Introduction
While mantle cell lymphoma (MCL) is characterized by a nearly 80% male predominance, the biological underpinnings of this observation are unknown.[1] Sex-steroid receptors are widely expressed in the hematopoietic system and directly influence hematopoietic differentiation and activity: androgen exposure down-regulates NK cells/macrophages in vitro, while estrogen exposure enhances differentiation of antigen presenting cells and expansion of regulatory T-cells [2–5] Sex-related differences in receptor expression include higher AR expression in leukocytes and macrophages from male donors [6–8]. Notably, disease-specific differences include hypermethylation of AR in follicular and some diffuse large B cell lymphomas (DLBCL), whereas in MCL AR is unmethylated allowing gene transcription [9–13].
We hypothesized that the male-biased incidence of MCL may reflect sex-related differences in AR signaling during MCL lymphomagenesis and that AR may represent a unique therapeutic target. We evaluated AR expression in MCL cell lines and human specimens, and determined whether the potent AR antagonist enzalutamide inhibited MCL proliferation.
Methods
Cell lines and human MCL specimens
All studies were approved by the institutional review board of the Hutchinson Center. Prostate cancer (PCa) and MCL cell lines were obtained from American Type Culture Collection (ATCC, Rockville, Maryland, USA). Buffy coat RNA was isolated from three MCL patients with circulating tumor cells for analysis of AR transcript expression. Formalin fixed samples from twelve archival MCL tumor specimens were obtained for AR immunohistochemistry (IHC).
RNA Isolation, quantitative RT-PCR and immunohistochemistry
RNA isolation, quantitative real time PCR (qRT-PCR), and IHC staining for AR (clone C-19, Santa Cruz Biotechnology, Dallas, TX) were carried out as previously described [14, 15]. Fold changes were determined by the 2−ΔΔCT method [16].
Proliferation assays
Cells were brought up in serum free media (DMEM/F12 with 5% charcoal-stripped FBS) and incubated under standard culture conditions (37°C with 5% CO2) for 24 hours before being plated in triplicate in serum free media at 0.25×106 cells/ml with the AR antagonist enzalutamide (10uM), the synthetic AR agonist R1881 (1nM) or the combination for 96 hours. Proliferation was quantified using the CyQUANT Assay Kit (Thermo-Fisher). Confirmatory experiments were repeated over a dose range of enzalutamide, and the influence of 10uM enzalutamide was evaluated over a dose range of R1881 using the CellTiter 96 Aqueous Cell Proliferation Assay Kit (Promega).
Statistical analysis
Unpaired t tests were used to compare AR expression in MCL vs non-MCL or PCa cell lines, and to compare mean levels of proliferation in cell lines treated with enzalutamide or R1881. Correlation of AR and PSA expression was assessed by Spearman rank correlation. P-value <0.05 was considered significant.
