Abstract
Introduction
This paper describes the rationale and methodology a study which investigates mind-body treatment versus pharmacotherapy for women with urgency urinary incontinence (UUI). To explore brain associations in UUI, a subset of patients will also undergo functional magnetic resonance imaging (fMRI). We hypothesize that hypnotherapy a mind-body intervention, will be at least as effective pharmacotherapy in treating UUI. We also hypothesize that fMRI findings will change following treatment, with changes potentially differing between groups.
Methods
The purpose of this manuscript is to recount the development and design challenges of a study evaluating the efficacy of hypnotherapy compared to conventional pharmacotherapy in UUI treatment. The study randomizes women to either of these treatments and outcome measures include bladder diaries and validated questionnaires. Sample size estimates, based on a non-inferiority test (alpha=.025, beta=0.20), after considering drop-out/loss to follow-up, indicated approximately 150 woman would be required to test the hypothesis that hypnotherapy is non-inferior to pharmacotherapy within a 5% non-inferiority margin. The study will also evaluate fMRI change in a subset of participants before and after therapy. Study challenges included designing a study with a mind-body therapy and a comparison treatment equally acceptable to participants, standardizing the interventions, confronting the reality that trials are time-consuming for participants and making appropriate accommodations.
Results
Study enrollment began March 2013 and is ongoing.
Conclusions
This manuscript details the design a of randomized controlled trial (RCT) comparing mind-body therapy to medications in treatment of UUI and describes the challenges encountered in its planning.
Keywords: Women with urgency urinary incontinence, hypnosis/hypnotherapy, anti-cholinergics, complementary alternative integrative medicine
Introduction
Urgency urinary incontinence (UUI) is common, economically burdensome and life-altering [1,2]. Medications, standard treatment for UUI, are limited by their side-effects relative to their efficacy [3]. UUI focus groups highlight patients’ “desperate quest for (its) cause…and cure”[4]. Inadequate understanding of UUI pathophysiology has critically hampered clinician’s ability to meet patient needs. Increased awareness that UUI is a functional disorder may enhance its understanding. Functional disorders, including irritable bowel syndrome (IBS) and UUI, exhibit increased visceral sensitivity to physiologic stimulation (e.g. bowel or bladder distension). This increased visceral sensitivity is of uncertain etiology, confounding the ability to treat these disorders.
Recently, brain imaging studies have reported differences in brain activity in patients with functional disorders, including those with IBS [5,6] and UUI [7,8,9], indicating existence of a brain-body connection. These findings raise the possibility that brain-body interventions may play a role in their treatment. Mind-body therapies have been reported to improve IBS symptoms [10,11] and results from our group’s hypnotherapy pilot study and reports by others also suggest mind-body treatments improve UUI [12,13]. Based on these findings, we designed a randomized controlled trial (RCT) to compare the efficacy of hypnotherapy to pharmacotherapy in treatment of women with UUI. Using functional magnetic resonance imagining (fMRI), this same study is also designed to assess potential brain changes following UUI treatment. The current article describes this study’s design and the challenges encountered in its implementation.
Methods
Study Aims and Hypotheses
This study has two components. The clinical component is an RCT comparing efficacy of hypnotherapy to medication in UUI treatment. We hypothesize that among patients with UUI, hypnotherapy is at least as effective as pharmacotherapy in diminishing symptom severity of UUI. The study’s translational component uses fMRI to investigate a subgroup of women enrolled in the RCT, with fMRI performed prior to and following treatment. We hypothesize that hypnotherapy will decrease abnormal brain activation in UUI patients and will modulate brain functional connectivity, and will investigate whether this normalization will be greater in hypnotherapy compared to pharmacotherapy. The fMRI methods and baseline findings have been previously published [14].
The primary aim of this study’s clinical component is to compare the effectiveness of hypnotherapy compared to pharmacotherapy based on 3-day voiding diaries following 8 weeks of treatment. Secondary clinical aims include evaluation of UUI symptom and sexual function change and potential change in other functional abnormalities (including IBS, interstitial cystitis) based on validated questionnaires at 8 weeks. All parameters will also be evaluated at 6 and 12 month follow-up.
The fMRI portion of this study is based on prior work which found that women with UUI differ in brain activation on fMRI compared to controls [7,8, 9]. FMRI uses brain oxygen level dependent (BOLD) signal on brain imaging as an indicator of neuronal activity. FMRI researchers commonly administer stimuli, or “tasks”, to elicit change in BOLD signal to compare differences between patients and controls [14]. UUI researchers have commonly used bladder filling as that task [7,8,14]. In addition to task oriented brain activation, specific brain regions also demonstrate similar fluctuations in BOLD signal over time that are intrinsic in nature [15]. Areas of the brain that show coherent neural activation and deactivation, are described as demonstrating “functional” connectivity”, and together constitute “functional networks”. One method of assessing these functional networks is to use brain sites that activate during a task as seed points and to then evaluate if these seed points co-activate with other sites when the brain is at rest. This is called “seed-based resting state connectivity analysis” [15]. Seed-based resting state connectivity analysis will be used in this study to compare network differences between patients and controls before and after treatment. One benefit of this resting state analysis is that it minimizes confounding effects of subject adaptation to a repeated task.
