Abstract
Background
The differential diagnosis of intractable reflux in children includes rumination syndrome but confirming the diagnosis using antroduodenal manometry is invasive, costly and requires anesthesia. High resolution esophageal manometry with impedance (HRM-MII) overcomes these limitations and the goal of this study is to validate the use of HRM-MII as a diagnostic tool for rumination and to describe the subtypes of pediatric rumination.
Methods
We reviewed the HRM-MII tracings of 21 children presenting with symptoms of intractable reflux in whom rumination was being considered. Patients underwent a standard and post-prandial HRM-MII. Peak intraluminal esophageal pressures, baseline gastric and thoracic pressures, and the timing of the R wave relative to LES relaxations and bolus flow were recorded. Chi square analyses were used for comparison of proportions and means were compared using t-tests or non-parametric equivalent.
Key Results
Forty one (55.5%) primary and 33 (44.5%) secondary rumination episodes were seen. Three types of primary rumination were identified: 1) LES relaxation without retrograde flow preceding the R wave (51% of episodes); 2) LES relaxation after the R wave (20% of episodes); and 3) R waves with no LES relaxation (29% of episodes). Eleven patients had rumination episodes with a peak gastric pressure <30 mm Hg. A total of 44 (60%) rumination episodes occurred during the standard HRM-MII, and 30 (40%) occurred during or after the meal.
Conclusions and Inferences
HRM-MII can accurately diagnose rumination in children. We identify three types of primary rumination which may provide insight into therapeutic response.
Keywords: Rumination, Impedance, High resolution esophageal manometry, pediatrics
Graphical abstract
Forty one (55.5%) primary and 33 (44.5%) secondary rumination episodes were seen in 18 patients. Three types of primary rumination were identified in children: 1) LES relaxation without retrograde flow preceding the R wave (51% of episodes); 2) LES relaxation after the R wave (20% of episodes); and 3) R waves with no LES relaxation (29% of episodes). Pediatric rumination can be accurately diagnosed using high resolution esophageal manometry with impedance.
Introduction
Rumination is a functional gastrointestinal disorder characterized by the recurrent regurgitation of gastric contents into the mouth with spitting or reswallowing (1-6). It was initially described in children with developmental disabilities, but is now recognized in many pediatric subgroups (1, 4). Even though the clinical history is suggestive of rumination, there is symptom overlap with intractable reflux so differentiating the two can be difficult but important because the therapies differ (2, 7, 8). Making a diagnosis of rumination in children is often problematic because children may be too young or lack the developmental skills to verbalize symptoms. (8). Furthermore, androduodenal (AD) manometry which allows for visualization of R waves which are pathognomonic for rumination is invasive, costly and requires catheter placement under anesthesia (2, 6, 8-10). Therefore, the diagnosis of rumination is often given presumptively rather than confirmed by testing which may lead to patient/parental questioning of the diagnosis and its associated therapies (2, 6, 8).
Studies in adults have shown that, in lieu of AD manometry, high resolution esophageal manometry (HRM) can be used to diagnose rumination by visualization of R waves when pressure sensors are placed into the stomach and esophagus(7, 11-13). The addition of impedance to HRM (HRM-MII) has also allowed for the visualization of bolus movement relative to the R waves, a significant advantage over standard AD manometry (11-13).
The use of HRM-MII is appealing in pediatrics because the study can be performed over an hour, it does not require anesthesia, and the patient/family can see the R waves which provides visual credibility for the diagnosis (7). HRM-MII may also provide insight into the mechanism of rumination in children. It is the goal of the present study to determine the feasibility of using HRM-MII to diagnose rumination in children and to determine if there are subtypes of pediatric rumination as described in adults.
Methods
Between between January 2013-December 2015, we studied 21 patients referred to the Center for Motility and Functional Gastrointestinal Disorders at Boston Children's Hospital for symptoms of intractable regurgitation despite acid suppression therapy. The protocol was approved by the IRB.
A prolonged HRM-MII was performed after an overnight fast using a catheter with 36 high resolution pressure ports and 12 impedance sensors Medtroncis (Minneapolis Minnesota) or Laborie (Williston, VT). All studies were performed while the patients were taking proton pump inhibitors. The catheter was introduced transnasally and placed such that there were a minimum of 5 pressure sensors in the stomach with the remaining sensors distributed throughout the esophagus with both the lower esophageal sphincter (LES) and upper esophageal sphincter (UES) visualized simultaneously. During the first 20 minutes of the study a standard esophageal manometry was performed in the sitting position. The patient was given 10 saline and 10 viscous swallows, with a volume of 5 ml each. After the standard manometry was completed, the patient was given a meal (brought by the family who determined it to be appropriate symptom-inducing meal) over 15 minutes. Then, with the catheter still in place, patients were observed for an additional 30 minutes after the meal. Symptoms were recorded during the study including pain, regurgitation, cough or any other sensations expressed by the patient.
Tracings were then blindly reviewed by two reviewers (RR, SN) for: (1) the presence of R waves, (2) the relationship of R waves to LES relaxations, and (3) the relationship of R waves to retrograde bolus movement into the esophagus visualized by MII.
