To the editor
Deleterious germline BRCA 1/2 mutation was described as an independent risk factor for carboplatin hypersensitivity reactions (HSR) (1). In 2013, Moon et al. observed in a retrospective study that most patients who developed carboplatin HSR had deleterious BRCA1/2 mutations (93%, 27/29), versus 50% (29/58) in patients without HSR (p<0.0001). The studied population was composed of 87 patients with ovarian, fallopian tube or primary peritoneal cancer. This study also found that patients with BRCA1/2 mutations developed a HSR to carboplatin with a lower cumulative dose (1).
Patients who are allergic to platinum-based agents can benefit from rapid drug desensitization (RDD). RDD to carboplatin protects patients against severe HSR, with over 90% of them presenting no or mild reactions during the procedure, including those with an initial anaphylactic reaction (2).
The overall prevalence of the BRCA1/2 mutation in tubo-ovarian cancer patients is 13 to 17% (3,4) and its prevalence in breast cancer patients treated at the Dana Farber Cancer Institute (DFCI) is 6.1% (5). BRCA 1/2 mutation prevalence among carboplatin allergic patients is unknown. We hypothesized that patients undergoing carboplatin RDD would present a high prevalence of BRCA 1/2 mutations, and evaluated the impact of BRCA mutations on the type of initial HSR and on reactions during RDD.
We reviewed 239 consecutive records of patients treated at the DFCI and enrolled in the Brigham and Women`s Hospital Desensitization Program between January 2011 to October 2015, who underwent RDD to carboplatin. BRCA 1/2 mutation status was reported in 138 patients, who had tubo-ovarian, peritoneal, breast or endometrial cancer.
Data are presented as mean ± standard deviation, unless otherwise stated. Categorical variables were compared using qui squared and Fisher’s exact test. Continuous variables were compared using t-test and p <0.05 was considered statistical significant.
The mean age was 60.6 ± 10 years old and no significant difference was found between BRCA positive (59.1± 8 yo) and negative patients (61.2± 11 yo). The majority of patients presented tubo-ovarian cancer (91.3%, n=126), followed by breast (3.6%, n=5), peritoneal (2.1%, n=3), peritoneal + ovarian (2.1%, n=3), and endometrial cancer (0.7%, n=1).
The overall prevalence of the BRCA mutation was 34% (n=47); 66% (n=31) with BRCA 1 and 34% (n=16) with BRCA 2 mutation.
The mean number of lifetime exposures prior to the allergic reaction in BRCA positive patients was 9.9 ± 4.5 versus 9.1 ± 4.7 for BRCA negative patients (p = 0.37).
BRCA positive patients had more initial immediate HSR than BRCA negative patients (Figure 1a, p=0.03). No statistical difference was found between BRCA positive and negative patients regarding the severity of the initial reaction (Figure 1b, p=0.22).
Figure 1.
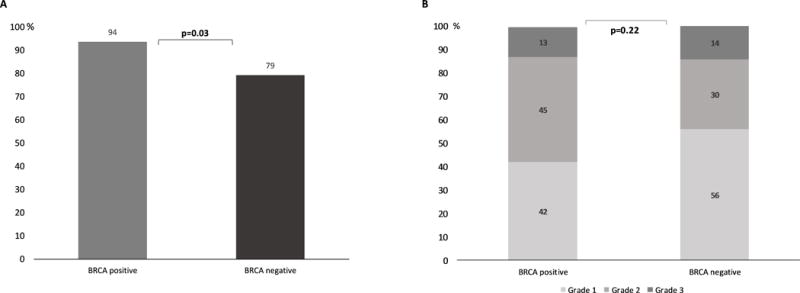
(A) Incidence of initial immediate hypersensitivity reactions among BRCA positive and negative patients. (B) Severity of initial hypersensitivity reactions to carboplatin (1: mild, 2: moderate and 3: severe) (9) among BRCA positive and negative patients.
The majority of patients were desensitized using the 12 steps/3 bags protocol (92%; n=127), while 8% (n=11) used the 16 steps/4 bags protocol.
Reactions during RDD occurred in 51% of patients with the BRCA1/2 mutation versus 27% of patients without the mutation (Figure 2a, p<0.01). The severity of RDD reactions had a similar distribution between groups (Figure 2b, p=0.72).
