Abstract
The metabolic syndrome (MetS) is defined as the concurrence of obesity-associated cardiovascular risk factors including abdominal obesity, impaired glucose tolerance, hypertriglyceridemia, decreased HDL cholesterol, and/or hypertension. Earlier conceptualizations of the MetS focused on insulin resistance as a core feature, and it is clearly coincident with the above list of features. Each component of the MetS is an independent risk factor for cardiovascular disease and the combination of these risk factors elevates rates and severity of cardiovascular disease, related to a spectrum of cardiovascular conditions including microvascular dysfunction, coronary atherosclerosis and calcification, cardiac dysfunction, myocardial infarction, and heart failure. While advances in understanding the etiology and consequences of this complex disorder have been made, the underlying pathophysiologic mechanisms remain incompletely understood, and it is unclear how these concurrent risk factors conspire to produce the variety of obesity-associated adverse cardiovascular diseases. In this review we highlight current knowledge regarding the pathophysiologic consequences of obesity and the MetS on cardiovascular function and disease, including considerations of potential physiologic and molecular mechanisms that may contribute to these adverse outcomes.
Keywords: Metabolic syndrome, obesity, atherosclerosis, cardiovascular disease, adipokines
INTRODUCTION
The association between visceral obesity, hypertension, and atherosclerosis was recognized as early as 1765 by Joannes Baptista Morgagni in his seminal work entitled ‘De sedibus et causis morborum per anatomen indagata’.(1) Later studies by Hitzenberger, Richter-Quitner, and Kylin in the early 1920’s further documented the co-incident relationships between metabolic abnormalities such as hyperglycemia, hypertension, and other maladies such as hyperuricaemia.(2) These pioneering efforts laid the groundwork for what is now commonly referred to as the “Metabolic Syndrome” (MetS), a clustering of inter-related and co-incident risk factors which include abdominal obesity, impaired glucose tolerance, hypertriglyceridemia, diminished high density lipoprotein (HDL) cholesterol, and/or hypertension.(3) The original conceptualization of this syndrome focused on a central role of insulin resistance,(3) and this is clearly a concurrent and associated feature. More recently the focus has been on the MetS as an epidemiologic tool related to cardiovascular disease risk, and therefore traditional cardiovascular disease risk factors have been adopted as the defining features. Although the precise definition of what clinically constitutes the MetS has generated considerable debate, it is well accepted that these co-morbidities represent a pathological state that substantially augments risk for the development of type 2 diabetes mellitus and atherosclerotic cardiovascular disease.(4)
As of 2014, the Centers for Disease Control and Prevention estimates that ~70% of adults in the United States are overweight or obese, with ~40% of these individuals considered obese (defined as body mass index (BMI) ≥30 kg/m2). The National Health and Nutritional Examination Survey (NHANES) estimates that ~30% of overweight and ~60% of obese men and women meet the criteria for a diagnosis of MetS;(5) in other words, a majority of obese people carry the concurrent risk features that identify them as carrying augmented risk of cardiovascular disease. Therefore, in parallel with the obesity epidemic, the MetS is a growing epidemic, affecting ~20% of adults in the Western world.(6) Each component of the MetS is an independent risk factor for cardiovascular disease,(4, 6) together producing a wide spectrum of vascular and cardiac diseases.(7-13) While some advances in understanding the etiology and consequences of this complex disorder have been made, the underlying mechanisms that translate these obesity-associated risk factors into the full spectrum of observed cardiovascular pathologies remain insufficiently explained. The purpose of this review is to highlight current knowledge regarding the pathophysiologic consequences of obesity and the MetS and to outline recent advances in potential mechanisms that may contribute to these adverse cardiovascular outcomes. The literature linking type 2 diabetes with cardiovascular outcomes will not be reviewed in detail, because type 2 diabetes exerts effects on cardiovascular disease distinct from those of the underlying obesity and MetS and this would detract from our focus on obesity/MetS. Previous reviews by Abel et al.,(14) Poirier et al.,(15) Jiamspripong et al.,(16) Mottillo et al.,(17) Bastien et al.,(18) and Grundy and colleagues.(4, 11, 19) have summarized current epidemiology or evaluated specific cardiac conditions in the connection between obesity/MetS and cardiovascular disease. Here we focus on physiologic and pathophysiologic aspects of obesity- and MetS-associated changes in hemodynamics, microvascular dysfunction, myocardial metabolism, atherosclerosis and calcification, and infarction and heart failure.
HEMODYNAMIC AND CARDIAC EFFECTS OF OBESITY AND THE METABOLIC SYNDROME
The observed association of obesity with hypertension prompted a body of work exploring causes and effects of obesity on the heart. Chronic increases in body weight and adiposity can lead to significant neuro-hormonal changes and adaptations in the cardiovascular system.(18, 20) These alterations include activation of the activation of the renin-angiotensin-aldosterone system,(21, 22) altered levels of adipocytokines,(23-27) and pro-inflammatory cytokines(28, 29), and activation of the sympathetic nervous system.(30-33) Sympathetic nervous system activation can contribute to commonly described increases in heart rate, renal sodium retention, circulating blood volume, ventricular end-diastolic volume (pre-load), cardiac output, and/or blood pressure.(18, 30, 34) More generally, over activation of the sympathetic nervous system can concurrently drive abnormalities of vascular and cardiac function (e.g. vasoconstriction, tachycardia) and abnormalities of metabolic balance (e.g. excess lipolysis driving fatty acid levels, cathechol-induced peripheral and hepatic insulin resistance) (33). The degree to which these changes result directly from adrenergic receptor activation versus secondary alterations in adipokines, cytokines, and renal salt and water retention remains an active area of investigation.
Although insulin resistance has been dropped from the clinical definition of the MetS, the fact remains that underlying insulin resistance contributes to many of the features of the MetS (dysglycemia, elevated fatty acid levels, hyperinsulinemia and potentially a contribution of hyperinsulinemia to sympathetic system activation, among others)(35, 36). The epidemiologic literature consistently demonstrates associations of hyperinsulinemia with obesity-related heart disease (37). Studies of the metabolic physiology of insulin resistance have led to a deeper understanding of the interplay of fuel selection, with excess fatty acids contributing in part to cellular resistance to glucose metabolism but also with impairment in insulin receptor and post-receptor signaling events and abnormalities in mitochondrial number and function all contributing to the overall phenomenon (38-40). The contributions of these factors to abnormalities in myocardial metabolism are reviewed below.
Obesity-associated changes in cardiac function have been described as the ‘cardiomyopathy of obesity’. Data from the Multi-Ethnic Study of Atherosclerosis (MESA) demonstrate a direct association between left ventricular end-diastolic volume and body mass index in men and in women.(41) This large population based study also found that left ventricular mass increased to a greater extent than ventricular volume in obesity.(23, 41) Although early studies into the ‘cardiomyopathy of obesity’ suggested that obesity resulted in volume overload and eccentric cardiac hypertrophy,(42, 43) more recent findings have established that the majority of obese subjects develop concentric left ventricular hypertrophy as well as mild (subclinical) diastolic and/or systolic dysfunction, with normal or elevated left ventricular ejection fraction.(41, 44-48) This can be associated with poor outcomes following cardiac intervention procedures (49). More sensitive measures of contractile function such as left ventricular fractional shortening, systolic velocity, and myocardial strain (circumferential and longitudinal) have been shown to be impaired in the setting of obesity and/or MetS.(45, 48, 50, 51) Alterations in load-independent measures of myocardial contractility (e.g. end-systolic pressure volume relationship) have also been reported in animal models of obesity/MetS(52-54) and in obese humans with essential hypertension.(55) Importantly, cardiac functional responses to physiologic perturbations (e.g. exercise),(56-58) pathologic conditions (e.g. myocardial ischemia)(59-61), or pharmacologic stimuli (e.g. catecholamines, glucagon-like-peptide-1 mimetics)(52, 62, 63) are also known to be significantly influenced by an obese/MetS phenotype.
The molecular mechanisms underlying this cardiac and hemodynamic phenotype are complex and incompletely characterized. Investigators in this field have identified relevant and important molecular pathways linking obesity to cardiac dysfunction. In particular, alterations in myocardial Ca2+ handling via changes in the functional expression of SERCA2A and ryanodine (RyR2) receptors(52, 57, 64) have been of interest, including a recent comprehensive paper demonstrating concurrent effects of RyR2 abnormalities to induced myocellular and β-cell dysfunction (65), affecting subcellular structures in addition to producing abnormalities in intracellular Ca2+ handling (66). This shared dependence of the myocardium and β-cell highlights the need for further studies in to the mechanisms underlying the dysfunctional regulation of Ca2+ in obesity/MetS. Also of note is a growing interest on modifications in the regulation of myocardial titin (which influences the passive and restoring force of the cardiac sarcomere and can contribute to hypertrophic signaling) as a potential target or mediator of obesity-associated cardiac dysfunction.(52, 67-71) There is of course the added possibility that progressive vascular disease further influences these changes through mechanisms specific to the atherosclerotic process, or relating to microvascular dysfunction, independent of the obese state.(7, 9, 72) These examples highlight the opportunities to better understand the pathophysiologic manifestations of obesity/MetS by exploring key shared cellular mechanisms.
MICROVASCULAR DYSFUNCTION IN OBESITY AND THE METABOLIC SYNDROME
In all vascular beds, the microcirculation is the primary site of blood flow regulation, through regulation of resistance to flow at the level of the microvasculature. Microvascular resistance is simultaneously modulated by a variety of intrinsic (myogenic) and extrinsic (endothelial, neural, hormonal, metabolic) mechanisms which collectively dictate overall tissue perfusion.(73) There is a strong body of evidence demonstrating that control of microvascular tone and microvascular density are significantly impacted by obesity status, and that the MetS is similarly associated with physiologically important alterations in the regulation of arteriolar resistance. For example, changes in microvascular structure and function in obesity/MetS have been shown to result in an overall imbalance between tissue oxygen delivery and metabolism in many vascular beds, including heart,(7, 9, 74, 75) kidney,(76) brain,(77) and skeletal muscle.(78, 79)
In the coronary circulation, microvascular dysfunction has been demonstrated as reductions in coronary vasodilator responsiveness to a variety of pharmacologic agonists.(75, 80-82) Importantly, diminished coronary flow reserve is a powerful predictor of major adverse cardiovascular events.(72) Furthermore, the MetS significantly impairs the balance between coronary blood flow and myocardial metabolism in response to exercise (local metabolic vasodilation),(83, 84) with alterations in coronary perfusion pressure (pressure-flow autoregulation),(85) and in response to cardiac ischemia (reactive hyperemia).(84) Importantly, these changes occur prior to any evidence of overt atherosclerotic disease and have been associated with diminished diastolic and systolic contractile function in obese/MetS in humans(47, 86, 87) and in animal models.(20, 57, 88) These findings indicate that underlying coronary microvascular dysfunction likely contributes to reductions in cardiac contractile function,(45, 47, 48, 51) to concentric ventricular hypertrophy,(41, 44-48, 89) and to the significant increases in risk of myocardial infarction(17) and cardiovascular mortality(8, 90, 91) observed in obese individuals with the MetS.