Results and Discussion
Androgen receptor (AR) expression in MCL cell lines and human tumors
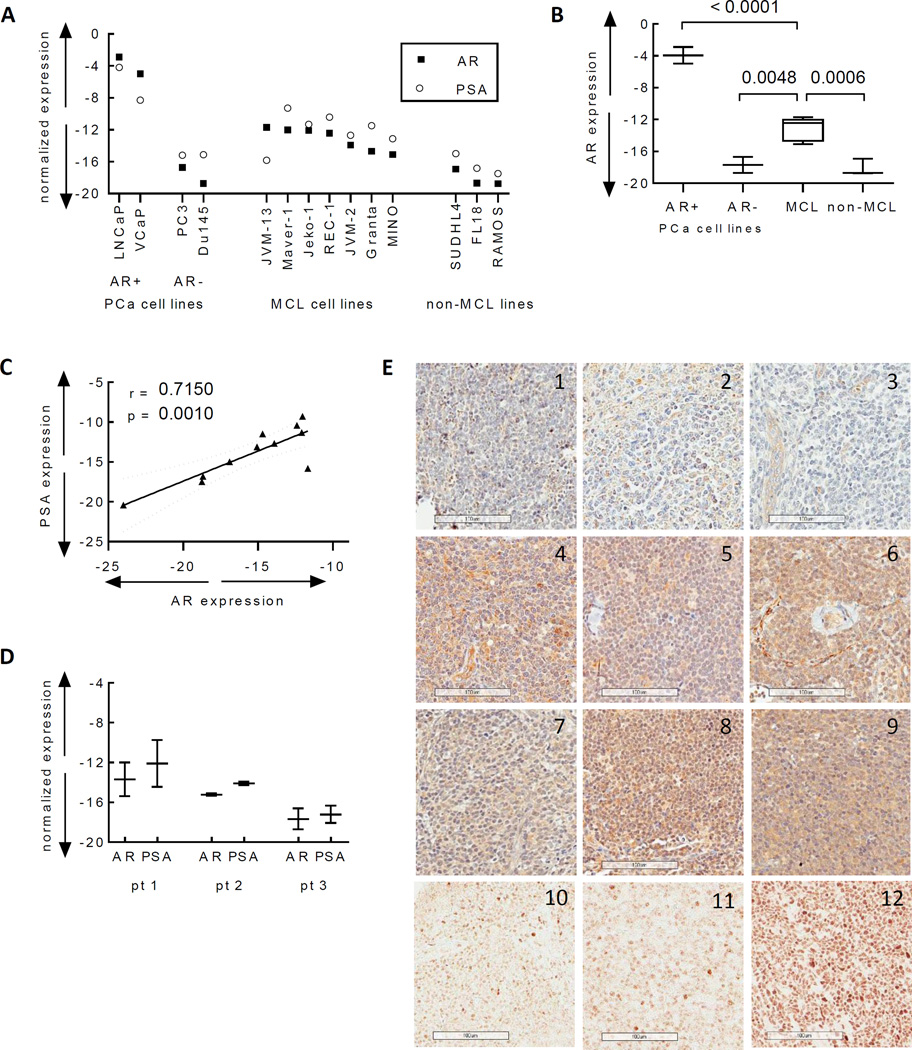
We quantified AR expression in seven MCL and three non-MCL B-NHL lines. The median difference in cycle threshold (dCT) for AR (normalized to the housekeeping gene RPL13A) was −12 in MCL lines vs −18 in non-MCL lines (p=0.006, Figure 1A–1B), representing a 64-fold higher level. AR in MCL lines was considerably lower than in AR-positive PCa lines (LNCaP and VCaP, p=<0.0001), yet clearly higher than AR-negative PCa cells (PC3 and DU145, median dCT −17, p= 0.0048).
Figure 1. Expression of the androgen receptor (AR) in MCL cell lines and tumors.
(A) Transcript expression of AR and the androgen-regulated gene PSA were measured by quantitative RT-PCR in each of the indicated prostate cancer (PCa), MCL, and non-MCL lymphoma cell lines (SUDHL4 (DLBCL), FL18 (follicular), and Ramos (Burkitt’s)). Data are represented as the difference in cycle thresholds (dCT) of AR or PSA vs the housekeeping gene RPL13A. (B) Box and whisker plot of the median AR expression in the AR positive and AR negative PCa cells compared to the MCL and non-MCL lymphoma cell lines. Horizontal lines indicate median values; white boxes denote the 75th (upper margin) and 25th percentiles (lower margin). Upper and lower bars indicate minimum and maximum values, respectively. Unpaired t tests were used to assess significance. (C) Spearman rank correlation between transcript levels of AR and PSA in the MCL and non-MCL lymphoma cell lines. (D) Box and whisker plot of the median AR and PSA expression in frozen tumor cells from three MCL patients. (E) Expression of AR by IHC in formalin fixed tumor biopsies from male and female (cases 1, 3 and 9) MCL patients. Samples 10–12 were stained without the hematoxylin counterstain to better appreciate nuclear staining. Images acquired with an Aperio Scanscope AT Turbo microscope using a Basler L301KC camera at 20× magnification (resolution 0.50 um/pixel). Scale bar = 100um.