Definitions
R waves were defined as simultaneous high amplitude spikes in pressure seen in both the stomach and the esophagus in the absence of cough. Peak intraluminal gastric and esophageal pressures 5 cm above the LES during the R waves were recorded. (12). Baseline gastric and thoracic pressures were also measured at least 30 seconds before an R wave, and 30 seconds after a swallow with its associated LES relaxation. Finally, the timing of the R wave relative to LES relaxation and to bolus flow detected by impedance was recorded. Successful rumination was defined as the presence of R waves and simultaneous retrograde bolus flow by MII across the LES (4).
Transient Lower Esophageal Relaxations (TLSERs) were defined as previously described (14). Impedance tracings were analyzed for the occurrence of reflux episodes according to previously published criteria (15). Briefly, a liquid reflux episode detected by impedance was defined as a retrograde drop in impedance by more than 50% of baseline in the distal 2 channels. A gas reflux episode was defined as a simultaneous increase in impedance in 2 consecutive channels to greater than 8000 ohms. (15).
Rumination episodes
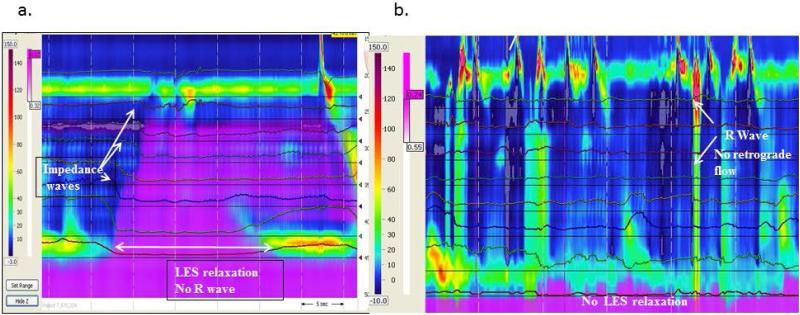
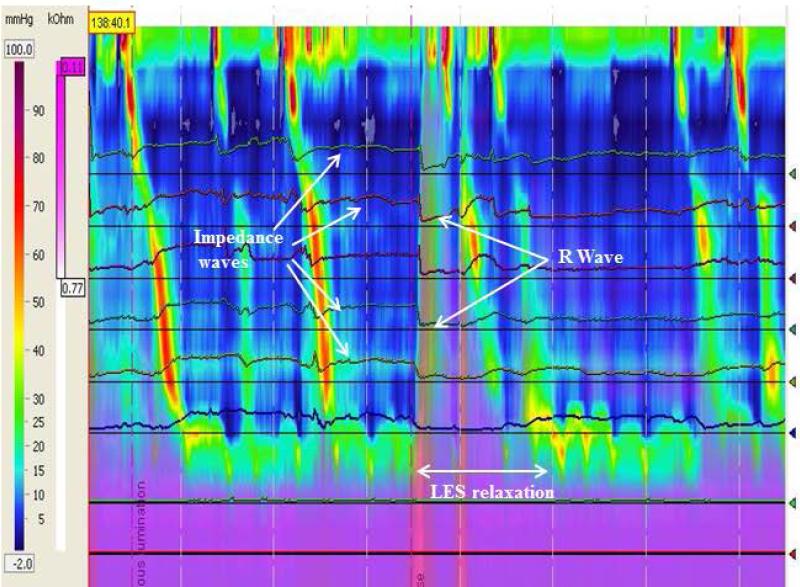
Rumination episodes were categorized as primary if the R wave triggered bolus movement into the esophagus (Figure 1-3) or secondary if the R wave occurred after the onset of bolus movement into the esophagus (Figure 4) (11, 12, 16). Supragastric belch was defined by the presence of an aboral movement of the diaphragm that creates a sub-atmospheric pressure in the esophageal body and concurrent UES relaxation (12). Unsuccessful rumination was defined as episodes in which there were clear R waves present without associated LES relaxation or bolus movement (Figure 5b) in the absence of a recorded cough. A gastroesophageal reflux episode was defined if there was evidence of LES relaxation with retrograde bolus, and no evidence of R waves (Figure 5a). (4, 11). Cough episodes were defined as simultaneous gastric and esophageal contractions with a concomitant clinical cough observed and noted during the study (4, 11).
Figure 1.
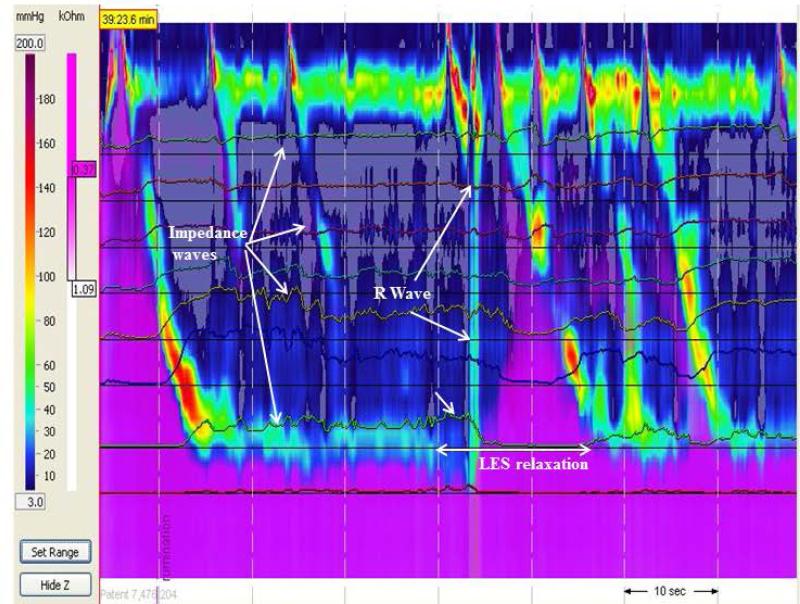
Primary rumination with LES relaxation prior to R wave (L-R-F subtype). Purple denotes fluid flow by impedance. There is no retrograde flow until the R wave occurs even though there is preceding LES relaxation.