Microvascular dysfunction in obesity has also been documented in other vascular beds such as the kidney, brain, and skeletal muscle, as noted above. Obesity and MetS augment renal vasoconstriction in response to angiotensin II,(92, 93) impair renal autoregulation,(94) and blunt myogenic afferent arteriolar constriction.(95) In contrast, diminished vasodilator responsiveness and increased myogenic activation is evident in isolated middle cerebral arteries and gracilis arterioles from obese Zucker rats.(77, 96, 97) This skeletal muscle microvasculopathy is associated with an enhanced rate of fatigue and decreased maximal force development of skeletal muscle,(79, 97, 98) which can be largely corrected through pharmacologic enhancement of perfusion.(98, 99) Renal, cerebral, and skeletal muscle circulations have also been shown to be adversely affected by obesity and the MetS, which produce diminish microvascular-capillary density.(9-13, 100, 101) Significant coronary vascular remodeling and altered vascular wall mechanics have also been documented in obese swine with MetS.(85) Aside from impaired oxygenation and tissue hypoxia, microvascular dysfunction in MetS has also been suggested to play a role in the development of glomerular injury, tubular atrophy, interstitial fibrosis,(76, 95) and exacerbation of injury in the setting of peripheral vascular disease(98) or stroke.(77)
A related aspect of microvascular dysfunction is impaired dilator/constrictor tone. It is apparent that underlying activation of the renin-angiotensin-aldosterone system, the sympathetic nervous system, and inflammatory pathways contributes to a diminished vasodilator and augmented vasoconstrictor phenotype of the MetS.(7) A hallmark of MetS-induced vascular disease is impaired endothelial function, which is associated not only with diminished bioavailability of nitric oxide but also increased production and/or vascular sensitivity to endothelial-dependent vasoconstrictors such as endothelin-1, prostaglandin H2, and thromboxane A2.(7, 20, 31) Endothelial dysfunction is pathologically important not only for the modulation of vascular resistance and tissue perfusion but also as a critical step in the initiation and progression of vascular atherogenesis.(102) MetS is also associated with alterations in the functional expression of, and electromechanical coupling between, voltage-dependent K+ and Ca2+ channels.(7, 83, 84, 88, 103, 104) Continued research to elucidate the precise mechanisms responsible for the deleterious impact of the MetS on microvascular function is needed, and stands to provide novel targets for directed therapies needed to treat the pathologic consequences of this multifactorial syndrome.
MYOCARDIAL METABOLISM IN OBESITY AND THE METABOLIC SYNDROME
As noted in the introduction, insulin resistance is a key underlying component of the pathophysiology of the MetS. This applies to the heart, which is subject to systemic alterations in fuel delivery as well as to the effects of systemically and locally produced regulatory factors such as hormones and adipokines. Here we review what is known about the dysregulation of myocardial metabolism in obesity/MetS and how this contributes to functional abnormalities that characterize this syndrome.
The myocardium is a metabolic omnivore. In other words, myocardium is capable of sustained function using fuels including, but not limited to, acetate, glucose or long-chain fatty acids. In health the heart preferentially consumes fatty acids, which provides the most energy per unit of fuel (i.e. moles of ATP per mole of fatty acid), but conversely requires more oxygen for each ATP unit generated (requiring 0.24 mole O2 per mole ATP generated) compared to glucose (0.16 mole O2 per mole ATP generated).(105) Like other tissues, the myocardium responds to insulin by shifting toward glucose uptake and glucose oxidation,(106) and interestingly the myocardium also responds to glucagon-like peptide 1 (GLP-1) to shift toward glucose uptake and oxidation.(52, 107, 108) Although the capacity of myocardial tissues to respond in this way is established, it is not clear how these actions contribute to the regulation of myocardial fuel selection in the course of normal physiology.
Abnormalities in myocardial fuel substrate selection in obesity have been described, as well as abnormalities in the responses to shifts in substrate availability, and abnormalities in the responses to hormonal controls. In obesity, the myocardium exhibits abnormally increased rates of fatty acid uptake and oxidation, and an impaired ability to shift away from this increased fatty acid utilization.(109, 110) Such abnormalities have been shown in animal models of insulin resistance,(111-113) in humans with obesity/insulin resistance,(46, 114, 115) and in obese humans with Type 2 diabetes.(116) This lipotoxicity effect is in addition to the previously described adverse effects of hyperglycemia on myocardial fuel selection (which combined exert ‘glucolipotoxicity’) (117), although the hyperglycemia is modest in MetS in the absence of concurrent diabetes. Notably, emerging data suggests a sexual dimorphism, with increased rates of fatty acid uptake and utilization in female animal models(110, 118) and in women.(115, 119, 120) The implications of this sexual difference in fuel selection on obesity-related myocardial disease are not yet known, but this phenomenon may help explain the relatively greater adverse effect of the development of diabetes on myocardial outcomes in women.(114)
Increased fatty acid uptake is not due simply to increased rates of fatty acid delivery in the setting of obesity. Rather, there is evidence for increased transport capacity and increased rates of fatty acid transport. The major sarcolemmal fatty acid transport proteins, CD36 and FATP, are over-expressed in animal models of obesity.(112, 121, 122) Lipoprotein lipase, functioning in adipocytes or in vasculature more proximal to the myocardium to liberate fatty acids from the circulating storage form of triacyglyerols, may also contribute to the detrimental phenotype of elevated fatty acid uptake.(123) Augmented fatty acid uptake in obesity is also not simply a neutral shift in fuel preference. Experimental studies limiting or augmenting fatty acid uptake have demonstrated that increased fatty acid uptake can lead to impairments in myocardial function, and that limiting fatty acid uptake can rescue the heart from this lipotoxic circumstance.(46, 124-128)
Distinct from questions of fatty acid availability and uptake, there is a question of whether insulin resistance contributes separately to abnormal myocardial metabolism in obesity. Many prior reports have linked obesity and insulin resistance with increased myocardial fatty acid uptake and utilization, as detailed above. Resistance to insulin action in the heart to drive glucose uptake is not consistently seen, particularly in human studies.(46, 129-132) Experimental evidence from animal models demonstrates impairment in insulin-stimulated myocardial glucose uptake, impairment in insulin’s effects to modulate blood flow, and impairment in insulin’s role as a regulator of cell growth and cell cycle in the setting of obesity or isolated myocardial fat loading.(127, 131, 133-135) Nevertheless, from the systemic viewpoint it appears that the actions of insulin in tissues outside the heart, acting to regulate fuel supply and other regulatory factors, exert larger effects on myocardial metabolism than its direct actions on the heart.(106, 129) It is therefore unclear whether myocardium-specific insulin resistance is a therapeutic target. Nevertheless, treatments that improve systemic insulin resistance can exert beneficial effects on myocardial metabolism, so systemic insulin resistance remains a valid overall target.(136-138)
Other features of obesity, such as abnormalities in adipokines (e.g. adiponectin and leptin) and increased systemic inflammation, may impact myocardial fuel selection or myocardial responses to injury.(139-141) Effects of adiponectin to directly modulate myocardial metabolism have been shown in experimental models,(142) and the importance of leptin for normal cardiac function and development is demonstrated by the abnormal cardiac phenotype of leptin-deficient animals.(143) The actions of these and other adipose-derived factors on the heart may reflect concurrent effects on systemic hemodynamics, vascular function, endothelial response to injury in addition any direct effects on myocardial metabolism.(27, 142, 144)
The implications of these metabolic abnormalities along with microvascular dysfunction and atherosclerotic vascular lesions on cardiovascular outcomes in obesity and the MetS are discussed separately below.
ATHEROSCLEROTIC DISEASE AND VASCULAR CALCIFICATION
Thirty years of focused research have shown that increasing degrees of obesity and the MetS are associated with accelerated atherosclerosis and a greater incidence of coronary heart disease.(145-150) These higher rates of atherosclerotic disease have been shown to result in an ~2-fold increase in risk of myocardial infarction(17) and a significantly elevated risk of cardiovascular mortality.(8, 90, 91) Further, this increase in cardiovascular risk is proportionally greater in women compared to men.(10, 17, 90) Recent findings from the PROSPECT (Providing Regional Observations to Study Predictors of Events in the Coronary Tree) study indicate compositional differences in coronary plaques underlie these increased event rates, with evidence for increased prevalence of adverse features (total plaque burden, necrotic core and calcium content, among others) in untreated, non-culprit lesions from patients with MetS or diabetes.(151) Increased prevalence and absolute progression of coronary artery calcification has also been demonstrated in the setting of the MetS and augmented cardiometabolic risk.(152-154)
The molecular mechanisms underlying the augmented initiation and progression of MetS-induced vascular disease remain an area of active research. Recent work has focused on the idea of adipose-derived hormones and cytokines (‘adipokines’) as molecular links between adiposity and vascular disease(155). Roles of adipokines in the regulation of many relevant features of obesity-associated cardiovascular disease have been described, including effects of adipokines on insulin sensitivity (leptin, adiponectin and resistin), inflammation (IL-8, monocyte chemotactic protein 1, leptin, chemerin), coagulation (plasminogen activator inhibitor-1), and vascular function and atherosclerosis (leptin, resistin, tumor necrosis factor-alpha, adiponectin,visfatin, omentin).(24, 156-163) Relevant fat depots also include the fat on the heart itself. Cardiac adipose tissue volume expands with obesity, but accumulates preferentially around coronary arteries, and atherosclerotic plaques occur predominantly in arteries encased by perivascular adipose tissue (PVAT).(164, 165 ) Recent work is evaluating an “outside-to-inside” signaling paradigm by which adipokines released from coronary perivascular adipose tissue (PVAT) are capable of influencing the development of vascular dysfunction and atherosclerosis in immediately adjacent vessels.(156, 166-170) Future studies are needed to better delineate the relative importance of systemically derived versus locally produced adipokines, to better understand the molecular mechanisms linking adipokines to vascular pathophysiology, and to assess whether these signaling pathways constitute therapeutic targets that may be of specific benefit in combating cardiovascular disease in obesity and MetS.
MYOCARDIAL INFARCTION AND HEART FAILURE IN OBESITY AND THE METABOLIC SYNDROME
Obesity and the MetS are associated with increased risk of heart disease, with two distinct diseases represented. First, obesity predisposes to congestive heart failure. Second, obesity is a contributor to risk of atherosclerotic heart disease, distinct from the effects of concurrent diabetes. The other components of the MetS are themselves epidemiologic risk factors for these conditions, working concurrent with the obesity effect to augment risk for each of these conditions.
The association of obesity with congestive heart failure is well recognized but remains unexplained. This condition was historically called ‘obesity cardiomyopathy’, and felt to arise due to effects of systemic and pulmonary hypertension, with contributory effects of obstructive sleep apnea.(20, 171) Unlike heart failure associated with atherosclerotic disease, diastolic dysfunction is a dominant aspect of the obesity-associated impairment in myocardial function.(172) Experimental and clinical trial evidence suggests that some of this dysfunction is associated with the aberrant fatty acid uptake phenotype of the obese heart, and amenable to improvement by reducing myocardial fatty acid uptake.(173-176) Epidemiologic evidence links hyperinsulinemia with heart failure,(177-179) and there is a body of experimental study evidence suggesting that insulin resistance in the heart is a contributor to the pathogenesis of heart failure.(129, 131) Unfortunately this literature is confusing in that insulin resistance is also described as a consequence of heart failure of various etiologies, with opposite systemic metabolic phenotypes to what is seen in obesity.(180-182) Emerging evidence suggests that impaired myocardial metabolic responses to GLP-1 may exist in obesity.(62, 183) GLP-1 derived treatments may represent a novel approach to metabolic modulation for heart failure,(184, 185) although the myocardial effects in populations with obesity will need to be carefully evaluated.
