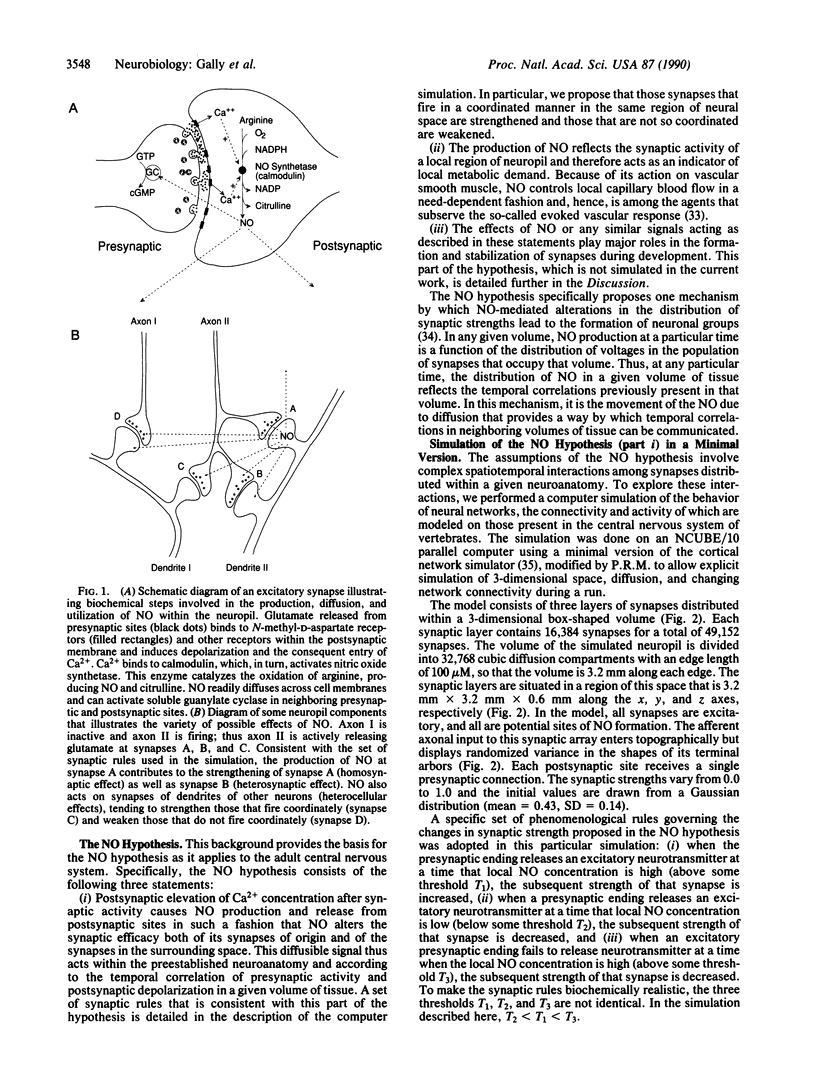
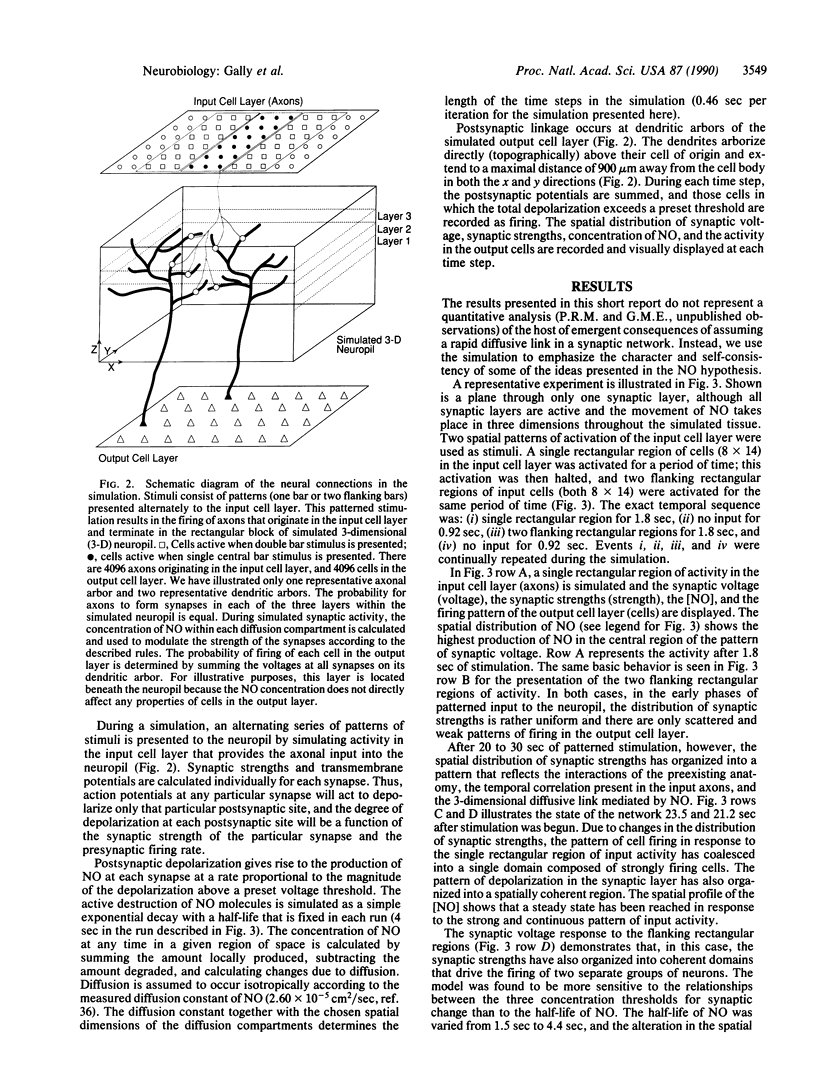
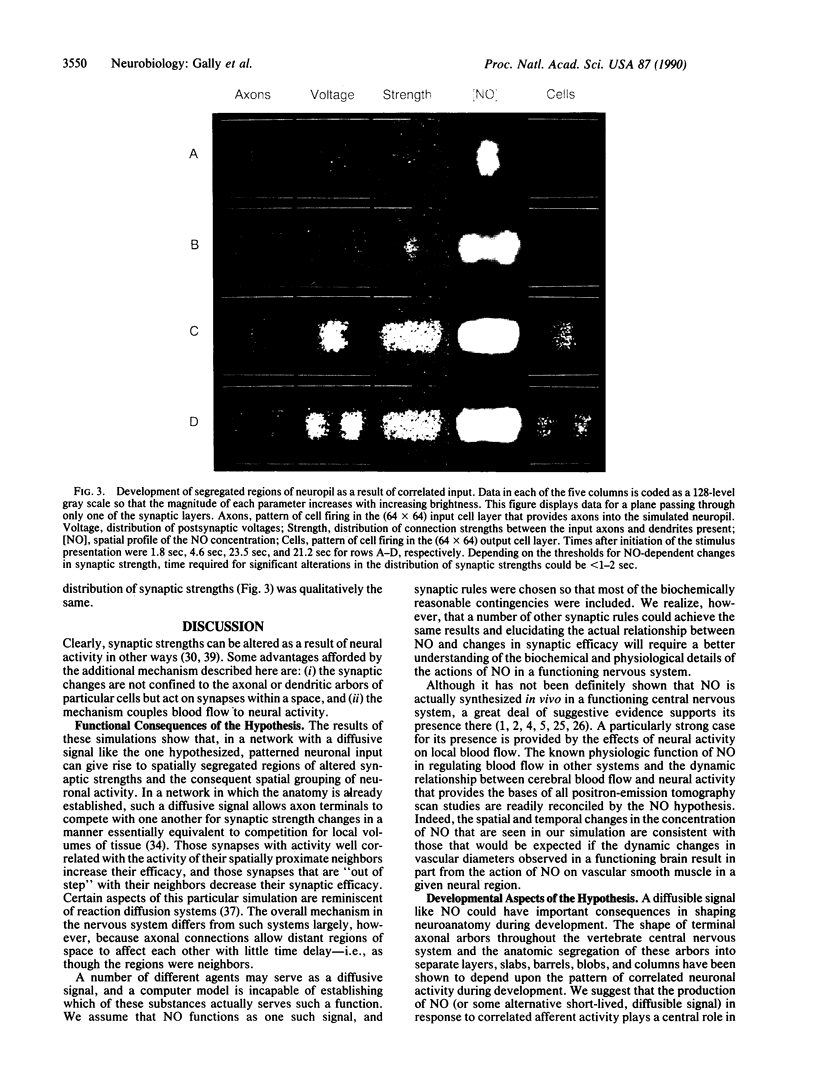
Abstract
Several observations suggest that the Ca2(+)-dependent postsynaptic release of nitric oxide (NO) may be important in the formation and function of the vertebrate nervous system. We explore here the hypothesis that the release of NO and its subsequent diffusion may be critically related to three aspects of nervous system function: (i) synaptic plasticity and long-term potentiation in certain regions of the adult nervous system, (ii) the control of cerebral blood flow in such regions, and (iii) the establishment and activity-dependent refinement of axonal projections during the later stages of development. In this paper, we detail and analyze the basic assumptions underlying this NO hypothesis and describe a computer simulation of a minimal version of the hypothesis. In the simulation, a 3-dimensional volume of neuropil is presented with patterned afferent input; NO is produced, diffuses, and is destroyed; and synaptic strengths are determined by a set of synaptic rules based on the correlation of synaptic depolarization and NO levels. According to the hypothesis, voltage-dependent postsynaptic release of this rapidly diffusing substance links the activities of neurons in a local volume of tissue, regardless of whether the neurons are directly connected by synapses. This property is demonstrated in the simulation, and it is this property that is exploited in the hypothesis to account for certain aspects of long-term potentiation and activity-dependent sharpening of axonal arbors.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonhoeffer T., Staiger V., Aertsen A. Synaptic plasticity in rat hippocampal slice cultures: local "Hebbian" conjunction of pre- and postsynaptic stimulation leads to distributed synaptic enhancement. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8113–8117. doi: 10.1073/pnas.86.20.8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990 Jan;87(2):682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Nitric oxide mediates glutamate-linked enhancement of cGMP levels in the cerebellum. Proc Natl Acad Sci U S A. 1989 Nov;86(22):9030–9033. doi: 10.1073/pnas.86.22.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüne B., Lapetina E. G. Activation of a cytosolic ADP-ribosyltransferase by nitric oxide-generating agents. J Biol Chem. 1989 May 25;264(15):8455–8458. [PubMed] [Google Scholar]
