STRUCTURED ABSTRACT
Study Design
Immunohistochemistry labeled pre- and post-synaptic structural markers to quantify excitatory and inhibitory synapses in the spinal superficial dorsal horn at 14 days after painful facet joint injury in the rat.
Objective
The objective of this study was to investigate the relationship between pain and synapse density in the spinal cord after facet injury.
Summary of Background Data
Neck pain is a major contributor to disability and often becomes chronic. The cervical facet joints are susceptible to loading-induced painful injury, initiating spinal central sensitization responses. Although excitatory synapse plasticity has been reported in the superficial dorsal horn early after painful facet injury, whether excitatory and/or inhibitory synapse density is altered at a time when pain is maintained is unknown.
Methods
Rats underwent either a painful C6/C7 facet joint distraction or sham surgery. Mechanical hyperalgesia was measured and immunohistochemistry techniques for synapse quantification were used to quantify excitatory and inhibitory synapse densities in the superficial dorsal horn at day 14. Logarithmic correlation analyses evaluated whether the severity of facet injury correlated with either behavioral or synaptic outcomes.
Results
Facet joint injury induces pain that is sustained until day 14 (p<0.001) and both significantly greater excitatory synapse density (p=0.042) and lower inhibitory synapse density (p=0.0029) in the superficial dorsal horn at day 14. Injury severity is significantly correlated with pain at days 1 (p=0.0011) and 14 (p=0.0002), but only with inhibitory, not excitatory, synapse density (p=0.0025) at day 14.
Conclusions
This study demonstrates a role for structural plasticity in both excitatory and inhibitory synapses in the maintenance of facet-mediated joint pain, and that altered inhibitory, but not excitatory, synapse density correlates to the severity of painful joint injury. Understanding the functional consequences of this spinal structural plasticity is critical to elucidate mechanisms of chronic joint pain.
Keywords: facet, joint, pain, injury, synaptogenesis, synapse, plasticity, central sensitization
INTRODUCTION
Neck pain is the fourth leading cause of years lived with disability worldwide [1] and becomes chronic, with a 12-month prevalence of 30–50% [2]. The cervical facet joints are common pain sources from trauma and with degeneration [2]. Cervical facet loading can activate and injure the afferents innervating the facet capsular ligament, inducing pain [3–7]. In parallel, facet joint injury initiates a host of peripheral and spinal responses that are characteristic of central sensitization, including increased afferent firing and spinal neuronal hyperexcitability, increases in proteins promoting excitatory signaling, and spinal synaptic structural plasticity [8–11]. The dynamic reorganization of pre-synaptic and post-synaptic terminals in the spinal cord during synaptic plasticity contributes to altered synaptic function during central sensitization [12,13] and is reported with chronic neuropathic pain [14–17]. Coincident with pain from nerve injury, the total number of dorsal horn synapses increases by up to 86% [14,15,18], and the number of dorsal horn excitatory synapses nearly triples [16,17]. Despite apparent links between synaptic structural plasticity, central sensitization, and chronic neuropathic pain, little is known about whether spinal synapse numbers change after a painful facet joint injury.
Modifications in both the excitatory and inhibitory synapse density and organization contribute to the altered synaptic function and central sensitization that occur when pain is maintained [11,13,19–25]. We have previously found that excitatory synaptogenesis plays a role in pain early after a facet joint injury in the rat, with ~50% more excitatory synapses in the superficial dorsal horn after a painful facet injury [11]. That increase in excitatory synapses coincides with neuronal hyperexcitability and increased spontaneous firing in the spinal cord [8,10]. While excitatory synaptogenesis characteristic of central sensitization is evident, it is unknown if structural alterations in excitatory synapses persist. Further, inhibitory synaptic changes, such as a loss of functional inhibitory interneurons in the dorsal horn or death of inhibitory interneurons, also contribute to central sensitization in chronic pain [13,19,26,27]. Indeed, structural changes in both inhibitory and excitatory synapses can contribute to the host of neuronal sensitization responses that are evident in the dorsal horn after painful facet injury [8,10,11]. Although excitatory synapse numbers have been investigated in joint pain [11], whether or not facet joint injury alters inhibitory synapses is unknown. Moreover, although changes in synapse structure have a role in chronic neuropathic pain [14–18], whether the extent of changes in the density of excitatory and/or inhibitory synapses at later times after painful injury relates to the injury severity has not been investigated for any pathophysiology of pain.