The association of obesity with atherosclerotic heart disease has been recognized for more than 50 years.(147) The ongoing obesity epidemic has made this component of population cardiovascular disease risk increasingly apparent, and urgent. The approach to cardiovascular disease prevention and management is not different in those with obesity, and is focused on prevention through management of risk factors such as blood pressure, smoking, and cholesterol (primarily with ‘statin’ class medications) and on revascularization when necessary. Weight loss interventions, including diet/exercise (‘lifestyle’) paradigms, pharmacologic treatment and surgically induced weight loss, can reverse the magnitude of cardiovascular risk that is represented in the MetS components (186-189). However, lifestyle change to induce weight loss failed to improve cardiovascular risk among a population with type 2 diabetes in the LookAHEAD study.(186) Similarly, medications that induce weight loss have not been shown to prevent future cardiovascular disease, and in some instances these medications also exert separate adverse cardiac effects.(187, 188) In contrast, surgically-induced weight loss studies suggest long-term survival benefits including diabetes remission and reduced rates of atherosclerotic coronary vascular disease, (186, 190, 191), along with improvements in obesity-associated derangements in cardiac microcirculation, structure, and function (192, 193). These discordant results suggest that either the degree of weight loss with non-surgical approaches was insufficient to achieve benefit, or that surgically induced weight loss confers weight-independent cardioprotection. Therapies targeting metabolic abnormalities for atherosclerotic heart disease in obesity are under investigation. Prior studies were focused on potential benefits of PPAR-gamma agonists (modulating fatty acid delivery and peripheral insulin resistance),(194) but this class of agents has since fallen out of favor with subsequent revelations of increased fluid retention rates, increased rates of bone loss, and uncertainty regarding net cardiovascular disease benefits. More recently the glucagon-like peptide 1 (GLP-1) mimetics have begun to be evaluated. These treatments, originally developed for management of glycemia in Type 2 diabetes, may have distinct beneficial effects to reduce rates of atherosclerotic disease and it is likely that studies of cardioprotection in obesity will be soon to follow.(183, 195-197)
INSIGHTS FROM PROTEOMIC AND GENOMIC STUDIES
As with the physiologic changes seen in obesity/MetS, the genetic and molecular factors underlying the cardiovascular perturbations in MetS are complex and inter-related. A considerable body of evidence has been produced identifying molecular changes associated with obesity and the individual components of the MetS. Modern high-throughput, comprehensive molecular methodologies (the so-called ‘omics’ methods) assessing genetics, nucleic acids, proteins, or metabolites hold the promise of providing insights into biologic processes with an opportunity to assess all concurrent molecular changes. These methods can provide powerful integrative insights into whole-body, tissue, and/or cellular physiology, but at the cost of producing a large number of inter-related results that can be challenging to interpret. Associations between select miR species and specific cardiovascular disease, along with associations with putative mechanistic mediators, have been observed for atrial fibrillation (198), and cerebrovascular disease (199), along with associations with pathophysiologic mediators of vascular disease including lipid metabolism (200)and endothelial dysfunction (201). A comprehensive review of the current state of knowledge linking miR and cardiovascular disease has recently been published (202). The literature specifically evaluating miR associations with cardiovascular disease in obesity/MetS is relatively sparse, and in the current context worthy of a detailed presentation.
Using microarrays, Phillip-Couderc et al. identified 63 genes that were differentially expressed in dog ventricles following 24 weeks of diet-induced obesity and hypertension.(203) Using hierarchical clustering analyses, they were able to identify groups of co-regulated genes and ascribe predicted functions to the products of the differentially expressed genes. The identified gene groupings were associated with many diverse cellular functions in the myocardium including regulation of cell proliferation and cell structure, key functional pathways such as calcium handling, response to cellular stress, and regulation of energy metabolism and mitochondrial function.(203) Of note, this study demonstrated that changes to the transcriptome took place continuously over the 24 week experimental timeline.
Nucleic acids are now well recognized to fill roles beyond information storage and transmission. Multiple functional RNA moieties have been discovered, including subsets with particular topological structures (e.g. shRNA), and others with sequence-specific actions to modulate transcription (siRNA) or post-transcriptional events (lncRNA, miRNA). One important group of post-transcriptional modulators, the microRNAs (miR), have been reported to contribute to the regulation of metabolic disease(204-206) and of cardiovascular disease.(207-209) Initial studies of the effects of miRs were associative and often focused on single miR species, but studies of the regulation of miRs, direct actions of miRs, and concurrent changes in multiple miR species are beginning to appear. For example, recent work by our lab(52) and others(204, 210) suggests that a complex system of factors (obesity/MetS, myocardial ischemia, pharmacologic therapy) alters the expression of miRs which in turn are linked with the regulation of cellular functions and/or pathophysiologic responses. The emerging paradigm is one in which miR expression changes are not necessarily inextricably related to a particular pathology (though some may be(210)), but instead are determined by multiple concurrent factors including underlying metabolic disease, cardiovascular pathology (e.g. atherosclerotic disease, ischemic heart disease, heart failure) and exposure to pharmacotherapy. For example a randomized, placebo-controlled, double-blinded study with 18 placebo and 17 metformin treated patients with type 2 diabetes mellitus found that metformin-specific changes in insulin resistance were associated with differential changes in circulating levels of miR-140-5p, miR-222, miR-142, and miR-192.(204) Work from our laboratory in Ossabaw swine assessed left ventricular miR expression changes in response to combinations of stimuli including diet-induced obesity, myocardial ischemia, and exendin-4, a GLP-1 mimetic.(52) We found that miR expression was regulated in complex ways, where some miR changes were related to the obese condition (e.g. miR133a-5p), while others were specific to the exendin treatment (e.g. miR15, miR let7).(52) These observations are associative; how these miRs are related to the physiologic effects or treatment responses is unknown at present. These observations highlight both the strengths and the challenges of exploring physiology using high-throughput measures.
As with other high-throughput integrative testing approaches, proteomic approaches promise to advance our understanding of physiology and pathophysiology. Many forms of protein mass-spectrometry allow for antibody-independent, label-free quantification and analysis of protein components within a tissue. Whole proteome analyses have previously been utilized to demonstrate obesity-specific changes in abundance and phosphorylation of proteins related to ion transport, mitochondrial metabolism, antioxidant function and cardiac contractile function.(211-215) Some work has been done exploring the proteomic responses to ischemia in non-obese models,(216-219) but very little work has been done to date exploring obesity-specific proteomic changes associated with myocardial ischemia or in relation to obesity-specific cardiac dysfunction. Our own work presents an initial foray into these questions.(52) In our studies in Ossabaw swine, the proteomic changes associated with obesity, ischemia and exendin-4 treatment provided intriguing novel observations of obesity-related changes in the structural protein titin and in multiple components of the myocelluar calcium-regulating machinery.(52) These results need confirmation and further exploration, but along with the miR observations underscore the value of these ‘omic’ approaches as tools for discovery.
SUMMARY AND FUTURE DIRECTIONS
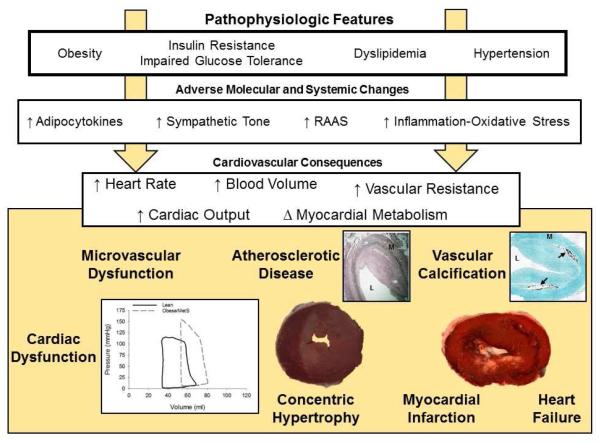
The MetS is defined as the concurrence of mutually associated cardiovascular risk factors including abdominal obesity, impaired glucose tolerance, hypertriglyceridemia, decreased HDL cholesterol, and/or hypertension. In association with these factors, many investigators have described the activation of the sympathetic nervous system, renin-angiotensin system, and increased levels of pro-inflammatory adipokines and cytokines which subsequently contribute to increases in heart rate, circulating blood volume, ventricular end-diastolic volume, cardiac output, and vascular resistance. This hemodynamic phenotype is associated with cardiovascular disease risk. The associated pathophysiologic changes include alterations in myocardial substrate metabolism, microvascular dysfunction, impaired oxygen supply/demand balance, cardiac (diastolic) contractile dysfunction, and concentric cardiac hypertrophy (summarized in the Figure). These changes are distinct from the pathophysiology in conduit vessels through the processes of traditional atherosclerosis and arterial calcification, which are also augmented and contribute to adverse cardiovascular outcomes. (17) Despite significant advances made to date, the physiological and molecular mechanisms of metabolic, functional and vascular disease in obesity/MetS remain poorly understood. The most obvious hurdle is the complicated, inter-dependent multi-factorial nature of the syndrome itself, making it difficult to disentangle relevant factors or specific combinations of factors. It is also possible that the most relevant features are separate from the defining cardiovascular disease risk factors and have yet to be identified. Adipokines have been suggested as one possible example of such integrative underlying factors, but the potential also exists for ‘omic’ approaches to provide insight into molecules and mechanisms of which we are not yet even aware. There is a clear need for ongoing exploration of these issues, to better understand and better treat obesity and MetS-associated cardiovascular disease, in order to better address the considerable public health implications of these conditions.
Figure.
Schematic diagram of the pathologic features, adverse molecular and systemic changes, and cardiovascular consequences of the metabolic syndrome. Clustering of causally inter-related risk factors including abdominal obesity, impaired glucose tolerance, hypertriglyceridemia, decreased HDL cholesterol, and/or hypertension is associated activation of the sympathetic nervous system, renin-angiotensin-aldosterone system (RAAS), and increased levels of pro-inflammatory adipokines and cytokines. These phenotypic changes subsequently contribute to increases in heart rate, circulating blood volume, cardiac output, vascular resistance, and changes in myocardial metabolism. The consequences of these changes include microvascular dysfunction, cardiac contractile dysfunction (augmented end-diastolic volume and systemic pressure development observed in left ventricular pressure-volume relationship (data from Sassoon et al.(52)), atherosclerotic disease (L = lumen; M = media; image of human coronary artery from Noblet et al.(170)), vascular calcification (arrow points to calcification; image provided by Dr. Michael Sturek with permission), concentric cardiac hypertrophy, myocardial infarction, and heart failure.
Acknowledgements
Supported by a National Institutes of Health grant, HL117620 (J. Tune and K. Mather, PI).
Abbreviations
- BMI
Body Mass Index
- MetS
Metabolic Syndrome
- NHANES
National Health and Nutritional Examination Survey
- miR
microRNA
- HDL
high-density lipoprotein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: All authors have read the journal’s policy on disclosure of potential conflicts of interest and declare that no competing interest exists.
None of the authors have financial or personal relationship with organizations that could potentially be perceived as influencing the described research. The work is solely that of the authors and no editorial support was used in the preparation of this manuscript. All authors have read the journal’s authorship agreement and the manuscript has been reviewed and approved by all named authors.