The translational aims of the fMRI portion of this study are to determine whether brain activation and connectivity change following treatment, to determine whether hypnotherapy is associated with greater modification of brain activation and connectivity than pharmacotherapy, and to evaluate whether fMRI changes are associated with treatment efficacy. We have reported the fMRI methodology and baseline differences in patients and controls [14], and will evaluate fMRI changes in patients following RCT interventions at 8 weeks.
Overall Study Design & Study Flow of Participants
Figure 1 illustrates UUI participants’ progression through this study. The medication group receives anti-cholinergic medication with weekly interaction with a pharmacotherapy counselor for 8 weeks. The hypnotherapy group receives weekly hypnotherapy sessions with a licensed clinical hypnotherapist for 8 weeks. Participants complete initial follow-up after 8 weeks of treatment. The medication group continues anti-cholinergics and the hypnotherapy group is encouraged to practice self-hypnosis with follow-up at 6 and 12 months. The study received IRB approval (#09-314) and all participants give written, informed consent.
Figure 1.
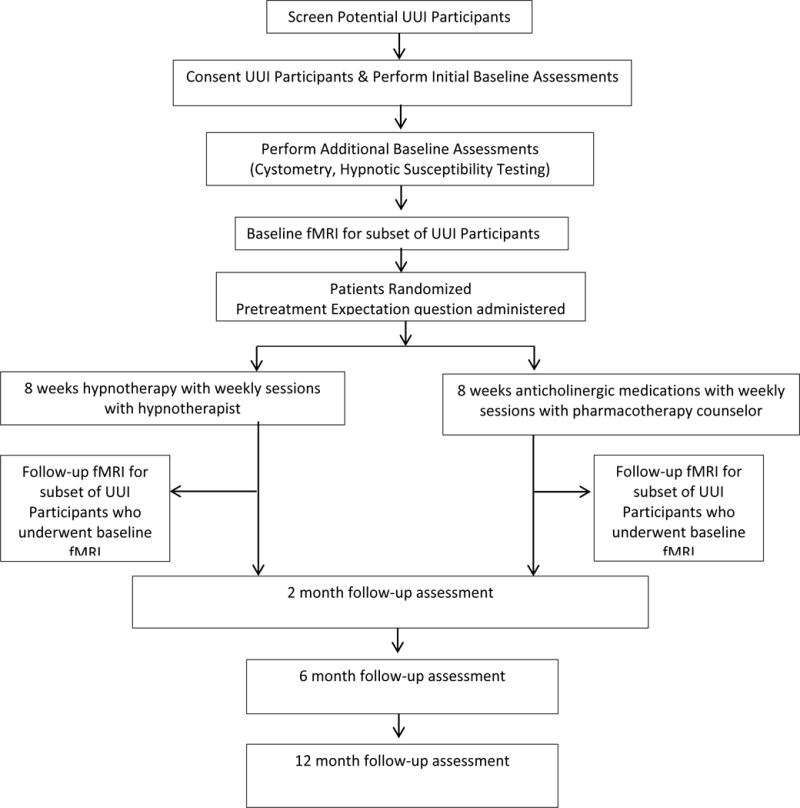
Participant Flow Diagram
Inclusion & Exclusion Criteria
The study targets women with idiopathic UUI who are naïve to therapy or with minimal exposure to medication therapy. Participants’ Inclusion/Exclusion Criteria are noted in Table 1.
Table 1.
Study Inclusion and Exclusion Criteria
| Randomized Clinical Trial |
| Inclusion Criteria |
|
| Exclusion Criteria |
|
|
|
| Functional MRI Study |
| Inclusion Criteria UUI Participants |
|
| Exclusion Criteria UUI Participants |
|
| Inclusion Criteria Controls |
|
| Exclusion Criteria Controls |
|
Participant Screening, Recruitment, Enrollment
Potential study candidates are recruited from the University of New Mexico Health Sciences Center Clinics and the community. Recruitment tools include study flyers, radio advertisements, community outreach seminars and informational sessions for primary care providers. Potential candidates are identified by clinicians and study coordinators and are administered the OAB Awareness Tool, a validated tool which clinically screens for OAB symptoms; participants are required to have a score <8 [16]. Coordinators fully discuss the study with candidates, provide them a copy of the consent and allow appropriate time to consider study participation. Upon consent, women are enrolled and baseline evaluations are administered.