Figure 3.
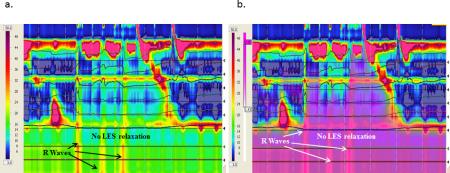
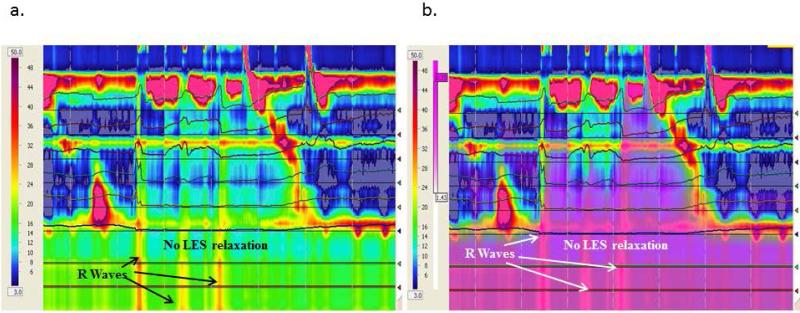
Primary rumination with R wave with no LES relaxation (N-R-F subtype). Purple denotes fluid flow by impedance. The figures represent the same rumination episodes without (a) and with (b) the purple impedance added to show bolus flow despite a closed LES.
Figure 4.
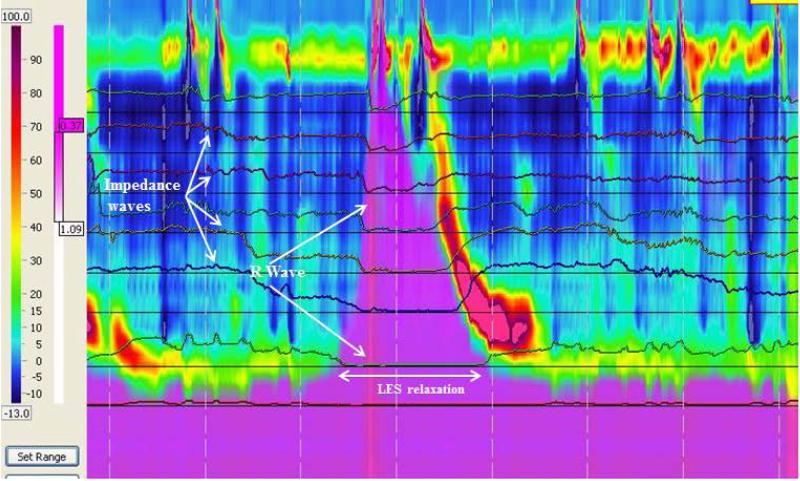
An example of secondary rumination. There is LES relaxation with retrograde flow before the R wave occurs.
Figure 5.
Examples of episodes where HRM-MII disproved rumination including reflux episodes with a TLESR and retrograde bolus flow (a) and generation of R waves without bolus flow. Purple represents liquid by impedance.
Each rumination event was scored for the following variables (12): (1) the presence of R waves; (2) The peak pressure amplitude and duration of the R wave in the abdomen (2 cm below the LES) and the thorax (3 cm above the LES); (3) the gastric-thoracic pressure gradients before and during the rumination episodes; (4) the time between R waves and esophageal bolus entry; (5) the time between LES relaxations and R waves; (6) the time from LES relaxation to bolus entry; (7) the sequence of bolus entry relative to gastric pressure increases; and (8) the maximum height of the retrograde flow.
Statistics
Data are presented as medians and range or mean and SE depending on the variable distribution. Statistical analysis was performed using SPSS version 23. Comparisons between proportion were used using chi-square, and between continuous variables paired tests, independent t-tests or non-parametric statistics when applicable. Differences were considered statistically significant when P < 0.05.
Results
Twenty one patients were included. There were 15 females. The mean age of the patients was 15 ± 4.9 years (range 7 to 18 years). Post-prandial regurgitation was present in 100% of patients, nausea in 30%, heartburn in 50%, intractable reflux in 70%, and chest pain in 20%. All patients continued to have symptoms despite twice daily PPI therapy. All had a grossly normal upper endoscopy with no visible erosions, normal esophageal biopsies with no eosinophilic infiltration, and a normal esophageal manometry by Chicago classification (17). Of the 18 patients with rumination, 9 patients had a normal gastric emptying scans performed, three had abnormal gastric emptying scans, and 6 patients did not have a gastric emptying scans part of their evaluation.
Rumination episodes were seen in 18/21 patients. Of the three patients without rumination, one patient had R waves but no retrograde bolus movement into the esophagus (unsuccessful rumination, Figure 5b) and two patients had LES relaxations with retrograde bolus movement without R waves (gastroesophageal reflux, Figure 5a). Therefore, in three patients, HRM-MII disproved the diagnosis of rumination. The following analysis includes only those 18 patients with documented rumination
Rumination episodes
There were 74 rumination episodes seen in 18 patients. The characteristics of the rumination episodes are seen in Table 1. No episodes of supragastric belching were seen. There were 41 (55.5%) primary and 33 (44.5%) secondary rumination episodes. Thirteen patients had both primary and secondary episodes of rumination, 3 had only primary episodes, and 2 had only secondary rumination episodes.