Expression of the canonical AR-regulated target gene, PSA (Figure 1A) and the tight correlation of PSA with AR (r=.715, p=0.001, Figure 1C) suggest AR expression within these MCL lines is capable of driving transactivation of target genes.
We next interrogated a limited number of frozen patient-derived MCL tumor cells. Two of three patients had AR and PSA levels similar to the MCL lines, while one was more similar to AR-negative PCa and non-MCL NHL lines (Figure 1D). As before, PSA and AR levels tracked together. A range of AR expression was also observed in biopsies from twelve patients (clinical characteristics summarized in Supplementary Table S1), ranging from no staining (patients 1–3), to predominantly cytoplasmic AR staining of uncertain significance (patients 4–7), to varying degrees of scattered nuclear AR staining (patients 8–12) (Figure 1E). There was no consistent association between AR expression and patient sex.
Impact of androgen-axis blockade on MCL proliferation
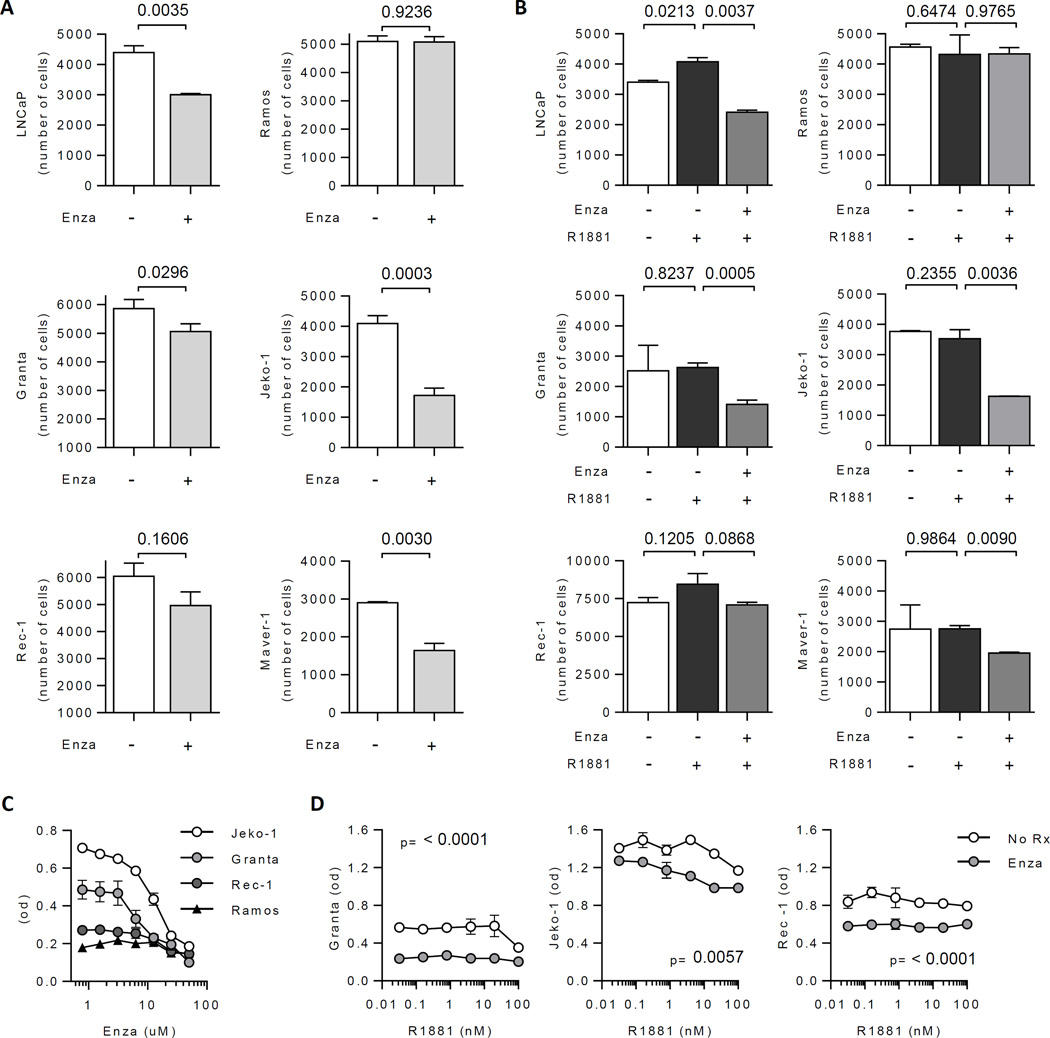
Treatment of four AR-positive MCL lines with the potent anti-androgen enzalutamide (10uM, similar to serum levels achieved with therapeutic dosing) showed statistically significant suppression in three lines (Grants, Jeko-1, Maver-1) with a trend toward significance in the fourth (Rec-1; p=0.16), while AR-negative Ramos cells showed no change (p=0.92) (Figure 2A)[17]. The AR agonist R1881 did not stimulate MCL growth (in contrast to stimulation of LNCaP growth), suggesting a possible ligand-independent function of AR in these cell lines, as has been reported in PCa cells [18].
Figure 2. Response of MCL cell lines to androgen receptor inhibition.
(A) Proliferation of the LNCaP prostate cancer cell line, the Ramos Burkitt’s cell line, and the Granta, Jeko-1, Rec-1 and Maver-1 MCL cell lines plated in charcoal stripped serum for 96 hours in the absence or presence of enzalutamide (10uM). Data are representative of 3 independent experiments. (B) Proliferation of the same cell lines in the presence of the synthetic androgen agonist R1881 (1nM) or the combination of R1881 and enzalutamide (10uM). (C) Proliferation of the indicated cell lines over a dose response of enzalutamide. (D) Proliferation of the Granta, Jeko-1 and Rec-1 MCL cell lines over a dose response of R1881 in the absence or presence of enzalutamide (10uM). Unpaired t tests were used to assess significance.
