. Epub 1999/09/04. [DOI] [PubMed] [Google Scholar]
- 43.Diserens K, Moreira T, Hirt L, Faouzi M, Grujic J, Bieler G, et al. Early mobilization out of bed after ischaemic stroke reduces severe complications but not cerebral blood flow: a randomized controlled pilot trial. Clin Rehabil. 2012;26(5):451–9. 10.1177/0269215511425541 [DOI] [PubMed] [Google Scholar]
- 44.Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009;10(12):861–72. Epub 2009/11/06. 10.1038/nrn2735 [DOI] [PubMed] [Google Scholar]
- 45.Krakauer JW, Carmichael ST, Corbett D, Wittenberg GF. Getting neurorehabilitation right: what can be learned from animal models? Neurorehabil Neural Repair. 2012;26(8):923–31. Epub 2012/04/03. 10.1177/1545968312440745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marigold DS, Eng JJ, Dawson AS, Inglis JT, Harris JE, Gylfadottir S. Exercise leads to faster postural reflexes, improved balance and mobility, and fewer falls in older persons with chronic stroke. J Am Geriatr Soc. 2005;53(3):416–23. 10.1111/j.1532-5415.2005.53158.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pfeifer M, Sinaki M, Geusens P, Boonen S, Preisinger E, Minne HW. Musculoskeletal rehabilitation in osteoporosis: a review. J Bone Miner Res. 2004;19(8):1208–14. Epub 2004/07/03. 10.1359/JBMR.040507 [DOI] [PubMed] [Google Scholar]
- 48.Dionyssiotis Y, Skarantavos G, Papagelopoulos P. Modern rehabilitation in osteoporosis, falls, and fractures. Clin Med Insights Arthritis Musculoskelet Disord. 2014;7:33–40. Epub 2014/06/26. 10.4137/CMAMD.S14077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee HC, Chang KC, Huang YC, Hung JW, Chiu HH, Chen JJ, et al. Readmission, mortality, and first-year medical costs after stroke. J Chin Med Assoc. 2013;76(12):703–14. 10.1016/j.jcma.2013.08.003 [DOI] [PubMed] [Google Scholar]
- 50.Tung YC, Jeng JS, Chang GM, Chung KP. Processes and outcomes of ischemic stroke care: the influence of hospital level of care. Int J Qual Health Care. 2015;27(4):260–6. 10.1093/intqhc/mzv038 [DOI] [PubMed] [Google Scholar]
- 51.Chen TT, Chen CP, Kuang SH, Wang V. Patient- and Hospital-Level Determinants of Rehabilitation for In-Patient Stroke Care: An Observation Analysis. Medicine (Baltimore). 2016;95(19):e3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Verheyden GS, Weerdesteyn V, Pickering RM, Kunkel D, Lennon S, Geurts AC, et al. Interventions for preventing falls in people after stroke. Cochrane Database Syst Rev. 2013;5:CD008728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simpson LA, Miller WC, Eng JJ. Effect of stroke on fall rate, location and predictors: a prospective comparison of older adults with and without stroke. PLoS One. 2011;6(4):e19431 Epub 2011/05/12. 10.1371/journal.pone.0019431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O'Loughlin JL, Robitaille Y, Boivin JF, Suissa S. Incidence of and risk factors for falls and injurious falls among the community-dwelling elderly. Am J Epidemiol. 1993;137(3):342–54. Epub 1993/02/01. [DOI] [PubMed] [Google Scholar]
- 55.Speechley M, Tinetti M. Falls and injuries in frail and vigorous community elderly persons. J Am Geriatr Soc. 1991;39(1):46–52. Epub 1991/01/01. [DOI] [PubMed] [Google Scholar]
- 56.Kanis J, Oden A, Johnell O. Acute and long-term increase in fracture risk after hospitalization for stroke. Stroke. 2001;32(3):702–6. [DOI] [PubMed] [Google Scholar]
- 57.Kapral MK, Fang J, Alibhai SM, Cram P, Cheung AM, Casaubon LK, et al. Risk of fractures after stroke: Results from the Ontario Stroke Registry. Neurology. 2016. Epub 2016/11/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Callaly EL, Ni Chroinin D, Hannon N, Sheehan O, Marnane M, Merwick A, et al. Falls and fractures 2 years after acute stroke: the North Dublin Population Stroke Study. Age Ageing. 2015;44(5):882–6. Epub 2015/08/14. 10.1093/ageing/afv093 [DOI] [PubMed] [Google Scholar]
- 59.Alexandru D, So W. Evaluation and management of vertebral compression fractures. Perm J. 2012;16(4):46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nevitt MC, Cummings SR. Type of fall and risk of hip and wrist fractures: the study of osteoporotic fractures. The Study of Osteoporotic Fractures Research Group. J Am Geriatr Soc. 1993;41(11):1226–34. [DOI] [PubMed] [Google Scholar]