Randomization & Masking
Approximately 150–160 women will be randomized to either hypnotherapy or pharmacotherapy after baseline assessments are completed. The randomization scheme is computer generated with varying permuted block sizes of 4–8, stratified for fMRI performance and baseline UUI severity (<4 UUI episodes versus ≥4 episodes on 3 day voiding diaries). Group assignments were placed in opaque, sealed envelopes at study initiation by individuals otherwise unassociated with the study. These are opened at randomization.
The interventions do not allow participant masking (i.e. participants know whether they are receiving medications or hypnotherapy). The study personnel coordinating study visits, hypnotherapists and medication counselors are not masked. The investigators, personnel responsible for primary data entry, and the statistician are masked to participant treatment. Data collected includes only participants’ research number in order to maintain masking. Double data entry is performed and data are reviewed for discrepancies which are reconciled quarterly.
RCT Interventions
During the baseline study visit all participants receive written information regarding standard behavioral therapy, including lifestyle modification and pelvic floor exercise.
Description of Hypnosis & Hypnotherapist Requirements
Hypnotherapy is, “The induction of a trance-like state to facilitate relaxation and…use of enhanced suggestibility to treat psychological and medical conditions and affect behavioral changes” [17]. Hypnosis is often induced by eye closure, followed by progressive muscular relaxation and deepening techniques. Suggestions for normalization of function and sensitivity are made using both imagery and conditioning techniques [18]. The American Psychological Association described ‘introduction’ and ‘suggestion’ as necessary components, stating, “Hypnosis typically involves an introduction…during which the patient is told that suggestions for imaginative experiences will be presented. The hypnotic induction is an extended initial suggestion for using one’s imagination, and may contain further elaborations of the introduction. The hypnotic procedure is used to encourage and evaluate responses to suggestions. When using hypnosis, one person (the subject) is guided by another (the hypnotist) to respond to suggestions for changes in subjective experience, alterations in perception, sensation, emotion, thought or behavior” [19, 20]. Investigators have suggested that hypnosis alters sensory awareness and cognitive processing in response to external stimuli and, on fMRI, modulates activation of the limbic cortex in response to those stimuli [21, 22]. All study hypnotherapists are members of the International Board of Hypnotherapy, are board certified, have completed > 400 hours of hypnotherapy training from a state licensed school and passed a written exam. The board requires members to adhere to their scope of practice and code of ethics, maintain independent liability coverage and complete annual continuing education requirements.
Hypnotherapists & Hypnotherapy Sessions
Participants are assigned to one of four clinical hypnotherapists affiliated with the study who administer the sessions in a standardized fashion (Table 2). The study’s Co-Investigator (RS), a physician, certified hypnotherapist and hypnotherapy instructor, co-authored the study’s hypnotherapy manual; study hypnotherapists received 4 hours of additional training from RS and are clinically overseen by him. Study sessions are administered as eight, one-hour sessions over 8 weeks in outpatient offices.
Table 2.
RCT Interventions
| Session | Hypnotherapy | Pharmacotherapy |
|---|---|---|
| Session #1 | Give study overview, explain bladder physiology, UUI pathophysiology, hypnotherapy principles, discuss any fears/concerns. Perform hypnosis; induction/relaxation/deepening/therapeutic suggestions/terminate hypnosis. Debrief. | Give study overview, explain bladder physiology, UUI pathophysiology, discuss medications & mechanism of action, discuss any fears/concerns. Medication instructions given and medication dispensed. |
| Session #2 | Review change/persistence in UUI symptoms & prior week’s experiences, address UUI associated emotions/life impact, introduce self-hypnosis. Proceed with hypnosis/debrief (See Session #1). | Review change/persistence in UUI symptoms & prior week’s experiences, tolerability of meds discussed. Coping mechanisms re: side effects discussed. Arrangements made for medication change if needed. |
| Session #3 | Provide/discuss digital recording specifically prepared for participant hypnotherapy home practice, emphasizes ego strengthening. Proceed with hypnosis/debrief (See Session #1). | Review change/persistence in UUI symptoms & prior week’s experiences, tolerability of meds discussed. Coping mechanisms re: side effects discussed. Arrangements made for medication change if needed. |
| Session #4 | Assist participant in developing a “healing script.” Proceed with hypnosis/debrief (See Session #1). | Same as above. Medication instructions given and medication dispensed. |
| Session #5 | Have patient develop own therapeutic suggestions. Proceed with hypnosis/debrief (See Session #1). | Same as Session #3. |
| Session #6 | Have participant reflect on their past including responses resulting in current UUI associated behavior; consider how to alter behaviors. Proceed with hypnosis/debrief (See Session #1). | Discuss urge incontinence triggers & participant encouraged to develop coping mechanisms. Same as above. |
| Session #7 | Focus on integration of inner & outer resources to improve health. Proceed with hypnosis/debrief (See Session #1). | Same as above. |
| Session #8 | Focus on healing imagery & continuation of self-hypnosis. Proceed with hypnosis/debrief (See Session #1). | Same as above. Counselor discusses potential setbacks & emphasizes these are temporary. Side-effects & coping mechanisms reviewed. Medications dispensed |
Description of Medications
Long acting oxybutynin 10 mg/day is initially dispensed to women randomized to pharmacotherapy. The alternate medication is long acting tolterodine 4mg/day. If the participant previously took oxybutynin with marginal success, tolterodine is given as an alternative. Participants may switch between these medications if they find the side-effects/efficacy of either medication unacceptable.
