Abstract
Sleep apnea is highly prevalent in patients with cardiovascular disease. These disordered breathing events are associated with a profile of perturbations that include intermittent hypoxia, oxidative stress, sympathetic activation, and endothelial dysfunction, all of which are critical mediators of cardiovascular disease. Evidence supports a causal association of sleep apnea with the incidence and morbidity of hypertension, coronary heart disease, arrhythmia, heart failure, and stroke. Several discoveries in the pathogenesis, along with developments in the treatment of sleep apnea, have accumulated in recent years. In this review, we discuss the mechanisms of sleep apnea, the evidence that addresses the links between sleep apnea and cardiovascular disease, and research that has addressed the effect of sleep apnea treatment on cardiovascular disease and clinical endpoints. Finally, we review the recent development in sleep apnea treatment options, with special consideration of treating patients with heart disease. Future directions for selective areas are suggested.
Keywords: central sleep apnea, hypertension, obstructive sleep apnea
Cardiovascular disease (CVD) accounted for >800,000 deaths (31% of all deaths) in 2013 in the United States, with an estimated 155,000 deaths in Americans <65 years of age (1). By 2030, total direct medical costs of CVD are projected to be $920 billion. The high prevalence of obstructive sleep apnea (OSA), which affects 34% of men and 17% of women and is largely undiagnosed (2), is a modifiable CVD risk factor. OSA is a cause of systemic hypertension (HTN) and is associated with an increased incidence of stroke, heart failure (HF), atrial fibrillation (AF), and coronary heart disease (CHD) (3). OSA, particularly when severe, is associated with increased all-cause and cardiovascular (CV) mortality (Central Illustration).
CENTRAL ILLUSTRATION. Potential Etiological Risk Factors for Sleep Apnea and the Downstream Consequences.
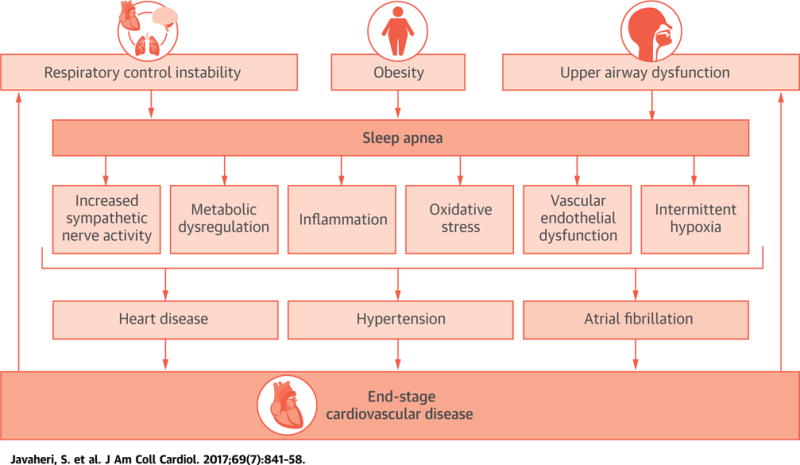
The illustration depicts the multietiological risk factors for sleep apnea and its downstream consequences, which include increased sympathetic nerve activity, metabolic dysregulation, inflammation, oxidative stress, vascular endothelial dysfunction, and intermittent hypoxia. These mechanistic pathways are critical for the pathogenesis of coronary heart disease, hypertension, and atrial fibrillation, all of which are etiological risk factors for end-stage cardiovascular disease. The figure is a schematic representation to illustrate important concepts, but does not fully depict the complex interactions between the various key variables.
A sleep apnea subtype, central sleep apnea (CSA) is rare in the general population, but is common in patients with HF, stroke, and AF (4,5). However, recent data suggest that CSA is also a risk factor for incident AF (6) and HF (7).
This review provides an update on sleep apnea and CVD. We hope to provide a catalyst for cardiologists to join with sleep physicians to conduct research, particularly clinical trials, that addresses the role of sleep apnea treatment in patients who are at high risk of or have existing CVD.
DEFINITIONS OF TYPES OF SLEEP APNEAS AND HYPOPNEAS
An apnea is the absence of inspiratory airflow for at least 10 s. A hypopnea is a lesser decrease in airflow, lasting 10 s or longer, and associated with a drop in arterial oxyhemoglobin saturation and or an electroencephalographic arousal. Apneas and hypopneas are classified as obstructive or central, but regardless, they result from an absence or reduction of brainstem neural output to upper airway muscles (e.g., genioglossus) and/or lower thoracic inspiratory pump muscles (diaphragm and intercostal muscles). The pattern of neural output determines the phenotype: OSA occurs when complete upper airway occlusion occurs (absent airflow, tongue falling backward) in the face of continued activity of inspiratory thoracic pump muscles (Figure 1) (8). In contrast, CSA (Figure 2) occurs when there is a transient reduction by the pontomedullary pacemaker in the generation of breathing rhythm, usually reflecting changes in the partial pressure of CO2 (PCO2), which can fall below the apneic threshold, a level of PCO2 below which breathing ceases (4,5). The 2 most common causes in adults are HF and opioid use (4).
FIGURE 1. Polysomnographic Example of OSA.
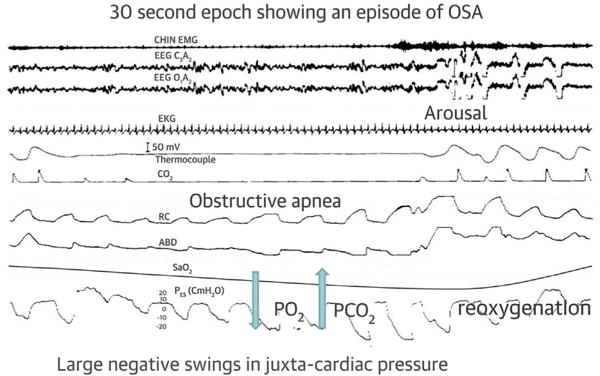
First tracing is chin electromyogram, second and third are electroencephalogram, fourth is electrocardiogram, fifth and sixth are airflow measured by thermocouple (fifth) and CO2 (sixth), seventh and eighth are rib cage (RC, seventh) and abdominal (ABD, eighth), ninth is oxyhemoglobin saturation measured by pulse oximetry, and tenth is esophageal pressure. Please note that during obstructive apnea, airflow is absent while breathing effort continues. Breathing resumes with the onset of arousal. Reprinted with permission from Javaheri (8). OSA = obstructive sleep apnea.
FIGURE 2. Polysomnographic Example of Hunter-Cheyne-Stokes Breathing.
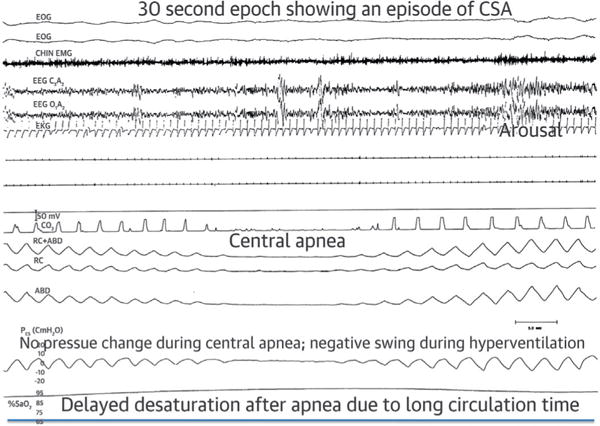
The first 2 tracings are electro-occulogram; otherwise tracings as in Figure 1. Note the smooth and gradual changes in the thoracoabdominal excursions and esophageal pressure in the crescendo and decrescendo arms of the cycle. There is an intervening central apnea, absence of naso-oral airflow, and excursions in pleural pressure, thorax, and abdomen. The arousal occurs at the peak of hyperventilation. Desaturation is delayed because of long circulation time in heart failure. Reprinted with permission from Dowdell WT, Javaheri S, McGinnis W. Cheyne-Stokes respiration presenting as sleep apnea syndrome: clinical and polysomnographic features. Am Rev Respir Dis 1990;141:871-9.
PATHOGENESIS OF OSA
Sleep has multiple pronounced effects on the respiratory system and control of breathing. Experimental studies in instrumented cats have shown decreased electrical activity in medullary inspiratory neurons with efferent output to the upper and lower respiratory muscles, reflected in decreased activity of diaphragm and dilator muscles of the upper airway (also observed in human studies) (9). With reduction in the activity of the genioglossus muscle at the onset of sleep, the tongue falls backward, and individuals with altered mechanical properties of the upper airway are prone to upper airway obstruction. Several anatomic processes may compromise the patency of the upper airway, including alterations in craniofacial structures, enlarged tonsils, upper airway edema, decreased lung volume, and most importantly, obesity (9,10).
The mechanisms linking obesity to OSA (2,9) are complex, although mostly due to direct mechanical effects on the respiratory system. These include fat deposits within the upper airway and reduction in lung volume, resulting in a loss of caudal traction on the upper airway (10).
Although obesity is the major risk factor for OSA, roughly 20% to 40% of OSA patients are not obese. In these individuals, nonanatomic factors (9,10), such as upper airway dilator muscle dysfunction, heightened chemosensitivity, and low arousal threshold (i.e., waking up from sleep prematurely, causing instability in ventilatory control), are important, and define various phenotypes of OSA that may be amenable to specific therapeutic options. Specifically, dilator muscle dysfunction should best respond to hypoglossal nerve stimulation, whereas heightened chemosensitivity and low arousal threshold may be amenable to pharmacotherapy to down-regulate ventilatory responses (nocturnal oxygen-attenuating hypoxic response), and hypnotics to increase the arousal threshold. With the push toward personalized medicine, individualizing therapy may be a viable approach for OSA, as the mechanism(s) underlying disease are better defined and consequently corrected. Much work is needed in this area.
Another risk factor that has received recent attention is that of fluid around the upper airway (5,11). In individuals with lower-extremity edema, excess fluid may accumulate in the pharyngeal area with cephalad transposition of fluid from lower extremities to the neck area when supine (5,11), rendering the upper airway susceptible to collapse during sleep. Redistribution of fluid to the lungs also may potentiate CSA, as discussed later in the text. Therapeutic approaches, such as diuretic agents, stockings, and exercise, have been shown to improve OSA and CSA (5,11).
PATHOGENESIS OF CSA
APNEIC THRESHOLD IN SLEEP AND INCREASED LOOP GAIN
Apneic threshold is the arterial PCO2 below which the ponto-medullary respiratory rhythm generator ceases, thereby silencing motor nerves innervating inspiratory thoracic pump muscles. Consequently, ventilation ceases and CSA occurs. Sleep unmasks a highly sensitive hypocapneic-induced apneic threshold, which in health at sea level, approximates the waking eupneic partial arterial pressure of carbon dioxide (PaCO2) (4). When ventilation increases in response to a transient spontaneous arousal or sigh, the subsequent ventilatory overshoot often elicits sufficient hypocapnia (i.e., in the −1 to −4 mm Hg PaCO2 range) to cause apneas. Once the central respiratory drive is withdrawn, apnea persists until PaCO2 rises above the apneic threshold.