Table 1.
Comparison between primary and secondary rumination episodes (means±SE unless indicated).
| Total Rumination Episodes | Primary Rumination | Secondary Rumination | p Value | |
|---|---|---|---|---|
| N (%) | 74 | 41 (55.4) | 33 (44.5) | 0.3 |
| LES baseline pressure (mmHg) | 26.5 ± 1.3 | 24.9 ± 1.3 | 27.8 ± 1.9 | 0.2 |
| LES pressure during rumination episode (mmHg) | 5.3± 1.1 | 7.2 ± 1.1 | 2.7 ± 0.9 | 0.04 |
| Gastric pressure before R wave (mmHg) | 10.8 ± 0.4 | 10.6 ± 3.5 | 11.1 ± 3.4 | 0.6 |
| Gastric pressure during R wave (mmHg) | 71.2± 4.7 | 62.1± 5.9 | 79.5 ± 6.7 | 0.05 |
| Duration of gastric R wave (sec) | 0.6 ± 0.1 | 0.5 ± 0.7 | 0.7 ±0.2 | 0.4 |
| Difference in baseline gastric pressure and Peak R wave pressure (mmHg) | 61.2±4.7 | 51.4 ± 6.1 | 68.9 ± 6.7 | 0.04 |
| Thoracic pressure before R wave (mmHg) | −0.2 ± 0.4 | −0.3 ±0.5 | 0.5 ± 0.6 | 0.5 |
| Thoracic pressure during R wave (mmHg) | 51.0± 4.3 | 40.5 ± 4.2 | 59.3 ± 6.7 | 0.02 |
| Difference in baseline thoracic pressure and peak R wave pressure (mmHg) | 51.0±4.4 | 40.7±4.4 | 59.1 ± 6.9 | 0.04 |
| Difference in gastric pressure and thoracic pressure during baseline (mmHg) | 10.7±0.4 | 10.9 ± 0.6 | 10.5 ± 0.6 | 0.8 |
| Difference in gastric pressure and thoracic pressure during rumination (mmHg) | 20.1 ± 2.8 | 20.1±3.4 | 21.6 ± 4.5 | 0.7 |
| Mean time from R wave to Bolus Entry (sec) | 0.8±0.2 | 0.2 ± 0.1 | 0.4 ± 0.2 | 0.02 |
| Mean time LES relaxation to bolus entry (sec) | 3.2±1.1 | 4.4 ±1.8 | 2.8±1.1 | 0.3 |
| Mean time from LES relaxation to R wave (sec) | 4±1.1 | 3.7±1.3 (only those with LES relaxation) | 4.4±1.9 | 0.7 |
| Time of bolus presence in the esophagus to R wave (sec) | 0.8±0.9 | 0.05 ± 0.1 | 1.5 ± 0.4 before the R wave | 0.003 |
| Duration of bolus presence in the esophagus (sec) | 4.4±0.8 | 5.9 ± 1.4 | 3.1 ±0.9 | 0.08 |
| Sensation before R wave (%) | 39 | 42 | 39 | 0.3 |
| UES opening during rumination (%) | 84 | 78 | 97 | 0.004 |
| Bolus reaching UES during rumination (%) | 64 | 58 | 78 | 0.06 |
| Rumination episodes during meal (%) | 44 | 44 | 36 | 0.3 |
A comparison primary and secondary rumination characteristics is shown in Table 1. During secondary rumination episodes, the LES nadir during relaxation was significantly lower and the peak R wave pressures in chest and stomach were higher compared to primary rumination episodes. Additionally, during secondary rumination, UES nadir pressures were lower and proximal bolus migration was higher compared to primary rumination.
In addition to categorizing ruminations episodes as primary versus secondary, we also discovered three distinct patterns of primary rumination 1) L-R-F (LES relaxation followed by R wave and bolus flow, Figure 1); 2) R-L-F (R wave followed by LES relaxation and bolus flow, Figure 2); and 3) N-R-F (no LES relaxation but R waves and bolus flow, Figure 3 a and b). A comparison of the main characteristics of each type of primary rumination episode is shown in Table 2. Not all patients had all types of primary rumination. Only 16 had primary rumination events. Of those 16, five had only type L-R-F primary rumination, two only had type R-L-F, and one had NR-F. The other 8 patients had a combination of primary rumination types. All patients had normal LES relaxations during swallows, with the mean LES residual pressure of 3.5±0.4 mm Hg during swallows. Those patients with type NRF had a nadir LES pressure of 16.0 + 2.4 mm Hg during rumination which is significantly higher than the LES residual pressures during swallowing.
Figure 2.
Primary rumination with R wave preceding LES relaxation (R-L-F subtype). Purple denotes fluid flow by impedance.
Table 2.
