Abstract
Purpose
To determine if ultra-wide-field fundus autofluorescence (UWFFAF) findings in acute zonal occult outer retinopathy (AZOOR) correlated well with perimetry, optical coherence tomography (OCT), and electroretinography (ERG) findings.
Methods
Retrospective observational study on 16 eyes of 10 subjects with AZOOR seen at a single referral center from October 2012 to March 2015 who had UWFFAF performed. Chi-square analysis was performed to compare categorical variables and Mann-Whitney U-test used for comparisons of non-parametric continuous variables.
Results
All eyes examined within 3 months of symptom onset (5 of 5 eyes) had diffusely hyperautofluorescent areas on UWFFAF. The remaining eyes contained hypoautofluorescent lesions with hyperautofluorescent borders. In 11/16 (68.8%) eyes, UWFFAF showed the full extent of lesions that would not have been possible with standard FAF centered on the fovea. There were 3 patterns of spread: centrifugal spread (7/16, 43.8%), centripetal spread (5/16, 31.3%), and centrifugal + centripetal spread (4/16, 25.0%). UWFFAF lesions corresponded well with perimetric, OCT, and ERG abnormalities.
Conclusions
UWFFAF along with OCT can be useful in the evaluation and monitoring of AZOOR patients.
Keywords: acute zonal occult outer retinopathy, AZOOR, fundus autofluorescence, optical coherence tomography, electroretinography
Introduction
Acute zonal occult outer retinopathy (AZOOR) is an idiopathic syndrome characterized by an acute onset of photopsias, scotoma, or both and is typically associated with a persistent loss of function involving one or more zones of the outer retina.1–3 AZOOR was first reported by Donald Gass in 1992 when he presented his observations on 13 patients at the Donders Lecture of the Netherlands Ophthalmological Society.1 AZOOR can involve one or both eyes and the condition usually stabilizes within 6 months of onset of symptoms although some cases continue to progress. During the acute stage, usually there is no ophthalmoscopically detectable fundus abnormality. However, some cases can present with a transient gray-white or white ring encompassing the involved area of retina, a presentation that has been referred to as acute annular outer retinopathy (AAOR).4–6 During the chronic stages, the affected areas typically manifest signs of atrophy of the retinal pigmented epithelium (RPE) and/or intraretinal pigment migration.1,2,7
The ophthalmic diagnostic tests used in the evaluation and monitoring of patients with AZOOR include perimetry, electroretinography (ERG), optical coherence tomography (OCT) and fundus autofluorescence (FAF). Visual field changes that have been reported in association with AZOOR include enlargement of the blind spot, central scotoma, paracentral scotoma, arcuate scotoma, annular scotoma, altitudinal hemianopia, and constriction of the peripheral field.1,2,8–10 Almost every patient with AZOOR demonstrates some degree of abnormality on full-field ERG (ffERG) and/or multi-focal ERG (mfERG).2,9,11,12 In addition, abnormalities in electrooculography have been reported in patients with AZOOR.13 Therefore, the diagnosis of AZOOR should be questioned in the setting of a normal electrophysiological test. The changes seen on OCT within the affected area of the retina in patients with AZOOR include irregularity of the RPE, absence or irregularity of the inner segment/outer segment (IS/OS) junction or the ellipsoid zone (EZ), attenuation of the outer nuclear layer, and decreased overall thickness of the retina.2,14–19
FAF findings in patients with AZOOR have been described in a limited number of reports. The most common finding on FAF imaging in AZOOR is a zone of hypoautofluorescence surrounded by a border of hyperautofluorescence.2,14,20–22 The hypoautofluorescence in the central portions of the AZOOR lesion is due to RPE atrophy while the hyperautofluorescence of the border of the lesion is attributed to accumulation of lipofuscin-like material at the edges of the lesion.22 However, in a few published cases there was no abnormality detected on FAF imaging.14,23 Ultra-wide-field FAF (UWFFAF) is an imaging modality which has become available recently for use in clinical practice that allows imaging of more than 200° or 82% of the retina.24,25 Because of the much larger area of retina imaged as compared to standard FAF that images up to 30° of the retina,26 or wide-field FAF that can image 50–60° of the retina,27 changes that involve the peripheral retina which could be missed by the latter tests are likely to be detected on UWFFAF. Therefore, we examined the question that UWFFAF imaging can correspond to abnormalities found on perimetry, OCT and ERG in patients with AZOOR, and that there is clinical utility of this test in the initial evaluation and monitoring of progression in patients with AZOOR.