Susceptibility to ventilatory instabilities depends critically on the “loop gain” of the respiratory control system. Loop gain defines the magnitude of a response (increased ventilation) to a disturbance (reduced breathing [4]). The higher the gain, the greater the ventilatory overshoot and undershoot, and the more likely the occurrence of continued ventilatory cycling and repeated apneas. The role of loop gain in mediating periodic breathing in HF has been reviewed elsewhere (4).
OVERNIGHT CONSEQUENCES OF SLEEP APNEA
Normal sleep provides a time of low physiological stress, which is advantageous to the CV system. In particular, during non-rapid-eye-movement sleep (approximately 80% of total sleep time), sympathetic activity decreases and parasympathetic activity increases, with lowering of blood pressure (BP) and heart rate (Figure 3). However, sleep-disordered breathing (SDB) disrupts normal sleep. Apneas and hypopneas, and the consequent compensatory hyperpneas, are associated with 4 acute adverse CV consequences (12): 1) arterial blood gas abnormalities, with intermittent hypoxemia–reoxygenation and fluctuations in PCO2; 2) excessive arousals; 3) decreased parasympathetic and increased sympathetic activity (9,12); and 4) large negative intrathoracic pressure swings (12) (Figure 4). These consequences are qualitatively similar for OSA and CSA, but more pronounced in OSA. The adverse consequences of SDB have been reviewed elsewhere (4,5,9,12). We emphasize, however, that the large negative pressure swings due to inspiratory effort against a closed upper airway are reflected in juxtacardiac pressure increasing the transmural pressure of all intrathoracic structures, including atria, ventricles, intrathoracic aorta, and pulmonary vascular beds, with adverse effects on these structures. The negative pressure increases left ventricular afterload, increasing myocardial oxygen consumption, and impedes stroke volume. Thin-walled atria are vulnerable to surrounding negative pressure, stretching easily, stimulating mechanoreceptors with activation of ion channels that facilitate development of atrial arrhythmias, specifically AF. These effects are most prominent in association with other apnea-related consequences, such as tissue hypoxia and increased sympathetic activity. Atrial stretch also results in secretion of atrial natriuretic peptide, causing nocturia, a symptom of OSA.
FIGURE 3. Normal Cardiovascular Changes in NREM and REM Sleep.
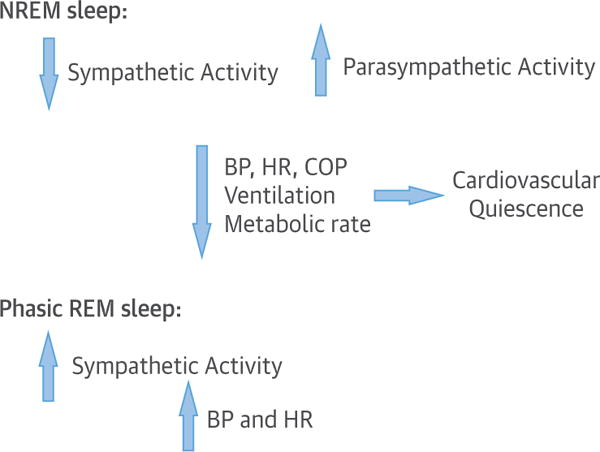
During NREM sleep sympathetic activity decreases whereas parasympathetic activity increases with consequent decrease in BP and HR. The reverse occurs in phasic REM sleep. BP = blood pressure; COP = cardiac output; HR = heart rate; NREM = non–rapid eye movement; REM = rapid eye movement.
FIGURE 4. Pathophysiological Consequences of Sleep Apnea and Hypopnea.
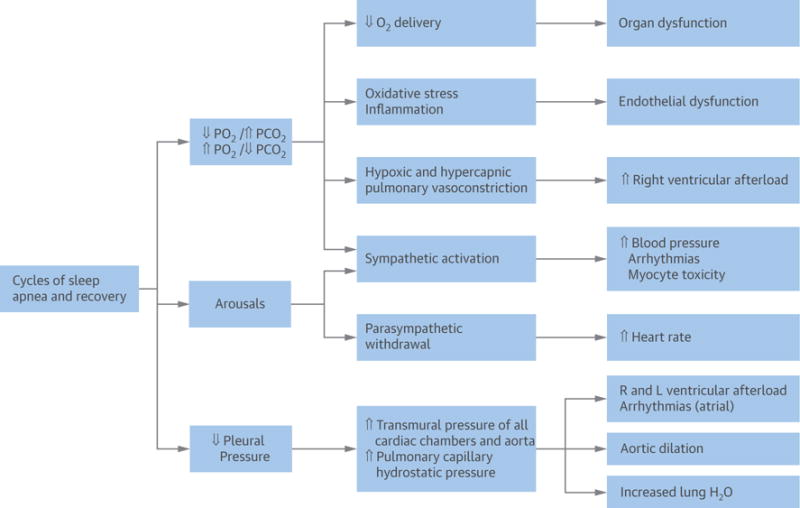
Pleural pressure (Ppl) is a surrogate of the pressure surrounding the heart and other vascular structures. [ = increased; Y = decreased. Reprinted with permission from Javaheri (8). CV = cardiovascular; H2O = water; L = left; O2 = oxygen; PCO2 = partial pressure of carbon dioxide in the blood; PO2 = partial pressure of oxygen in the blood; R = right.
MOLECULAR SIGNATURES OF SLEEP APNEA IN RELATION TO CARDIOCEREBROVASCULAR DISORDERS
OSA is a disorder associated with oxidative stress, up-regulation of redox-sensitive genes, and inflammatory cascade (13,14). Multiple studies of biomarkers related to CVD in patients with OSA assess how they change with treatment (13–17). Much of this work has focused on nuclear factor (NF)-kappaB– mediated pathways. Rapid reoxygenation at the end of apneas/hypopneas leads to production of free radicals, resulting in oxidative stress and up-regulation of nuclear factor-kappaB. There is some evidence that treatment of OSA with continuous positive airway pressure (CPAP) can reduce levels of inflammatory mediators, such as interleukin-6, tumor necrosis factor-α, and C-reactive protein (17).
Another molecular signature of OSA is increased catecholamines, consistent with perturbations in the autonomic nervous system. With effective treatment of OSA, catecholamine levels decline, with reversal upon withdrawal of CPAP (18).
Many CVD-related biomarkers are also elevated in obesity, and disentangling obesity from OSA-related effects is a challenge, especially given the high correlation between the apnea-hypopnea index (AHI) and body mass index. Obesity may magnify the effects of OSA because macrophages in fat are likely the target cells for the effects of chronic intermittent hypoxia, leading to increases in inflammatory biomarkers; thus, OSA and obesity may have synergistic effects. The results of a randomized controlled study in obese patients (19) showed that combined treatment with weight loss and CPAP reduced BP more than either therapy alone; however, C-reactive protein levels were only reduced in association with weight loss. The Icelandic Sleep Apnea Cohort (16) reported that intercellular adhesion molecule levels increase over 2 years in the most obese individuals with untreated OSA, whereas levels decrease in regular CPAP users. Thus, OSA may result in a progressive inflammatory state, which may be 1 mechanism of the vascular damage that occurs from OSA. Inflammatory consequences of OSA may vary with degree of obesity.
METABOLIC DYSFUNCTION AND OSA
OSA is independently associated with metabolic syndrome and insulin resistance, with an associated risk for incident CV events (20). Three randomized controlled trials (RCTs) (21–23) have demonstrated that treatment of OSA with CPAP improves insulin sensitivity. In 1 RCT, supervised CPAP treatment for 8 h over 2 weeks nightly significantly improved insulin sensitivity and glucose response in intravenous and oral glucose tolerance tests (21). In another RCT of pre-diabetic patients, the insulin sensitivity index improved significantly in those with severe OSA treated with CPAP (22), and there was a significant correlation between hours of CPAP use and improvement in insulin sensitivity, emphasizing the critical importance of adherence (21). Thus, current evidence suggests that OSA is associated with insulin resistance, and CPAP treatment may improve insulin sensitivity in pre-diabetic patients.
The effects of CPAP therapy of OSA on full-blown diabetes remain to be elucidated (24). Large-scale RCTs of CPAP are needed to establish the role of OSA in proatherogenic dyslipidemia.
OSA AND RELATED CVD: EFFECT OF CPAP THERAPY
OSA is strongly associated with a number of CVDs (Figure 5) including HTN, resistant HTN, transient ischemic attack (TIA), stroke, pulmonary hypertension (PHTN), HF, CHD, AF, myocardial ischemia, myocardial infarction (MI), and sudden death. The epidemiology of these associations has been extensively reviewed (3,5,13,14,20). In the following sections, we discuss trials of CPAP, the most effective therapy of OSA. We also discuss adjunct therapy of OSA.
FIGURE 5. Prevalence (%) of OSA in CVD.
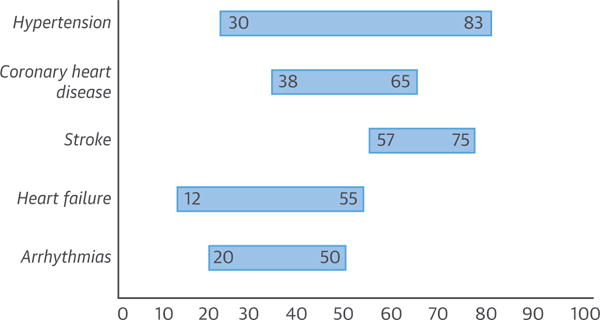
The lower limit is invariably using an AHI of ≥15/h, indicating presence of moderate-to-severe OSA. The upper part of the range relates to a lower threshold of ≥5/h. CVD = cardiovascular disease; OSA = obstructive sleep apnea.
CPAP AND SYSTEMIC HTN
The effect of CPAP on HTN has been widely investigated, and the available evidence from multiple RCTs and several recent meta-analyses demonstrates that CPAP significantly reduces BP in OSA patients. Studies using 24-h BP monitoring (25–32) consistently report drops of 2 to 2.5 mm Hg and 1.5 to 2 mm Hg in systolic blood pressure (SBP) and diastolic blood pressure (DBP), respectively, compared with subtherapeutic or conservative treatment (Figure 6), with greater reductions in patients with resistant HTN (between 4.7 to 7.2 mm Hg and 2.9 to 4.9 mm Hg for SBP and DBP, respectively) (Figure 7) (33–39). CPAP has also been demonstrated to reverse nondipper or riser nocturnal BP patterns in OSA patients.
FIGURE 6. Effect of CPAP Therapy on BP in Patients With Hypertension.