Comparison between types of primary rumination.
| Type L-R-F (LES relaxation-R wave-Bolus Flow) | Type R-L-F (R wave-LES relaxation-Bolus Flow) | Type N-R-F (no LES relaxation-R wave-Bolus Flow) | |
|---|---|---|---|
| Number of Primary Rumination Episodes (%) | 21 (51) | 8 (20) | 12 (29) |
| LES baseline pressure (mmHg) | 29.1 ± 2.7 | 22.7 ± 3.1 | 29.3 ± 4.4 |
| LES pressure during rumination episode (mmHg) | 7.2 ± 1.9 | 6.5 ± 1.8 | 16.0 ± 2.4 |
| Gastric pressure before R wave (mmHg) | 10.0 ± 0.6 | 14.0 ± 1.1 | 10.7 ± 8.3 |
| Gastric pressure during R wave (mmHg) | 82 ±8.6 | 89.8 ±16.7 | 71.3 ± 14.2 |
| Duration of gastric R wave (sec) | 0.7 ± 0.2 | 0.5± 0.2 | 0.8 ± 0.4 |
| Difference between baseline gastric pressure and peak R wave gastric pressure (mmHg) | 72.9 ±8.8 | 76.1± 17.0 | 61.0 ± 14.0 |
| Thoracic pressure before R wave (mmHg) | −0.6 ± 0.9 | 3.3± 1.2 | −0.3± 1.0 |
| Thoracic pressure during R wave (mmHg) | 55.0 ± 8.6 | 71.5± 16.4 | 61.0 ± 15.0 |
| Difference between baseline thoracic pressure and peak R wave thoracic pressure (mmHg) | 55.6 ± 8.6 | 70.0± 19.1 | 61.1 ± 15.1 |
| Difference between baseline gastric pressure and thoracic pressure at baseline (mmHg) | 10.5 ± 1.1 | 10.6± 1.3 | 11.0± 0.7 |
| Difference between gastric pressure and thoracic pressure during rumination (mmHg) | 27.0 ± 5.3 | 18.4± 4.7 | 10.6± 0.7 |
| Mean time from R wave to bolus entry (sec) | 0.2 ± 0.1 | 0.5± 0.3 | 0.1+ 0.1 |
| Mean time LES relaxation to bolus entry (sec) | 6.0 ± 2.0 | −0.2± 0.2 | No relaxation |
| Mean time from LES relaxation to start of R wave (sec) | 6.0 ±2.5 before R wave | 0.3 ± 0.5 after R wave | No relaxation |
| Time from bolus presence in the esophagus to R wave (sec) | 17 episodes simultaneous 4.35 + 1.84 sec with 4 episodes after R | 0.5 ±0.3 after R wave | 0.1 ± 0.1 after R wave |
| Duration of bolus presence in the esophagus (sec) | 4.3± 7.8 | 2.6± 3.4 | 1.1± 1.8 |
| Percentage of episodes sensed by patient prior to rumination | 33 | 62 | 33 |
| UES opening during rumination (%) | 86 | 75 | 64 |
| Bolus reaching UES during rumination (%) | 52 | 88 | 46 |
| During meal (%)* | 33 | 87 | 33 |
P=0.02
Characteristics of the R waves
In 11 patients, the pressure of the R wave was < 30 mmHg (range 19-27). Given that the adult definition stipulates that the R waves needs to have 30 mmHg (12), we compared the rumination episode characteristics between those with a R wave pressure of < 30 mm Hg and those with R waves > 30 mm Hg. As expected there was a significant difference in the peak R wave between both groups (23 ± 1.2 mm Hg vs. 80 ± 4.7 mm Hg, p < 0.004). There was no difference in the duration of the R wave, the type of rumination, or the sequence of esophageal events (p>0.1). The only other significant difference we found was in the time it took for the retrograde bolus to start after the R wave (0.9 ± 0.5 vs. 0.13 ± 0.11 sec; p<0.04)
Comparison between rumination events during stationary manometry vs during a meal
A total of 44 (60%) rumination episodes occurred during the standard HRM-MII, and 30 (40%) occurred after the meal. In 9/18 (50%) patients, the rumination events occurred only during the standard HRM-MII, in 3/18 (17%) patients, the rumination events occurred during both the standard HRM-MII and after the meal, and, in 6/18 (33%) patients, the rumination events occurred only after the meal.
Table 3 compares the rumination characteristics of episodes that occurred during the standard HRM-MII to those that occurred after the solid meal; as seen in the table, rumination episodes occurring after the meal were accompanied by more preceding LES relaxations and with lower R wave pressures and duration as compared with those that occurred after liquid swallows.
Table 3.
Differences in rumination characteristics that occur after liquid swallows and after a meal.