Reference List
- 1.Enzi G, Busetto L, Inelmen EM, Coin A, Sergi G. Historical perspective: visceral obesity and related comorbidity in Joannes Baptista Morgagni's 'De sedibus et causis morborum per anatomen indagata'. Int J Obes Relat Metab Disord. 2003;27(4):534–5. doi: 10.1038/sj.ijo.0802268. [DOI] [PubMed] [Google Scholar]
- 2.Sarafidis PA, Nilsson PM. The metabolic syndrome: a glance at its history. J Hypertens. 2006;24(4):621–6. doi: 10.1097/01.hjh.0000217840.26971.b6. [DOI] [PubMed] [Google Scholar]
- 3.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595–607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 4.Sperling LS, Mechanick JI, Neeland IJ, Herrick CJ, Despres JP, Ndumele CE, et al. The CardioMetabolic Health Alliance: Working Toward a New Care Model for the Metabolic Syndrome. J Am Coll Cardiol. 2015;66(9):1050–67. doi: 10.1016/j.jacc.2015.06.1328. [DOI] [PubMed] [Google Scholar]
- 5.Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003-2006. Natl Health Stat Report. 2009;(13):1–7. [PubMed] [Google Scholar]
- 6.O'Neill S, O'Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev. 2015;16(1):1–12. doi: 10.1111/obr.12229. [DOI] [PubMed] [Google Scholar]
- 7.Berwick ZC, Dick GM, Tune JD. Heart of the matter: coronary dysfunction in metabolic syndrome. J Mol Cell Cardiol. 2012;52(4):848–56. doi: 10.1016/j.yjmcc.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288(21):2709–16. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 9.Knudson JD, Dincer UD, Bratz IN, Sturek M, Dick GM, Tune JD. Mechanisms of coronary dysfunction in obesity and insulin resistance. Microcirculation. 2007;14(4-5):317–38. doi: 10.1080/10739680701282887. [DOI] [PubMed] [Google Scholar]
- 10.Galassi A, Reynolds K, He J. Metabolic syndrome and risk of cardiovascular disease: a meta-analysis. Am J Med. 2006;119(10):812–9. doi: 10.1016/j.amjmed.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 11.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Curr Opin Cardiol. 2006;21(1):1–6. doi: 10.1097/01.hco.0000200416.65370.a0. [DOI] [PubMed] [Google Scholar]
- 12.Hunt KJ, Resendez RG, Williams K, Haffner SM, Stern MP, San Antonio Heart S. National Cholesterol Education Program versus World Health Organization metabolic syndrome in relation to all-cause and cardiovascular mortality in the San Antonio Heart Study. Circulation. 2004;110(10):1251–7. doi: 10.1161/01.CIR.0000140762.04598.F9. [DOI] [PubMed] [Google Scholar]
- 13.Ritchie SA, Connell JMC. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr Metab Cardiovas. 2007;17(4):319–26. doi: 10.1016/j.numecd.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Physiol Rev. 2008;88(2):389–419. doi: 10.1152/physrev.00017.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poirier P, Eckel RH. Obesity and cardiovascular disease. Curr Atheroscler Rep. 2002;4(6):448–53. doi: 10.1007/s11883-002-0049-8. [DOI] [PubMed] [Google Scholar]
- 16.Jiamsripong P, Mookadam M, Alharthi MS, Khandheria BK, Mookadam F. The metabolic syndrome and cardiovascular disease: part 2. Prev Cardiol. 2008;11(4):223–9. doi: 10.1111/j.1751-7141.2008.00002.x. [DOI] [PubMed] [Google Scholar]
- 17.Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56(14):1113–32. doi: 10.1016/j.jacc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 18.Bastien M, Poirier P, Lemieux I, Despres JP. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog Cardiovasc Dis. 2014;56(4):369–81. doi: 10.1016/j.pcad.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 19.Grundy SM. Metabolic syndrome update. Trends Cardiovasc Med. 2016;26(4):364–73. doi: 10.1016/j.tcm.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Alpert MA. Obesity cardiomyopathy: pathophysiology and evolution of the clinical syndrome. Am J Med Sci. 2001;321(4):225–36. doi: 10.1097/00000441-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Whaley-Connell A, Pavey BS, Chaudhary K, Saab G, Sowers JR. Renin-angiotensin-aldosterone system intervention in the cardiometabolic syndrome and cardio-renal protection. Ther Adv Cardiovasc Dis. 2007;1(1):27–35. doi: 10.1177/1753944707082697. [DOI] [PubMed] [Google Scholar]
- 22.Cooper SA, Whaley-Connell A, Habibi J, Wei Y, Lastra G, Manrique C, et al. Renin-angiotensin-aldosterone system and oxidative stress in cardiovascular insulin resistance. Am J Physiol Heart Circ Physiol. 2007;293(4):H2009–23. doi: 10.1152/ajpheart.00522.2007. [DOI] [PubMed] [Google Scholar]
- 23.Shah RV, Abbasi SA, Heydari B, Rickers C, Jacobs DR, Jr., Wang L, et al. Insulin resistance, subclinical left ventricular remodeling, and the obesity paradox: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2013;61(16):1698–706. doi: 10.1016/j.jacc.2013.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knudson JD, Dick GM, Tune JD. Adipokines and coronary vasomotor dysfunction. Exp Biol Med (Maywood) 2007;232(6):727–36. [PubMed] [Google Scholar]
- 25.Tune JD, Considine RV. Effects of leptin on cardiovascular physiology. J Am Soc Hypertens. 2007;1(4):231–41. doi: 10.1016/j.jash.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beltowski J. Central vs. peripheral leptin excess in the pathogenesis of obesity-associated hypertension. J Hypertens. 2008;26(4):827–8. doi: 10.1097/HJH.0b013e3282f47688. [DOI] [PubMed] [Google Scholar]
- 27.Collins S. A heart-adipose tissue connection in the regulation of energy metabolism. Nat Rev Endocrinol. 2014;10(3):157–63. doi: 10.1038/nrendo.2013.234. [DOI] [PubMed] [Google Scholar]
- 28.Keaney JF, Jr., Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, et al. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23(3):434–9. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 29.Stapleton PA, James ME, Goodwill AG, Frisbee JC. Obesity and vascular dysfunction. Pathophysiology. 2008;15(2):79–89. doi: 10.1016/j.pathophys.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reaven GM, Lithell H, Landsberg L. Hypertension and associated metabolic abnormalities--the role of insulin resistance and the sympathoadrenal system. N Engl J Med. 1996;334(6):374–81. doi: 10.1056/NEJM199602083340607. [DOI] [PubMed] [Google Scholar]
- 31.Grassi G, Seravalle G, Quarti-Trevano F, Scopelliti F, Dell'Oro R, Bolla G, et al. Excessive sympathetic activation in heart failure with obesity and metabolic syndrome: characteristics and mechanisms. Hypertension. 2007;49(3):535–41. doi: 10.1161/01.HYP.0000255983.32896.b9. [DOI] [PubMed] [Google Scholar]
- 32.Straznicky NE, Grima MT, Sari CI, Karapanagiotidis S, Wong C, Eikelis N, et al. The relation of glucose metabolism to left ventricular mass and function and sympathetic nervous system activity in obese subjects with metabolic syndrome. J Clin Endocrinol Metab. 2013;98(2):E227–37. doi: 10.1210/jc.2012-3277. [DOI] [PubMed] [Google Scholar]
- 33.Ciccarelli M, Santulli G, Pascale V, Trimarco B, Iaccarino G. Adrenergic receptors and metabolism: role in development of cardiovascular disease. Front Physiol. 2013;4:265. doi: 10.3389/fphys.2013.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res. 2015;116(6):991–1006. doi: 10.1161/CIRCRESAHA.116.305697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laws A, Reaven GM. Insulin resistance and risk factors for coronary heart disease. Baillieres Clin Endocrinol Metab. 1993;7(4):1063–78. doi: 10.1016/s0950-351x(05)80245-9. [DOI] [PubMed] [Google Scholar]
- 36.Reaven G. Is insulin resistance: the link between TG-rich lipoproteins and excess death? J Intern Med. 2011;270(6):600–1. doi: 10.1111/j.1365-2796.2011.02460.x. author reply 2-3. [DOI] [PubMed] [Google Scholar]
- 37.Reaven G. All obese individuals are not created equal: insulin resistance is the major determinant of cardiovascular disease in overweight/obese individuals. Diab Vasc Dis Res. 2005;2(3):105–12. doi: 10.3132/dvdr.2005.017. [DOI] [PubMed] [Google Scholar]
- 38.Samuel VT, Shulman GI. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J Clin Invest. 2016;126(1):12–22. doi: 10.1172/JCI77812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1(7285):785–9. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 40.McGarry JD. What if Minkowski had been ageusic? An alternative angle on diabetes. Science. 1992;258(5083):766–70. doi: 10.1126/science.1439783. [DOI] [PubMed] [Google Scholar]
- 41.Turkbey EB, McClelland RL, Kronmal RA, Burke GL, Bild DE, Tracy RP, et al. The impact of obesity on the left ventricle: the Multi-Ethnic Study of Atherosclerosis (MESA) JACC Cardiovasc Imaging. 2010;3(3):266–74. doi: 10.1016/j.jcmg.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amad KH, Brennan JC, Alexander JK. The cardiac pathology of chronic exogenous obesity. Circulation. 1965;32(5):740–5. doi: 10.1161/01.cir.32.5.740. [DOI] [PubMed] [Google Scholar]
- 43.Alexander JK. Chronic heart disease due to obesity. J Chronic Dis. 1965;18(9):895–8. doi: 10.1016/0021-9681(65)90136-0. [DOI] [PubMed] [Google Scholar]
- 44.Litwin SE. Cardiac remodeling in obesity: time for a new paradigm. JACC Cardiovasc Imaging. 2010;3(3):275–7. doi: 10.1016/j.jcmg.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 45.Avelar E, Cloward TV, Walker JM, Farney RJ, Strong M, Pendleton RC, et al. Left ventricular hypertrophy in severe obesity - Interactions among blood pressure, nocturnal hypoxemia, and body mass. Hypertension. 2007;49(1):34–9. doi: 10.1161/01.HYP.0000251711.92482.14. [DOI] [PubMed] [Google Scholar]
- 46.Peterson LR, Herrero P, Schechtman KB, Racette SB, Waggoner AD, Kisrieva-Ware Z, et al. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation. 2004;109(18):2191–6. doi: 10.1161/01.CIR.0000127959.28627.F8. [DOI] [PubMed] [Google Scholar]
- 47.Peterson LR, Waggoner AD, Schechtman KB, Meyer T, Gropler RJ, Barzilai B, et al. Alterations in left ventricular structure and function in young healthy obese women: assessment by echocardiography and tissue Doppler imaging. J Am Coll Cardiol. 2004;43(8):1399–404. doi: 10.1016/j.jacc.2003.10.062. [DOI] [PubMed] [Google Scholar]
- 48.Wong CY, O'Moore-Sullivan T, Leano R, Byrne N, Beller E, Marwick TH. Alterations of left ventricular myocardial characteristics associated with obesity. Circulation. 2004;110(19):3081–7. doi: 10.1161/01.CIR.0000147184.13872.0F. [DOI] [PubMed] [Google Scholar]
- 49.Sardu C, Carreras G, Katsanos S, Kamperidis V, Pace MC, Passavanti MB, et al. Metabolic syndrome is associated with a poor outcome in patients affected by outflow tract premature ventricular contractions treated by catheter ablation. BMC Cardiovasc Disord. 2014;14:176. doi: 10.1186/1471-2261-14-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aurigemma GP, Silver KH, Priest MA, Gaasch WH. Geometric changes allow normal ejection fraction despite depressed myocardial shortening in hypertensive left ventricular hypertrophy. J Am Coll Cardiol. 1995;26(1):195–202. doi: 10.1016/0735-1097(95)00153-q. [DOI] [PubMed] [Google Scholar]