The objective of this study was to investigate the relationship between pain and synapse numbers in the spinal cord after painful facet injury. Since painful facet injury alters excitatory synapse numbers early after injury, we hypothesize that synaptic changes are also present late after injury, when pain persists. We further hypothesize that a loss of inhibitory synapses may contribute to pain persistence. We used a model of cervical facet joint injury in the rat that produces pain within 1 day and lasting for nearly 4 weeks [28]. Immunolabeling of spinal dorsal horn tissue at day 14 was performed with bassoon, a pre-synaptic structural marker, and one of the proteins homer1 or gephyrin, to label post-synaptic excitatory and inhibitory synapses, respectively; the co-localization of pre-and post-synaptic markers was quantified [11,29]. Additional assessments were performed to evaluate whether the synaptic structural changes, as well as pain, correlate with the degree of injury severity.
MATERIALS AND METHODS
All procedures were approved by our Institutional Animal Care and Use Committee and carried out according to the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain [30]. Studies used male Holtzman rats weighing 332–370g (Harlan Laboratories) under inhalation isoflurane anesthesia (4% induction, 2–3% maintenance). Rats underwent either a bilateral C6/C7 facet joint injury distraction to produce behavioral sensitivity (n=6) or a sham procedure (n=5), using previously described methods [10,11,31]. A midline incision over the back of the neck exposed the overlying paraspinal musculature that was separated to reveal the C4-T1 vertebrae. The C6 and C7 laminae were attached to a custom loading device using microforceps. For the injury group, the C6/C7 bilateral facet joints were distracted to 0.70mm at 15mm/s by displacing the C6 vertebra rostrally while holding C7 fixed [10,11,31]. A separate sham group underwent the same surgical procedures and microforceps attachment but no distraction (0mm) was applied across the joint.
To quantify injury severity, bead markers were placed on the right C6/C7 facet capsular ligament forming a grid for tracking ligament strains during distraction injury. A Phantom v4.3 CCD camera (Vision Research) recorded bead motions at 500Hz during the joint distraction. ProAnalyst software (Xcitex, Inc.) was used to digitize bead locations, and the displacements relative to the unloaded reference configuration were calculated. The ligament’s maximum principal strain (MPS) was computed using LS-DYNA (LSTC) [11,31,32] for each rat as the metric of severity of the applied joint injury.
To measure pain responses, behavioral sensitivity was assessed by measuring mechanical hyperalgesia in the forepaws of each rat prior to surgery (baseline) and at post-operative days 1,3,5,7,10, and 14. A tester blinded to procedures measured the paw withdrawal threshold (PWT) in the bilateral forepaws in response to stimulation with a series of von Frey filaments (Stoelting) of increasing strength (1.4,2,4,6,8,10,15, and 26g) [8,10,11]. Each filament was separately applied five times to each forepaw, and a positive response was recorded if the rat exhibited licking, shaking, or withdrawing the forepaw in response to stimulation. Once a positive response was recorded for two consecutive filaments, the lower strength filament was taken as the PWT for that testing session. Three rounds of testing were completed on each day, separated by at least 10 minutes, and all rounds were averaged across rats for both the left and right PWTs. The average withdrawal thresholds were compared between injury and sham groups using a repeated-measures ANOVA with post-hoc Tukey HSD tests. All statistical analyses were performed with α=0.05 using JMPPro11 (SAS Institute).
To investigate the number of synapses in the spinal cord at day 14, spinal cord tissue was immunolabeled for pre- and post-synaptic markers. Excitatory and inhibitory synapses were distinguished by co-labeling for the pre-synaptic marker, bassoon, and either the excitatory post-synaptic marker, homer1, or the inhibitory post-synaptic marker, gephyrin. To harvest spinal cord tissue, rats were anesthetized at day 14 after behavioral testing with sodium pentobarbital (65mg/kg, i.p.) and transcardially perfused with 250ml of PBS and 250ml of 4% paraformaldehyde (PFA). Spinal cord at C6 was harvested, post-fixed in 4% PFA overnight, and cryopreserved in 30% sucrose in PBS for seven days at 4°C. Samples were freeze-mounted in OCT medium (Fisher Scientific) and 6–8 axial cryosections (14μm each) from each rat were mounted on Superfrost Plus slides (Fisher Scientific). Samples were co-labeled for bassoon (guinea pig, 1:1000, Synaptic Systems), with either homer1 (rabbit, 1:200, Synaptic Systems), or gephyrin (mouse, 1:500, Synaptic Systems). Sections were blocked in 10% normal goat serum in PBS for 2 hours at room temperature and incubated overnight at 4°C with the primary antibodies in 10% goat serum with 0.3% Triton-X PBS. Sections were then incubated for 2 hours at room temperature with the appropriate fluorescent secondary antibodies: goat anti-guinea pig Alexa488 for bassoon, goat anti-rabbit Alexa568 for homer1, or goat anti-mouse Alexa546 for gephyrin (1:1000; Invitrogen). After rinsing in PBS and deionized water, slides were air-dried and cover-slipped with Fluorogel (EMS).