| Liquid swallow | After solid meal | P value | |
|---|---|---|---|
| Age | 15.7 + 1.9 | 13.8 + 4.2 | 0.9 |
| Number of Episodes | 44 | 30 | 0.4 |
| Number of Primary Rumination Episodes | 23 | 18 | 0.5 |
| Number of Secondary Rumination Episodes | 21 | 12 | |
| LES Pressure Prior to Rumination (mmHg) | 26.9 + 1.8 | 26.0 + 1.4 | 0.4 |
| Time from R Wave to Bolus Entry (sec) | −0.12 +0.2 | 0.1 +0.1 | 0.5 |
| Time from LES relaxation to start of R wave (sec) | 6.5 + 1.8 | 0.36 + 0.2 | 0.006 |
| Time from LES Relaxation to Bolus Entry (sec) | 5.4 + 1.7 | 0.04 + 0.7 | 0.01 |
| Time from Bolus to Peak R wave Pressure (sec) | 1.2 + 0.4 | 0.3+0.2 | 0.06 |
| Gastric pressure before Rumination (mmHg) | 10 + 0.5 | 12.0 + 0.6 | 0.013 |
| Thoracic pressure before Rumination (mmHg) | −0.64+0.5 | 1.0+0.7 | 0.049 |
| Peak Gastric Pressure during R Wave (mmHg) | 79.6 +7.3 | 61.4 + 3.9 | 0.03 |
| Peak Thoracic Pressure during R Wave (mmHg) | 55.2+ 6.9 | 54.5 + 3.8 | 0.3 |
| Duration of R Wave (sec) | 0.9 + 0.2 | 0.1 + 0.1 | 0.001 |
| % of Rumination Episodes with LES relaxation before R | 40% | 73% | 0.03 |
| % of Rumination Episodes associated with UES Relaxation | 64% | 68% | 0.5 |
| Gastric pressures during R wave < 30 mmHg | 18% | 10% | 0.2 |
| Subtypes of Primary Rumination (N) | |||
| L-R-F | 79 | 57 | <0.006 |
| RLF | 2 | 26 | |
| NRF | 18 | 16 |
Discussion
This is the first study to (1) prove that HRM-MII is a useful tool to diagnose (or exclude rumination) in children; and (2) define rumination subtypes relative to LES relaxations. Our study suggests that the majority of rumination episodes in children immediately follow an LES relaxation, even when it is not associated with a reflux event, a finding that has not been previously described either in adults or children. This latter finding may be of particular importance as therapies which target LES relaxations may be beneficial in subtypes of rumination patients.
The diagnosis of rumination can be difficult (2, 8) with patients often undergoing extensive and costly work up including AD manometry (2, 8). AD manometry is only performed in a few tertiary centers, is invasive, and does not establish the relationship between the generated abdominal pressure and the retrograde bolus flow into the esophagus. Therefore the present study is important because it highlights that HRM-MII can be used to successfully diagnose (and even disprove) rumination in children referred for intractable regurgitation. This ability to prove or disprove a diagnosis highlights the value of HRM-MII which not only can detect R waves but also can detect bolus flow using impedance; in our series, 15% of patients with a possible diagnosis of rumination actually has significant reflux or abdominal contractions without the presence of bolus movement into the esophagus, a condition that would have been missed by antroduodenal manometry or HRM without impedance.
Current adult definitions of primary or secondary rumination are based on the timing of R wave relative to bolus flow. In adults, secondary rumination is common, making up as much as 86% of all rumination episodes (7, 11, 12) and, in these episodes, the pathophysiology is more clear; patients sense bolus movement into the esophagus which then triggers R waves (4, 7, 11, 12). In our study, unlike in adult studies, secondary rumination episodes only made up 45% of pediatric rumination episodes so clearly a better understanding of the pathophysiology of primary rumination becomes more important. We identified three distinct subtypes of primary rumination: (1) L-R-F: those with LES relaxation prior to R waves and bolus flow; (2) R-L-F.: those with LES relaxation following R waves and bolus flow; and (3) N-R-F: R waves without LES relaxation. We found that almost 50% of the primary rumination episodes occurred after an isolated LES relaxation event. This is much more rare in adults; a single adult study reports that this pattern is only seen in 11% of rumination events (11). Because these LRF rumination episodes occurred after LES relaxation without bolus presence in the esophagus, we consider these primary rumination episodes based on the published adult definition requiring esophageal bolus presence (12); unfortunately in the landmark adult paper establishing this definition, there is a clear description of R waves and retrograde bolus movement but the interrelationship between the LES relaxations and those events not described (12). However, we recognize these LRF episodes may represent a similar mechanism to secondary rumination and clearly additional studies are needed to see if these two subpopulations respond to therapies identically.
These differences suggest that the mechanisms underlying rumination in children may be different than adults and we hypothesize that these LES relaxation events trigger sensory feedback independently of bolus flow which results in the need to ruminate. Studies in adults support a sensory mechanism for rumination; patients may have increased gastric sensitivity and other associated esophageal sensory abnormalities (4, 18). In fact a previous study showed that during esophageal HRM studies 72% of adult patients spontaneously reported sensory based symptoms such as dyspepsia before the appearance of R waves with resolution of symptoms once gastric contents reach the mouth (18).
These sensory abnormalities may also be associated with gastric motor abnormalities. Studies in adult suggest that gastric distension may results in more LES relaxations, and a lower LES relaxation nadir in patients that ruminate. (18). Other studies suggest that there is poor postprandial gastric accommodation and a disruption of normal abdominothoracic activity after meals which result in increased numbers of LES relaxations (6). Therefore rumination patients may have a hypersensitive response to LES relaxation, either related to the relaxation itself or due to an LES relaxation-triggered common cavity phenomena that generates the R wave as an attempt to relieve abnormal sensation, or dyspeptic symptoms (4, 11). Because the patients experience relief of symptoms with the regurgitation of gastric contents, they unconsciously adopt a sequence of learned behaviors that produces the rumination event to relieve symptoms (2).
Further supporting this possibility that LES relaxations trigger an uncomfortable sensation which triggers the urge to ruminate, we found that 40% of patients experienced some type of sensation before the R wave occurred across all types of rumination. In 6 patients with primary rumination in which there was LES relaxation prior to generation of R waves (type L-R-F), the patients described a sensation of pressure before the R wave, and then produced the R wave and retrograde flow to relieve the pressure. In all cases the discomfort disappeared.