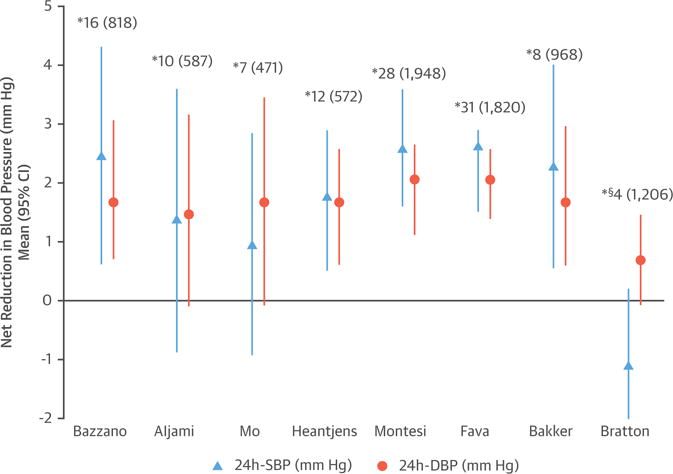
Summary of different meta-analyses of RCTs. Positive figures mean improvement in BP level with CPAP (net changes) *Number of studies included (number of patients included). §Patients without daytime hypersomnia. BP = blood pressure; CI = confidence interval; CPAP = continuous positive airway pressure; DBP = diastolic blood pressure; RCT = randomized controlled trial; SBP = systolic blood pressure.
FIGURE 7. Effect of CPAP Therapy on BP in Patients With Resistant Hypertension.
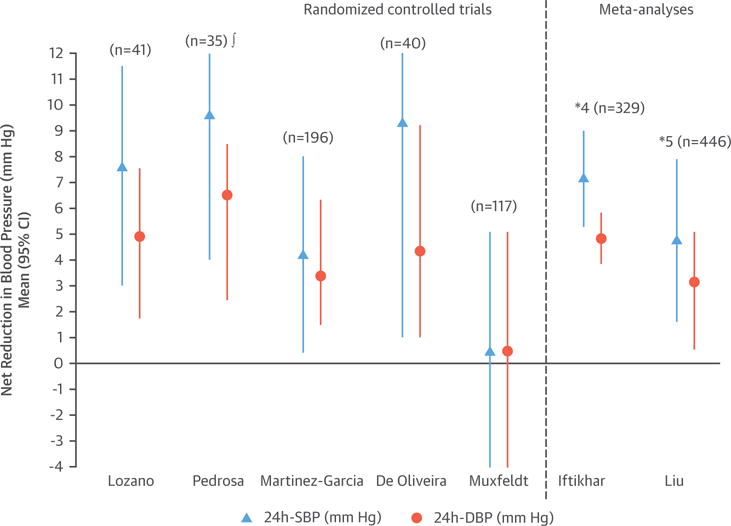
The figure shows 5 randomized controlled trials and 2 meta-analyses. The differences between the 2 meta-analyses depend on the most updated references included in the 2015 meta-analysis. Positive figures mean improvement in BP level with CPAP (net changes) *Number of studies included (number of patients included). !Daytime BP values. Abbreviations as in Figure 6.
Because long-term reductions of 2 to 3 mm Hg in SBP are associated with a 4% to 8% reduction in the future risk of stroke and CHD, long-term treatment of OSA in hypertensive patients could eventually reduce incident CV burden.
There are multiple reasons why the antihypertensive effect of CPAP is limited. First, essential HTN is quite common in the general population, and should be equally prevalent in OSA patients. CPAP may not decrease BP if the underlying cause of HTN is unrelated. Second, CPAP therapy can only reverse certain intermediate mechanisms associated with OSA. For example, CPAP reduces sympathetic tone, thereby decreasing BP (18). However, other pathophysiological mechanisms underlying HTN, such as those associated with obesity, salt intake, and volume overload, may be unaffected by CPAP. Third, OSA and HTN are chronic disorders, and if OSA-induced HTN has been longstanding, with consequent remodeling of the vascular bed and/or increased set point for BP on the basis of prevailing BP regulatory mechanisms (e.g., baroreflex), CPAP may not be able to reduce BP much, certainly in the short term. Given this issue, for ethical reasons, RCTs have a follow-up of <1 year. Therefore, it is unknown whether BP may fall further in long-term follow-up.
Overall, highly variable BP improvement is expected with CPAP therapy, as the drop correlates with OSA severity, baseline HTN, and hours of CPAP use. Depending on these characteristics, some study patients might have greater antihypertensive benefits from CPAP, whereas others—for example, those with less severe OSA—might not. The level of adherence needed to obtain a significant reduction in BP levels is unknown, although a minimum threshold of 4 h, and optimally >5 to 6 h/night, is needed. In resistant HTN, a linear correlation has been observed between the number of hours of CPAP use and drop in BP measures (decreases of 1.9 and 1.0 mm Hg in SBP and DBP, respectively, for each additional hour of CPAP use) (37), suggesting that greater CPAP adherence would be associated with better BP control. This dose-dependent relationship is similar to that for insulin sensitivity (22,23) and other CV outcomes, as discussed later in the text.
A recent study (40) reported that BP response to CPAP in OSA patients with resistant HTN can be predicted by measuring the plasma levels of 3 specific microribonucleic acids, which may help to personalize this treatment in the future.
There is evidence that the combination of antihypertensive drugs (41) or weight loss (19) with CPAP therapy could have a synergistic effect in reducing BP in OSA patients, supporting the multidimensional pathophysiology of HTN in this population. When hypertensive patients with and without OSA were treated with losartan for 6 weeks, the BP drop (measured by 24-h ambulatory BP monitoring) was significantly less in those with OSA than without. Importantly, when CPAP was added at the end of 6 weeks of losartan and used at least 4 h nightly for the next 6 weeks, BP decreased markedly in those with OSA (41). The combination of CPAP and weight loss may also have a synergistic effect, leading to larger BP reductions in OSA patients (19).
CPAP AND PHTN
About 10% of patients with OSA have PHTN (defined as the mean pulmonary artery pressure ≥25 mm Hg) (42,43), and multiple observational studies demonstrate that treating OSA with CPAP improves PHTN (42,43). In the only small randomized crossover study (therapeutic vs. sham CPAP) (44), 12 weeks of treatment resulted in a significant decrease in pulmonary artery systolic pressure (from a mean of 30 to 24 mm Hg). The reduction was greatest (8.5 mm Hg) in patients with baseline PHTN (pulmonary artery systolic pressure ≥30 mm Hg by echocardiography). The American College of Cardiology/American Heart Association expert consensus document recommends polysomnography to rule out OSA for all patients with PHTN (45). The recommendation comes from the idea that targeted therapy of OSA could either improve or prevent further deterioration in central hemodynamics.
CPAP AND STROKE/TIA
Sleep apnea is highly prevalent in patients with stroke or TIA, and OSA also is associated with increased risk for incident stroke (46–48). There is some evidence that early CPAP therapy has positive effects on long-term survival in ischemic stroke patients with moderate-to-severe OSA (46–48). Additionally, consistent with observational studies (46–48), in a recent large RCT (49), those OSA patients who were adherent to CPAP therapy exhibited reduced risk of incident cerebral events.
The American Heart Association/American Stroke Association guideline states: “A sleep study might be considered for patients with an ischemic stroke or TIA on the basis of the very high prevalence of sleep apnea in this population and the strength of the evidence that the treatment of sleep apnea improves outcomes in the general population” (50).
CPAP AND ARRHYTHMIAS
Two small RCTs showed that treatment of OSA with CPAP reduces the mean 24-h heart rate, as well as the frequency of premature ventricular beats during sleep (51,52). Data from observational studies show that CPAP treatment is associated with a significantly decreased recurrence rate of AF, even after electrical cardioversion or ablative therapies, and that patients are less likely to progress to more permanent forms of AF and have significantly reduced occurrence of paroxysmal AF compared with untreated patients (53–56).
In a meta-analysis (57) of 698 CPAP users and 549 non-CPAP users, those with OSA treated with CPAP after AF intervention had a 44% reduced AF risk; younger, more obese, and male patients benefited the most. On the basis of this evidence, a recent expert consensus document on AF identified OSA as a risk factor for AF recurrence after surgical and catheter ablation, and recommended its treatment (58). Although the reproducibility of these findings from observational studies is compelling, data from clinical trials are needed.
CPAP, CHD, CV MORTALITY, AND COMPOSITE CV OUTCOMES
Multiple observational studies demonstrate that untreated OSA is associated with CV morbidity and mortality, and that treatment with CPAP improves the need for revascularization, incident CHD, and survival (59–62).
To date, 3 RCTs investigated the effect of CPAP on CV outcomes (Figure 8), with the main endpoint a composite outcome of different fatal and nonfatal CV events. In the first RCT (63), 725 nonsleepy patients (Epworth Sleepiness Scale [ESS] <11 of 24), free of CVD and with moderate-to-severe OSA (AHI ≥20/h), were randomized to CPAP (n = 357) or conservative treatment (n = 366), and were followed for a median of 4 years. CPAP did not result in a significant reduction in the incidence of the primary composite outcome. However, in an adherence analysis, patients who used CPAP for ≥4 h/night did achieve a CV benefit (incidence density ratio 0.72; 95% confidence interval [CI]: 0.52 to 0.98; p = 0.04).
FIGURE 8. Effect of CPAP Treatment on CV Risk.
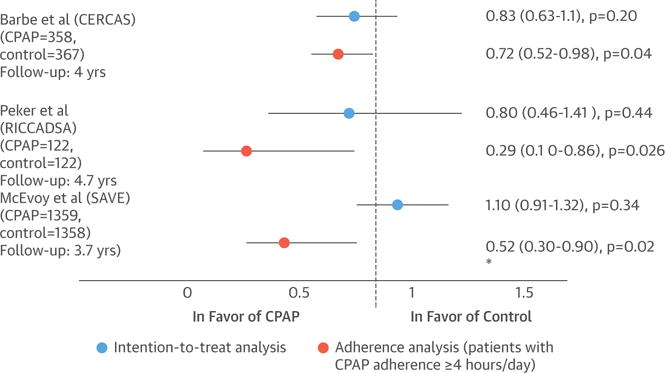
The figure shows the incidence risk for the primary composite endpoints in 3 RCTs (49,63,64) in the CPAP compared with the control group (hazard ratio or incidence density ratio, 95% CI) in the intention-to-treat analysis and in the adherence analysis (patients with CPAP adherence ≥4 h/day). *In the McEvoy study (49), the significant CV improvement in patients who used CPAP ≥4 h/day was only achieved in the risk of a cerebrovascular event, but not in the primary composite outcome. CV = cardiovascular; other abbreviations as in Figure 6.
Peker et al. (64) randomized 244 nonsleepy (ESS <10) patients with newly revascularized CHD and moderate-to-severe OSA (AHI >15/h) to autotitrating positive airway pressure (PAP) (n = 122) or no PAP (n = 122) for a median of 57 months. The incidence of the primary composite CV endpoint did not differ between the 2 groups. However, adjusted on-treatment analysis showed a significant CV risk reduction in those who used CPAP for >4 h, compared with those who used the device <4 h/night or did not receive treatment (adjusted hazard ratio: 0.29; 95% CI: 0.10 to 0.86; p = 0.026).