- 51.Almeida AL, Teixido-Tura G, Choi EY, Opdahl A, Fernandes VR, Wu CO, et al. Metabolic syndrome, strain, and reduced myocardial function: multi-ethnic study of atherosclerosis. Arq Bras Cardiol. 2014;102(4):327–35. doi: 10.5935/abc.20140040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sassoon DJ, Goodwill AG, Noblet JN, Conteh AM, Herring BP, McClintick JN, et al. Obesity alters molecular and functional cardiac responses to ischemia/reperfusion and glucagon-like peptide-1 receptor agonism. Basic Res Cardiol. 2016;111(4):43. doi: 10.1007/s00395-016-0563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bender SB, DeMarco VG, Padilla J, Jenkins NT, Habibi J, Garro M, et al. Mineralocorticoid receptor antagonism treats obesity-associated cardiac diastolic dysfunction. Hypertension. 2015;65(5):1082–8. doi: 10.1161/HYPERTENSIONAHA.114.04912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zibadi S, Vazquez R, Moore D, Larson DF, Watson RR. Myocardial lysyl oxidase regulation of cardiac remodeling in a murine model of diet-induced metabolic syndrome. Am J Physiol Heart Circ Physiol. 2009;297(3):H976–82. doi: 10.1152/ajpheart.00398.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garavaglia GE, Messerli FH, Nunez BD, Schmieder RE, Grossman E. Myocardial contractility and left ventricular function in obese patients with essential hypertension. Am J Cardiol. 1988;62(9):594–7. doi: 10.1016/0002-9149(88)90662-5. [DOI] [PubMed] [Google Scholar]
- 56.Pinto TE, Gusso S, Hofman PL, Derraik JG, Hornung TS, Cutfield WS, et al. Systolic and diastolic abnormalities reduce the cardiac response to exercise in adolescents with type 2 diabetes. Diabetes Care. 2014;37(5):1439–46. doi: 10.2337/dc13-2031. [DOI] [PubMed] [Google Scholar]
- 57.Dincer UD, Araiza A, Knudson JD, Shao CH, Bidasee KR, Tune JD. Dysfunction of cardiac ryanodine receptors in the metabolic syndrome. J Mol Cell Cardiol. 2006;41(1):108–14. doi: 10.1016/j.yjmcc.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 58.Milia R, Velluzzi F, Roberto S, Palazzolo G, Sanna I, Sainas G, et al. Differences in hemodynamic response to metaboreflex activation between obese patients with metabolic syndrome and healthy subjects with obese phenotype. Am J Physiol-Heart C. 2015;309(5):H779–H89. doi: 10.1152/ajpheart.00250.2015. [DOI] [PubMed] [Google Scholar]
- 59.Mozaffari MS, Schaffer SW. Myocardial ischemic-reperfusion injury in a rat model of metabolic syndrome. Obesity (Silver Spring) 2008;16(10):2253–8. doi: 10.1038/oby.2008.356. [DOI] [PubMed] [Google Scholar]
- 60.Hoshida S, Yamashita N, Otsu K, Kuzuya T, Hori M. Cholesterol feeding exacerbates myocardial injury in Zucker diabetic fatty rats. Am J Physiol Heart Circ Physiol. 2000;278(1):H256–62. doi: 10.1152/ajpheart.2000.278.1.H256. [DOI] [PubMed] [Google Scholar]
- 61.Thakker GD, Frangogiannis NG, Bujak M, Zymek P, Gaubatz JW, Reddy AK, et al. Effects of diet-induced obesity on inflammation and remodeling after myocardial infarction. Am J Physiol Heart Circ Physiol. 2006;291(5):H2504–14. doi: 10.1152/ajpheart.00322.2006. [DOI] [PubMed] [Google Scholar]
- 62.Moberly SP, Mather KJ, Berwick ZC, Owen MK, Goodwill AG, Casalini ED, et al. Impaired cardiometabolic responses to glucagon-like peptide 1 in obesity and type 2 diabetes mellitus. Basic Res Cardiol. 2013;108(4):365. doi: 10.1007/s00395-013-0365-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carroll JF, Jones AE, Hester RL, Reinhart GA, Cockrell K, Mizelle HL. Reduced cardiac contractile responsiveness to isoproterenol in obese rabbits. Hypertension. 1997;30(6):1376–81. doi: 10.1161/01.hyp.30.6.1376. [DOI] [PubMed] [Google Scholar]
- 64.Okatan EN, Durak AT, Turan B. Electrophysiological basis of metabolic-syndrome-induced cardiac dysfunction. Can J Physiol Pharmacol. 2016;94(10):1064–73. doi: 10.1139/cjpp-2015-0531. [DOI] [PubMed] [Google Scholar]
- 65.Santulli G, Pagano G, Sardu C, Xie W, Reiken S, D'Ascia SL, et al. Calcium release channel RyR2 regulates insulin release and glucose homeostasis. J Clin Invest. 2015;125(5):1968–78. doi: 10.1172/JCI79273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lavorato M, Huang TQ, Iyer VR, Perni S, Meissner G, Franzini-Armstrong C. Dyad content is reduced in cardiac myocytes of mice with impaired calmodulin regulation of RyR2. J Muscle Res Cell Motil. 2015;36(2):205–14. doi: 10.1007/s10974-015-9405-5. [DOI] [PubMed] [Google Scholar]
- 67.Hamdani N, Franssen C, Lourenco A, Falcao-Pires I, Fontoura D, Leite S, et al. Myocardial titin hypophosphorylation importantly contributes to heart failure with preserved ejection fraction in a rat metabolic risk model. Circ Heart Fail. 2013;6(6):1239–49. doi: 10.1161/CIRCHEARTFAILURE.113.000539. [DOI] [PubMed] [Google Scholar]
- 68.Hamdani N, Krysiak J, Kreusser MM, Neef S, Dos Remedios CG, Maier LS, et al. Crucial role for Ca2(+)/calmodulin-dependent protein kinase-II in regulating diastolic stress of normal and failing hearts via titin phosphorylation. Circ Res. 2013;112(4):664–74. doi: 10.1161/CIRCRESAHA.111.300105. [DOI] [PubMed] [Google Scholar]
- 69.Hamdani N, Bishu KG, von Frieling-Salewsky M, Redfield MM, Linke WA. Deranged myofilament phosphorylation and function in experimental heart failure with preserved ejection fraction. Cardiovasc Res. 2013;97(3):464–71. doi: 10.1093/cvr/cvs353. [DOI] [PubMed] [Google Scholar]
- 70.Kruger M, Linke WA. The Giant Protein Titin: A Regulatory Node That Integrates Myocyte Signaling Pathways. Journal of Biological Chemistry. 2011;286(12):9905–12. doi: 10.1074/jbc.R110.173260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zile MR, Baicu CF, Ikonomidis JS, Stroud RE, Nietert PJ, Bradshaw AD, et al. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: contributions of collagen and titin. Circulation. 2015;131(14):1247–59. doi: 10.1161/CIRCULATIONAHA.114.013215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Murthy VL, Naya M, Foster CR, Gaber M, Hainer J, Klein J, et al. Association Between Coronary Vascular Dysfunction and Cardiac Mortality in Patients With and Without Diabetes Mellitus. Circulation. 2012;126(15):1858–U178. doi: 10.1161/CIRCULATIONAHA.112.120402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Laughlin MH, Davis MJ, Secher NH, van Lieshout JJ, Arce-Esquivel AA, Simmons GH, et al. Peripheral circulation. Compr Physiol. 2012;2(1):321–447. doi: 10.1002/cphy.c100048. [DOI] [PubMed] [Google Scholar]
- 74.Prakash R, Mintz JD, Stepp DW. Impact of obesity on coronary microvascular function in the Zucker rat. Microcirculation. 2006;13(5):389–96. doi: 10.1080/10739680600745919. [DOI] [PubMed] [Google Scholar]
- 75.Di Carli MF, Charytan D, McMahon GT, Ganz P, Dorbala S, Schelbert HR. Coronary circulatory function in patients with the metabolic syndrome. J Nucl Med. 2011;52(9):1369–77. doi: 10.2967/jnumed.110.082883. [DOI] [PubMed] [Google Scholar]
- 76.Singh AK, Kari JA. Metabolic syndrome and chronic kidney disease. Curr Opin Nephrol Hypertens. 2013;22(2):198–203. doi: 10.1097/MNH.0b013e32835dda78. [DOI] [PubMed] [Google Scholar]
- 77.Chantler PD, Shrader CD, Tabone LE, D'Audiffret AC, Huseynova K, Brooks SD, et al. Cerebral Cortical Microvascular Rarefaction in Metabolic Syndrome is Dependent on Insulin Resistance and Loss of Nitric Oxide Bioavailability. Microcirculation. 2015;22(6):435–45. doi: 10.1111/micc.12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hodnett BL, Hester RL. Regulation of muscle blood flow in obesity. Microcirculation. 2007;14(4-5):273–88. doi: 10.1080/10739680701282143. [DOI] [PubMed] [Google Scholar]
- 79.Frisbee JC. Impaired skeletal muscle perfusion in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol. 2003;285(5):R1124–34. doi: 10.1152/ajpregu.00239.2003. [DOI] [PubMed] [Google Scholar]
- 80.Pirat B, Bozbas H, Simsek V, Yildirir A, Sade LE, Gursoy Y, et al. Impaired coronary flow reserve in patients with metabolic syndrome. Atherosclerosis. 2008;201(1):112–6. doi: 10.1016/j.atherosclerosis.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 81.Schindler TH, Cardenas J, Prior JO, Facta AD, Kreissl MC, Zhang ML, et al. Relationship between increasing body weight, insulin resistance, inflammation, adipocytokine leptin, and coronary circulatory function. Journal of the American College of Cardiology. 2006;47(6):1188–95. doi: 10.1016/j.jacc.2005.10.062. [DOI] [PubMed] [Google Scholar]
- 82.Teragawa H, Morita K, Shishido H, Otsuka N, Hirokawa Y, Chayama K, et al. Impaired myocardial blood flow reserve in subjects with metabolic syndrome analyzed using positron emission tomography and N-13 labeled ammonia. Eur J Nucl Med Mol Imaging. 2010;37(2):368–76. doi: 10.1007/s00259-009-1307-6. [DOI] [PubMed] [Google Scholar]
- 83.Berwick ZC, Dick GM, Moberly SP, Kohr MC, Sturek M, Tune JD. Contribution of voltage-dependent K(+) channels to metabolic control of coronary blood flow. J Mol Cell Cardiol. 2012;52(4):912–9. doi: 10.1016/j.yjmcc.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Borbouse L, Dick GM, Payne GA, Berwick ZC, Neeb ZP, Alloosh M, et al. Metabolic syndrome reduces the contribution of K+ channels to ischemic coronary vasodilation. Am J Physiol Heart Circ Physiol. 2010;298(4):H1182–9. doi: 10.1152/ajpheart.00888.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Trask AJ, Katz PS, Kelly AP, Galantowicz ML, Cismowski MJ, West TA, et al. Dynamic micro- and macrovascular remodeling in coronary circulation of obese Ossabaw pigs with metabolic syndrome. J Appl Physiol (1985) 2012;113(7):1128–40. doi: 10.1152/japplphysiol.00604.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gong HP, Tan HW, Fang NN, Song T, Li SH, Zhong M, et al. Impaired left ventricular systolic and diastolic function in patients with metabolic syndrome as assessed by strain and strain rate imaging. Diabetes Res Clin Pr. 2009;83(3):300–7. doi: 10.1016/j.diabres.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 87.Wong CY, O'Moore-Sullivan T, Fang ZY, Haluska B, Leano R, Marwick TH. Myocardial and vascular dysfunction and exercise capacity in the metabolic syndrome. Am J Cardiol. 2005;96(12):1686–91. doi: 10.1016/j.amjcard.2005.07.091. [DOI] [PubMed] [Google Scholar]
- 88.Borbouse L, Dick GM, Payne GA, Payne BD, Svendsen MC, Neeb ZP, et al. Contribution of BK(Ca) channels to local metabolic coronary vasodilation: Effects of metabolic syndrome. Am J Physiol Heart Circ Physiol. 2010;298(3):H966–73. doi: 10.1152/ajpheart.00876.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Owan T, Litwin SE. Is there a cardiomyopathy of obesity? Curr Heart Fail Rep. 2007;4(4):221–8. doi: 10.1007/s11897-007-0016-3. [DOI] [PubMed] [Google Scholar]
- 90.Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, Somers VK, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49(4):403–14. doi: 10.1016/j.jacc.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 91.Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24(4):683–9. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 92.Stepp DW, Boesen EI, Sullivan JC, Mintz JD, Hair CD, Pollock DM. Obesity augments vasoconstrictor reactivity to angiotensin II in the renal circulation of the Zucker rat. Am J Physiol Heart Circ Physiol. 2007;293(4):H2537–42. doi: 10.1152/ajpheart.01081.2006. [DOI] [PubMed] [Google Scholar]