Medication Counselor & Medication Counseling Sessions
Sessions are administered in eight sessions over 8 weeks with the medication counselor in an outpatient setting (Table 2). The counselor, a research coordinator with medical background whose only responsibility to the study is to administer medication counseling, received 4 hours of counseling instruction from a study investigator (YK). The sessions are administered according to the study’s pharmacotherapy counseling manual. Sessions take as long as required for subjects to feel that they have received adequate counseling. Participants may choose in person visits or phone follow-up visits. The initial session takes approximately 40–60 minutes, with follow-up contact times dependent on the patient’s needs (typically 10–20 minutes). In-person visits are mandatory for medication dispensing visits (weeks 1, 4 and 8).
Description of fMRI Procedures
Approximately 60–70 UUI patients will participate in the baseline and 8 week follow-up fMRI study. (Figure 1). Details of the procedures, including the fMRI task, data acquisition and processing, and analytic methods have been previously published [14]. In brief, prior to fMRI, bedside cystometry is performed. During the cystometry visit, fMRI participants undergo an abbreviated version of the bladder filling and emptying “task”, with task volume based on cystometric strong desire to void volumes. The abbreviated task is performed at cystometry visits to allow patients to understand what will happen during fMRI, minimizing patient anxiety. The fMRI is performed at a separate visit. On the day of the fMRI participants undergo scanning during rest for the “resting state connectivity analysis” and during task performance [14].
Outcome Measures & Other Measured Variables
RCT Outcome Measures
RCT study outcomes are measured prior to randomization, after 2 months of treatment and at six and twelve month follow-up. The primary outcome measure is the between group (pharmacotherapy versus hypnotherapy) change in UUI episodes at two months based on 3-day voiding diary. Diaries are evaluated, as outlined in the study manual, by at least 2 research coordinators. Disagreement in diary interpretation are adjudicated at a meeting attended by at least 3 research personnel. Secondary outcomes include between group differences in UUI episodes on voiding diary at 6 and 12 months, between group differences in UUI symptoms and sexual function based on validated questionnaires (OABq-SF [23], Incontinence Severity Index [24], Patient Perception of Bladder Condition (PPBC) [25], Prolapse & Incontinence Sexual Questionnaire-12 [26]) at 2,6, and 12 months. Other functional abnormalities which may co-occur with UUI will be measured, including Interstitial Cystitis (IC/BPS) and irritable bowel syndrome (IBS) based on validated questionnaires (the Bladder Pain Interstitial Cystitis Symptom Scale [27] including an additional visual analogue scale which documents the duration of symptoms, Colo-Rectal Anal Distress Inventory-8 [28], and the IBS Diagnostic Module [29] administered at baseline and 2/6/12 months. The questionnaires are further described in Table 3.
Table 3.