Methods
Study Subjects
New patients diagnosed with AZOOR and seen by the authors at a single referral center during the period from October 2012 to March 2015 who had UWFFAF done were included in the study. The diagnosis of AZOOR was made on the basis of the presence of photopsias, subjective loss of visual field, or both, in association with one or more contiguous zones of outer retinopathy (as evidence by changes on perimetry, OCT or ERG) in the absence of any other identifiable etiology by ophthalmic examination, by electrophysiology or by laboratory testing (including tests for syphilis and tuberculosis). Of 14 patients who were identified as having a diagnosis of AZOOR, 10 patients (16 eyes) met the criteria for AZOOR diagnosis required for inclusion in this study. The study was approved by the Institutional Review Board of Oregon Health & Science University. The study was also compliant with the Health Insurance Portability and Accountability Act and was conducted in accordance to the tenets of the Declaration of Helsinki. Identifying information was removed from all data, and given the retrospective nature of the study, obtaining informed consent was not required.
Collection of Demographic and Clinical Data
Demographic and clinical data were collected by reviewing the electronic medical records of the study subjects, including: age, gender, race, nature and duration of symptoms, the presence of any antecedent illness, best-corrected visual acuity (BCVA), presence of relative afferent pupillary defect, presence of cells in the anterior chamber or in the vitreous, and findings on ophthalmoscopy. Bilateral disease was designated if patients had bilateral symptoms and at least 2 imaging modalities confirming involvement of both eyes, or if patients had only unilateral symptoms but 2 imaging modalities confirmed AZOOR-like lesions in the contralateral eye, defined as a contiguous zone or zones of outer retinal and/or RPE changes.
Ophthalmic Imaging and Perimetry
All patients underwent both UWFFAF imaging and pseudocolor (red and green only) imaging using the Optos ultra-wide-field imaging device (Optos, Marlborough, MA). The UWFFAF images were acquired using a scanning laser ophthalmoscope technology with a green laser of 532 nm as the excitation light and with signal emitted from the retina detected from a raster scan after passing through an emission filter with a bandpass of 570 to 780 nm. OCT scans of the retina were obtained with spectral-domain technology (SD-OCT) using the Spectralis system (Heidelberg Engineering, Calsbad, CA). FAF images were also obtained on the Spectralis system with excitation at 488 nm and with an emission filter with a bandpass of 500 to 680 nm. Static and kinetic visual field measurements were obtained with the Humphrey Field Analyzer II (Carl Zeiss Meditec, Dublin, CA) or the Octopus 900 system (Haag-Streit, Mason, OH) by following the instructions of each manufacturer. An improvement in UWFFAF was defined as a change from hyperautofluorescent to normal autofluorescence, whereas worsening was defined as the development of new hyperautofluorescent or hypoautofluorescent lesions, and a stable UWFFAF was defined as an image that appeared the same in terms of lesion extent and degree of autofluorescence. Lesion spread was also documented based on the proposition by Gass that the inciting agent gains access to the photoreceptors via the disruption in the retinal pigmented epithelium (RPE) at the optic nerve and at the ora serrata.28 Based on this supposition, and the obligatory zonal process of AZOOR, centripetal spread was designated if the lesion appeared to arise in the peripheral retina and then spread posteriorly towards the posterior pole, whereas centrifugal spread was defined as a lesion that appeared to arise near the optic disc or in the macular region and then spread peripherally in a concentric, contiguous pattern. Centripetal + centrifugal spread was designated if both patterns were seen but were non-contiguous (separated by an unaffected zone). Correlation between UWFFAF and perimetry findings was designated if the location of autofluorescence abnormalities anatomically corresponded to the location of the visual field defect (e.g. if there was temporal peripheral hyperautofluorescence, it correlated with perimetry if there was corresponding nasal peripheral perimetric constriction).