- 93.Ahmed SB, Fisher ND, Stevanovic R, Hollenberg NK. Body mass index and angiotensin-dependent control of the renal circulation in healthy humans. Hypertension. 2005;46(6):1316–20. doi: 10.1161/01.HYP.0000190819.07663.da. [DOI] [PubMed] [Google Scholar]
- 94.Fujiwara K, Hayashi K, Matsuda H, Kubota E, Honda M, Ozawa Y, et al. Altered pressure-natriuresis in obese Zucker rats. Hypertension. 1999;33(6):1470–5. doi: 10.1161/01.hyp.33.6.1470. [DOI] [PubMed] [Google Scholar]
- 95.Hayashi K, Kanda T, Homma K, Tokuyama H, Okubo K, Takamatsu I, et al. Altered renal microvascular response in Zucker obese rats. Metabolism. 2002;51(12):1553–61. doi: 10.1053/meta.2002.36311. [DOI] [PubMed] [Google Scholar]
- 96.Butcher JT, Goodwill AG, Stanley SC, Frisbee JC. Differential impact of dilator stimuli on increased myogenic activation of cerebral and skeletal muscle resistance arterioles in obese zucker rats. Microcirculation. 2013;20(7):579–89. doi: 10.1111/micc.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Frisbee JC, Butcher JT, Frisbee SJ, Olfert IM, Chantler PD, Tabone LE, et al. Increased peripheral vascular disease risk progressively constrains perfusion adaptability in the skeletal muscle microcirculation. Am J Physiol Heart Circ Physiol. 2016;310(4):H488–504. doi: 10.1152/ajpheart.00790.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Frisbee JC, Goodwill AG, Frisbee SJ, Butcher JT, Wu F, Chantler PD. Microvascular perfusion heterogeneity contributes to peripheral vascular disease in metabolic syndrome. J Physiol. 2016;594(8):2233–43. doi: 10.1113/jphysiol.2014.285247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Butcher JT, Goodwill AG, Stanley SC, Frisbee JC. Blunted temporal activity of microvascular perfusion heterogeneity in metabolic syndrome: a new attractor for peripheral vascular disease? Am J Physiol Heart Circ Physiol. 2013;304(4):H547–58. doi: 10.1152/ajpheart.00805.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Beltowski J. Role of leptin in blood pressure regulation and arterial hypertension. J Hypertens. 2006;24(5):789–801. doi: 10.1097/01.hjh.0000222743.06584.66. [DOI] [PubMed] [Google Scholar]
- 101.Setty S, Sun W, Tune JD. Coronary blood flow regulation in the prediabetic metabolic syndrome. Basic Res Cardiol. 2003;98(6):416–23. doi: 10.1007/s00395-003-0418-7. [DOI] [PubMed] [Google Scholar]
- 102.Ross R, Glomset JA. Atherosclerosis and the arterial smooth muscle cell: Proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science. 1973;180(4093):1332–9. doi: 10.1126/science.180.4093.1332. [DOI] [PubMed] [Google Scholar]
- 103.Bender SB, Tune JD, Borbouse L, Long X, Sturek M, Laughlin MH. Altered mechanism of adenosine-induced coronary arteriolar dilation in early-stage metabolic syndrome. Exp Biol Med (Maywood) 2009;234(6):683–92. doi: 10.3181/0812-RM-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Borbouse L, Dick GM, Asano S, Bender SB, Dincer UD, Payne GA, et al. Impaired function of coronary BK(Ca) channels in metabolic syndrome. Am J Physiol Heart Circ Physiol. 2009;297(5):H1629–37. doi: 10.1152/ajpheart.00466.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lewandowski E, Ingwall J. The Physiological Chemistry of Energy Production in the Heart. In: Schlant R, RW A, O'Rourke R, Roberts R, Sonnenblick E, editors. Hurst's The Heart. 8th McGraw-Hill, Inc.; New York, NYU: 1994. pp. 153–64. [Google Scholar]
- 106.Iliadis F, Kadoglou N, Didangelos T. Insulin and the heart. Diabetes Res Clin Pract. 2011;93(Suppl 1):S86–91. doi: 10.1016/S0168-8227(11)70019-5. [DOI] [PubMed] [Google Scholar]
- 107.Bhashyam S, Fields AV, Patterson B, Testani JM, Chen L, Shen YT, et al. Glucagon-like peptide-1 increases myocardial glucose uptake via p38alpha MAP kinase-mediated, nitric oxide-dependent mechanisms in conscious dogs with dilated cardiomyopathy. Circ Heart Fail. 2010;3(4):512–21. doi: 10.1161/CIRCHEARTFAILURE.109.900282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhao T, Parikh P, Bhashyam S, Bolukoglu H, Poornima I, Shen YT, et al. Direct effects of glucagon-like peptide-1 on myocardial contractility and glucose uptake in normal and postischemic isolated rat hearts. J Pharmacol Exp Ther. 2006;317(3):1106–13. doi: 10.1124/jpet.106.100982. [DOI] [PubMed] [Google Scholar]
- 109.Lopaschuk GD, Folmes CD, Stanley WC. Cardiac energy metabolism in obesity. Circ Res. 2007;101(4):335–47. doi: 10.1161/CIRCRESAHA.107.150417. [DOI] [PubMed] [Google Scholar]
- 110.Rider OJ, Cox P, Tyler D, Clarke K, Neubauer S. Myocardial substrate metabolism in obesity. Int J Obes (Lond) 2013;37(7):972–9. doi: 10.1038/ijo.2012.170. [DOI] [PubMed] [Google Scholar]
- 111.Carley AN, Atkinson LL, Bonen A, Harper ME, Kunnathu S, Lopaschuk GD, et al. Mechanisms responsible for enhanced fatty acid utilization by perfused hearts from type 2 diabetic db/db mice. Arch Physiol Biochem. 2007;113(2):65–75. doi: 10.1080/13813450701422617. [DOI] [PubMed] [Google Scholar]
- 112.Coort SL, Bonen A, van der Vusse GJ, Glatz JF, Luiken JJ. Cardiac substrate uptake and metabolism in obesity and type-2 diabetes: role of sarcolemmal substrate transporters. Molecular and cellular biochemistry. 2007;299(1-2):5–18. doi: 10.1007/s11010-006-9372-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Oakes ND, Thalen P, Aasum E, Edgley A, Larsen T, Furler SM, et al. Cardiac metabolism in mice: tracer method developments and in vivo application revealing profound metabolic inflexibility in diabetes. Am J Physiol Endocrinol Metab. 2006;290(5):E870–81. doi: 10.1152/ajpendo.00233.2005. [DOI] [PubMed] [Google Scholar]
- 114.McGill JB, Peterson LR, Herrero P, Saeed IM, Recklein C, Coggan AR, et al. Potentiation of abnormalities in myocardial metabolism with the development of diabetes in women with obesity and insulin resistance. J Nucl Cardiol; 2011;2011:421–9. doi: 10.1007/s12350-011-9362-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Peterson LR, Saeed IM, McGill JB, Herrero P, Schechtman KB, Gunawardena R, et al. Sex and type 2 diabetes: obesity-independent effects on left ventricular substrate metabolism and relaxation in humans. Obesity (Silver Spring) 2012;20(4):802–10. doi: 10.1038/oby.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mather KJ, Hutchins GD, Perry K, Territo W, Chisholm R, Acton A, et al. Assessment of myocardial metabolic flexibility and work efficiency in human type 2 diabetes using 16- [18F]fluoro-4-thiapalmitate, a novel PET fatty acid tracer. Am J Physiol Endocrinol Metab. 2016;310(6):E452–60. doi: 10.1152/ajpendo.00437.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Toth PP, Raghavan VA. Glucolipotoxicity and the heart. Heart Fail Clin. 2012;8(4):xvii–xviii. doi: 10.1016/j.hfc.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 118.Banke NH, Yan L, Pound KM, Dhar S, Reinhardt H, De Lorenzo MS, et al. Sexual dimorphism in cardiac triacylglyceride dynamics in mice on long term caloric restriction. J Mol Cell Cardiol. 2012;52(3):733–40. doi: 10.1016/j.yjmcc.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Peterson LR, Herrero P, Coggan AR, Kisrieva-Ware Z, Saeed I, Dence C, et al. Type 2 diabetes, obesity, and sex difference affect the fate of glucose in the human heart. Am J Physiol Heart Circ Physiol. 2015;308(12):H1510–6. doi: 10.1152/ajpheart.00722.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Taegtmeyer H, Algahim MF. Obesity and cardiac metabolism in women. JACC Cardiovasc Imaging. 2008;1(4):434–5. doi: 10.1016/j.jcmg.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Coort SLM, Luiken JJFP, van der Vusse GJ, Bonen A, Glatz JFC. Increased FAT (fatty acid translocase)/CD36-mediated long-chain fatty acid uptake in cardiac myocytes from obese Zucker rats. Biochem Soc T. 2004;32:83–5. doi: 10.1042/bst0320083. Pt 1. [DOI] [PubMed] [Google Scholar]
- 122.Luiken JJ, Arumugam Y, Dyck DJ, Bell RC, Pelsers MM, Turcotte LP, et al. Increased rates of fatty acid uptake and plasmalemmal fatty acid transporters in obese Zucker rats. J Biol Chem. 2001;276(44):40567–73. doi: 10.1074/jbc.M100052200. [DOI] [PubMed] [Google Scholar]
- 123.Kim MS, Wang Y, Rodrigues B. Lipoprotein lipase mediated fatty acid delivery and its impact in diabetic cardiomyopathy. Biochimica et biophysica acta. 2012;1821(5):800–8. doi: 10.1016/j.bbalip.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 124.Dirkx E, Schwenk RW, Glatz JF, Luiken JJ, van Eys GJ. High fat diet induced diabetic cardiomyopathy. Prostaglandins Leukot Essent Fatty Acids. 2011;85(5):219–25. doi: 10.1016/j.plefa.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 125.Keung W, Ussher JR, Jaswal JS, Raubenheimer M, Lam VH, Wagg CS, et al. Inhibition of carnitine palmitoyltransferase-1 activity alleviates insulin resistance in diet-induced obese mice. Diabetes. 2013;62(3):711–20. doi: 10.2337/db12-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Luiken JJ. Sarcolemmal fatty acid uptake vs. mitochondrial beta-oxidation as target to regress cardiac insulin resistance. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2009;34(3):473–80. doi: 10.1139/H09-040. [DOI] [PubMed] [Google Scholar]
- 127.Mazumder PK, O'Neill BT, Roberts MW, Buchanan J, Yun UJ, Cooksey RC, et al. Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes. 2004;53(9):2366–74. doi: 10.2337/diabetes.53.9.2366. [DOI] [PubMed] [Google Scholar]
- 128.Pulinilkunnil T, Kienesberger PC, Nagendran J, Sharma N, Young ME, Dyck JR. Cardiac-specific adipose triglyceride lipase overexpression protects from cardiac steatosis and dilated cardiomyopathy following diet-induced obesity. Int J Obes (Lond) 2014;38(2):205–15. doi: 10.1038/ijo.2013.103. [DOI] [PubMed] [Google Scholar]
- 129.Abel ED, O'Shea KM, Ramasamy R. Insulin resistance: metabolic mechanisms and consequences in the heart. Arterioscler Thromb Vasc Biol. 2012;32(9):2068–76. doi: 10.1161/ATVBAHA.111.241984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hafstad AD, Solevag GH, Severson DL, Larsen TS, Aasum E. Perfused hearts from Type 2 diabetic (db/db) mice show metabolic responsiveness to insulin. Am J Physiol Heart Circ Physiol. 2006;290(5):H1763–9. doi: 10.1152/ajpheart.01063.2005. [DOI] [PubMed] [Google Scholar]
- 131.Mellor KM, Bell JR, Ritchie RH, Delbridge LM. Myocardial insulin resistance, metabolic stress and autophagy in diabetes. Clinical and experimental pharmacology & physiology. 2013;40(1):56–61. doi: 10.1111/j.1440-1681.2012.05738.x. [DOI] [PubMed] [Google Scholar]
- 132.Yokoyama I, Yonekura K, Ohtake T, Kawamura H, Matsumoto A, Inoue Y, et al. Role of insulin resistance in heart and skeletal muscle F-18 fluorodeoxyglucose uptake in patients with non-insulin-dependent diabetes mellitus. J Nucl Cardiol. 2000;7(3):242–8. doi: 10.1016/s1071-3581(00)70013-4. [DOI] [PubMed] [Google Scholar]
- 133.Montessuit C, Lerch R. Regulation and dysregulation of glucose transport in cardiomyocytes. Biochimica et biophysica acta. 2013;1833(4):848–56. doi: 10.1016/j.bbamcr.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 134.Watanabe T, Saotome M, Nobuhara M, Sakamoto A, Urushida T, Katoh H, et al. Roles of mitochondrial fragmentation and reactive oxygen species in mitochondrial dysfunction and myocardial insulin resistance. Experimental cell research. 2014;323(2):314–25. doi: 10.1016/j.yexcr.2014.02.027. [DOI] [PubMed] [Google Scholar]