| Questionnaire Name | Questionnaire Abbreviation | Description | Scoring |
|---|---|---|---|
| Overactive Bladder Questionnaire Short Form | OAB-qSF | This validated questionnaire for Overactive Bladder consists of symptom and quality of life components.* **It received a grade A designation from the International Continence Society *** | Scores range from 0–100 Quality of life: higher scores represent better quality of life Symptoms: higher scores represent greater symptoms |
| Incontinence Severity Index (also called the Sandvik Incontinence Severity Index) | ISI | Two item questionnaire which is a validated measure of incontinence severity.**** | Range in scores 0–12 0=no incontinence 1–2=slight 3–6=moderate 8–9=severe 12=very severe |
| Patient Perception of Bladder Condition | PPBC | Validated single item global measure of bladder symptoms. Minimally important difference ***** | Change in scores baseline to follow-up Major improvement≤ −2 Minor improvement=−1 No change=0 deterioration≥+1 |
| Prolapse and Incontinence Sexual Questionnaire-12 (short form) | PISQ-12 | Validated questionnaire measuring sexual function in sexually active heterosexual women with prolapse or incontinence | Range in scores 0–48 Higher scores represent better sexual function |
| Bladder Pain Interstitial Cystitis Symptom Scale | BPIC-SS | Validated questionnaire measuring bladder pain symptoms | Range 0–38 Mild symptoms=15.6 Moderate symptoms=17.1 Severe symptoms symptoms ≥28 |
| Colorectal-anal Distress Inventory-8 | CRADI-8 | The bowel subscale of the Pelvic Floor Distress Inventory Short-Form, validated in women with bowel symptoms and pelvic floor disorders. | Range 0–10 Higher score represents more distress |
| Irritable Bowel Syndrome Diagnostic Module (Rome III IBS Module) | IBS Diagnostic Module | Validated questionnaire to diagnosis IBS and type of IBS. Scores are not tallied; branching logic arrives at a diagnosis. | To diagnosis IBS: MUST answer:
MUST meet criteria of 2 of 3 questions below:
IBS Constipation:
IBS Diarrhea:
IBS Mixed:
IBS U:
|
Other Variables Measured
Patient characteristics are obtained at enrollment, including age, parity, surgical and medical history. Body mass index and vital signs are recorded. The pelvic organ prolapse quantitation (POP-Q) exam is performed if POP-Q results are unavailable within the last year. A clinical hypnotherapist administers the Stanford Hypnotic Susceptibility Scale [30] to all participants, the results of which will be analyzed at study completion to evaluate whether hypnotic susceptibility affects treatment efficacy. A trained research nurse performs bedside cystometric testing prior to randomization. The participants enrolled in the fMRI portion of the study undergo fMRI at a separate visit prior to randomization. Immediately following randomization and before treatment, all participants answer the question, ”I expect that my treatment will improve my urgency urinary incontinence problems” with 5 answers ranging from “strongly disagree” to “strongly agree” to measure the association between participant expectations and treatment efficacy. At treatment follow-up, participants complete the statement, “My treatment met my expectations for treating my urgency urinary incontinence problems,” answers ranging from “strongly disagree” to “strongly agree”.
Analytic Strategies
RCT Sample size calculation
Investigators performed sample size calculations for change in UUI episodes and change in OABq-SF scores based on pilot data and the literature,[3,12] utilizing a non-inferiority design for the primary outcome (UUI episode changes in hypnotherapy versus pharmacotherapy). A one-sided non-inferiority test with alpha = 0.25 and a non-inferiority margin of 5% was used. If μ_h is the population mean percent reduction in UUI episodes for hypnotherapy, and μ_m is the population mean percent reduction in UUI episodes for medication, investigators will test the hypothesis (H_0:μ_h-μ_m≤−5) against the one-sided alternative (H_0:μ_h-μ_m>−5) using a significance level of 0.025. If the null hypothesis is rejected, investigators may conclude hypnotherapy is not inferior to medication, on average, by more than 5%, and in fact may be superior to medication therapy, in percent reduction in incontinence episodes. The test may be performed by computing the lower 97.5% one-sided confidence bound for μ_h-μ_m. If the lower confidence bound exceeds −5% investigators will conclude non-inferiority of hypnotherapy. Superiority will be concluded if the lower bound exceeds zero. Assuming drop-out, withdrawal, missing data rates as high as 33%, approximately 52 subjects will be available for analysis in both groups. If μ_h-μ_m ≥ 9%, sample size would provide power ≥ 80% for non-inferiority testing. Improvement between groups will be compared at baseline and 2, 6, 12 month follow-up.
RCT Analysis
Parameters will be compared using a generalized linear mixed model analysis. If baseline differences between groups are found, appropriate variables will be added to the analysis as covariates. Primary analyses will be performed using intention to treat. Per protocol analysis will additionally be performed. Similar analyses will be performed comparing group differences in change in questionnaire scores.
Analysis for fMRI
Whole brain voxel wise analysis will be performed evaluating BOLD signal during bladder filling of pretreatment UUI and controls as described previously [14]. Task related differences between UUI and control brain activation were observed in multiple sites including the cingulate cortex and insula. For fMRI evaluation at 2 month follow-up compared to baseline, investigators will measure between and within group change in activation and connectivity. A linear mixed effects model will be used.
Results
Study enrollment began March 2013 and is ongoing. Analysis awaits initial 2 month study completion, projected to occur the end of 2016 with 1 year follow-up occurring in 2017. The control versus UUI patient baseline fMRI findings have been reported [14].
Discussion
The current manuscript describes the study’s methodology, the difficulties encountered and approaches for overcoming these difficulties.
Study Challenges
Feasibility & Recruitment Challenges
A major challenge was to design a study with differing interventions (behavioral versus pharmacologic) but acceptable to potential participants. Hypnotherapy provides significant personal contact with the hypnotherapist. We strengthened the interpersonal contact associated with the pharmacotherapy treatment arm with the addition of a medication counselor. The delivery of routine/standard-of-care behavioral therapy for both treatment arms was made more comparable by; providing all participants with a life-style handouts at study entry (reviewing fluid intake, timed voiding, urge suppression strategies), and discussing life-style modifications weekly with hypnotherapy and medication counselors.