The SAVE (Continuous Positive Airway Pressure Treatment of Obstructive Sleep Apnea to Prevent Cardiovascular Disease) study (49) enrolled 2,717 nonsleepy or mildly sleepy (ESS <15) patients age 45 to 75 years, with prior history of coronary or cerebrovascular disease and OSA, defined by an oxygen desaturation index ≥12/h, diagnosed by means of a 2-channel screening device. Patients were allocated to CPAP or usual-care treatment for a mean of 3.7 years. The incidence of a composite CV outcome was similar in the CPAP and control groups (p = 0.34). One-to-one propensity-score matching was performed to compare 561 patients who were adherent to CPAP therapy with 561 patients in the usual-care group. In this sensitivity analysis, a lower risk of a composite endpoint of cerebral events was found in the group of patients who used CPAP for at least 4 h/day (hazard ratio: 0.52; 95% CI: 0.30 to 0.90; p = 0.02).
This study has several limitations. First, SDB was diagnosed by means of a screening device on the basis of oximetry and nasal pressure recordings. Investigators excluded patients with a pattern of Cheyne-Stokes respiration on the ApneaLink (ResMed Corporation, San Diego, California) nasal pressure recording, but this device may not accurately detect Cheyne-Stokes respiration. Furthermore, this device could not differentiate between central and obstructive events. It is likely that patients with CSA were included, particularly considering that CSA is common in patients with stroke and HF associated with CHD. It is known that CSA is unlikely to respond to CPAP, and that these patients have a poorer CV prognosis. Second, the low average CPAP use of 3.3 h/day raises concern that the results were negative because of poor adherence. Third, patients with the most severe OSA were excluded (those with ESS >15 and hypoxemia, defined as oxygen saturation <80% for >10% of recording time). In fact, the mean AHI was 29/h, and the mean ODI was 28/h, values within the moderate OSA severity range. Hard CV outcomes have been associated with severe OSA, but are less associated with mild or moderate OSA; thus, it can be argued that many patients in this study with only mild-to-moderate OSA were not at an increased risk of CV outcomes. Fourth, 66% of the cohort was Asian in under-resourced areas, with some concern as to whether the findings can be generalized to other races in other settings that were less represented in the study. There is 1 ongoing RCT (65) that will shed more light on the true role of CPAP therapy in hard CV endpoints.
NOCTURNAL MYOCARDIAL ISCHEMIA, MI, AND SUDDEN DEATH
OSA patients with severe SDB and marked hypoxemia are at risk for nocturnal cardiac ischemia. OSA has been associated with onset of MI during the night/early morning (66). It is reasonable to consider that the increased likelihood of nocturnal nonfatal MI may also be accompanied by an increased risk of fatal MI and sudden death. In >10,000 individuals, the presence of OSA and significant desaturation was associated with an almost 2-fold increase in risk of sudden death, independent of known risk factors (67). Comparing the time of death of individuals with and without SDB, those with the disorder died mostly between 12:00 AM and 6:00 AM (68) suggesting that untreated OSA in patients with established CHD would likely lead to a poorer prognosis.
SUMMARY OF CPAP THERAPY AND CARDIOVASCULAR DISEASE
High-quality evidence recommends CPAP therapy in patients with HTN and OSA, especially in patients with resistant HTN. For other CVD and cerebral events, CPAP is not effective unless it is used for ≥4 h of sleep. The available evidence for arrhythmias is consistent with adverse effects of OSA and beneficial effects of CPAP, but these data come from observational studies.
ADJUNCT THERAPY IN OSA
Although CPAP therapy is very effective in the treatment of OSA, by itself it has limited metabolic effects, and poor adherence further compromises its effectiveness, as evidenced by recent RCTs (49,63,64). Incorporation of exercise and weight loss as adjunct therapy has important health benefits beyond CPAP therapy, and could be potentially synergistic.
EXERCISE
Exercise training and physical activity attenuate OSA (69). In a meta-analysis of 5 studies enrolling a total of 129 participants, the pooled estimate of mean pre- to post-exercise reduction in AHI was –6.3 events/h (95% CI: –8.5 to –4.0; p < 0.001) (about a 32% reduction), and occurred without significant weight loss (69). Multifactorial mechanisms (69) could include decreased rostral fluid redistribution (5,11,70); stabilization of chemo-receptor sensitivity; and improved nasal resistance, sleep quality, weight loss, and strength of pharyngeal dilator muscles (69). In regard to the latter, a meta-analysis involving 120 adults concluded that selective oropharyngeal exercises reduced snoring and AHI by 50%, from about 25 to 12/h (71). Oropharyngeal exercise may also decrease the amount of tongue fat.
WEIGHT LOSS
Weight reduction, when applicable, should be a core element in OSA treatment. In a randomized trial discussed earlier, weight loss provided an incremental BP reduction in CPAP-adherent participants (19). In contrast, use of CPAP often results in a small amount of weight gain, due in part to a reduction in nocturnal metabolic rate related to OSA work of breathing (eliminated by CPAP), with dietary intake and eating behavior having the greatest effects leading to a positive energy balance and weight gain (72). These findings highlight the importance of lifestyle modifications combined with CPAP (19), and ongoing follow-up of patients after CPAP is initiated to monitor weight and other health behaviors. Even though weight loss is an important component of long-term management of OSA and has significant respiratory and cardiometabolic effects, weight loss does not necessarily cure OSA, even after bariatric surgery, and requires follow-up sleep study after weight stabilization.
HF COMORBID WITH SLEEP APNEA
Both OSA and CSA are common in HF patients, and could lead to progression and decompensation of HF, as reviewed in detail elsewhere (4,5,73). Regarding CSA, John Hunter first described the Cheyne-Stokes breathing pattern. Hunter noted recurrent cycles of smooth and gradual crescendo–decrescendo changes in tidal volume with an intervening central apnea in a patient with HF who probably had AF (Figure 2). For this reason, we refer to this pattern of breathing as Hunter-Cheyne-Stokes breathing (HCSB). HCSB is unique to patients with HF, as it has a long cycle time (Figure 2), reflecting the prolonged circulation time, a pathological feature of heart failure with reduced ejection fraction (HFrEF) (4,73).
Although HCSB with central apneas or hypopneas is common in HF, patients frequently experience the coexistence of both OSA and CSA; however, generally 1 variant predominates (73). Overall, moderate-to-severe SDB (AHI ≥15/h) is highly prevalent in various pathological conditions associated with symptomatic or asymptomatic left ventricular dysfunction (Figure 9) discussed later in the text.
FIGURE 9. Sleep Apnea is Prevalent in Left Ventricular Dysfunction.
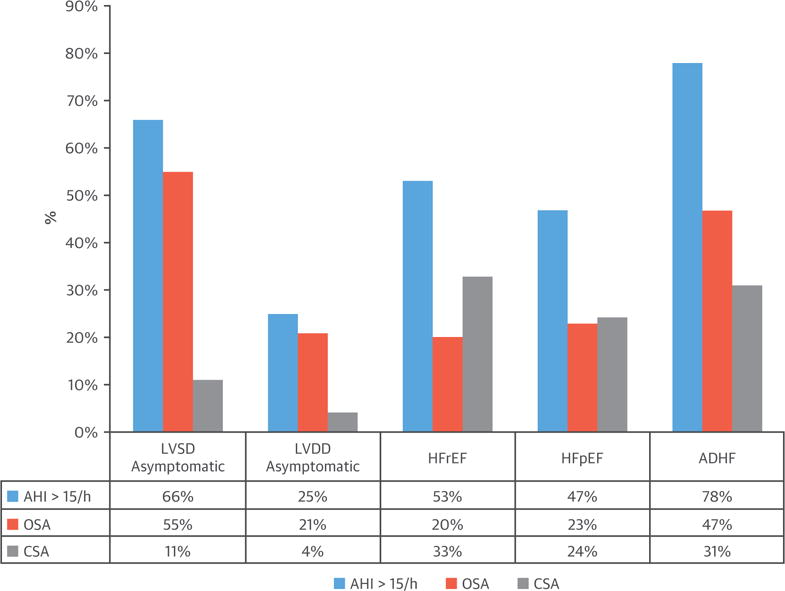
Prevalence (%) of moderate-to-severe sleep apnea (AHI ≥15) in asymptomatic left ventricular systolic dysfunction (LVSD) or left ventricular diastolic dysfunction (LVDD), heart failure with preserved ejection fraction (HFpEF) or heart failure with reduced ejection fraction (HFrEF), and acutely decompensated heart failure (ADHF). AHI = apnea-hypopnea index; CSA = central sleep apnea; OSA = obstructive sleep apnea.
PREVALENCE OF SLEEP APNEA IN ASYMPTOMATIC LEFT VENTRICULAR DYSFUNCTION
Using polysomnography, Lanfranchi et al. (74) showed that 66% of individuals with asymptomatic left ventricular systolic dysfunction experience moderate-to-severe sleep apnea: 55% with CSA and 11% with OSA (Figure 9). The prevalence is lower in heart failure with preserved ejection fraction (HFpEF), at about 25% (4% CSA), although the prevalence of diastolic dysfunction increases with the severity of OSA (75).
What is the clinical significance of a high prevalence of SDB in asymptomatic left ventricular dysfunction? Because asymptomatic left ventricular dysfunction is a predictor of incident symptomatic HF, undiagnosed SDB may contribute to the progression from an asymptomatic to a symptomatic condition. In this context, 2 prospective longitudinal studies demonstrated that OSA was independently a significant predictor of incident HF in men (76) and women (77). Similarly, the presence of CSA/HCSB has been shown to predict incident HF in a prospective study of 2,865 participants (7). This suggests that HCSB is not simply a marker of more severe HF, but may precede the onset of clinical HF, possibly by making those with subclinical ventricular dysfunction more likely to decompensate.
PREVALENCE OF SLEEP APNEA IN HFrEF, HFpEF, AND ACUTE HF
Combining the results of several studies (4,8,73), 53% of 1,607 patients with HFrEF had moderate-to-severe sleep apnea (defined as an AHI ≥15), of whom an estimated 34% had CSA and 19% had OSA (Figure 9). These findings are consistent with a recent study of 963 well-treated HFrEF patients, showing that 58% had moderate-to-severe SDB (AHI ≥15), with 46% classified as CSA and 16% OSA (78). However, there is considerable variation in the reported prevalence of these 2 forms of sleep apnea (73), with underestimation of prevalence of OSA in some studies due to misclassification of hypopneas (obstructive or central) as a component of AHI.
The prevalence of sleep apnea in HFpEF is similar to that in HFrEF. Combining 2 studies (79,80) with a total of 263 consecutive patients, 47% had sleep apnea (AHI ≥15): 24% OSA and 23% CSA (Figure 9). However, with decompensation, the prevalence increases considerably. In 1,117 consecutive HFrEF patients hospitalized with acute HF who underwent in-hospital polygraphy, 334 patients were identified with CSA (31%) and 525 with OSA (47%) (Figure 9) (81).
TREATMENT OF SDB IN HF
Depending on the predominant phenotype of sleep apnea, treatment strategies are different. However, a number of approaches are applicable to either type of sleep apnea.
GENERAL BENEFICIAL MEASURES
Optimization of cardiopulmonary function
Before obtaining an outpatient sleep study, it is often beneficial to use guided medical therapy of HF to improve cardiopulmonary function and minimize volume overload (5,11). Treatment of volume overload is important because fluid from lower extremities translocated cephalad can result in narrowing of the upper airway and precipitates obstructive events (5,11). In contrast, fluid translocation into the lungs promotes CSA (5) by increasing pulmonary capillary pressure (82).