Electroretinography
Full-field ERGs were obtained according to a previously described protocol that complied with the standards published by the International Society for Clinical Electrophysiology of Vision (ISCEV).29–31 Multi-focal ERG testing was done for each eye separately using the VERIS Multifocal System (Electro-Diagnostic Imaging, Redwood City, CA) with 6.0.3 Science™ software using a Burian-Allen bipolar contact lens electrode according to the ISCEV guidelines with a protocol that used 103 hexagons, a bandpass of 10 to 300 Hz with the test split into 8 fifty-second test segments.32,33
Results
Demographic and fundus exam findings of AZOOR patients
There were a total of 10 subjects and 16 affected eyes included in the study (Table 1). Out of the 16 affected eyes, 14 eyes also had ERGs and perimetry done at least once. There were 3 females and 7 males. The mean age of the study subjects was 38.3 years (range 14–76) and 50% of the subjects were Caucasian. All subjects had a negative syphilis serology, and tuberculosis testing, when available, was negative. None of the subjects had pulmonary findings on chest X-ray to suggest an alternative etiology. Four subjects (40.0%) had bilateral presentation both by symptoms and by findings on imaging, while 6 subjects (60.0%) had bilateral presentation based on changes in at least two imaging modalities. All bilateral subjects had varying degrees of asymmetry. The duration of symptoms at presentation ranged from 1 week to 8 years (Table 2). All subjects experienced subjective loss of visual field, while photopsias were present in 7 subjects. Only one case, who presented within one week of the onset of symptoms, reported an antecedent flu-like illness one week prior to the onset of visual symptoms (subject 7). The BCVA at presentation ranged from 20/20 to 20/200. Half of the subjects had myopia while the other half had hyperopia or emmetropia (Table 2). Vitreous cells were present in 5 of 16 (31.3%) eyes. There were pigmentary changes in the fundus (RPE atrophy and/or intraretinal pigment migration) in all 10 eyes that had symptoms for 6 months or more (Table 2). Eyes that presented acutely had either disc edema and/or outer retinal lesions or no acute lesions visible on fundus examination. One eye (subject 3, Figure 2) that had symptoms for only one week had a wedge-shaped area of RPE atrophy extending from the optic nerve, proximal to newer outer retinal lesions extending into the fovea, but that subject also had an episode of similar self-resolving symptoms in the affected eye four years prior to presentation that may have resulted in the observed RPE atrophy. There was a peripapillary annular outer retinal ring in both eyes of one subject who presented one week after the onset of symptoms (subject 7) which resolved over time (Figure 5A and B). There were multifocal pinpoint grayish RPE lesions in one subject who presented with acute symptoms (subject 10, Figure 1) which may represent a concomitant multifocal choroiditis without panuveitis.
Table 1.