Another challenge for this and any behavioral study is the time-consuming nature of the interventions. It is difficult for women to commit to eight weekly treatment sessions. Time demands could deter enrollment. We address this problem by; 1) having hypnotherapists available for sessions during evenings and week-ends 2) having the medication counselor contact patients at times patients deem convenient, offering phone calls if patients prefer. Due to these efforts, enrollment continues within its projected time-frame. Unlike many studies which evaluate behavioral or pharmacotherapy interventions, this study will follow participants for one year. The aim is to evaluate whether the interventions have longer term acceptability and durability.
Group Assignment Challenges
As with any RCT, participants cannot choose their intervention. Studies cannot rid participants of their pre-treatment biases or pre-conceptions regarding differences in treatment efficacy. Participants complete an expectation of treatment success question following randomization and prior to treatment to ascertain if pre-treatment expectations are associated with treatment success.
Standardization of Treatments
To address this issue, both treatment interventions are clearly outlined in Treatment Manuals which were reviewed with the interventionists (hypnotherapists, medication counsellors) at study initiation. Interventionists received equal amounts of educational time and instruction regarding these. Pharmacotherapy and hypnotherapy sessions are recorded and audited by study personnel who are uninvolved with study design and analysis. Auditors evaluate the sessions using fidelity checklists to ensure the interventionists include the important elements of treatment.
FMRI Challenges
Design and implementation of the fMRI task presented a unique set of challenges, as discussed more fully in a previous manuscript [14]. To allay unnecessary participant anxiety and decrease likelihood that fluid infusions would result in incontinence, participants underwent simulation of the task following cystometric testing. For the occasional patient observed by the research nurse to have undue anxiety during the simulation, the fluid volumes were adjusted to pre-determined lower volumes outlined in the manual of operations. Participants also undergo repeated education regarding what to expect in the scanner on the day of the fMRI. FMRI personnel use a script to inform the patients what to expect just prior to performance of the fMRI. The study coordinator accompanies the patient to the fMRI scanner, remaining in the scan room to decrease patient anxiety.
Potential Study Benefits
This study has a number of potential benefits. Women in this study, similar to enrollees in other trials, benefit from the individualized attention and education they receive as study participants. Study coordinators and counselors have repeated contact with all study participants. The 6 and 12 month follow-up will evaluate whether these benefits are durable over the longer term.
This study is, to our knowledge, the only trial which compares this novel treatment, hypnosis, to a standard treatment, anti-cholinergic medication. A 1982 case series of 50 women with UUI reported that following 12 hypnotherapy sessions, 29 (or 58%) were symptom-free, an additional 14 (or 28%) were improved and 7 (or 14%) remained unchanged [13]. Despite these reported improvements, an appropriately powered RCT utilizing hypnotherapy in UUI is lacking. Should this endeavor find that hypnotherapy treats UUI as effectively as standard therapy, it could expand therapeutic options for women with this chronic condition. Furthermore, it could pioneer efforts to treat UUI with other mind-body therapies.
The fMRI portion of the study could also benefit UUI clinicians and patients by helping these groups understand how the mind is affected by UUI and understand the role neuroplasticity plays in this condition. It may highlight fMRI similarities or differences associated with a mind-body therapy compared to pharmacotherapy.
This study does have several limitations which include participant’s time required for the RCT, potential lack of availability of board certified hypnotherapists, and the possibility that fMRI changes will not be found in patients following treatment. Strengths of the study, as noted above, include the standardization of both interventions, the plan to follow participants for one year and it RCT-design comparing hypnotherapy to pharmacotherapy in UUI.
In summary, this manuscript elucidates the problems encountered in this study’s design and implementation, comparing a complementary alternative/integrative health therapy to traditional therapy. Its lessons may assist others in designing future mind/body research.
Acknowledgments
Funding:
Research reported in this publication was supported by the1) National Center for Complementary & Integrative Health of the National Institutes of Health under Award Number R01AT007171 2)National Center for Research Resources and the National Center for Advancing Translational Sciences of the National Institutes of Health through Grant Number 8UL1TR000041, The University of New Mexico Clinical and Translational Science Center.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The study is registered with ClinicalTrials.gov; https://clinicaltrials.gov ID#: NCT01829425
Disclosures:
The authors below have the additional disclosures; Rebecca Rogers MD: UpToDate author, American Board Obstetrics and Gynecology, American College Obstetrics and Gynecology author, International Urogynecologic Association editor.