Intensive HF therapy must be executed carefully, otherwise adverse consequences, such as renal failure, may occur and improvement in quality of life and reverse remodeling may not ensue (83). Regarding device therapy, a meta-analysis (84) of cardiac resynchronization therapy trials in patients with HFrEF demonstrated improvement in CSA, but not OSA.
Exercise
As discussed earlier in the text, several studies indicate that supervised exercise attenuates OSA. Exercise training of patients with HF also attenuates SDB, particularly OSA (85).
TREATMENT OF OSA: IMPACT OF THERAPY ON SYMPATHETIC ACTIVITY, LVEF, READMISSION, AND MORTALITY
CPAP has been successfully used to treat OSA in patients with HF, both acutely (86) and chronically (87–89), with beneficial CV benefits.
One important issue is the augmentation of the adrenergic state imposed by SDB in patients with HFrEF (Figure 10). Small RCTs have shown that CPAP treatment decreases vascular (87) and myocardial sympathetic nerve function (88), improves myocardial energetics in the case of severe OSA (88), and increases left ventricular ejection fraction (LVEF) (89,90), although not consistently (88). OSA is independently associated with excess hospital readmission and mortality (81,91). In the largest observational study of about 30,000 Medicare beneficiaries newly diagnosed with HF (92), treatment of SDB was associated with decreased readmission, health care cost, and mortality (Figure 11) (91,92).
FIGURE 10. Effects of Sleep Apnea and HF on Central Sympathetic Outflow.
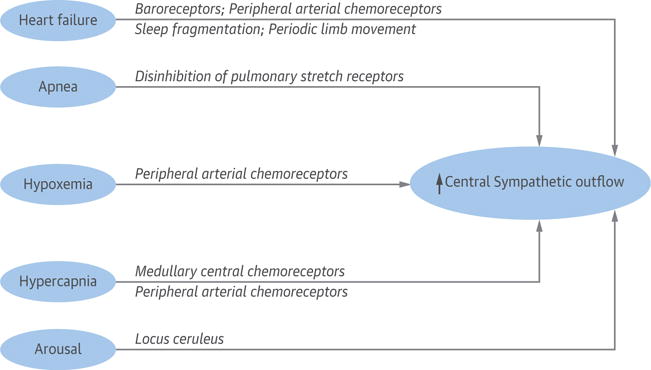
Heart failure is a hyperadrenergic state. Sleep apnea further contributes to increased central sympathetic outflow. Locus ceruleus is the brainstem arousal network, and norepinephrine is the neurotransmitter. HF = heart failure.
FIGURE 11. Comparative Survival of 258 HF Patients Treated for Sleep Apnea and 30,000 Patients Not Tested for Sleep Apnea.
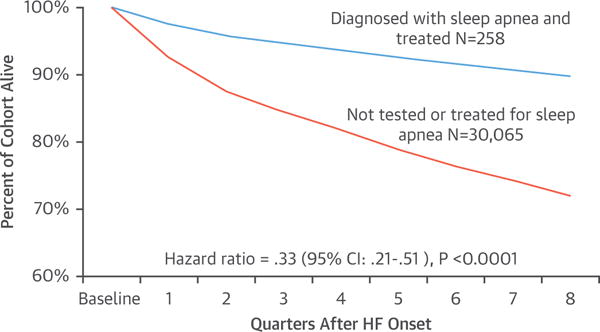
Adapted with permission from Javaheri et al. (92). HF = heart failure.
With regard to OSA comorbid with HFpEF, there is 1 randomized trial (93) showing reversal of diastolic dysfunction with CPAP.
TREATMENT OF CSA: IMPACT OF THERAPY ON SYMPATHETIC ACTIVITY, LVEF, READMISSION, AND MORTALITY
Intensive therapy of HF with pharmacotherapy and cardiac resynchronization therapy can improve cardiac function, periodic breathing, and CSA (5,73,84).
In contrast to OSA, CPAP is only partially effective in CSA (about 50% of patients) (86,94), and may be harmful in those whose CSA is not suppressed (94). Medications including theophylline and acetazolamide have been used in small RCTs, and have been extensively reviewed previously (73). Cardiac transplantation virtually eliminates CSA (95), but OSA develops in a large number of patients who gain weight (Figure 12). We will discuss 3 therapeutic options: positive airway pressure, nasal oxygen, and phrenic nerve stimulation (PNS).
FIGURE 12. Prevalence of OSA in 45 of 60 Consecutive Cardiac Transplant Recipients.
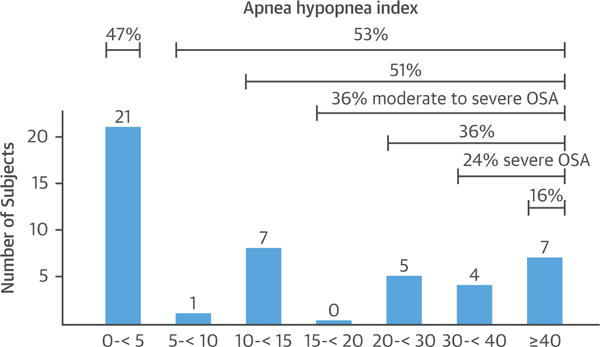
Those who developed sleep apnea had gained the most weight after transplantation. Adapted with permission from Javaheri et al. (95). CI = confidence interval; HF = heart failure; OSA = obstructive sleep apnea.
Similar to OSA, CSA also imposes an augmented hyperadrenergic state in HFrEF (96) (Figure 10), as measured by overnight urinary and morning plasma norepinephrine levels (97), and microneurography (98,99). Importantly, several randomized trials have demonstrated that attenuation of CSA by CPAP decreases plasma and urinary norepinephrine and nocturnal minute ventilation (surrogate of work of breathing), improving respiratory muscle strength and fatigue (97,100,101). The decrease in urinary catecholamines has also been confirmed in randomized trials comparing therapeutic adaptive servo-ventilation (ASV) to sham ASV (102) and nocturnal oxygen to sham (room air from a concentrator) (103). Consistent with a number of previous studies (reviewed by Javaheri et al. [73]), in the largest and most recent mortality study of 963 well-treated patients with HFrEF, those with CSA had the worst survival after accounting for several cofounders (78). These data are consistent with CSA as a negative prognostic indicator (104). Surprisingly, the recent ASV trial, SERVE-HF (Treatment of Predominant Central Sleep Apnoea by Adaptive Servo Ventilation in Patients With Heart Failure) (105), designed specifically to treat CSA with ASV, not only did not show survival benefit, but was associated with excess CV mortality. The ASV algorithm provides an anticyclical pressure support such that when the patient is hypoventilating the support is augmented, and vice versa (106).
The SERVE-HF investigators (105) cited 2 explanations for their findings: 1) increased PAP compromised cardiac output; or 2) CSA serves as a compensatory mechanism with protective effects, as previously hypothesized (104). As detailed elsewhere (107), there are additional reasons that may account for the observed increased mortality. One concern is that the results may not be applicable to the modern generation of ASV devices. SERVE-HF tested an early-generation ASV device (106), which may have sub-optimally treated CSA and OSA, and its application of pressure may have caused excessive ventilation, as well as adverse hemodynamic responses in vulnerable subsets of patients (107). Given results of the SERVE-HF trial, ASV is contraindicated to treat CSA in patients with low ejection fraction. Research with new-generation ASV devices is needed, with one in progress (ADVENT-HF [Effect of Adaptive Servo Ventilation (ASV) on Survival and Hospital Admissions in Heart Failure]; NCT01128816).
SUPPLEMENTAL NASAL OXYGEN
The uncertainty over use of pressure devices in patients with reduced ejection fraction has opened the door for other therapeutic options devoid of increasing intrathoracic pressure. We briefly review 2 options: nasal oxygen and PNS.
Systematic studies in patients with HFrEF (73,108) have shown that nocturnal nasal oxygen improves CSA, with randomized studies showing that therapy improves maximal exercise capacity; decreases overnight urinary norepinephrine excretion and muscle sympathetic nerve activity; and improves ventricular arrhythmias, LVEF, and quality of life (reviewed by Javaheri [73,108]). The potential benefits of supplemental oxygen include: improvement in oxygen stores; stabilization of breathing pattern; reduction in loop gain; and improvement in hypoxemia-related effects. The use of oxygen in HF, particularly to overcome hypoxemia, is also supported by data showing that degree of overnight hypoxemia is among the most significant determinants of HF-related mortality (78,109). However, it is important to note that use of high levels of oxygen in normoxic HF patients (when awake) has been shown to cause unwanted hyperoxia, increased systemic vascular resistance, and impaired ventricular function (110). RCTs are needed to determine the role of oxygen in treating CSA in HFrEF (108).
TRANSVENOUS UNILATERAL PNS
Transvenous unilateral PNS has been used to treat CSA. The phrenic nerve can be stimulated through the wall of either the right brachiocephalic vein or the left pericardiophrenic vein (111,112). In the recently completed RCT (112), 151 patients with CSA were implanted and randomized to stimulation or no stimulation for 6 months. With stimulation, multiple measures of sleep apnea severity (AHI, central apnea index, arousal index, and oxygen desaturation index), quality of life, and daytime sleepiness improved significantly. The most common side effect was therapy-related discomfort that was resolved with reprogramming in all but 1 patient. During the 6 months of therapy, 2 deaths occurred in the treatment group during daytime when stimulation was off, and 2 deaths occurred in the control group. Local side effects, such as infection and dislodgment, occurred in 13 of 151 implanted patients.
FUTURE DIRECTIONS
CARDIOVASCULAR DISEASE
RCTs are needed to provide unbiased assessments of the role of sleep apnea intervention on incident CVD, and CVD-associated morbidity and mortality. RCTs are designed to overcome potential biases in observational studies, ensuring that groups who are treated are otherwise comparable to control groups. However, RCTs present several challenges in design and implementation that need to be addressed in future trials. Studies need to be sufficiently large, and of sufficient duration to detect clinically and statistically significant differences in event rates. Because there are no optimal control conditions for CPAP, outcomes should be objectively collected and prospectively ascertained to minimize bias. Trials require careful implementation of methods for optimizing treatment adherence across the duration of interventions, aiming to treat sleep apnea every night and all night. Low adherence to CPAP has been a major limitation of the 3 major trials (49,63,64) discussed earlier in the text. Moreover, conventional methods for assessing adherence only consider average hours of use per night. For CVD, it may be that selective nonuse during early morning hours, when rapid-eye-movement sleep dominates and hypoxemia may be most profound, may contribute to specific CVD-related stress (113). Failure to treat sleep apnea during these critical periods may account for reduced treatment efficacy.
Future trials may benefit from more comprehensive approaches to CPAP adherence that account for both mechanical issues and behavior change. Careful attention to mask and pressure comfort, claustrophobia, nasal clogging, naso-oral dryness, and oral leak are critical. However, social and behavioral factors also are important, including bed partner support and self-efficacy. Motivational education, patient engagement using real-time electronic feedback, and peer support are promising strategies for improving adherence.