Figure 2.
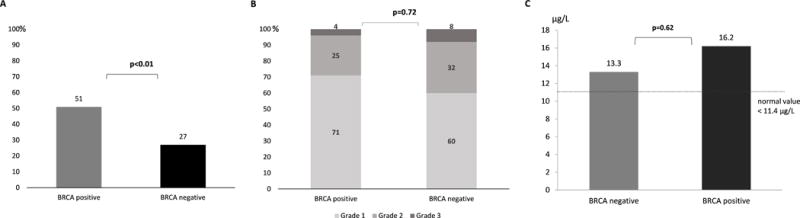
(A) Incidence of immediate hypersensitivity reactions during rapid drug desensitization (RDD) to carboplatin among BRCA positive and negative patients. (B) Grades of rapid drug desensitization reactions (RDD) among BRCA positive and negative patients. (C) Mean tryptase levels in immediate hypersensitivity reactions during rapid drug desensitization (RDD) to carboplatin among BRCA positive and negative patients (normal value < 11.4 μg/L).
Serum tryptase levels were obtained during initial HSR or RDD reaction in 14 BRCA negative patients and in 15 BRCA positive patients. In both groups mean tryptase levels were elevated (BRCA positive: 13.4 ± 13.1 μg/L, BRCA negative: 13.6 ± 13.4 μg/L; normal value < 11.4 μg/L). Tryptase levels obtained during RDD reactions were higher in BRCA positive patients (16.2 ± 12.9 μg/L) when compared to BRCA negative patients (13.3 ± 12 μg/L), but this difference was not statistically significant (Figure 2c, p=0.62).
Skin tests were positive in 94% (n=44) of BRCA positive patients versus 89% (n=81) for BRCA negative patients (p=0.33). When subdividing skin tests in negative, or positive at prick 10mg/dL, intradermal (ID) 1mg/ml or ID 10mg/dl levels, BRCA positive and negative patients presented a similar distribution (p=0.33).
Our studied population (n=138) was found to be comparable to the patients who were not evaluated for BRCA mutation (n=101) in terms of age, gender, grade of initial HSR, number of lifetime exposures to carboplatin, frequency of desensitization reactions and severity of these reactions.
BRCA 1/2 mutation was highly represented in patients with gynecologic malignancies undergoing RDD to carboplatin. The average prevalence of this mutation in tubo-ovarian cancer population is 13 to 17% (3), but when considering only allergic patients to carboplatin enrolled in the RDD program we identified a prevalence of 34%. This corroborates the finding of the BRCA mutation as an independent risk factor for HSR to carboplatin (1), since its prevalence is notably increased in our population.
We found that BRCA 1/2 positive patients receiving carboplatin presented more immediate HSR as their initial clinical presentation than BRCA negative patients (Figure 1a). This observation prompts enhanced surveillance during carboplatin administration for this group of patients, since immediate HSRs to carboplatin can manifest as anaphylaxis and require rapid medical intervention due to potential severity.
In terms of severity of initial immediate reactions, we found that the distribution between mild, moderate and severe reactions was similar between the groups (p=0.22), even though grade 1 HSRs were more slightly prevalent in BRCA negative patients and grade 2 HSRs in BRCA positive patients (Figure 1b).
Although Moon et al. reported that BRCA positive patients tend to develop carboplatin HSR with a lower cumulative dose, in our population the mean number of lifetime exposures was similar for both groups (1).
Patients who presented BRCA 1/2 mutations were found to be at a higher risk of immediate HSRs during RDD (Figure 2a, p<0.01); but in terms of severity of reactions, BRCA positive patients presented the same distribution as negative patients (Figure 2b, p=0.72), highlighting the safety of RDD for carboplatin-allergic patients.
Tryptase was elevated in reactions occurring during RDD in both groups (Figure 2c; normal value < 11.4 μg/L). This finding confirms the role of serum tryptase as a good method to evaluate drug-induced immediate HSR, when obtained in the first 30 to 120 minutes of the reaction (6,7).