Robert Sapien MD: International Board of Hypnotherapy President, Board Member Global Hypnotherapy Advancement Foundation
Timothy Simmerman-Sierra: International Board of Hypnotherapy, Board Member Global Hypnotherapy Advancement Foundation
Contributions:
Y. Komesu: Protocol Development, Data Collection, Manuscript writing/editing
L. Ketai: Protocol Development, Data Collection Manuscript writing/editing
R. Sapien: Protocol Development, Manuscript writing/editing
R. Rogers: Protocol Development, Data Collection, Manuscript writing/editing
R. Schrader: Protocol Development, Data Analysis, Manuscript writing/editing
T. Simmerman: Protocol Development, Manuscript writing/editing
A. Mayer: Protocol Development, Data Collection, Manuscript writing/editing
References
- 1.Coyne KS, Wein A, Nicholson S, Kvasz M, Chen CI, Milsom I. Comorbidities and personal burden of urgency urinary incontinence: a systematic review. Int J Clin Pract. 2013;67(10):1015–33. doi: 10.1111/ijcp.12164. [DOI] [PubMed] [Google Scholar]
- 2.Milsom I, Coyne KS, Nicholson S, Kvasz M, Chen CI, Wein AJ. Global prevalence and economic burden of urgency urinary incontinence: a systematic review. Eur Urol. 2014;65(1):79–95. doi: 10.1016/j.eururo.2013.08.031. [DOI] [PubMed] [Google Scholar]
- 3.Shamliyan T, Wyman J, Kane RL. Nonsurgical Treatments for Urinary Incontinence in Adult Women: Diagnosis and Comparative Effectiveness. Rockville, MD: Agency for Healthcare Research and Quality; Apr, 2012. (Comparative Effectiveness Review No. 36. (Prepared by the University of Minnesota Evidence-based Practice Center under Contract No. HHSA 290-2007-10064-I.) AHRQ Publication No. 11(12)-EHC074-EF). Available at: www.effectivehealthcare.ahrq.gov/reports/final.cfm. Last accessed 6/1/2016. [PubMed] [Google Scholar]
- 4.Anger JT, Nissim HA, Le TX, Smith AL, Lee U, Sarkisian C, et al. Women’s experience with severe overactive bladder symptoms and treatment: insight revealed from patient focus groups. Neurourol Urodyn. 2011;30(7):295–9. doi: 10.1002/nau.21004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hubbard CS, Hong J, Jiang Z, Ebrat B, Suyenobu B, Smith S, et al. Increased attentional network functioning related to symptom severity measures in females with irritable bowel syndrome. Neurogastroenterol Motil. 2015;27(9):1282–94. doi: 10.1111/nmo.12622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larsson MB, Tillisch K, Craig AD, Engström M, Labus J, Naliboff B, et al. Brain responses to visceral stimuli reflect visceral sensitivity thresholds in patients with irritable bowel syndrome. Gastroenterology. 2012;142(3):463–472. doi: 10.1053/j.gastro.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griffiths D, Derbyshire S, Stenger A, Resnik N. Brain control of normal and overactive bladder. J Urol. 2005;174(5):1862–7. doi: 10.1097/01.ju.0000177450.34451.97. [DOI] [PubMed] [Google Scholar]
- 8.Nardos R, Karstens L, Carpenter S, Aykes K, Krisky C, Stevens C, et al. Abnormal functional connectivity in women with urgency urinary incontinence: Can we predict disease presence and severity in individual women using Rs-fcMRI. Neurourol Urodyn. 2015 May 1; doi: 10.1002/nau.22767. [DOI] [PubMed] [Google Scholar]
- 9.Komesu YM, Ketai LH, Mayer AR, Teshiba TM, Rogers RG. Functional MRI of the Brain in Women with Overactive Bladder: Brain Activation During Urinary Urgency. Female Pelvic Med Reconstr Surg. 2011;17(1):50–54. doi: 10.1097/SPV.0b013e3182065507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaylord SA, Palsson OS, Garland EL, Faurot KR, Coble RS, Mann JD, Frey W, Leniek K, Whitehead WE. Mindfulness training reduces the severity of irritable bowel syndrome in women: results of a randomized controlled trial. Am J Gastroenterol. 2011;106(9):1678–88. doi: 10.1038/ajg.2011.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller V, Carruthers HR, Hasa SS, Archibold S, Whorwell PJ. Hypnotherapy for irritable bowel syndrome: an audit of one thousand adult patients. Aliment Pharmacol Ther. 2015;41(9):844–55. doi: 10.1111/apt.13145. [DOI] [PubMed] [Google Scholar]