Notably, concurrent presence of R1881 did not dampen the suppressive effect of enzalutamide on MCL proliferation (Figure 2B), suggesting that circulating androgen levels in male patients (12–25nM for testosterone) would not abrogate activity of enzalutamide in vivo. Importantly, most MCL cells lines (but not Ramos) showed a dose-dependent response to AR inhibition (Figure 2C) that was maintained over a wide androgen concentration (0.1–100uM, Figure 2D). Of the four MCL lines evaluated, Jeko-1 is derived from a female donor and demonstrated a robust response to AR inhibition, suggesting possible anti-tumor efficacy in both male and female patients.
This first report of AR activity in MCL is hypothesis generating. Many questions remain regarding the potential role of AR in MCL pathogenesis and AR-associated inhibition of MCL proliferation. The ectopic expression of cyclin D1 in MCL is unlikely to be involved in driving AR activity, as the primary Cyclin D isoform in MCL tumors is D1a which negatively regulates AR activity [19].
The behavior of established MCL cell lines may not accurately reflect developmental pathobiology. Thus, while proliferation of MCL lines in vitro was not stimulated by ligand-mediated AR activation this does not preclude an influence of circulating androgens on tumor pathogenesis in male patients. Conversely, while gender-related differences favoring AR activity may underlie the male predominance of this disease, this does not preclude the possibility that AR activity plays a role in female MCL patients, as suggested by sensitivity of the female-derived Jeko-1 cells to enzalutamide.
Castration resistant prostate tumors express AR splice variants (ARVs) which lack the ligand binding domain (LBD), rendering them resistant to enzalutamide which binds AR in the LBD [20, 21]. We did not detect transcripts encoding the common AR variant 7 (ARv7) in MCL cell lines or in circulating MCL tumor cells (data not shown), and the MCL lines were clearly responsive to enzalutamide. However, ARVs in human MCL tumors could associate with a lack of response or development of resistance to enzalutamide.