Excitatory and inhibitory synapses were counted using the co-localization of bassoon- and homer1-positive immunolabeled puncta, or bassoon- and gephyrin-positive immunolabeled puncta, respectively, using confocal imaging and synapse quantification methods [11,29]. To ensure consistency in sampling, a stack of nine confocal images was acquired in the superficial dorsal horn (laminae I–II) of each section at 0.33μm increments up to 3μm depth based on the cytoarchitecture of the dorsal horn using the 63X objective of a Zeiss LSM510 confocal microscope (1024×1024 pixels). The maximum intensity projection (MIP) of each set of three sequential images (i.e. images 1–3, images 4–6, images 7–9) was identified using a custom MATLAB program. Since the middle projection image (MIP from images 4–6; corresponding to 1–2μm depth) had the most consistent and robust immunolabeling, that MIP was used to quantify synaptic puncta [11]. Co-localization of the pre- and post-synaptic puncta was identified using the Puncta Analyzer in ImageJ (NIH) using previously published methods [11,29]. Although each MIP corresponded to 0.02mm2 of the superficial dorsal horn, each image included regions without tissue. A customized MATLAB program (VersionR2015a Mathworks) quantified the tissue area per image and co-localized excitatory or inhibitory puncta were normalized to that tissue area for each image to calculate synapse density (synapse number per tissue area). The total synapse density was calculated for each rat by adding the excitatory synapse density and the inhibitory synapse density for that rat. Synapse densities for each rat were also normalized to the average synapse density of the sham group, for excitatory, inhibitory, and total densities separately. Excitatory, inhibitory, and total synapse densities were compared between groups using separate Student’s t-tests.
Logarithmic regression models were used to investigate whether synapse density at day 14 after facet injury is sensitive to the severity of the applied facet joint distraction, and if the degree of mechanical hyperalgesia (PWT) at day 1 and day 14 is correlated with injury severity. For these analyses, the mean MPS across the facet capsule was taken as the degree of injury severity for each rat and as the independent variable in separate analyses with inhibitory synapse density, excitatory synapse density, day 1 PWT, or day 14 PWT. Since sham surgery does not impose any tissue strain, a MPS of 0% was used in the regression. As such, separate logarithmic fits were applied and the corresponding coefficient of determination (R2) for each correlation was calculated as a measure of goodness-of-fit. The significance of each model fit was assessed using an F-test. All logarithmic correlation analyses were performed using JMPPro11.
RESULTS
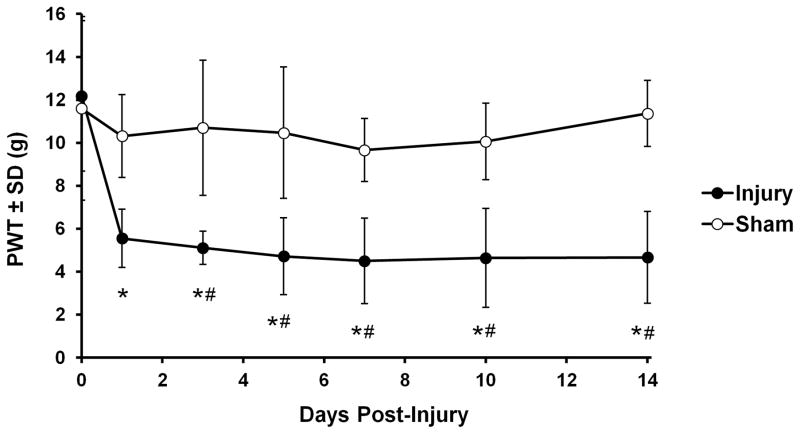
The average joint distraction applied to the injury group was 0.69±0.08mm, resulting in a mean MPS across the capsule surface of 24.37±7.87%. Overall, facet joint injury induces pain that is sustained until day 14 (p<0.001). Injury significantly decreases the PWT from baseline on all days (p≤0.0007), which is also lower than sham at each day except day 1 (p≤0.037) (Figure 1). Sham procedures do not change PWT from baseline (Figure 1). There is no difference in PWT between groups at baseline.
Figure 1.
The paw withdrawal threshold (PWT) significantly decreases after a C6/C7 facet joint distraction injury (n=6 rats) but is unchanged from baseline (day 0) for sham (n=5 rats). PWT is significantly lower than baseline on all days of testing (*p<0.0007) and lower than PWTs for sham (#p<0.037) on all days except day 1.