As alternatives to CPAP, such as nocturnal supplemental oxygen, oral appliances, or hypoglossal nerve stimulation, are identified, there is also opportunity to conduct comparative effectiveness trials. These trials may more easily be conducted in “real-world” settings, and may allow for broader patient enrollment, such as patients with sleepiness, who generally have been excluded from studies with “no treatment” arms (49,63,64). Symptomatic patients may better adhere to therapy, and also are a group who may be at high risk for adverse outcomes.
RCTs are necessary across a number of other CV disorders. Current studies show a high prevalence of SDB in asymptomatic left ventricular systolic and diastolic dysfunction. It remains to be established in RCTs if treatment of OSA or CSA aborts or delays incident HF in subjects with preclinical left ventricular dysfunction or, conversely, if treatment of left ventricular dysfunction improves SDB, breaking a vicious cycle.
In patients with HFrEF, studies have demonstrated reversal of adverse consequences of OSA, including diastolic dysfunction, with CPAP. Randomized, adequately-powered trials are needed to confirm the results of largely observational studies showing that treatment with CPAP lowers hospital readmission and improves survival in such patients. With regard to HFpEF, a small RCT showed improvement in left ventricular diastolic dysfunction with CPAP treatment of OSA (93). This is an important observation that should lead to a large randomized trial, as so far there has not been a therapy for HFpEF showing reverse cardiac remodeling.
Results of the SERVE-HF trial suggest that treatment of CSA with ASV could be harmful. However, the ASV used in the trial was an older-generation device with algorithm shortcomings (106). Another trial using state-of-the-art ASV is in progress. Nocturnal oxygen therapy and PNS are 2 treatment options devoid of increasing intrathoracic pressure. The pivotal study on PNS has been published (112). Time has passed for an RCT with oxygen therapy (108). These RCTs with PNS and oxygen should be powered to determine important cardiovascular outcomes.
Many studies implicate untreated SDB in the development and recurrence of AF; the field is ripe for the conduct of RCTs with well-phenotyped participants, followed closely for intervention adherence, to examine the effect of SDB treatment on AF outcomes.
PHENOTYPING
Pathogenesis underlying OSA is variable. With the recent push toward personalized medicine, individualizing therapy may be a viable approach for OSA if the exact mechanism(s) underlying disease can be defined and corrected. For example, in patients with dysfunction in the upper airway dilator muscles, if well-defined, hypoglossal nerve stimulation of the upper airway muscles could be effective. Elevated loop gain can be effectively minimized using oxygen or acetazolamide. If certain sedative/hypnotic agents could raise the arousal threshold, they would be predicted to stabilize breathing in carefully selected individuals with low arousal threshold. An elevated arousal threshold can allow the accumulation of respiratory stimuli (carbon dioxide, negative intrathoracic pressure) that activate upper airway dilator muscles, and thus stabilize breathing. In individuals in whom fluid accumulation around the upper airway is playing an important role in compromising the upper airway, diuretic therapy can be helpful to improve apnea, albeit to a modest extent. In certain individuals, multiple abnormalities likely underlie OSA pathogenesis, and thus, combination therapy may be required to eliminate SDB.
Acknowledgments
The authors thank Mr. Alex Mackey for his excellent technical contribution in preparing the figures.
Dr. Shahrokh Javaheri is a consultant for Respicardia, Philips, and Leva Nova Group. Dr. Barbe has received research grants from ResMed and Oxigen Salus; and has received a speaker’s fee from Philips. Dr. Khayat has received research grants through The Ohio State University from Philips Respironics. Dr. Mehra’s institution has received positive airway pressure machines and equipment from Philips Respironics and ResMed for research; and she has received royalties from Up to Date. Dr. Somers’ institution has received a gift from the Phillips Respironics Foundation for the study of sleep and cardiovascular disease; he was a consultant for Respicardia, ResMed, Sorin Inc., Biosense Webster, U-Health, Philips, Ronda Grey, Dane Garvin, and GlaxoSmithKline; and is working with Mayo Health Solutions and their industry partners on intellectual property related to sleep and cardiovascular disease.
ABBREVIATIONS AND ACRONYMS
- AF
atrial fibrillation
- AHI
apnea-hypopnea index
- CHD
coronary heart disease
- CPAP
continuous positive airway pressure
- CSA
central sleep apnea
- CVD
cardiovascular disease
- HF
heart failure
- HTN
hypertension
- OSA
obstructive sleep apnea
- TIA
transient ischemic attack
Footnotes
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. for the American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–14. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Javaheri S, Drager LF, Lorenzi-Filho G. Sleep and cardiovascular disease: present and future. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practices of Sleep Medicine. 6th. Philadelphia, PA: Elsevier; 2017. pp. 1222–8. [Google Scholar]
- 4.Javaheri S, Dempsey JA. Central sleep apnea. Compr Physiol. 2013;3:141–63. doi: 10.1002/cphy.c110057. [DOI] [PubMed] [Google Scholar]
- 5.Lyons OD, Bradley TD. Heart failure and sleep apnea. Can J Cardiol. 2015;31:898–908. doi: 10.1016/j.cjca.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 6.May AM, Blackwell T, Stone PH, et al. for the MrOS Sleep (Outcomes of Sleep Disorders in Older Men) Study Group Central sleep-disordered breathing predicts incident atrial fibrillation in older men. Am J Respir Crit Care Med. 2016;193:783–91. doi: 10.1164/rccm.201508-1523OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Javaheri S, Blackwell T, Ancoli-Israel S, et al. for the Osteoporotic Fractures in Men Study Research Group Sleep-disordered breathing and incident heart failure in older men. Am J Respir Crit Care Med. 2016;193:561–8. doi: 10.1164/rccm.201503-0536OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Javaheri S. Cardiovascular Diseases. In: Kryger MH, Avidan AY, Berry RB, editors. Atlas of Clinical Sleep Medicine. 2nd. Philadelphia, PA: Saunders; 2014. pp. 316–28. [Google Scholar]
- 9.Dempsey JA, Veasey SC, Morgan BJ, et al. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet. 2014;383:736–47. doi: 10.1016/S0140-6736(13)60734-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mendelson M, Lyons OD, Yadollahi A, et al. Effects of exercise training on sleep apnoea in patients with coronary artery disease: a randomised trial. Eur Respir J. 2016;48:142–50. doi: 10.1183/13993003.01897-2015. [DOI] [PubMed] [Google Scholar]
- 12.Somers V, Javaheri S. Cardiovascular effects of sleep-related breathing disorders. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practices of Sleep Medicine. 6th. Philadelphia, PA: Elsevier; 2017. pp. 1243–52. [Google Scholar]
- 13.Bauters F, Rietzschel ER, Hertegonne KB, et al. The link between obstructive sleep apnea and cardiovascular disease. Curr Atheroscler Rep. 2016;18:1. doi: 10.1007/s11883-015-0556-z. [DOI] [PubMed] [Google Scholar]
- 14.Baltzis D, Bakker JP, Patel SR, et al. Obstructive sleep apnea and vascular diseases. Compr Physiol. 2016;6:1519–28. doi: 10.1002/cphy.c150029. [DOI] [PubMed] [Google Scholar]
- 15.De Luca Canto G, Pachêco-Pereira C, Aydinoz S, et al. Diagnostic capability of biological markers in assessment of obstructive sleep apnea: a systematic review and meta-analysis. J Clin Sleep Med. 2015;11:27–36. doi: 10.5664/jcsm.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pak VM, Keenan BT, Jackson N, et al. Adhesion molecule increases in sleep apnea: beneficial effect of positive airway pressure and moderation by obesity. Int J Obes (Lond) 2015;39:472–9. doi: 10.1038/ijo.2014.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baessler A, Nadeem R, Harvey M, et al. Treatment for sleep apnea by continuous positive airway pressure improves levels of inflammatory markers—a meta-analysis. J Inflamm (Lond) 2013;10:13. doi: 10.1186/1476-9255-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kohler M, Stoewhas AC, Ayers L, et al. Effects of continuous positive airway plressure therapy withdrawal in patients with obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2011;184:1192–9. doi: 10.1164/rccm.201106-0964OC. [DOI] [PubMed] [Google Scholar]
- 19.Chirinos JA, Gurubhagavatula I, Teff K, et al. CPAP, weight loss, or both for obstructive sleep apnea. N Engl J Med. 2014;370:2265–75. doi: 10.1056/NEJMoa1306187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drager LF, Polotsky VY, O’Donnell CP, et al. Translational approaches to understanding metabolic dysfunction and cardiovascular consequences of obstructive sleep apnea. Am J Physiol Heart Circ Physiol. 2015;309:H1101–11. doi: 10.1152/ajpheart.00094.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pamidi S, Wroblewski K, Stepien M, et al. Eight hours of nightly continuous positive airway pressure treatment of obstructive sleep apnea improves glucose metabolism in patients with prediabetes. A randomized controlled trial. Am J Respir Crit Care Med. 2015;192:96–105. doi: 10.1164/rccm.201408-1564OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weinstock TG, Wang X, Rueschman M, et al. A controlled trial of CPAP therapy on metabolic control in individuals with impaired glucose tolerance and sleep apnea. Sleep. 2012;35:617–25B. doi: 10.5665/sleep.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salord N, Fortuna AM, Monasterio C, et al. A randomized controlled trial of continuous positive airway pressure on glucose tolerance in obese patients with obstructive sleep apnea. Sleep. 2016;39:35–41. doi: 10.5665/sleep.5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pamidi S, Tasali E. Continuous positive airway pressure for improving glycemic control in type 2 diabetes: where do we stand? Am J Respir Crit Care Med. 2016;194:397–9. doi: 10.1164/rccm.201604-0698ED. [DOI] [PubMed] [Google Scholar]
- 25.Bratton DJ, Stradling JR, Barbé F, et al. Effect of CPAP on blood pressure in patients with minimally symptomatic obstructive sleep apnoea: a meta-analysis using individual patient data from four randomised controlled trials. Thorax. 2014;69:1128–35. doi: 10.1136/thoraxjnl-2013-204993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bakker JP, Edwards BA, Gautam SP, et al. Blood pressure improvement with continuous positive airway pressure is independent of obstructive sleep apnea severity. J Clin Sleep Med. 2014;10:365–9. doi: 10.5664/jcsm.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alajmi M, Mulgrew AT, Fox J, et al. Impact of continuous positive airway pressure. therapy on blood pressure in patients with obstructive sleep apnea hypopnea: a meta-analysis of randomized controlled trials. Lung. 2007;185:67–72. doi: 10.1007/s00408-006-0117-x. [DOI] [PubMed] [Google Scholar]