- Changeux J. P., Danchin A. Selective stabilisation of developing synapses as a mechanism for the specification of neuronal networks. Nature. 1976 Dec 23;264(5588):705–712. doi: 10.1038/264705a0. [DOI] [PubMed] [Google Scholar]
- Cline H. T., Debski E. A., Constantine-Paton M. N-methyl-D-aspartate receptor antagonist desegregates eye-specific stripes. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4342–4345. doi: 10.1073/pnas.84.12.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin A. C., Errington M. L., Bliss T. V. Long-term potentiation of the perforant path in vivo is associated with increased glutamate release. Nature. 1982 Jun 10;297(5866):496–498. doi: 10.1038/297496a0. [DOI] [PubMed] [Google Scholar]
- Garthwaite J., Charles S. L., Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature. 1988 Nov 24;336(6197):385–388. doi: 10.1038/336385a0. [DOI] [PubMed] [Google Scholar]
- Garthwaite J., Garthwaite G., Palmer R. M., Moncada S. NMDA receptor activation induces nitric oxide synthesis from arginine in rat brain slices. Eur J Pharmacol. 1989 Oct 17;172(4-5):413–416. doi: 10.1016/0922-4106(89)90023-0. [DOI] [PubMed] [Google Scholar]
- Garthwaite J., Southam E., Anderton M. A kainate receptor linked to nitric oxide synthesis from arginine. J Neurochem. 1989 Dec;53(6):1952–1954. doi: 10.1111/j.1471-4159.1989.tb09266.x. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Vavrin Z., Taintor R. R. L-arginine is required for expression of the activated macrophage effector mechanism causing selective metabolic inhibition in target cells. J Immunol. 1987 Jan 15;138(2):550–565. [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Binocular interaction in striate cortex of kittens reared with artificial squint. J Neurophysiol. 1965 Nov;28(6):1041–1059. doi: 10.1152/jn.1965.28.6.1041. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N., LeVay S. Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond B Biol Sci. 1977 Apr 26;278(961):377–409. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J. Endothelium-derived nitric oxide: actions and properties. FASEB J. 1989 Jan;3(1):31–36. doi: 10.1096/fasebj.3.1.2642868. [DOI] [PubMed] [Google Scholar]