While the LES relaxation may play an important role in the sensation that triggers a rumination episode, we also found that up to a third of primary rumination episodes occurred without true LES relaxations. We did see subtle drops in LES pressures but no complete LES relaxation and this was unexpected based on studies in healthy adult volunteers which actually show increases LES pressures with rises in abdominal pressure (19). This finding of rumination through a closed LES is a novel finding and in fact, adult studies have documented the importance of LES relaxation as a prerequisite to regurgitation of gastric contents (18, 20). It is possible that those patients in which regurgitation of gastric contents occurs without LES relaxation represent a different type of underlying pathophysiology, and may have more behavioral or psychiatric comorbidities as a trigger rather than a sensory dysregulation.
The subtyping of rumination events may allow for improved predictions for which patients will respond favorably to different therapies. (4, 5, 11) While limited adult and pediatric studies suggest that response to behavioral therapy or diaphragmatic breathing is generally positive in up to 50% of patients (2, 4, 6, 11), some patients do not respond and some patients are ineligible for behavioral therapies because of developmental/neurologic comorbidities. (2, 4, 5). We postulate that the presence/absence of LES relaxations and their type of association with the R waves may predict response to therapy; for example, in the subgroup of patients in whom LES relaxations precede the R wave, mediations that modulate LES relaxation may be beneficial. In fact recent evidence in adults has shown that the use of baclofen, an agonist of the γ aminobutyric acid (GABA)B acid receptor, increases LES basal pressures and decreases TLSERs and swallowing rate; this may decrease symptoms and reduce retrograde bolus flow in patients with rumination (21). Baclofen has similarly been shown, in pediatric GERD patients, (22) to decrease TLSERs and improve gastric emptying both of which may be beneficial in the patients with LES relaxation-predominant rumination. (23).
Apart from the novel subtypes of primary rumination that we describe in this study, there are two other important points to highlight in our results. First, we report that children are able to ruminate with gastric pressures as low as 20 mmHg, so adult centers performing manometries in children will need to revise their definition of rumination episodes for the pediatric patients. (12, 16). Second, we found that more than a third of rumination episodes only occurred after administration of a meal so patients would have been incorrectly diagnosed 33% of the time if only stationary manometry had been performed. Our findings are similar to some previous studies in adults in which rumination was demonstrated in 48% after the water swallows, 15% after the first 200-mL water swallow and in 37% after the test meal (11). The fact that a solid meal is necessary to produce rumination episodes is not a surprising finding given the abnormal gastric accommodation that has been described in these patients.(6, 18). Therefore, for all children with suspected rumination, HRM-MII should include both a standard manometry as well as a post-meal manometry.
One of the limitations to our study is that we studies patients only for 30 minutes after a meal so some episodes of rumination may have been missed with this length of observation. For the three patients in whom rumination was not seen, it is possible that longer observation periods may been needed to capture episodes. Additionally, the predominant types of ruminations episodes may shift as the time away from the meal lengthens and this merits additional study.
In conclusion, we have shown the HRM-MII is an ideal tool to diagnose rumination in children because of its short duration and noninvasive nature. We have documented both primary and secondary rumination in children and most importantly, we suggest a new classification of primary rumination based on the R wave presence relative to LES relaxation. Future studies are needed to determine if these subtypes predict therapeutic response.
Key Points.
While high resolution esophageal manometry has been used to diagnose rumination in adults, its utility in pediatrics is not known.
We show that HRM-MII is useful to diagnose rumination in children and we identify three novel subtypes of primary rumination in children.
The high frequency of rumination events preceded by lower esophageal sphincter relaxations raises the possibility that medications that modulate LES relaxations may improve symptoms of rumination in children
Acknowledgements
Funding This work was supported by the Translational Research Program at Boston Children's Hospital (RR), the NASPGHAN Foundation/AstraZeneca Research Award for Diseases of the Upper Tract (RR) and R01 DK097112-01 (RR) from the National Institutes of Health.
Abbreviations
- TLESR
Transient lower esophageal sphincter relaxation
- HRM-MII
High resolution esophageal manometry with impedance
- LES
Lower esophageal sphincter
Footnotes
Authors’ contribution:
RR participated in the design of the study, performed the manometry studies, performed the analyses, drafted the manuscript and approved of the final version.
LR performed the manometry studies, drafted the manuscript and approved of the final version.
SN participated in the design of the study, performed the manometry studies, performed the analyses, drafted the manuscript and approved of the final version.