- 135.Zhang L, Keung W, Samokhvalov V, Wang W, Lopaschuk GD. Role of fatty acid uptake and fatty acid beta-oxidation in mediating insulin resistance in heart and skeletal muscle. Biochimica et biophysica acta. 2010;1801(1):1–22. doi: 10.1016/j.bbalip.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 136.Lautamaki R, Airaksinen KE, Seppanen M, Toikka J, Luotolahti M, Ball E, et al. Rosiglitazone improves myocardial glucose uptake in patients with type 2 diabetes and coronary artery disease: a 16-week randomized, double-blind, placebo-controlled study. Diabetes. 2005;54(9):2787–94. doi: 10.2337/diabetes.54.9.2787. [DOI] [PubMed] [Google Scholar]
- 137.Naoumova RP, Kindler H, Leccisotti L, Mongillo M, Khan MT, Neuwirth C, et al. Pioglitazone improves myocardial blood flow and glucose utilization in nondiabetic patients with combined hyperlipidemia: a randomized, double-blind, placebo-controlled study. J Am Coll Cardiol. 2007;50(21):2051–8. doi: 10.1016/j.jacc.2007.07.070. [DOI] [PubMed] [Google Scholar]
- 138.Pelzer T, Jazbutyte V, Arias-Loza PA, Segerer S, Lichtenwald M, Law MP, et al. Pioglitazone reverses down-regulation of cardiac PPARgamma expression in Zucker diabetic fatty rats. Biochem Biophys Res Commun. 2005;329(2):726–32. doi: 10.1016/j.bbrc.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 139.Berg G, Schreier L, Miksztowicz V. Circulating and adipose tissue matrix metalloproteinases in cardiometabolic risk environments: pathophysiological aspects. Horm Mol Biol Clin Investig. 2014;17(2):79–87. doi: 10.1515/hmbci-2013-0069. [DOI] [PubMed] [Google Scholar]
- 140.Mangge H, Almer G, Truschnig-Wilders M, Schmidt A, Gasser R, Fuchs D. Inflammation, adiponectin, obesity and cardiovascular risk. Curr Med Chem. 2010;17(36):4511–20. doi: 10.2174/092986710794183006. [DOI] [PubMed] [Google Scholar]
- 141.Nakamura K, Fuster JJ, Walsh K. Adipokines: A link between obesity and cardiovascular disease. Journal of Cardiology. 2014;63(3-4):250–9. doi: 10.1016/j.jjcc.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Maia-Fernandes T, Roncon-Albuquerque R, Jr., Leite-Moreira AF. Cardiovascular actions of adiponectin: pathophysiologic implications. Revista portuguesa de cardiologia : orgao oficial da Sociedade Portuguesa de Cardiologia = Portuguese journal of cardiology : an official journal of the Portuguese Society of Cardiology. 2008;27(11):1431–49. [PubMed] [Google Scholar]
- 143.Ren J, Ma H. Impaired cardiac function in leptin-deficient mice. Curr Hypertens Rep. 2008;10(6):448–53. doi: 10.1007/s11906-008-0084-0. [DOI] [PubMed] [Google Scholar]
- 144.Sweeney G. Cardiovascular effects of leptin. Nat Rev Cardiol. 2010;7(1):22–9. doi: 10.1038/nrcardio.2009.224. [DOI] [PubMed] [Google Scholar]
- 145.Grundy SM. Obesity, metabolic syndrome, and coronary atherosclerosis. Circulation. 2002;105(23):2696–8. doi: 10.1161/01.cir.0000020650.86137.84. [DOI] [PubMed] [Google Scholar]
- 146.McGill HC, Jr., McMahan CA, Herderick EE, Zieske AW, Malcom GT, Tracy RE, et al. Obesity accelerates the progression of coronary atherosclerosis in young men. Circulation. 2002;105(23):2712–8. doi: 10.1161/01.cir.0000018121.67607.ce. [DOI] [PubMed] [Google Scholar]
- 147.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67(5):968–77. doi: 10.1161/01.cir.67.5.968. [DOI] [PubMed] [Google Scholar]
- 148.Rimm EB, Stampfer MJ, Giovannucci E, Ascherio A, Spiegelman D, Colditz GA, et al. Body-Size and Fat Distribution as Predictors of Coronary Heart-Disease among Middle-Aged and Older Us Men. American Journal of Epidemiology. 1995;141(12):1117–27. doi: 10.1093/oxfordjournals.aje.a117385. [DOI] [PubMed] [Google Scholar]
- 149.Olijhoek JK, van der Graaf Y, Banga JD, Algra A, Rabelink TJ, Visseren FL, et al. The metabolic syndrome is associated with advanced vascular damage in patients with coronary heart disease, stroke, peripheral arterial disease or abdominal aortic aneurysm. Eur Heart J. 2004;25(4):342–8. doi: 10.1016/j.ehj.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 150.Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. 2005;28(7):1769–78. doi: 10.2337/diacare.28.7.1769. [DOI] [PubMed] [Google Scholar]
- 151.Marso SP, Mercado N, Maehara A, Weisz G, Mintz GS, McPherson J, et al. Plaque composition and clinical outcomes in acute coronary syndrome patients with metabolic syndrome or diabetes. JACC Cardiovasc Imaging. 2012;5(3 Suppl):S42–52. doi: 10.1016/j.jcmg.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 152.Wong ND, Nelson JC, Granston T, Bertoni AG, Blumenthal RS, Carr JJ, et al. Metabolic syndrome, diabetes, and incidence and progression of coronary calcium: the Multiethnic Study of Atherosclerosis study. JACC Cardiovasc Imaging. 2012;5(4):358–66. doi: 10.1016/j.jcmg.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ellison RC, Zhang Y, Wagenknecht LE, Eckfeldt JH, Hopkins PN, Pankow JS, et al. Relation of the metabolic syndrome to calcified atherosclerotic plaque in the coronary arteries and aorta. Am J Cardiol. 2005;95(10):1180–6. doi: 10.1016/j.amjcard.2005.01.046. [DOI] [PubMed] [Google Scholar]
- 154.Yamazoe M, Hisamatsu T, Miura K, Kadowaki S, Zaid M, Kadota A, et al. Relationship of Insulin Resistance to Prevalence and Progression of Coronary Artery Calcification Beyond Metabolic Syndrome Components: Shiga Epidemiological Study of Subclinical Atherosclerosis. Arterioscler Thromb Vasc Biol. 2016;36(8):1703–8. doi: 10.1161/ATVBAHA.116.307612. [DOI] [PubMed] [Google Scholar]
- 155.Lau DCW, Dhillon B, Yan HY, Szmitko PE, Verma S. Adipokines: molecular links between obesity and atheroslcerosis. Am J Physiol-Heart C. 2005;288(5):H2031–H41. doi: 10.1152/ajpheart.01058.2004. [DOI] [PubMed] [Google Scholar]
- 156.Payne GA, Kohr MC, Tune JD. Epicardial perivascular adipose tissue as a therapeutic target in obesity-related coronary artery disease. Brit J Pharmacol. 2012;165(3):659–69. doi: 10.1111/j.1476-5381.2011.01370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Knudson JD, Dincer UD, Zhang C, Swafford AN, Jr., Koshida R, Picchi A, et al. Leptin receptors are expressed in coronary arteries, and hyperleptinemia causes significant coronary endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2005;289(1):H48–56. doi: 10.1152/ajpheart.01159.2004. [DOI] [PubMed] [Google Scholar]
- 158.Zhang H, Park Y, Wu J, Chen X, Lee S, Yang J, et al. Role of TNF-alpha in vascular dysfunction. Clin Sci (Lond) 2009;116(3):219–30. doi: 10.1042/CS20080196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gruen ML, Hao M, Piston DW, Hasty AH. Leptin requires canonical migratory signaling pathways for induction of monocyte and macrophage chemotaxis. Am J Physiol Cell Physiol. 2007;293(5):C1481–8. doi: 10.1152/ajpcell.00062.2007. [DOI] [PubMed] [Google Scholar]
- 160.Goralski KB, McCarthy TC, Hanniman EA, Zabel BA, Butcher EC, Parlee SD, et al. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. Journal of Biological Chemistry. 2007;282(38):28175–88. doi: 10.1074/jbc.M700793200. [DOI] [PubMed] [Google Scholar]
- 161.Dick GM, Katz PS, Farias M, III, Morris M, James J, Knudson JD, et al. Resistin impairs endothelium-dependent dilation to bradykinin, but not acetylcholine, in the coronary circulation. Am J Physiol Heart Circ Physiol. 2006;291(6):H2997–H3002. doi: 10.1152/ajpheart.01035.2005. [DOI] [PubMed] [Google Scholar]
- 162.Yamawaki H, Hara N, Okada M, Hara Y. Visfatin causes endothelium-dependent relaxation in isolated blood vessels. Biochem Biophys Res Commun. 2009;383(4):503–8. doi: 10.1016/j.bbrc.2009.04.074. [DOI] [PubMed] [Google Scholar]
- 163.Noblet JN, Goodwill AG, Sassoon DJ, Kiel AM, Tune JD. Leptin augments coronary vasoconstriction and smooth muscle proliferation via a Rho-kinase-dependent pathway. Basic Res Cardiol. 2016;111(3):25. doi: 10.1007/s00395-016-0545-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ding J, Hsu FC, Harris TB, Liu Y, Kritchevsky SB, Szklo M, et al. The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Clin Nutr. 2009;90(3):499–504. doi: 10.3945/ajcn.2008.27358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Greif M, Becker A, von Ziegler F, Lebherz C, Lehrke M, Broedl UC, et al. Pericardial Adipose Tissue Determined by Dual Source CT Is a Risk Factor for Coronary Atherosclerosis. Arterioscl Throm Vas. 2009;29(5):781–U369. doi: 10.1161/ATVBAHA.108.180653. [DOI] [PubMed] [Google Scholar]
- 166.Owen MK, Noblet JN, Sassoon DJ, Conteh AM, Goodwill AG, Tune JD. Perivascular adipose tissue and coronary vascular disease. Arterioscler Thromb Vasc Biol. 2014;34(8):1643–9. doi: 10.1161/ATVBAHA.114.303033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cheng KH, Chu CS, Lee KT, Lin TH, Hsieh CC, Chiu CC, et al. Adipocytokines and proinflammatory mediators from abdominal and epicardial adipose tissue in patients with coronary artery disease. Int J Obes (Lond) 2008;32(2):268–74. doi: 10.1038/sj.ijo.0803726. [DOI] [PubMed] [Google Scholar]
- 168.McKenney ML, Schultz KA, Boyd JH, Byrd JP, Alloosh M, Teague SD, et al. Epicardial adipose excision slows the progression of porcine coronary atherosclerosis. J Cardiothorac Surg. 2014;9:2. doi: 10.1186/1749-8090-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chatterjee TK, Aronow BJ, Tong WS, Manka D, Tang Y, Bogdanov VY, et al. Human coronary artery perivascular adipocytes overexpress genes responsible for regulating vascular morphology, inflammation, and hemostasis. Physiol Genomics. 2013;45(16):697–709. doi: 10.1152/physiolgenomics.00042.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Noblet JN, Owen MK, Goodwill AG, Sassoon DJ, Tune JD. Lean and Obese Coronary Perivascular Adipose Tissue Impairs Vasodilation via Differential Inhibition of Vascular Smooth Muscle K+ Channels. Arterioscler Thromb Vasc Biol. 2015;35(6):1393–400. doi: 10.1161/ATVBAHA.115.305500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Alpert MA, Hashimi MW. Obesity and the heart. Am J Med Sci. 1993;306(2):117–23. doi: 10.1097/00000441-199308000-00011. [DOI] [PubMed] [Google Scholar]
- 172.Kunju SU, Badarudeen S, Schwarz ER. Impact of obesity in patients with congestive heart failure. Rev Cardiovasc Med. 2009;10(3):142–51. doi: 10.3909/ricm0463. [DOI] [PubMed] [Google Scholar]
- 173.Chess DJ, Stanley WC. Role of diet and fuel overabundance in the development and progression of heart failure. Cardiovasc Res. 2008;79(2):269–78. doi: 10.1093/cvr/cvn074. [DOI] [PubMed] [Google Scholar]
- 174.Khan RS, Chokshi A, Drosatos K, Jiang H, Yu S, Harris CR, et al. Fish oil selectively improves heart function in a mouse model of lipid-induced cardiomyopathy. J Cardiovasc Pharmacol. 2013;61(4):345–54. doi: 10.1097/FJC.0b013e318283d845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kolwicz SC, Jr., Purohit S, Tian R. Cardiac metabolism and its interactions with contraction, growth, and survival of cardiomyocytes. 2013 doi: 10.1161/CIRCRESAHA.113.302095. cartographers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Liu L, Trent CM, Fang X, Son NH, Jiang H, Blaner WS, et al. Cardiomyocyte-specific Loss of Diacylglycerol Acyltransferase 1 (DGAT1) Reproduces the Abnormalities in Lipids Found in Severe Heart Failure. J Biol Chem. 2014;289(43):29881–91. doi: 10.1074/jbc.M114.601864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bahrami H, Bluemke DA, Kronmal R, Bertoni AG, Lloyd-Jones DM, Shahar E, et al. Novel metabolic risk factors for incident heart failure and their relationship with obesity: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;51(18):1775–83. doi: 10.1016/j.jacc.2007.12.048. [DOI] [PubMed] [Google Scholar]