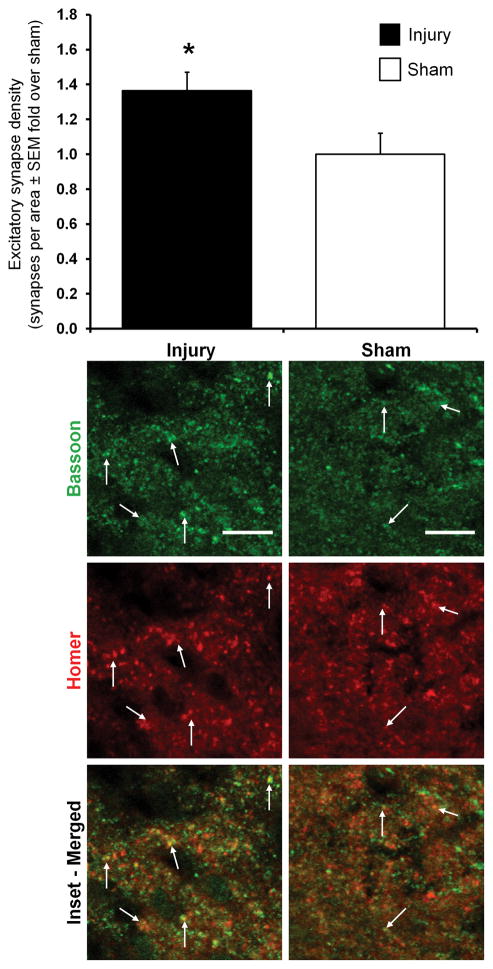
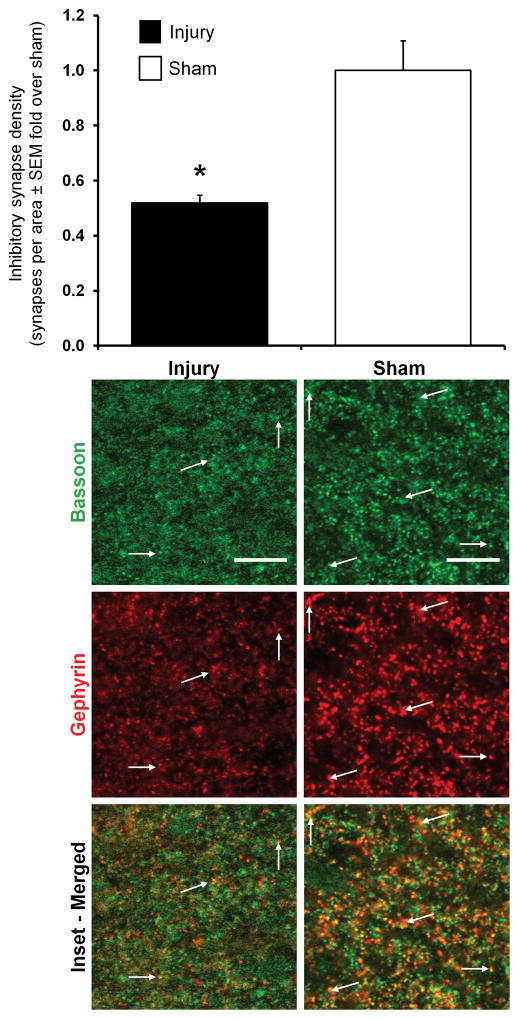
Both excitatory and inhibitory synapses are identified in the C6 spinal cord of both groups at day 14 (Figures 2 & 3). Normalized total synapse density in the superficial dorsal horn after painful injury (0.58±0.07) decreases to nearly half of sham (1.00±0.24) levels (p=0.013), with the number of total synapses/area in the superficial dorsal horn of injured rats (1205±145) being significantly lower than that of shams (2085±505). Yet, the number of inhibitory synapses/area in the superficial dorsal horn (range of 895–2362) is an order of magnitude larger than the number of excitatory synapses (range of 93–224) in that same region. Relative to the density of synapses evident after a sham procedure, painful injury induces both significantly greater excitatory synapse density (p=0.042) and significantly lower inhibitory synapse density (p=0.0029) in the superficial dorsal horn (Figures 2 & 3).
Figure 2.
Immunofluorescent labeling and quantification of excitatory synapse density in the superficial dorsal horn of the C6 spinal cord at day 14. The excitatory synapse density in the superficial dorsal horn of the spinal cord is significantly greater after painful facet injury than after sham (*p=0.042). Groups are shown as fold-change over sham±SEM. Arrows point to excitatory synapses that are identified by the co-localization (yellow) of bassoon (green) and homer1 (red). The scale bars represent 10μm and apply to all images.
Figure 3.
Immunofluorescent labeling and quantification of inhibitory synapse density in the superficial dorsal horn of the C6 spinal cord at day 14. The inhibitory synapse density in the superficial dorsal horn of the spinal cord is significantly lower after painful facet injury than after sham (*p=0.0029). Inhibitory synapses are counted as the co-localization (yellow) of bassoon (green) and gephyrin (red) positively-labeled puncta and are indicated in the representative images of the superficial dorsal horn. Group changes are shown relative to sham±SEM; the scale bars represent 10μm and apply to all images.