Like other NHL, MCL cell lines and human tumors express both alpha and beta estrogen receptors (Figure S1)[7, 22, 23]. While inhibition of MCL proliferation by enzalutamide may occur via direct AR inhibition, it may also occur indirectly via inhibition of AR cross-talk with other steroid receptors such as ER, as observed in breast cancer models [24, 25].
Our studies show that AR is expressed in MCL cells and that AR-axis blockade yields reproducible suppression of MCL proliferation in vitro. The range of AR expression in human tumor samples suggests the hypothesis that targeting the AR-axis may have clinical efficacy in a subset of MCL patients, leading to initiation of an NCCN-supported pilot study of enzalutamide in patients with relapsed or refractory MCL (NCT02489123). Should enzalutamide show efficacy, understanding mechanisms of response and resistance will be critical for optimizing patient selection and designing combination treatment strategies.
Supplementary Material
Highlights.
Androgen receptor (AR) expression is increased in mantle cell lymphoma (MCL)
AR-axis blockade with enzalutamide results in suppression of MCL proliferation
AR-axis blockade may be a novel treatment strategy in MCL; clinical trials are ongoing
Acknowledgments
Grant Funding
K24 CA184039-02, Listwin Family Foundation and Gregory Pilot Fund, Lymphoma Research Foundation Mantle Cell Lymphoma Initiative, T-32 (CA009515), and donations from Don and Debbie Hunkins, Mary Aileen Wright Memorial Foundation, and Frank and Betty Vandermeer.
AKG: Consulting/honoraria: Sanofi, Seattle Genetics, Gilead, Janssen. Research Funding: Gilead, Janssen, Merck, Teva, Pfizer, BMS, Takeda
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship Contributions
- Conception and design: EM, AKG
- Development of methodology: EM, SM, SF, AKG
- Acquisition of data: EM, SM, SF, AZ, KLE
- Analysis and interpretation of data: PSM, EM, KLE, AKG
- Writing, review and/or revision of the manuscript: All
- Administrative, technical, or material support: EM, OWP, AKG
- Study supervision: EM, AKG
Disclosure of Conflicts of Interest
EM: Nothing to disclose
PM: Nothing to disclose
SM: Nothing to disclose
SF: Nothing to disclose
AZ: Nothing to disclose
KLE: Nothing to disclose
OWP: Nothing to disclose
References
- 1.Zhou Y, et al. Incidence trends of mantle cell lymphoma in the United States between 1992 and 2004. Cancer. 2008;113(4):791–798. doi: 10.1002/cncr.23608. [DOI] [PubMed] [Google Scholar]
- 2.Danel L, et al. Distribution of androgen and estrogen receptors among lymphoid and haemopoietic cell lines. Leuk Res. 1985;9(11):1373–1378. doi: 10.1016/0145-2126(85)90125-0. [DOI] [PubMed] [Google Scholar]
- 3.Khetawat G, et al. Human megakaryocytes and platelets contain the estrogen receptor beta and androgen receptor (AR): testosterone regulates AR expression. Blood. 2000;95(7):2289–2296. [PubMed] [Google Scholar]
- 4.Kovats S. Estrogen receptors regulate an inflammatory pathway of dendritic cell differentiation: mechanisms and implications for immunity. Horm Behav. 2012;62(3):254–262. doi: 10.1016/j.yhbeh.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein SL. Immune cells have sex and so should journal articles. Endocrinology. 2012;153(6):2544–2550. doi: 10.1210/en.2011-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sader MA, et al. Androgen receptor gene expression in leucocytes is hormonally regulated: implications for gender differences in disease pathogenesis. Clin Endocrinol (Oxf) 2005;62(1):56–63. doi: 10.1111/j.1365-2265.2004.02173.x. [DOI] [PubMed] [Google Scholar]