- 178.Ingelsson E, Sundstrom J, Arnlov J, Zethelius B, Lind L. Insulin resistance and risk of congestive heart failure. JAMA. 2005;294(3):334–41. doi: 10.1001/jama.294.3.334. [DOI] [PubMed] [Google Scholar]
- 179.Li C, Ford ES, McGuire LC, Mokdad AH. Association of metabolic syndrome and insulin resistance with congestive heart failure: findings from the Third National Health and Nutrition Examination Survey. J Epidemiol Community Health. 2007;61(1):67–73. doi: 10.1136/jech.2006.048173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Nishimura M, Murase M, Hashimoto T, Kobayashi H, Yamazaki S, Imai R, et al. Insulin resistance and impaired myocardial fatty acid metabolism in dialysis patients with normal coronary arteries. Kidney Int. 2006;69(3):553–9. doi: 10.1038/sj.ki.5000100. [DOI] [PubMed] [Google Scholar]
- 181.Paternostro G, Pagano D, Gnecchi-Ruscone T, Bonser RS, Camici PG. Insulin resistance in patients with cardiac hypertrophy. Cardiovasc Res. 1999;42(1):246–53. doi: 10.1016/s0008-6363(98)00233-8. [DOI] [PubMed] [Google Scholar]
- 182.Tuunanen H, Engblom E, Naum A, Scheinin M, Nagren K, Airaksinen J, et al. Decreased myocardial free fatty acid uptake in patients with idiopathic dilated cardiomyopathy: evidence of relationship with insulin resistance and left ventricular dysfunction. Journal of cardiac failure. 2006;12(8):644–52. doi: 10.1016/j.cardfail.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 183.Gejl M, Sondergaard HM, Stecher C, Bibby BM, Moller N, Botker HE, et al. Exenatide alters myocardial glucose transport and uptake depending on insulin resistance and increases myocardial blood flow in patients with type 2 diabetes. J Clin Endocrinol Metab. 2012;97(7):E1165–9. doi: 10.1210/jc.2011-3456. [DOI] [PubMed] [Google Scholar]
- 184.Nikolaidis LA, Mankad S, Sokos GG, Miske G, Shah A, Elahi D, et al. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004;109(8):962–5. doi: 10.1161/01.CIR.0000120505.91348.58. [DOI] [PubMed] [Google Scholar]
- 185.Vest AR. Incretin-related drug therapy in heart failure. Current heart failure reports. 2015;12(1):24–32. doi: 10.1007/s11897-014-0232-6. [DOI] [PubMed] [Google Scholar]
- 186.Wing R, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, et al. Cardiovascular Effects of Intensive Lifestyle Intervention in Type 2 Diabetes. New Engl J Med. 2013;369(2):145–54. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.James WP, Caterson ID, Coutinho W, Finer N, Van Gaal LF, Maggioni AP, et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med. 2010;363(10):905–17. doi: 10.1056/NEJMoa1003114. [DOI] [PubMed] [Google Scholar]
- 188.Comerma-Steffensen S, Grann M, Andersen CU, Rungby J, Simonsen U. Cardiovascular effects of current and future anti-obesity drugs. Curr Vasc Pharmacol. 2014;12(3):493–504. doi: 10.2174/1570161112666140423223529. [DOI] [PubMed] [Google Scholar]
- 189.Lavie CJ, Milani RV, Artham SM, Patel DA, Ventura HO. The obesity paradox, weight loss, and coronary disease. Am J Med. 2009;122(12):1106–14. doi: 10.1016/j.amjmed.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 190.Sjostrom L, Peltonen M, Jacobson P, Ahlin S, Andersson-Assarsson J, Anveden A, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA. 2014;311(22):2297–304. doi: 10.1001/jama.2014.5988. [DOI] [PubMed] [Google Scholar]
- 191.Busetto L, De Stefano F, Pigozzo S, Segato G, De Luca M, Favretti F. Long-term cardiovascular risk and coronary events in morbidly obese patients treated with laparoscopic gastric banding. Surg Obes Relat Dis. 2014;10(1):112–20. doi: 10.1016/j.soard.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 192.Shin SH, Lee YJ, Heo YS, Park SD, Kwon SW, Woo SI, et al. Beneficial Effects of Bariatric Surgery on Cardiac Structure and Function in Obesity. Obes Surg. 2016 doi: 10.1007/s11695-016-2330-x. [DOI] [PubMed] [Google Scholar]
- 193.Valenta I, Dilsizian V, Quercioli A, Jungling FD, Ambrosio G, Wahl R, et al. Impact of obesity and bariatric surgery on metabolism and coronary circulatory function. Curr Cardiol Rep. 2014;16(1):433. doi: 10.1007/s11886-013-0433-8. [DOI] [PubMed] [Google Scholar]
- 194.Jay MA, Ren J. Peroxisome proliferator-activated receptor (PPAR) in metabolic syndrome and type 2 diabetes mellitus. Curr Diabetes Rev. 2007;3(1):33–9. doi: 10.2174/157339907779802067. [DOI] [PubMed] [Google Scholar]
- 195.Chen WR, Hu SY, Chen YD, Zhang Y, Qian G, Wang J, et al. Effects of liraglutide on left ventricular function in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am Heart J. 2015;170(5):845–54. doi: 10.1016/j.ahj.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 196.Lonborg J, Vejlstrup N, Kelbaek H, Botker HE, Kim WY, Mathiasen AB, et al. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur Heart J. 2012;33(12):1491–9. doi: 10.1093/eurheartj/ehr309. [DOI] [PubMed] [Google Scholar]
- 197.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016;375(4):311–22. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Santulli G, Iaccarino G, De Luca N, Trimarco B, Condorelli G. Atrial fibrillation and microRNAs. Front Physiol. 2014;5:15. doi: 10.3389/fphys.2014.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Volny O, Kasickova L, Coufalova D, Cimflova P, Novak J. microRNAs in Cerebrovascular Disease. Adv Exp Med Biol. 2015;888:155–95. doi: 10.1007/978-3-319-22671-2_9. [DOI] [PubMed] [Google Scholar]
- 200.Novak J, Olejnickova V, Tkacova N, Santulli G. Mechanistic Role of MicroRNAs in Coupling Lipid Metabolism and Atherosclerosis. Adv Exp Med Biol. 2015;887:79–100. doi: 10.1007/978-3-319-22380-3_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Santulli G. MicroRNAs and Endothelial (Dys) Function. J Cell Physiol. 2016;231(8):1638–44. doi: 10.1002/jcp.25276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wronska A, Kurkowska-Jastrzebska I, Santulli G. Application of microRNAs in diagnosis and treatment of cardiovascular disease. Acta Physiol (Oxf) 2015;213(1):60–83. doi: 10.1111/apha.12416. [DOI] [PubMed] [Google Scholar]
- 203.Philip-Couderc P, Smih F, Hall JE, Pathak A, Roncalli J, Harmancey R, et al. Kinetic analysis of cardiac transcriptome regulation during chronic high-fat diet in dogs. Physiol Genomics. 2004;19(1):32–40. doi: 10.1152/physiolgenomics.00001.2004. [DOI] [PubMed] [Google Scholar]
- 204.Ortega FJ, Mercader JM, Moreno-Navarrete JM, Rovira O, Guerra E, Esteve E, et al. Profiling of circulating microRNAs reveals common microRNAs linked to type 2 diabetes that change with insulin sensitization. Diabetes Care. 2014;37(5):1375–83. doi: 10.2337/dc13-1847. [DOI] [PubMed] [Google Scholar]
- 205.Fernandez-Hernando C, Ramirez CM, Goedeke L, Suarez Y. MicroRNAs in metabolic disease. Arterioscler Thromb Vasc Biol. 2013;33(2):178–85. doi: 10.1161/ATVBAHA.112.300144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Grueter CE, van Rooij E, Johnson BA, DeLeon SM, Sutherland LB, Qi X, et al. A cardiac microRNA governs systemic energy homeostasis by regulation of MED13. Cell. 2012;149(3):671–83. doi: 10.1016/j.cell.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hinkel R, Penzkofer D, Zuhlke S, Fischer A, Husada W, Xu QF, et al. Inhibition of microRNA-92a protects against ischemia/reperfusion injury in a large-animal model. Circulation. 2013;128(10):1066–75. doi: 10.1161/CIRCULATIONAHA.113.001904. [DOI] [PubMed] [Google Scholar]
- 208.Ganesan J, Ramanujam D, Sassi Y, Ahles A, Jentzsch C, Werfel S, et al. MiR-378 controls cardiac hypertrophy by combined repression of mitogen-activated protein kinase pathway factors. Circulation. 2013;127(21):2097–106. doi: 10.1161/CIRCULATIONAHA.112.000882. [DOI] [PubMed] [Google Scholar]
- 209.Fiedler J, Thum T. MicroRNAs in myocardial infarction. Arterioscler Thromb Vasc Biol. 2013;33(2):201–5. doi: 10.1161/ATVBAHA.112.300137. [DOI] [PubMed] [Google Scholar]
- 210.Thome JG, Mendoza MR, Cheuiche AV, La Porta VL, Silvello D, Dos Santos KG, et al. Circulating microRNAs in obese and lean heart failure patients: A case-control study with computational target prediction analysis. Gene. 2015;574(1):1–10. doi: 10.1016/j.gene.2015.07.068. [DOI] [PubMed] [Google Scholar]
- 211.Wende AR. Post-translational modifications of the cardiac proteome in diabetes and heart failure. Proteomics Clin Appl. 2016;10(1):25–38. doi: 10.1002/prca.201500052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.de Weger RA, Schipper ME, Siera-de Koning E, van der Weide P, van Oosterhout MF, Quadir R, et al. Proteomic profiling of the human failing heart after left ventricular assist device support. J Heart Lung Transplant. 2011;30(5):497–506. doi: 10.1016/j.healun.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 213.Chugh S, Suen C, Gramolini A. Proteomics and mass spectrometry: what have we learned about the heart? Curr Cardiol Rev. 2010;6(2):124–33. doi: 10.2174/157340310791162631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Cieniewski-Bernard C, Mulder P, Henry JP, Drobecq H, Dubois E, Pottiez G, et al. Proteomic analysis of left ventricular remodeling in an experimental model of heart failure. J Proteome Res. 2008;7(11):5004–16. doi: 10.1021/pr800409u. [DOI] [PubMed] [Google Scholar]
- 215.Arab S, Gramolini AO, Ping P, Kislinger T, Stanley B, van Eyk J, et al. Cardiovascular proteomics: tools to develop novel biomarkers and potential applications. J Am Coll Cardiol. 2006;48(9):1733–41. doi: 10.1016/j.jacc.2006.06.063. [DOI] [PubMed] [Google Scholar]
- 216.Barallobre-Barreiro J, Didangelos A, Schoendube FA, Drozdov I, Yin X, Fernandez-Caggiano M, et al. Proteomics analysis of cardiac extracellular matrix remodeling in a porcine model of ischemia/reperfusion injury. Circulation. 2012;125(6):789–802. doi: 10.1161/CIRCULATIONAHA.111.056952. [DOI] [PubMed] [Google Scholar]
- 217.Liu T, Chen L, Kim E, Tran D, Phinney BS, Knowlton AA. Mitochondrial proteome remodeling in ischemic heart failure. Life Sci. 2014;101(1-2):27–36. doi: 10.1016/j.lfs.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Littlejohns B, Heesom K, Angelini GD, Suleiman MS. The effect of disease on human cardiac protein expression profiles in paired samples from right and left ventricles. Clin Proteomics. 2014;11(1):34. doi: 10.1186/1559-0275-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Mitra A, Basak T, Ahmad S, Datta K, Datta R, Sengupta S, et al. Comparative Proteome Profiling during Cardiac Hypertrophy and Myocardial Infarction Reveals Altered Glucose Oxidation by Differential Activation of Pyruvate Dehydrogenase E1 Component Subunit beta. J Mol Biol. 2015;427(11):2104–20. doi: 10.1016/j.jmb.2014.10.026. [DOI] [PubMed] [Google Scholar]