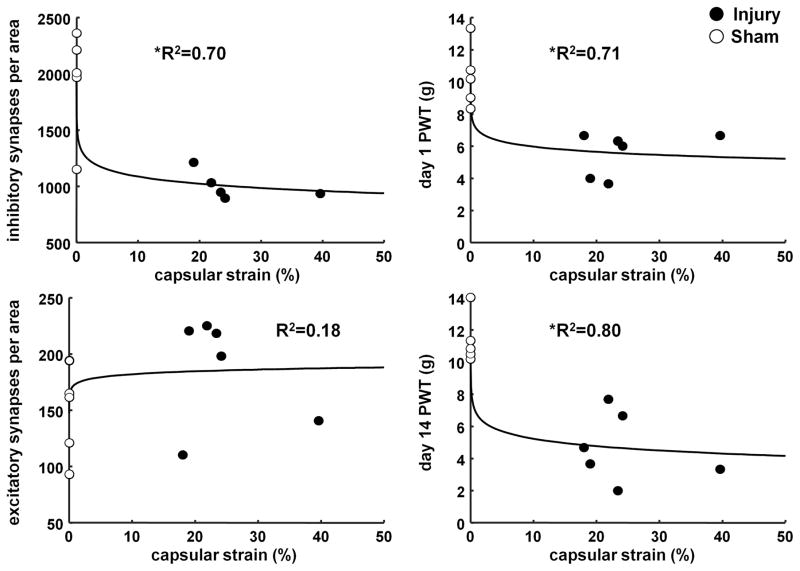
Logarithmic regressions reveal significant relationships between capsular strain (i.e. injury severity) and pain at early (day 1) and late (day 14) time points (Figure 4). Yet, both types of synapses do not exhibit the same relationship with applied injury. MPS is significantly correlated to inhibitory synapse density (p=0.0025, R2=0.70), with greater strain related to lower inhibitory synapse density (Figure 4). Although this relationship with inhibitory synapse density is significant, excitatory synapse density is not correlated to injury severity (p=0.19, R2=0.18) (Figure 4). Not surprisingly, PWT at day 1 (p=0.0011, R2=0.71) and day 14 (p=0.0002, R2=0.80) are significantly related to the applied injury severity, with greater MPS corresponding to lower PWT (more sensitivity) (Figure 4).
Figure 4.
Correlations between the mean maximum principal strain (MPS) and the inhibitory and excitatory synapse density at day 14, as well as the paw withdrawal threshold (PWT) at each of day 1 and 14. The logarithmic regression fit with inhibitory synapse density at day 14 (p=0.0025), PWT at day 1 (p=0.0011), and PWT at day 14 (p=0.0002) are all significant. The coefficient of determination (R2) for those correlations shows a very good goodness of fit. Yet, excitatory synapse density at day 14 is not significantly correlated with MPS (p=0.19) and has a low R2.
DISCUSSION
Facet injury induces pain that is sustained for at least 14 days, when changes in both excitatory and inhibitory synapse densities are evident in the dorsal horn (Figures 1–3). Although the total synapse density decreases by nearly half after painful injury, similar to the decrease in inhibitory synapse density (Figure 3), excitatory synapse density increases (Figure 2). Either an increase in excitatory, or decrease in inhibitory, synapse density could lead to increased spinal signaling [11,13,19–25]; only inhibitory synapse density is significantly associated with the severity of the applied joint injury (Figure 4).
These spinal synaptic changes after painful facet injury are consistent with central sensitization responses that maintain pain. The increase in excitatory synapse density occurring at day 14 (1.36±0.11-fold; Figure 2) is similar to those increases reported after painful nerve injury [16,17]. The greater excitatory synapse density at this later time after painful facet injury is also consistent with the 1.56-fold increase in excitatory synapses at day 7 after this same injury, concomitant with increased spinal neuron firing [8,10,11]. This increase in excitatory synapses could reflect low-threshold Aβ-fibers sprouting from the deep laminae (III–V) into the superficial laminae [12,33–35]. Aβ-fiber sprouting can form aberrant connections with the nociceptive Aδ-fiber and C-fiber neurons in the superficial laminae [22–25]. This structural reorganization activates up to 81% of the nociceptive-specific neurons in laminae II by normally non-noxious stimuli after nerve injury [23]. However, the induction of fiber sprouting after painful facet injury was not measured here and synapses were quantified using structural markers at only one time point. Nonetheless, the increased excitatory synapse density after painful facet injury (Figure 2) is aligned with the previously reported increases in excitatory post-synaptic currents in dorsal horn neurons after painful peripheral nerve injury [22–25]. Electrophysiological studies are needed to quantify the excitability of nociceptive-specific neurons at this late time after painful facet injury.