- 28.Fava C, Dorigoni S, Dalle Vedove F, et al. Effect of CPAP on blood pressure in patients with OSA/hypopnea: a systematic review and meta-analysis. Chest. 2014;145:762–71. doi: 10.1378/chest.13-1115. [DOI] [PubMed] [Google Scholar]
- 29.Montesi SB, Edwards BA, Malhotra A, et al. The effect of continuous positive airway pressure treatment on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Clin Sleep Med. 2012;8:587–96. doi: 10.5664/jcsm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bazzano LA, Khan Z, Reynolds K, et al. Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea. Hypertension. 2007;50:417–23. doi: 10.1161/HYPERTENSIONAHA.106.085175. [DOI] [PubMed] [Google Scholar]
- 31.Haentjens P, Van Meerhaeghe A, Moscariello A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials. Arch Intern Med. 2007;167:757–64. doi: 10.1001/archinte.167.8.757. [DOI] [PubMed] [Google Scholar]
- 32.Mo L, He QY. Effect of long-term continuous positive airway pressure ventilation on blood pressure in patients with obstructive sleep apnea hypopnea syndrome: a meta-analysis of clinical trials. Zhonghua Yi Xue Za Zhi. 2007;87:1177–80. [PubMed] [Google Scholar]
- 33.Liu L, Cao Q, Guo Z, et al. Continuous positive airway pressure in patients with obstructive sleep apnea and resistant hypertension: a meta-analysis of randomized controlled trials. J Clin Hypertens (Greenwich) 2016;18:153–8. doi: 10.1111/jch.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muxfeldt ES, Margallo V, Costa LM, et al. Effects of continuous positive airway pressure treatment on clinic and ambulatory blood pressures in patients with obstructive sleep apnea and resistant hypertension: a randomized controlled trial. Hypertension. 2015;65:736–42. doi: 10.1161/HYPERTENSIONAHA.114.04852. [DOI] [PubMed] [Google Scholar]
- 35.Iftikhar IH, Valentine CW, Bittencourt LR, et al. Effects of continuous positive airway pressure on blood pressure in patients with resistant hypertension and obstructive sleep apnea: a meta-analysis. J Hypertens. 2014;32:2341–50. doi: 10.1097/HJH.0000000000000372. discussion 2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Oliveira AC, Martinez D, Massierer D, et al. The antihypertensive effect of positive airway pressure on resistant hypertension of patients with obstructive sleep apnea: a randomized, double-blind, clinical trial. Am J Respir Crit Care Med. 2014;190:345–7. doi: 10.1164/rccm.201403-0479LE. [DOI] [PubMed] [Google Scholar]
- 37.Martínez-García MA, Capote F, Campos-Rodríguez F, et al. for the Spanish Sleep Network Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: The HIPARCO randomized clinical trial. JAMA. 2013;310:2407–15. doi: 10.1001/jama.2013.281250. [DOI] [PubMed] [Google Scholar]
- 38.Pedrosa RP, Drager LF, de Paula LK, et al. Effects of OSA treatment on BP in patients with resistant hypertension: a randomized trial. Chest. 2013;144:1487–94. doi: 10.1378/chest.13-0085. [DOI] [PubMed] [Google Scholar]
- 39.Lozano L, Tovar JL, Sampol G, et al. Continuous positive airway pressure treatment in sleep apnea patients with resistant hypertension: a randomized, controlled trial. J Hypertens. 2010;28:2161–8. doi: 10.1097/HJH.0b013e32833b9c63. [DOI] [PubMed] [Google Scholar]
- 40.Sánchez-de-la-Torre M, Khalyfa A, Sánchez-de-la-Torre A, et al. for the Spanish Sleep Network Precision medicine in patients with resistant hypertension and obstructive sleep apnea: blood pressure response to continuous positive airway pressure treatment. J Am Coll Cardiol. 2015;66:1023–32. doi: 10.1016/j.jacc.2015.06.1315. [DOI] [PubMed] [Google Scholar]
- 41.Thunström E, Manhem K, Rosengren A, et al. Blood pressure response to losartan and continuous positive airway pressure in hypertension and obstructive sleep apnea. Am J Respir Crit Care Med. 2016;193:310–20. doi: 10.1164/rccm.201505-0998OC. [DOI] [PubMed] [Google Scholar]
- 42.Javaheri S, Javaheri S, Javaheri A. Sleep apnea, heart failure and pulmonary hypertension. Curr Heart Fail Rep. 2013;10:315–20. doi: 10.1007/s11897-013-0167-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nieto FJ, Young T, Peppard PE, et al. Systemic and pulmonary hypertension in obstructive sleep apnea. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practices of Sleep Medicine. 6th. Philadelphia, PA: Elsevier; 2017. pp. 1253–63. [Google Scholar]
- 44.Arias MA, García-Río F, Alonso-Fernández A, et al. Pulmonary hypertension in obstructive sleep apnoea: effects of continuous positive airway pressure: a randomized, controlled cross-over study. Eur Heart J. 2006;27:1106–13. doi: 10.1093/eurheartj/ehi807. [DOI] [PubMed] [Google Scholar]
- 45.McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association. J Am Coll Cardiol. 2009;53:1573–619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 46.Lyons OW, Ryan CM. Sleep apnea and stroke. Can J Cardiol. 2015;31:918–27. doi: 10.1016/j.cjca.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 47.Parra O, Sánchez-Armengol Á, Capote F, et al. Efficacy of continuous positive airway pressure treatment on 5-year survival in patients with ischaemic stroke and obstructive sleep apnea: a randomized controlled trial. J Sleep Res. 2015;24:47–53. doi: 10.1111/jsr.12181. [DOI] [PubMed] [Google Scholar]
- 48.Kim Y, Koo YS, Lee HY, et al. Can continuous positive airway pressure reduce the risk of stroke in obstructive sleep apnea patients? A systematic review and meta-analysis PLoS One. 2016;11:e0146317. doi: 10.1371/journal.pone.0146317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McEvoy RD, Antic NA, Heeley E, et al. for the SAVE Investigators and Coordinators CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375:919–31. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 50.Kernan WN, Ovbiagele B, Black HR, et al. for the American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for health-care professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160–236. doi: 10.1161/STR.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 51.Craig S, Pepperell JC, Kohler M, et al. Continuous positive airway pressure treatment for obstructive sleep apnoea reduces resting heart rate but does not affect dysrhythmias: a randomised controlled trial. J Sleep Res. 2009;18:329–36. doi: 10.1111/j.1365-2869.2008.00726.x. [DOI] [PubMed] [Google Scholar]
- 52.Ryan CM, Usui K, Floras JS, et al. Effect of continuous positive airway pressure on ventricular ectopy in heart failure patients with obstructive sleep apnoea. Thorax. 2005;60:781–5. doi: 10.1136/thx.2005.040972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fein AS, Shvilkin A, Shah D, et al. Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol. 2013;62:300–5. doi: 10.1016/j.jacc.2013.03.052. [DOI] [PubMed] [Google Scholar]
- 54.Neilan TG, Farhad H, Dodson JA, et al. Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. J Am Heart Assoc. 2013;2:e000421. doi: 10.1161/JAHA.113.000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Naruse Y, Tada H, Satoh M, et al. Concomitant obstructive sleep apnea increases the recurrence of atrial fibrillation following radiofrequency catheter ablation of atrial fibrillation: clinical impact of continuous positive airway pressure therapy. Heart Rhythm. 2013;10:331–7. doi: 10.1016/j.hrthm.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 56.Holmqvist F, Guan N, Zhu Z, et al. for the ORBIT-AF Investigators Impact of obstructive sleep apnea and continuous positive airway pressure therapy on outcomes in patients with atrial fibrillation-results from the outcomes registry for better informed treatment of atrial fibrillation (ORBIT-AF) Am Heart J. 2015;169:647–54.e2. doi: 10.1016/j.ahj.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 57.Qureshi WT, Nasir UB, Alqalyoobi S, et al. Meta-analysis of continuous positive airway pressure as a therapy of atrial fibrillation in obstructive sleep apnea. Am J Cardiol. 2015;116:1767–73. doi: 10.1016/j.amjcard.2015.08.046. [DOI] [PubMed] [Google Scholar]
- 58.Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Heart Rhythm. 2012;9:632–96.e21. doi: 10.1016/j.hrthm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 59.Peker Y, Franklin KA, Hedner J. Coronary artery disease and obstructive sleep apnea. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practices of Sleep Medicine. 6th. Philadelphia, PA: Elsevier; 2017. pp. 1264–70. [Google Scholar]
- 60.Wu X, Lv S, Yu X, et al. Treatment of OSA reduces the risk of repeat revascularization after percutaneous coronary intervention. Chest. 2015;147:708–18. doi: 10.1378/chest.14-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 62.Campos-Rodriguez F, Martinez-Garcia MA, de la Cruz-Moron I, et al. Cardiovascular mortality in women with obstructive sleep apnea with or without continuous positive airway pressure treatment: a cohort study. Ann Intern Med. 2012;156:115–22. doi: 10.7326/0003-4819-156-2-201201170-00006. [DOI] [PubMed] [Google Scholar]
- 63.Barbé F, Durán-Cantolla J, Sánchez-de-la-Torre M, et al. for the Spanish Sleep and Breathing Network Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307:2161–8. doi: 10.1001/jama.2012.4366. [DOI] [PubMed] [Google Scholar]
- 64.Peker Y, Glantz H, Eulenburg C, et al. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea: the RICCADSA randomized controlled trial. Am J Respir Crit Care Med. 2016;194:613–20. doi: 10.1164/rccm.201601-0088OC. [DOI] [PubMed] [Google Scholar]
- 65.Esquinas C, Sánchez-de-la Torre M, Aldomá A, et al. for the Spanish Sleep Network Rationale and methodology of the impact of continuous positive airway pressure on patients with ACS and nonsleepy OSA: the ISAACC trial. Clin Cardiol. 2013;36:495–501. doi: 10.1002/clc.22166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kuniyoshi FH, Garcia-Touchard A, Gami AS, et al. Day-night variation of acute myocardial infarction in obstructive sleep apnea. J Am Coll Cardiol. 2008;52:343–6. doi: 10.1016/j.jacc.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gami AS, Olson EJ, Shen WK, et al. Obstructive sleep apnea and the risk of sudden cardiac death: a longitudinal study of 10,701 adults. J Am Coll Cardiol. 2013;62:610–6. doi: 10.1016/j.jacc.2013.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gami AS, Howard DE, Olson EJ, et al. Day-night pattern of sudden cardiac death in obstructive sleep apnea. N Engl J Med. 2005;352:1206–14. doi: 10.1056/NEJMoa041832. [DOI] [PubMed] [Google Scholar]