Skin tests were positive in 94% of BRCA positive patients versus 89% in BRCA negative. Although not significant, it suggests that some of the reactions in the BRCA negative population are not IgE-mediated.
Whether BRCA 1/2 mutations can influence the expression of Th2 genes and increase specific IgE to carboplatin and/or increase signal transductions pathways during mast cell activation in response to carboplatin is not known (8).
In conclusion, patients with BRCA 1/2 mutations should be especially closely monitored throughout their carboplatin treatment and during RDD so that potential anaphylactic reactions can be rapidly treated.
Clinical Implications Box.
BRCA positive patients undergoing rapid drug desensitization (RDD) to carboplatin presented more initial immediate hypersensitivity reactions (HSR) (p=0.03) and reacted more during RDD (p<0.01) than BRCA negative patients. Carriers of the mutation should therefore be closely monitored during RDD.
Contributor Information
Violeta Régnier Galvão, Division of Rheumatology, Immunology and Allergy, Department of Medicine, Brigham and Women’s Hospital, Boston, Massachusetts. 75 Francis St, Boston, MA 02115; Division of Clinical Immunology and Allergy, University of São Paulo Medical School, São Paulo, Brazil.
Elizabeth Phillips, Division of Infectious Diseases, Department of Medicine, Vanderbilt University Medical Center, Tennessee, USA. 1161 21st Avenue South, Nashville, TN 37232-2582; Institute for Immunology and Infectious diseases, Murdoch University, Perth, Australia.
Pedro Giavina-Bianchi, Division of Clinical Immunology and Allergy, University of São Paulo Medical School, São Paulo, Brazil. Avenida Doutor Arnaldo, 455 – Cerqueira César, São Paulo – SP, 01246-904, Brazil.
Mariana Castells, Division of Rheumatology, Immunology and Allergy, Department of Medicine, Brigham and Women’s Hospital, Boston, Massachusetts. 75 Francis St, Boston, MA 02115. Phone: 617-525-1265.
References
- 1.Moon DH, Lee J-M, Noonan AM, Annunziata CM, Minasian L, Houston N, et al. Deleterious BRCA1/2 mutation is an independent risk factor for carboplatin hypersensitivity reactions. Br J Cancer. 2013 Aug 20;109(4):1072–8. doi: 10.1038/bjc.2013.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sloane D, Govindarajulu U, Harrow-Mortelliti J, Barry W, Hsu FI, Hong D, et al. Safety, Costs, and Efficacy of Rapid Drug Desensitizations to Chemotherapy and Monoclonal Antibodies. J Allergy Clin Immunol Pract. 2016 May-Jun;4(3):497–504. doi: 10.1016/j.jaip.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 3.Pal T, Permuth-Wey J, Betts JA, Krischer JP, Fiorica J, Arango H, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005 Dec 15;104(12):2807–16. doi: 10.1002/cncr.21536. [DOI] [PubMed] [Google Scholar]
- 4.Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011 Jun 30;474(7353):609–15. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tung N, Lin NU, Kidd J, Allen BA, Singh N, Wenstrup RJ, et al. Frequency of Germline Mutations in 25 Cancer Susceptibility Genes in a Sequential Series of Patients With Breast Cancer. J Clin Oncol. 2016 May 1;34(13):1460–8. doi: 10.1200/JCO.2015.65.0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz LB. Tryptase, a mediator of human mast cells. J Allergy Clin Immunol. 1990 Oct;86(4 Pt 2):594–8. doi: 10.1016/s0091-6749(05)80222-2. [DOI] [PubMed] [Google Scholar]
- 7.Galvão VR, Castells MC. Hypersensitivity to biological agents-updated diagnosis, management, and treatment. J Allergy Clin Immunol Pract. 2015 Apr;3(2):175–85. doi: 10.1016/j.jaip.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Jiang Q, Greenberg RA. Deciphering the BRCA1 Tumor Suppressor Network. J Biol Chem. 2015 Jun 5; doi: 10.1074/jbc.R115.667931. jbc.R115.667931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown SG. Clinical features and severity grading of anaphylaxis. J Allergy Clin Immunol. 2004 Aug;114(2):371–6. doi: 10.1016/j.jaci.2004.04.029. [DOI] [PubMed] [Google Scholar]