The decrease in inhibitory synapse density that is observed late after facet injury (Figure 3) may contribute to pain maintenance. Normally, densely distributed inhibitory interneurons in the superficial dorsal horn release GABA and/or glycine to decrease excitatory signaling and mediate inhibition in the spinal cord [19,36]. A loss of those inhibitory interneurons, and their associated ability to modulate excitatory signaling, could potentiate excitatory transmission [27]. In this study, inhibitory synapses were quantified using gephyrin (Figure 3), a post-synaptic protein known to interact with both GABA and glycine receptors [37]. Decreased gephryin labeling could, therefore, indicate a loss of those receptors or degeneration of the inhibitory interneuron synapses that populate the superficial dorsal horn [38]. Either inhibitory receptor loss or synapse degeneration would lead to disinhibition and the presence of mechanical hyperalgesia (Figure 1). Indeed, spinal administration of either GABA or glycine antagonists alone is sufficient to cause behavioral sensitivity [39–41].
Mechanically-induced facet injury increases spinal neuronal evoked-firing and behavioral sensitivity very soon after injury, both of which depend on the severity of the injury [8,11,31]. The severity of facet injury (i.e. strain) correlates with both the extent of behavioral sensitivity and inhibitory synapse density evident at day 14 (Figure 4), suggesting that both structural changes are induced by the same mechanical injury. Indeed, in previous work with this model facet capsular strains between 20–30% consistently induce behavioral sensitivity [11,42]. In contrast, the relationship between excitatory synapse density and injury severity does not follow pain behavior (Figure 4), suggesting that the plasticity of spinal inhibitory connections may be more sensitive to injurious strain than excitatory connections. Furthermore, a loss of functional inhibitory synapses, may itself drive central sensitization [13,19]. Taken together with results of the current study, it is possible that disinhibition via the decrease in inhibitory synapses is primarily responsible for pain maintenance after joint injury.
Altered synapse densities in this study were examined in the spinal cord at the level (C6) of the imposed facet injury and only at one time point. Although the number of synapses in the spinal dorsal horn have been shown to vary across spinal levels in the lumbar spine after a sciatic nerve injury, with increases only at L5 but not at its adjacent levels [14,15], the responses in the cervical region and for joint-mediated pain have not been explicitly examined to date. Certainly, a full spatiotemporal map of synaptic plasticity following joint injury would provide more insight about the extent to which synapse density changes after joint injury; however, the practicality of synapse examination at more frequent time points is not technically pragmatic and may unnecessarily use animals to produce negative findings. For example, since synaptic plasticity in the central nervous system is not established until 3–5 days after a peripheral nerve injury [43], examining time points before that time when new synapses develop may not be adequately long to allow for synaptic plasticity. Of note, excitatory synapses are increased at day 7 in this same injury model [11]. Since the time course for synaptic density changes in joint pain is not known, it is possible that they may occur along a different time period than those after nerve injury.
Overall, this study provides the first evidence of structural synaptic plasticity in facet-mediated joint pain persistence and demonstrates that the plasticity of inhibitory, but not excitatory, synapses is correlated to the severity of painful injury. Interestingly, although mechanically-induced joint injury increases excitatory and decreases inhibitory synapse density in parallel with pain, only inhibitory synapses correlate to injury severity with pain. Given the complex role of synaptic structural plasticity, injury, and pain development, understanding the functional consequences of both inhibitory and excitatory synapses in pain maintenance is critical to understanding joint pain.
Acknowledgments
The National Institutes of Health (#AR056288) and the Catherine Sharpe Foundation grant funds were received in support of this work.