Summary statistics in AZOOR patients
| Demographics | 10 patients; 16 affected eyes | P value |
|---|---|---|
| Mean age in years (range, SD) | 38.3 (14–76, 21.34) | NA |
| Female sex n (%) | 3/10 patients (30%) | NA |
| Mean follow-up in months (range, SD) |
12.7 (0–29, 10.3) | NA |
| Ethnicity n Caucasian (%) | 5/10 patients (50%) | NA |
| NA | ||
| Ophthalmologic findings | ||
| Initial BCVA (logmaror Snellen mean, SD) |
0.2 or 20/30 (0.3) | |
| Follow up BCVA (logmaror Snellen mean, SD) |
0.2 or 20/30 (0.4) | p=0.4 (compared to initial BCVA) |
| % Bilateral by symptoms | 4/10 (40%) | NA |
| % Bilateral by at least 2 imaging modalities |
6/10 (60%) | NA |
| % eyes with anterior vitreous cell | 5/16 (31.3%) | NA |
| % eyes with CME | 1/16 (6.3%) | NA |
| % eyes with retinal vasculitis (among eyes who had FA done) |
3/13 (23.1%) | NA |
| % eyes centrifugal spread | 7/16 (43.8%) | NA |
| Mean LogMAR or Snellen BCVA (SD) |
0.3 or 20/40 (0.4) | NA |
| % eyes centripetal spread | 5/16 (31.2%) | NA |
| Mean LogMAR or Snellen BCVA (SD) |
0.1 20/25 (0.2) | p=0.88 (compared to mean logmar BCVA of centrifugal spread) |
| % eyes centrifugal + centripetal spread |
4/16 (25%) | NA |
| % eyes with hyper-AF lesions in setting of acute presentation (within first 3 months of symptoms) |
6/6 (100%) | NA |
| % eyes with UWFFAF findings outside central 50 degrees |
11/16 (68.8%) | NA |
| % eyes with UWFFAF findings only outside central 50 degrees |
2/16 (12.5%) | NA |
SD: standard deviation; BCVA: best-corrected visual acuity; CME: cystoid macular edema; FA: fluorescein angiography; hyper-AF (hyperautofluorescent); UWFFAF: ultra widefield fundus autofluorescence; LogMAR: the logarithm of the minimal angle of resolution
Table 2.
Demographic and initial clinical features of AZOOR patients
| Subject No. |
Age, years |
Gender | Race | Affected eye |
Duration* | Photopsia | Scotoma | Antecedent illness |
BCVA | Refractive error |
RAPD | AC/vitreous cells |
Fundus findings |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 14 | Male | Mixed | OD | A few years |
Present | Present† | Unknown | 20/30 | Low hyperopia |