- Katz L. C., Constantine-Paton M. Relationships between segregated afferents and postsynaptic neurones in the optic tectum of three-eyed frogs. J Neurosci. 1988 Sep;8(9):3160–3180. doi: 10.1523/JNEUROSCI.08-09-03160.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt A., Bear M. F., Singer W. Blockade of "NMDA" receptors disrupts experience-dependent plasticity of kitten striate cortex. Science. 1987 Oct 16;238(4825):355–358. doi: 10.1126/science.2443978. [DOI] [PubMed] [Google Scholar]
- Knowles R. G., Palacios M., Palmer R. M., Moncada S. Formation of nitric oxide from L-arginine in the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5159–5162. doi: 10.1073/pnas.86.13.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarewicz J. W., Wroblewski J. T., Palmer M. E., Costa E. Activation of N-methyl-D-aspartate-sensitive glutamate receptors stimulates arachidonic acid release in primary cultures of cerebellar granule cells. Neuropharmacology. 1988 Jul;27(7):765–769. doi: 10.1016/0028-3908(88)90088-3. [DOI] [PubMed] [Google Scholar]
- Lynch G. S., Gribkoff V. K., Deadwyler S. A. Long term potentiation is accompanied by a reduction in dendritic responsiveness to glutamic acid. Nature. 1976 Sep 9;263(5573):151–153. doi: 10.1038/263151a0. [DOI] [PubMed] [Google Scholar]
- Lynch G., Larson J., Kelso S., Barrionuevo G., Schottler F. Intracellular injections of EGTA block induction of hippocampal long-term potentiation. Nature. 1983 Oct 20;305(5936):719–721. doi: 10.1038/305719a0. [DOI] [PubMed] [Google Scholar]
- Malinow R., Miller J. P. Postsynaptic hyperpolarization during conditioning reversibly blocks induction of long-term potentiation. Nature. 1986 Apr 10;320(6062):529–530. doi: 10.1038/320529a0. [DOI] [PubMed] [Google Scholar]