Footnotes
Level of Evidence: N/A
References
- 1.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2163–96. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hogg-Johnson S, van der Velde G, Carroll LJ, et al. The burden and determinants of neck pain in the general population: results of the bone and joint decade 2000–2010 task force on neck pain and its associated disorders. Spine (Phila Pa 1986) 2008;33(4 Suppl):S39–51. doi: 10.1097/BRS.0b013e31816454c8. [DOI] [PubMed] [Google Scholar]
- 3.Chen C, Lu Y, Kallakuri S, et al. Distribution of A- δ and C-fiber receptors in the cervical facet joint capsule. J Bone Joint Surg. 2006;88:1807–16. doi: 10.2106/JBJS.E.00880. [DOI] [PubMed] [Google Scholar]
- 4.Lu Y, Chen C, Kallakuri S, et al. Neural response of cervical facet joint capsule to stretch: a study of whiplash pain mechanism. Stapp Car Crash J. 2005;49:49–65. doi: 10.4271/2005-22-0003. [DOI] [PubMed] [Google Scholar]
- 5.Kallakuri S, Singh A, Lu Y, et al. Tensile stretching of cervical facet joint capsule and related axonal changes. Eur Spine J. 2008;17:556–63. doi: 10.1007/s00586-007-0562-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee KE, Davis MB, Winkelstein BA. Capsular ligament involvement in the development of mechanical hyperalgesia after facet joint loading: behavioral and inflammatory outcomes in a rodent model of pain. J Neurotrauma. 2008;25:1383–93. doi: 10.1089/neu.2008.0700. [DOI] [PubMed] [Google Scholar]
- 7.Lee KE, Thinnes JH, Gokhin DS, et al. A novel rodent neck pain model of facet-mediated behavioral hypersensitivity: implications for persistent pain and whiplash injury. J Neurosci Methods. 2004;137:151–9. doi: 10.1016/j.jneumeth.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 8.Quinn KP, Dong L, Golder FJ, et al. Neuronal hyperexcitability in the dorsal horn after painful facet joint injury. Pain. 2010;151:414–21. doi: 10.1016/j.pain.2010.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crosby ND, Weisshaar CL, Winkelstein BA. Spinal neuronal plasticity is evident within 1 day after a painful cervical facet joint injury. Neurosci Lett. 2013;542:102–6. doi: 10.1016/j.neulet.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crosby ND, Gilliland TM, Winkelstein BA. Early afferent activity from the facet joint after painful trauma to its capsule potentiates neuronal excitability and glutamate signaling in the spinal cord. Pain. 2014;155:1878–87. doi: 10.1016/j.pain.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crosby ND, Zaucke F, Kras JV, et al. Thrombospondin-4 and excitatory synaptogenesis promote spinal sensitization after painful mechanical joint injury. Exp Neurol. 2015;264:111–20. doi: 10.1016/j.expneurol.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woolf CJ, Shortland P, Coggeshall RE. Peripheral nerve injury triggers central sprouting of myelinated afferents. Nature. 1992;355:75–8. doi: 10.1038/355075a0. [DOI] [PubMed] [Google Scholar]
- 13.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin JY, Peng B, Yang ZW, et al. Number of synapses increased in the rat spinal dorsal horn after sciatic nerve transection: a stereological study. Brain Res Bull. 2011;84:430–3. doi: 10.1016/j.brainresbull.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Peng B, Lin JY, Shang Y, et al. Plasticity in the synaptic number associated with neuropathic pain in the rat spinal dorsal horn: a stereological study. Neurosci Lett. 2010;486:24–8. doi: 10.1016/j.neulet.2010.09.037. [DOI] [PubMed] [Google Scholar]
- 16.Li KW, Yu YP, Zhou C, et al. Calcium channel α2δ1 proteins mediate trigeminal neuropathic pain states associated with aberrant excitatory synaptogenesis. J Biol Chem. 2014;289:7025–37. doi: 10.1074/jbc.M114.548990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park J, Trinh VN, Sears-Kraxberger I, et al. Synaptic ultrastructure changes in trigeminocervical complex posttrigeminal nerve injury. J Comp Neurol. 2016;524:309–22. doi: 10.1002/cne.23844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaken RJP, Joosten EAJ, Knuwer M, et al. Synaptic plasticity in the substantia gelatinosa in a model of chronic neuropathic pain. Neurosci Lett. 2010;469:30–3. doi: 10.1016/j.neulet.2009.11.038. [DOI] [PubMed] [Google Scholar]
- 19.Basbaum AI, Bautista DM, Scherrer G, et al. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–84. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim DS, Li KW, Boroujerdi A, et al. Thrombospondin-4 contributes to spinal sensitization and neuropathic pain states. J Neurosci. 2012;32:8977–87. doi: 10.1523/JNEUROSCI.6494-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang HH, Zhang XQ, Wang WY, et al. Increased synaptophysin is involved in inflammation-induced heat hyperalgesia mediated by cyclin-dependent kinase 5 in rats. PLoS One. 2012;7:e46666. doi: 10.1371/journal.pone.0046666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woodbury CJ, Kullmann FA, McIlwrath SL, et al. Identity of myelinated cutaneous sensory neurons projecting to nocireceptive laminae following nerve injury in adult mice. J Comp Neurol. 