None | Vitreous cells |
A contiguous zone of RPE atrophy and pigment migration involving the peripapillary area and the mid- peripheral retina |
| OS | A few years |
Present | Present† | Unknown | 20/30 | Low hyperopia |
None | Vitreous cells |
A contiguous zone of RPE atrophy and pigment migration involving the peripapillary area and the mid- peripheral retina |
||||
| 2 | 20 | Female | White | OS | 3 months | Present | Present | None | 20/30 | Low myopia | None | None | History of transient disc edema; peripapillary RPE changes |
| 3 | 24 | Female | White | OD | 2 weeks‡ | Present | Present | None | 20/70 | High myopia |
None | None | An irregularwedge-shaped zone of RPE atrophy extending from the superotemopraloptic disc, outer retinal whitish dots extending from peripapillary region from edge of RPE atrophy |
| OS | 1 week | Present | Present | None | 20/20 | High myopia |
None | None | Normal appearance | ||||
| 4 | 29 | Male | Asian | OD | 6 months | Present | Present | None | 20/20 | Low myopia | None | None | A contiguous peripapillary zone of RPE atrophy with pigment migration at the edge of the lesion |
| 5 | 40 | Male | Mixed | OD | 8 years | Absent | Present | Unknown | 20/200 | Moderate myopia |
None | None | RPE mottling in the macula; pigmentary changes in the periphery |
| OS | 8 years | Absent | Present | Unknown | 20/200 | Moderate myopia |
None | None | RPE mottling in the macula; pigmentary changes in the periphery |
||||
| 6 | 76 | Female | Asian | OD | >1 year | Absent | Present† | Unknown | 20/40 | Low hyperopia |
None | None | Zone of RPE changes in inferonasal quadrant and macula |
| OS | > 1 year | Absent | Absent | Unknown | 20/30 | Low hyperopia |
None | None | Peripapillary wedge of RPE atrophy |
||||
| 7 | 17 | Male | White | OD | 1 week | Present | Present | Flu-like illness |
20/20 | Low hyperopia |
None | None | Peripapillaryouter retinal ring; mild disc fullness and hyperemia |
| OS | 1 week | Present | Present | 20/20 | Low hyperopia |
None | None | Peripapillary pale ring; mild disc fullness and hyperemia |
|||||