- Marletta M. A., Yoon P. S., Iyengar R., Leaf C. D., Wishnok J. S. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988 Nov 29;27(24):8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- Mattson M. P., Lee R. E., Adams M. E., Guthrie P. B., Kater S. B. Interactions between entorhinal axons and target hippocampal neurons: a role for glutamate in the development of hippocampal circuitry. Neuron. 1988 Nov;1(9):865–876. doi: 10.1016/0896-6273(88)90134-1. [DOI] [PubMed] [Google Scholar]
- Morris R. G., Anderson E., Lynch G. S., Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. 1986 Feb 27-Mar 5Nature. 319(6056):774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Murad F., Mittal C. K., Arnold W. P., Katsuki S., Kimura H. Guanylate cyclase: activation by azide, nitro compounds, nitric oxide, and hydroxyl radical and inhibition by hemoglobin and myoglobin. Adv Cyclic Nucleotide Res. 1978;9:145–158. [PubMed] [Google Scholar]
- Sandman C. A., O'Halloran J. P., Isenhart R. Is there an evoked vascular response? Science. 1984 Jun 22;224(4655):1355–1357. doi: 10.1126/science.6729458. [DOI] [PubMed] [Google Scholar]
- Schmidt J. T. Long-term potentiation and activity-dependent retinotopic sharpening in the regenerating retinotectal projection of goldfish: common sensitive period and sensitivity to NMDA blockers. J Neurosci. 1990 Jan;10(1):233–246. doi: 10.1523/JNEUROSCI.10-01-00233.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sretavan D. W., Shatz C. J. Prenatal development of cat retinogeniculate axon arbors in the absence of binocular interactions. J Neurosci. 1986 Apr;6(4):990–1003. doi: 10.1523/JNEUROSCI.06-04-00990.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sretavan D. W., Shatz C. J., Stryker M. P. Modification of retinal ganglion cell axon morphology by prenatal infusion of tetrodotoxin. Nature. 1988 Dec 1;336(6198):468–471. doi: 10.1038/336468a0. [DOI] [PubMed] [Google Scholar]
- Stryker M. P., Harris W. A. Binocular impulse blockade prevents the formation of ocular dominance columns in cat visual cortex. J Neurosci. 1986 Aug;6(8):2117–2133. doi: 10.1523/JNEUROSCI.06-08-02117.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. H., Errington M. L., Lynch M. A., Bliss T. V. Arachidonic acid induces a long-term activity-dependent enhancement of synaptic transmission in the hippocampus. Nature. 1989 Oct 26;341(6244):739–742. doi: 10.1038/341739a0. [DOI] [PubMed] [Google Scholar]