- 69.Iftikhar IH, Kline CE, Youngstedt SD. Effects of exercise training on sleep apnea: a meta-analysis. Lung. 2014;192:175–84. doi: 10.1007/s00408-013-9511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Redolfi S, Bettinzoli M, Venturoli N, et al. Attenuation of obstructive sleep apnea and overnight rostral fluid shift by physical activity. Am J Respir Crit Care Med. 2015;191:856–8. doi: 10.1164/rccm.201412-2192LE. [DOI] [PubMed] [Google Scholar]
- 71.Camacho M, Certal V, Abdullatif J, et al. Myofunctional therapy to treat obstructive sleep apnea: a systematic review and meta-analysis. Sleep. 2015;38:669–75. doi: 10.5665/sleep.4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tachikawa R, Ikeda K, Minami T, et al. Changes in energy metabolism after continuous positive airway pressure for obstructive sleep apnea. Am J Respir Crit Care Med. 2016;194:729–38. doi: 10.1164/rccm.201511-2314OC. [DOI] [PubMed] [Google Scholar]
- 73.Javaheri S. Heart failure. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practices of Sleep Medicine. 6th. Philadelphia, PA: Elsevier; 2017. pp. 1271–85. [Google Scholar]
- 74.Lanfranchi PA, Somers VK, Braghiroli A, et al. Central sleep apnea in left ventricular dysfunction: prevalence and implications for arrhythmic risk. Circulation. 2003;107:727–32. doi: 10.1161/01.cir.0000049641.11675.ee. [DOI] [PubMed] [Google Scholar]
- 75.Wachter R, Lüthje L, Klemmstein D, et al. Impact of obstructive sleep apnoea on diastolic function. Eur Respir J. 2013;41:376–83. doi: 10.1183/09031936.00218211. [DOI] [PubMed] [Google Scholar]
- 76.Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: The Sleep Heart Health Study. Circulation. 2010;122:352–60. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roca GQ, Redline S, Claggett B, et al. Sex-specific association of sleep apnea severity with subclinical myocardial injury, ventricular hypertrophy, and heart failure risk in a community-dwelling cohort: The Atherosclerosis Risk in Communities–Sleep Heart Health Study. Circulation. 2015;132:1329–37. doi: 10.1161/CIRCULATIONAHA.115.016985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oldenburg O, Wellmann B, Buchholz A, et al. Nocturnal hypoxaemia is associated with increased mortality in stable heart failure patients. Eur Heart J. 2016;37:1695–703. doi: 10.1093/eurheartj/ehv624. [DOI] [PubMed] [Google Scholar]
- 79.Bitter T, Faber L, Hering D, et al. Sleep-disordered breathing in heart failure with normal left ventricular ejection fraction. Eur J Heart Fail. 2009;11:602–8. doi: 10.1093/eurjhf/hfp057. [DOI] [PubMed] [Google Scholar]
- 80.Herrscher TE, Akre H, Øverland B, et al. High prevalence of sleep apnea in heart failure outpatients: even in patients with preserved systolic function. J Card Fail. 2011;17:420–5. doi: 10.1016/j.cardfail.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 81.Khayat R, Jarjoura D, Porter K, et al. Sleep disordered breathing and post-discharge mortality in patients with acute heart failure. Eur Heart J. 2015;36:1463–9. doi: 10.1093/eurheartj/ehu522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chenuel BJ, Smith CA, Skatrud JB, et al. Increased propensity for apnea in response to acute elevations in left atrial pressure during sleep in the dog. J Appl Physiol (1985) 2006;101:76–83. doi: 10.1152/japplphysiol.01617.2005. [DOI] [PubMed] [Google Scholar]
- 83.Gandhi PU, Szymonifka J, Motiwala SR, et al. Characterization and prediction of adverse events from intensive chronic heart failure management and effect on quality of life: results from the Pro-B-Type Natriuretic Peptide Outpatient-Tailored Chronic Heart Failure Therapy (PROTECT) study. J Card Fail. 2015;21:9–15. doi: 10.1016/j.cardfail.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 84.Lamba J, Simpson CS, Redfearn DP, et al. Cardiac resynchronization therapy for the treatment of sleep apnoea: a meta-analysis. Europace. 2011;13:1174–9. doi: 10.1093/europace/eur128. [DOI] [PubMed] [Google Scholar]
- 85.Ueno LM, Drager LF, Rodrigues AC, et al. Effects of exercise training in patients with chronic heart failure and sleep apnea. Sleep. 2009;32:637–47. doi: 10.1093/sleep/32.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Javaheri S. Effects of continuous positive airway pressure on sleep apnea and ventricular irritability in patients with heart failure. Circulation. 2000;101:392–7. doi: 10.1161/01.cir.101.4.392. [DOI] [PubMed] [Google Scholar]
- 87.Usui K, Bradley TD, Spaak J, et al. Inhibition of awake sympathetic nerve activity of heart failure patients with obstructive sleep apnea by nocturnal continuous positive airway pressure. J Am Coll Cardiol. 2005;45:2008–11. doi: 10.1016/j.jacc.2004.12.080. [DOI] [PubMed] [Google Scholar]
- 88.Hall AB, Ziadi MC, Leech JA, et al. Effects of short-term continuous positive airway pressure on myocardial sympathetic nerve function and energetics in patients with heart failure and obstructive sleep apnea: a randomized study. Circulation. 2014;130:892–901. doi: 10.1161/CIRCULATIONAHA.113.005893. [DOI] [PubMed] [Google Scholar]
- 89.Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348:1233–41. doi: 10.1056/NEJMoa022479. [DOI] [PubMed] [Google Scholar]
- 90.Mansfield DR, Gollogly C, Kaye DM. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am J Respir Crit Care Med. 2004;169:361–6. doi: 10.1164/rccm.200306-752OC. [DOI] [PubMed] [Google Scholar]
- 91.Javaheri S, Brown LK, Randerath WJ. Positive airway pressure therapy with adaptive servo-ventilation: part 2. Chest. 2014;146:858–68. doi: 10.1378/chest.13-1776. [DOI] [PubMed] [Google Scholar]
- 92.Javaheri S, Caref EB, Chen E, et al. Sleep apnea testing and outcomes in a large cohort of Medicare beneficiaries with newly diagnosed heart failure. Am J Respir Crit Care Med. 2011;183:539–46. doi: 10.1164/rccm.201003-0406OC. [DOI] [PubMed] [Google Scholar]
- 93.Arias MA, García-Río F, Alonso-Fernández A, et al. Obstructive sleep apnea syndrome affects left ventricular diastolic function: effects of nasal continuous positive airway pressure in men. Circulation. 2005;112:375–83. doi: 10.1161/CIRCULATIONAHA.104.501841. [DOI] [PubMed] [Google Scholar]
- 94.Artz M, Floras JS, Logan AG, et al. for the CANPAP Investigators Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP) Circulation. 2007;115:3173–80. doi: 10.1161/CIRCULATIONAHA.106.683482. [DOI] [PubMed] [Google Scholar]
- 95.Javaheri S, Abraham WT, Brown C, et al. Prevalence of obstructive sleep apnea and periodic limb movement in 45 subjects with heart transplantation. Eur Heart J. 2004;25:260–6. doi: 10.1016/j.ehj.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 96.Costanzo MR, Khayat R, Ponikowski P, et al. Mechanisms and clinical consequences of untreated central sleep apnea in heart failure. J Am Coll Cardiol. 2015;65:72–84. doi: 10.1016/j.jacc.2014.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Naughton MT, Benard DC, Liu PP, et al. Effects of nasal CPAP on sympathetic activity in patients with heart failure and central sleep apnea. Am J Respir Crit Care Med. 1995;152:473–9. doi: 10.1164/ajrccm.152.2.7633695. [DOI] [PubMed] [Google Scholar]
- 98.van de Borne P, Oren R, Abouassaly C, et al. Effect of Cheyne-Stokes respiration on muscle sympathetic nerve activity in severe congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1998;81:432–6. doi: 10.1016/s0002-9149(97)00936-3. [DOI] [PubMed] [Google Scholar]
- 99.Andreas S, Bingeli C, Mohacsi P, et al. Nasal oxygen and muscle sympathetic nerve activity in heart failure. Chest. 2003;123:366–71. doi: 10.1378/chest.123.2.366. [DOI] [PubMed] [Google Scholar]
- 100.Naughton MT, Benard DC, Rutherford R, et al. Effect of continuous positive airway pressure on central sleep apnea and nocturnal PCO2 in heart failure. Am J Respir Crit Care Med. 1994;150:1598–604. doi: 10.1164/ajrccm.150.6.7952621. [DOI] [PubMed] [Google Scholar]
- 101.Granton JT, Naughton MT, Benard DC, et al. CPAP improves inspiratory muscle strength in patients with heart failure and central sleep apnea. Am J Respir Crit Care Med. 1996;153:277–82. doi: 10.1164/ajrccm.153.1.8542129. [DOI] [PubMed] [Google Scholar]
- 102.Pepperell JC, Maskell NA, Jones DR, et al. A randomized controlled trial of adaptive ventilation for Cheyne-Stokes breathing in heart failure. Am J Respir Crit Care Med. 2003;168:1109–14. doi: 10.1164/rccm.200212-1476OC. [DOI] [PubMed] [Google Scholar]
- 103.Staniforth AD, Kinnear WJ, Starling R, et al. Effect of oxygen on sleep quality, cognitive function and sympathetic activity in patients with chronic heart failure and Cheyne-Stokes respiration. Eur Heart J. 1998;19:922–8. doi: 10.1053/euhj.1997.0861. [DOI] [PubMed] [Google Scholar]
- 104.Naughton MT. Cheyne-stokes respiration: friend or foe? Thorax. 2012;67:357–60. doi: 10.1136/thoraxjnl-2011-200927. [DOI] [PubMed] [Google Scholar]
- 105.Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373:1095–105. doi: 10.1056/NEJMoa1506459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Javaheri S, Brown LK, Randerath WJ. Positive airway pressure therapy with adaptive servoventilation: part 1: operational algorithms. Chest. 2014;146:514–23. doi: 10.1378/chest.13-1776. [DOI] [PubMed] [Google Scholar]
- 107.Javaheri S, Brown LK, Randerath W, et al. SERVE-HF: more questions than answers. Chest. 2016;149:900–4. doi: 10.1016/j.chest.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 108.Javaheri S. Pembrey’s dream: the time has come for a long-term trial of nocturnal supplemental nasal oxygen to treat central sleep apnea in congestive heart failure. Chest. 2003;123:322–5. doi: 10.1378/chest.123.2.322. [DOI] [PubMed] [Google Scholar]
- 109.Bodez D, Guellich A, Kharoubi M, et al. Prevalence, severity, and prognostic value of sleep apnea syndromes in cardiac amyloidosis. Sleep. 2016;39:1333–41. doi: 10.5665/sleep.5958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Haque WA, Boehmer J, Clemson BS, et al. Hemodynamic effects of supplemental oxygen administration in congestive heart failure. J Am Coll Cardiol. 1996;27:353–7. doi: 10.1016/0735-1097(95)00474-2. [DOI] [PubMed] [Google Scholar]
- 111.Ponikowski P, Javaheri S, Michalkiewicz D, et al. Transvenous phrenic nerve stimulation for the treatment of central sleep apnoea in heart failure. Eur Heart J. 2012;33:889–94. doi: 10.1093/eurheartj/ehr298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Costanzo MR, Ponikowski P, Javaheri S, et al. for the remedé System Pivotal Trial Study Group Randomised controlled trial of transvenous neurostimulation for central sleep apnoea. Lancet. 2016;388:974–82. doi: 10.1016/S0140-6736(16)30961-8. [DOI] [PubMed] [Google Scholar]
- 113.Mokhlesi B, Finn LA, Hagen EW, et al. Obstructive sleep apnea during REM sleep and hypertension. Results of the Wisconsin Sleep Cohort. Am J Respir Crit Care Med. 2014;190:1158–67. doi: 10.1164/rccm.201406-1136OC. [DOI] [PMC free article] [PubMed] [Google Scholar]