- 61.Palvanen M, Kannus P, Parkkari J, Pitkajarvi T, Pasanen M, Vuori I, et al. The injury mechanisms of osteoporotic upper extremity fractures among older adults: a controlled study of 287 consecutive patients and their 108 controls. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2000;11(10):822–31. [DOI] [PubMed] [Google Scholar]
- 62.Lofman O, Hallberg I, Berglund K, Wahlstrom O, Kartous L, Rosenqvist AM, et al. Women with low-energy fracture should be investigated for osteoporosis. Acta Orthop. 2007;78(6):813–21. 10.1080/17453670710014608 [DOI] [PubMed] [Google Scholar]
- 63.Bliuc D, Alarkawi D, Nguyen TV, Eisman JA, Center JR. Risk of subsequent fractures and mortality in elderly women and men with fragility fractures with and without osteoporotic bone density: the Dubbo Osteoporosis Epidemiology Study. J Bone Miner Res. 2015;30(4):637–46. Epub 2014/11/02. 10.1002/jbmr.2393 [DOI] [PubMed] [Google Scholar]
- 64.Johansson J, Nordstrom A, Nordstrom P. Greater Fall Risk in Elderly Women Than in Men Is Associated With Increased Gait Variability During Multitasking. J Am Med Dir Assoc. 2016;17(6):535–40. Epub 2016/03/24. 10.1016/j.jamda.2016.02.009 [DOI] [PubMed] [Google Scholar]
- 65.Jalayondeja C, Sullivan PE, Pichaiyongwongdee S. Six-month prospective study of fall risk factors identification in patients post-stroke. Geriatr Gerontol Int. 2014;14(4):778–85. Epub 2014/03/29. 10.1111/ggi.12164 [DOI] [PubMed] [Google Scholar]
- 66.Scheffer AC, Schuurmans MJ, van Dijk N, van der Hooft T, de Rooij SE. Fear of falling: measurement strategy, prevalence, risk factors and consequences among older persons. Age Ageing. 2008;37(1):19–24. Epub 2008/01/16. 10.1093/ageing/afm169 [DOI] [PubMed] [Google Scholar]
- 67.Cheng PT, Wu SH, Liaw MY, Wong AM, Tang FT. Symmetrical body-weight distribution training in stroke patients and its effect on fall prevention. Archives of physical medicine and rehabilitation. 2001;82(12):1650–4. Epub 2001/12/06. 10.1053/apmr.2001.26256 [DOI] [PubMed] [Google Scholar]
- 68.Green J, Forster A, Bogle S, Young J. Physiotherapy for patients with mobility problems more than 1 year after stroke: a randomised controlled trial. Lancet (London, England). 2002;359(9302):199–203. Epub 2002/01/29. [DOI] [PubMed] [Google Scholar]
- 69.Bernhardt J, Dewey H, Thrift A, Collier J, Donnan G. A very early rehabilitation trial for stroke (AVERT): phase II safety and feasibility. Stroke; a journal of cerebral circulation. 2008;39(2):390–6. Epub 2008/01/05. [DOI] [PubMed] [Google Scholar]
- 70.Kwok T, Mok F, Chien WT, Tam E. Does access to bed-chair pressure sensors reduce physical restraint use in the rehabilitative care setting? J Clin Nurs. 2006;15(5):581–7. Epub 2006/04/25. 10.1111/j.1365-2702.2006.01354.x [DOI] [PubMed] [Google Scholar]
- 71.Mayo NE, Gloutney L, Levy AR. A randomized trial of identification bracelets to prevent falls among patients in a rehabilitation hospital. Archives of physical medicine and rehabilitation. 1994;75(12):1302–8. Epub 1994/12/01. [PubMed] [Google Scholar]
- 72.von Koch L, de Pedro-Cuesta J, Kostulas V, Almazan J, Widen Holmqvist L. Randomized controlled trial of rehabilitation at home after stroke: one-year follow-up of patient outcome, resource use and cost. Cerebrovascular diseases (Basel, Switzerland). 2001;12(2):131–8. Epub 2001/08/08. [DOI] [PubMed] [Google Scholar]
- 73.Batchelor F, Hill K, Mackintosh S, Said C. What works in falls prevention after stroke?: a systematic review and meta-analysis. Stroke; a journal of cerebral circulation. 2010;41(8):1715–22. Epub 2010/07/10. [DOI] [PubMed] [Google Scholar]
- 74.Batchelor FA, Hill KD, Mackintosh SF, Said CM, Whitehead CH. Effects of a multifactorial falls prevention program for people with stroke returning home after rehabilitation: a randomized controlled trial. Archives of physical medicine and rehabilitation. 2012;93(9):1648–55. Epub 2012/04/17. 10.1016/j.apmr.2012.03.031 [DOI] [PubMed] [Google Scholar]
- 75.Woolcott JC, Richardson KJ, Wiens MO, Patel B, Marin J, Khan KM, et al. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med. 2009;169(21):1952–60. 10.1001/archinternmed.2009.357 [DOI] [PubMed] [Google Scholar]
- 76.Harnod T, Wang YC, Kao CH. Association of Migraine and Sleep-Related Breathing Disorder: A Population-Based Cohort Study. Medicine (Baltimore). 2015;94(36):e1506. Epub 2015/09/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cheng CL, Kao YH, Lin SJ, Lee CH, Lai ML. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf. 2011;20(3):236–42. 10.1002/pds.2087 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.