- 12.Komesu YM, Sapien RE, Rogers RG, Ketai LH. Hypnotherapy for treatment of overactive bladder: a randomized controlled trial pilot study. Female Pelvic Med Reconstr Surg. 2011;17(6):308–13. doi: 10.1097/SPV.0b013e31823a08d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freeman RM, Baxby K. Hypnotherapy for incontinence caused by the unstable detrusor. British Med J. 1982;284:1831–1834. doi: 10.1136/bmj.284.6332.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ketai LH, Komesu YM, Dodd AB, Rogers RG, Ling JM, Mayer AR. Urgency urinary incontinence and the interoceptive network: a functional magnetic resonance imaging study. Am J Obstet Gynecol. 2016 May 9; doi: 10.1016/j.ajog.2016.04.056. pii:S0002-9378(16)30193-4.doi:10.1016.04.056.[Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huettel SA, Song AW, McCarthy G. Statistical Analysis II: Advanced Approaches. In: Huettel Song McCarthy., editor. Functional magnetic resonance imaging. 2nd. Sunderland, MA: Sinauer Associates; 2009. pp. 377–399. [Google Scholar]
- 16.Coyne KS, Zyczynski T, Margolis MK, Elinoff V, Roberts RG. Validation of an overactive bladder awareness tool for use in primary care settings. Adv Ther. 2005;22(4):381–94. doi: 10.1007/BF02850085. [DOI] [PubMed] [Google Scholar]
- 17.Ernst E, Pittler M, Wider B. The desktop guide to complementary and alternative medicine: an evidence-based approach. 2nd. Edinburgh: Mosby; 2006. p. 480. [Google Scholar]
- 18.Jones H, Cooper P, Miller V, Brooks N, Whorwell PJ. Treatment of non-cardiac chest pain: a controlled trial of hypnotherapy. Gut. 2006;55(10):1403–8. doi: 10.1136/gut.2005.086694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green JP, Barabasz AF, Barrett D, Montgomery GH. Forging ahead: the 2003 APA Division 30 definition of hypnosis. Int J of Clin and Exper Hypnosis. 53:259–64. doi: 10.1080/00207140590961321. [DOI] [PubMed] [Google Scholar]
- 20.Barnier AJ, Nash MR. Introduction: a roadmap for explanation, a working definition. In: Nash MR, Barnier AJ, editors. The Oxford Handbook of Hypnosis Theory, Research and Practice. Oxford University Press; New York, New York: 2008. pp. 1–18. [Google Scholar]
- 21.Egner T, Jamieson G, Gruzelier J. Hypnosis decouples cognitive control from conflict monitoring processes of the frontal lobe. NeuroImage. 2005;27:969–78. doi: 10.1016/j.neuroimage.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Faymonville M-E, Roediger L, Del Fiore G, Delgueldre C, Phillips C, Lamy M, Luxen A, Maquet P, Laureys S. Increased cerebral functional connectivity underlying the antinociceptive effects of hypnosis. Cogn Brain Res. 2003;17:255–62. doi: 10.1016/s0926-6410(03)00113-7. [DOI] [PubMed] [Google Scholar]
- 23.Coyne KS, Thompson CL, Lai J-S, Sexton An Overactive Bladder Symptom and Health-Related Quality of Life Short-Form: Validation of the OAB-q SF. Neurourol and Urodyn. 2015;34:255–263. doi: 10.1002/nau.22559. [DOI] [PubMed] [Google Scholar]
- 24.Sandvik H, Espuna H, Hunskaar S. Validity of the incontinence severity indes: comparison with pad-weighing tests. Int Urogynecol J. 2006;17:520–24. doi: 10.1007/s00192-005-0060-z. [DOI] [PubMed] [Google Scholar]
- 25.Coyne KS, Matza LS, Kopp Z, Abrams P. The Validation of the Patient Perception of Bladder Condition (PPBC):A Single-Item Global Measure for Patients with Overactive Bladder. Euro Urol. 2006;49:1079–1086. doi: 10.1016/j.eururo.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Rogers RG, Coates KW, Kammerer-Doak D, Khalsa S, Qualls C. A short form of the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12) Int Urogynecol J. 2003;14:164–8. doi: 10.1007/s00192-003-1063-2. [DOI] [PubMed] [Google Scholar]
- 27.Humphrey L, Arbuckle R, Moldwin R, Nordling J, van de Merwe JP, Meunier J, Crook T, Abraham L. The Bladder Pain/Interstitial Cystitis Symptom Score: Development, Validation, and Identification of a Cut Score. Euro Urol. 2011;61:271–279. doi: 10.1016/j.eururo.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Barber MD, Walters MD, Bump RC. Short forms of two condition-specific quality-of-life questionnaires for women with pelvic floor disorders (PFDI-10 and PFIQ-7) Am J Obstet Gynecol. 2005;193:103–13. doi: 10.1016/j.ajog.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 29.Rome Foundation website: www.theromefoundation.org/questionnaires/questionnaire accessed from website: http://theromefoundation.org/products/copyright-and-licensing/rome-foundation-materials-for-academic-download/ Last accessed June 3, 2016
- 30.Stanford Hypnotic Susceptibility Scale: last accessed from website June 3, 2016 at http://socrates.berkeley.edu/~kihlstrm/PDFfiles/Hypnotizability/SHSSC%20Script.pdf