- 7.Shim GJ, et al. Differential expression of oestrogen receptors in human secondary lymphoid tissues. J Pathol. 2006;208(3):408–414. doi: 10.1002/path.1883. [DOI] [PubMed] [Google Scholar]
- 8.Samy TS, et al. Androgen and estrogen receptors in splenic T lymphocytes: effects of flutamide and trauma-hemorrhage. Shock. 2000;14(4):465–470. doi: 10.1097/00024382-200014040-00008. [DOI] [PubMed] [Google Scholar]
- 9.Liu ZJ, et al. Androgen receptor CpG island methylation status in human leukemia cancer cells. Cancer Invest. 2009;27(2):156–162. doi: 10.1080/07357900802208590. [DOI] [PubMed] [Google Scholar]
- 10.McDonald HL, et al. Involvement of the X chromosome in non-Hodgkin lymphoma. Genes Chromosomes Cancer. 2000;28(3):246–257. [PubMed] [Google Scholar]
- 11.Danel L, et al. Characterization of a specific androgen receptor in non-Hodgkin's lymphoma cells. J Steroid Biochem. 1984;21(4):421–426. doi: 10.1016/0022-4731(84)90305-4. [DOI] [PubMed] [Google Scholar]
- 12.Shi H, et al. Oligonucleotide-based microarray for DNA methylation analysis: principles and applications. J Cell Biochem. 2003;88(1):138–143. doi: 10.1002/jcb.10313. [DOI] [PubMed] [Google Scholar]
- 13.Yang H, et al. The androgen receptor gene is preferentially hypermethylated in follicular non-Hodgkin's lymphomas. Clin Cancer Res. 2003;9(11):4034–4042. [PubMed] [Google Scholar]
- 14.Montgomery RB, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68(11):4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X, et al. Androgen receptor variants occur frequently in castration resistant prostate cancer metastases. PLoS One. 2011;6(11):e27970. doi: 10.1371/journal.pone.0027970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Scher HI, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1–2 study. Lancet. 2010;375(9724):1437–1446. doi: 10.1016/S0140-6736(10)60172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denmeade SR, et al. Dissociation between androgen responsiveness for malignant growth. vs. expression of prostate specific differentiation markers PSA, hK2, and PSMA in human prostate cancer models. Prostate. 2003;54(4):249–257. doi: 10.1002/pros.10199. [DOI] [PubMed] [Google Scholar]
- 19.Dreyling MH, et al. Alterations of the cyclin D1/p16-pRB pathway in mantle cell lymphoma. Cancer Res. 1997;57(20):4608–4614. [PubMed] [Google Scholar]
- 20.Yu Z, et al. Rapid Induction of Androgen Receptor Splice Variants by Androgen Deprivation in Prostate Cancer. Clin Cancer Res. 2014 doi: 10.1158/1078-0432.CCR-13-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, et al. Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer Res. 2013;73(2):483–489. doi: 10.1158/0008-5472.CAN-12-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yakimchuk K, et al. Effect of ligand-activated estrogen receptor beta on lymphoma growth in vitro and in vivo. Leukemia. 2011;25(7):1103–1110. doi: 10.1038/leu.2011.68. [DOI] [PubMed] [Google Scholar]
- 23.Wang LH, et al. Activation of estrogen receptor blocks interleukin-6-inducible cell growth of human multiple myeloma involving molecular cross-talk between estrogen receptor and STAT3 mediated by co-regulator PIAS3. J Biol Chem. 2001;276(34):31839–31844. doi: 10.1074/jbc.M105185200. [DOI] [PubMed] [Google Scholar]
- 24.Claessens F, Tilley W. Androgen signalling and steroid receptor crosstalk in endocrine cancers. Endocr Relat Cancer. 2014;21(4):E3–E5. doi: 10.1530/ERC-14-0274. [DOI] [PubMed] [Google Scholar]
- 25.McNamara KM, et al. Complexities of androgen receptor signalling in breast cancer. Endocr Relat Cancer. 2014;21(4):T161–T181. doi: 10.1530/ERC-14-0243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.