Disclosures:
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Hyams JS, Di Lorenzo C, Saps M, Shulman RJ, Staiano A, van Tilburg M. Functional Disorders: Children and Adolescents. Gastroenterology. 2016 doi: 10.1053/j.gastro.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Mousa HM, Montgomery M, Alioto A. Adolescent rumination syndrome. Current gastroenterology reports. 2014;16:398. doi: 10.1007/s11894-014-0398-9. [DOI] [PubMed] [Google Scholar]
- 3.Rasquin A, Di Lorenzo C, Forbes D, et al. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2006;130:1527–1537. doi: 10.1053/j.gastro.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tack J, Blondeau K, Boecxstaens V, Rommel N. Review article: the pathophysiology, differential diagnosis and management of rumination syndrome. Alimentary pharmacology & therapeutics. 2011;33:782–788. doi: 10.1111/j.1365-2036.2011.04584.x. [DOI] [PubMed] [Google Scholar]
- 5.Blondeau K, Boecxstaens V, Rommel N, et al. Baclofen improves symptoms and reduces postprandial flow events in patients with rumination and supragastric belching. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2012;10:379–384. doi: 10.1016/j.cgh.2011.10.042. [DOI] [PubMed] [Google Scholar]
- 6.Barba E, Burri E, Accarino A, et al. Biofeedback-guided control of abdominothoracic muscular activity reduces regurgitation episodes in patients with rumination. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2015;13:100–106. e101. doi: 10.1016/j.cgh.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 7.Rommel N, Tack J, Arts J, Caenepeel P, Bisschops R, Sifrim D. Rumination or belching-regurgitation? Differential diagnosis using oesophageal impedance-manometry. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2010;22:e97–104. doi: 10.1111/j.1365-2982.2009.01431.x. [DOI] [PubMed] [Google Scholar]
- 8.Chial HJ, Camilleri M, Williams DE, Litzinger K, Perrault J. Rumination syndrome in children and adolescents: diagnosis, treatment, and prognosis. Pediatrics. 2003;111:158–162. doi: 10.1542/peds.111.1.158. [DOI] [PubMed] [Google Scholar]
- 9.Camilleri M, Bharucha AE, di Lorenzo C, et al. American Neurogastroenterology and Motility Society consensus statement on intraluminal measurement of gastrointestinal and colonic motility in clinical practice. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2008;20:1269–1282. doi: 10.1111/j.1365-2982.2008.01230.x. [DOI] [PubMed] [Google Scholar]
- 10.Di Lorenzo C, Hillemeier C, Hyman P, et al. Manometry studies in children: minimum standards for procedures. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2002;14:411–420. doi: 10.1046/j.1365-2982.2002.00347.x. [DOI] [PubMed] [Google Scholar]
- 11.Tucker E, Knowles K, Wright J, Fox MR. Rumination variations: aetiology and classification of abnormal behavioural responses to digestive symptoms based on high-resolution manometry studies. Alimentary pharmacology & therapeutics. 2013;37:263–274. doi: 10.1111/apt.12148. [DOI] [PubMed] [Google Scholar]
- 12.Kessing BF, Bredenoord AJ, Smout AJ. Objective manometric criteria for the rumination syndrome. The American journal of gastroenterology. 2014;109:52–59. doi: 10.1038/ajg.2013.428. [DOI] [PubMed] [Google Scholar]
- 13.Kessing BF, Smout AJ, Bredenoord AJ. Current diagnosis and management of the rumination syndrome. Journal of clinical gastroenterology. 2014;48:478–483. doi: 10.1097/MCG.0000000000000142. [DOI] [PubMed] [Google Scholar]
- 14.Holloway RH, Boeckxstaens GE, Penagini R, Sifrim D, Smout AJ. Objective definition and detection of transient lower esophageal sphincter relaxation revisited: is there room for improvement? Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2012;24:54–60. doi: 10.1111/j.1365-2982.2011.01812.x. [DOI] [PubMed] [Google Scholar]
- 15.Rosen R, Lord C, Nurko S. The sensitivity of multichannel intraluminal impedance and the pH probe in the evaluation of gastroesophageal reflux in children. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2006;4:167–172. doi: 10.1016/s1542-3565(05)00854-2. [DOI] [PubMed] [Google Scholar]
- 16.Kessing BF, Govaert F, Masclee AA, Conchillo JM. Impedance measurements and high-resolution manometry help to better define rumination episodes. Scandinavian journal of gastroenterology. 2011;46:1310–1315. doi: 10.3109/00365521.2011.605467. [DOI] [PubMed] [Google Scholar]
- 17.Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2015;27:160–174. doi: 10.1111/nmo.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thumshirn M, Camilleri M, Hanson RB, Williams DE, Schei AJ, Kammer PP. Gastric mechanosensory and lower esophageal sphincter function in rumination syndrome. The American journal of physiology. 1998;275:G314–321. doi: 10.1152/ajpgi.1998.275.2.G314. [DOI] [PubMed] [Google Scholar]
- 19.Mittal RK, Fisher M, McCallum RW, Rochester DF, Dent J, Sluss J. Human lower esophageal sphincter pressure response to increased intra-abdominal pressure. The American journal of physiology. 1990;258:G624–630. doi: 10.1152/ajpgi.1990.258.4.G624. [DOI] [PubMed] [Google Scholar]
- 20.Smout AJ, Breumelhof R. Voluntary induction of transient lower esophageal sphincter relaxations in an adult patient with the rumination syndrome. The American journal of gastroenterology. 1990;85:1621–1625. [PubMed] [Google Scholar]
- 21.Bredenoord AJ. Management of belching, hiccups, and aerophagia. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2013;11:6–12. doi: 10.1016/j.cgh.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Vadlamudi NB, Hitch MC, Dimmitt RA, Thame KA. Baclofen for the treatment of pediatric GERD. Journal of pediatric gastroenterology and nutrition. 2013;57:808–812. doi: 10.1097/MPG.0b013e3182a2747b. [DOI] [PubMed] [Google Scholar]
- 23.Omari TI, Benninga MA, Sansom L, Butler RN, Dent J, Davidson GP. Effect of baclofen on esophagogastric motility and gastroesophageal reflux in children with gastroesophageal reflux disease: a randomized controlled trial. The Journal of pediatrics. 2006;149:468–474. doi: 10.1016/j.jpeds.2006.05.029. [DOI] [PubMed] [Google Scholar]