2008;508:500–9. doi: 10.1002/cne.21693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okamoto M, Baba H, Goldstein PA, et al. Functional reorganization of sensory pathways in the rat spinal dorsal horn following peripheral nerve injury. J Physiol. 2001;532:241–50. doi: 10.1111/j.1469-7793.2001.0241g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohno T, Moore KA, Baba H, et al. Peripheral nerve injury alters excitatory synaptic transmission in lamina II of the rat dorsal horn. J Physiol. 2003;548:131–8. doi: 10.1113/jphysiol.2002.036186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bester H, Beggs S, Woolf CJ. Changes in tactile stimuli-induced behavior and c-Fos expression in the superficial dorsal horn and in parabrachial nuclei after sciatic nerve crush. J Comp Neurol. 2000;428:45–61. doi: 10.1002/1096-9861(20001204)428:1<45::aid-cne5>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 26.Moore KA, Kohno T, Karchewski LA, et al. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci. 2002;22:6724–31. doi: 10.1523/JNEUROSCI.22-15-06724.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scholz J, Broom DC, Youn DH, et al. Blocking caspase activity prevents transsynaptic neuronal apoptosis and the loss of inhibition in lamina II of the dorsal horn after peripheral nerve injury. J Neurosci. 2005;25:7317–23. doi: 10.1523/JNEUROSCI.1526-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rothman SM, Hubbard RD, Lee KE, et al. Detection, transmission, and perception of pain. In: Slipman CW, Derby R, Simeone FA, Mayer TG, editors. Interventional Spine: An Algorithmic Approach. Philadelphia, PA: Saunders; 2008. pp. 29–38. [Google Scholar]
- 29.Ippolito DM, Eroglu C. Quantifying synapses: an immunocytochemistry-based assay to quantify synapse number. J Vis Exp. 2010;45:e2270. doi: 10.3791/2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–10. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 31.Dong L, Winkelstein BA. Simulated whiplash modulates expression of the glutamatergic system in the spinal cord suggesting spinal plasticity is associated with painful dynamic cervical facet loading. J Neurotrauma. 2010;174:163–74. doi: 10.1089/neu.2009.0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quinn KP, Lee KE, Ahaghotu CC, et al. Structural changes in the cervical facet capsular ligament: potential contributions to pain following subfailure loading. Stapp Car Crash J. 2007;51:1–19. doi: 10.4271/2007-22-0008. [DOI] [PubMed] [Google Scholar]
- 33.Koerber HR, Mirnics K, Brown PB, et al. Central sprouting and functional plasticity of regenerated primary afferents. J Neurosci. 1994;14:3655–71. doi: 10.1523/JNEUROSCI.14-06-03655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woolf CJ, Shortland P, Reynolds M, et al. Reorganization of central terminals of myelinated primary afferents in the rat dorsal horn following peripheral axotomy. J Comp Neurol. 1995;360:121–34. doi: 10.1002/cne.903600109. [DOI] [PubMed] [Google Scholar]
- 35.Shortland P, Kinman E, Molander C. Sprouting of A-fibre primary afferents into lamina II in two rat models of neuropathic pain. Eur J Pain. 1997;1:215–27. doi: 10.1016/s1090-3801(97)90107-5. [DOI] [PubMed] [Google Scholar]
- 36.Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–9. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 37.Tretter V, Mukherjee J, Maric HM, et al. Gephyrin, the enigmatic organizer at GABAergic synapses. Front Cell Neurosci. 2012;6:1–16. doi: 10.3389/fncel.2012.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci. 2010;11:823–36. doi: 10.1038/nrn2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malan TP, Mata HP, Porreca F. Spinal GABA(A) and GABA(B) receptor pharmacology in a rat model of neuropathic pain. Anesthesiology. 2002;96:1161–7. doi: 10.1097/00000542-200205000-00020. [DOI] [PubMed] [Google Scholar]
- 40.Sivilotti L, Woolf CJ. The contribution of GABAA and glycine receptors to central sensitization: disinhibition and touch-evoked allodynia in the spinal cord. J Neurophysiol. 1994;72:169–79. doi: 10.1152/jn.1994.72.1.169. [DOI] [PubMed] [Google Scholar]
- 41.Yaksh TL. Behavioral and autonomic correlates of the tactile evoked allodynia produced by spinal glycine inhibition: effects of modulatory receptor systems and excitatory amino acid antagonists. Pain. 1989;37:111–23. doi: 10.1016/0304-3959(89)90160-7. [DOI] [PubMed] [Google Scholar]
- 42.Dong L, Quindlen JC, Lipschutz DE, et al. Whiplash-like facet joint loading initiates glutamatergic responses in the DRG and spinal cord associated with behavioral hypersensitivity. Brain Res. 2012;1461:51–63. doi: 10.1016/j.brainres.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lo FS, Zhao S, Erzurumlu RS. Astrocytes promote peripheral nerve injury-induced reactive synaptogenesis in the neonatal CNS. J Neurophysiol. 2011;106:2876–87. doi: 10.1152/jn.00312.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]