| 8 | 59 | Male | Mixed | OD | 6 years | Present | Present | Unknown | 20/25 | Moderate myopia |
None | Vitreous cells |
Atrophy in inferior macula; bone spicules in the periphery |
| OS | 6 years | Present | Present | Unknown | 20/25 | Moderate myopia |
None | Vitreous cells |
Atrophy in inferior macula; bone spicules in the periphery |
||||
| 9 | 62 | Male | White | OD | 1 year | Present | Present | None | 20/25 | Low hyperopia |
None | None | Irregular wedge-shaped zone of RPE atrophy extending off of inferotemporal optic disc |
| 10 | 42 | Male | White | OD | 1 month | Present | Present | None | 20/20 | Emmetropia | None | Vitreous cells |
Zone of RPE changes in superonasal periphery |
: Time interval from onset of symptoms in the affected eye to the time when the first ultra-wide-field fundus autofluorescence imaging was carried out.
: Reported as decreased peripheral vision
: This patient had a history of photopsias in the right eye four years earlier
Abbreviations: RAPD, relative afferent pupillary defect; AC, anterior chamber; RPE, retinal pigmented epithelium
Figure 2.
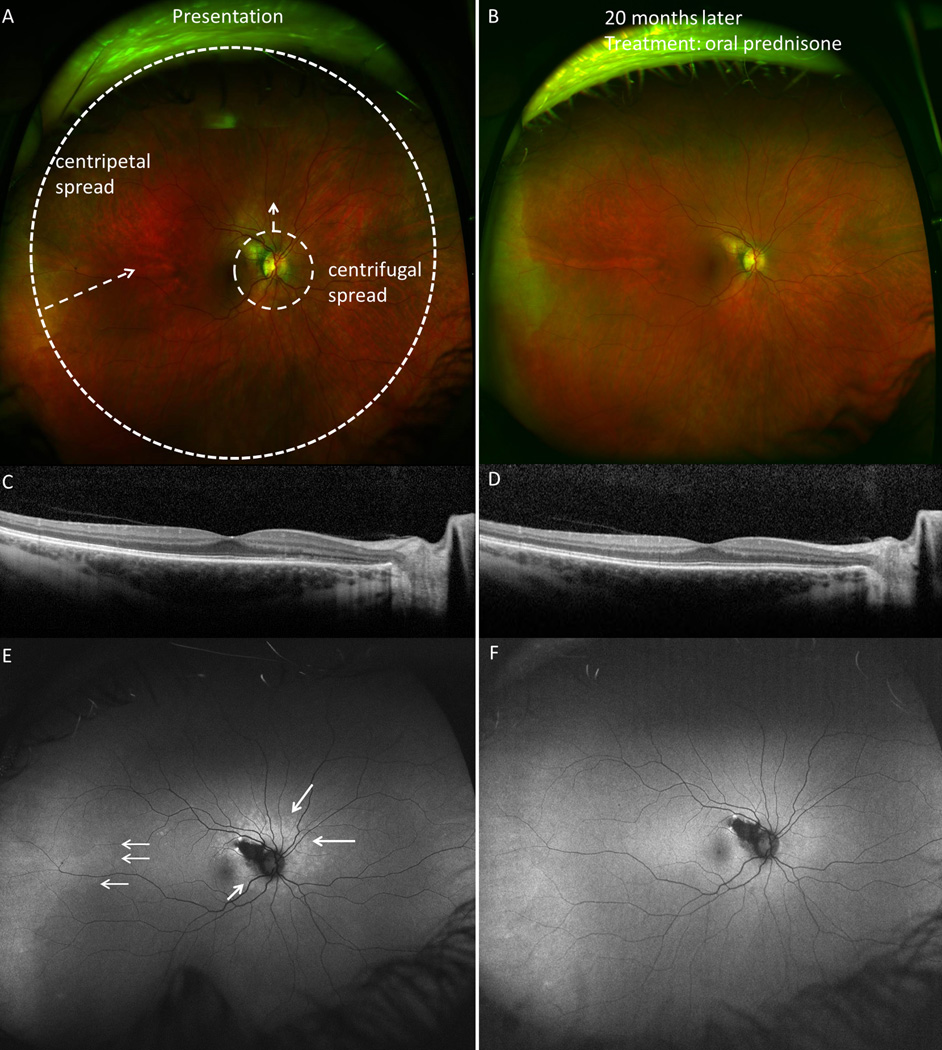
Centrifugal and centripetal spread at presentation and follow-up in subject 3. A. Fundus image of the right eye demonstrating pinpoint zone of hypopigmented outer retinal lesions surrounding optic nerve. B. Resolution of acute changes seen 20 months later, after a course of oral prednisone. C. Spectral domain optical coherence tomography (SD-OCT) image shows ellipsoid zone (EZ) and external limiting membrane (ELM) disruption in the nasal parafovea. D. SD-OCT at 20 months shows normalization of EZ and ELM disruptions outside area of retinal pigment epithlium atrophy. E. Ultra-wide-field fundus autofluorescence (UWFFAF) shows both centrifugal spread of the hyperautofluorescent lesions and centripetal spread from the temporal periphery (white arrows), which resolve on follow-up UWFFAF (F). Refer to Table 3 for associated perimetric findings.
Figure 5.
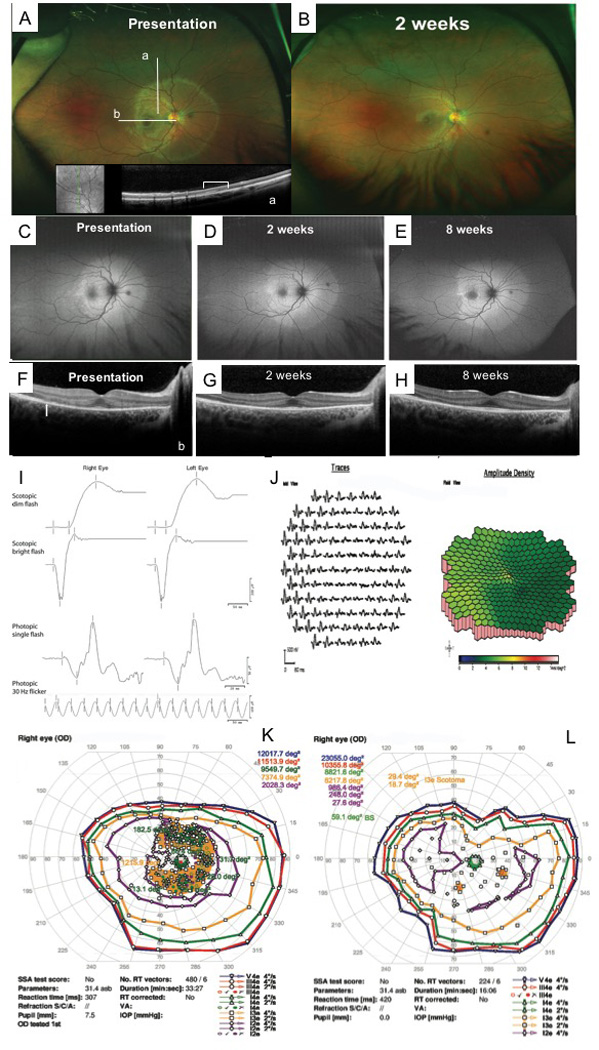
Acute annular outer retinopathy presentation in subject 7. A. Fundus image of the right eye taken at initial presentation and (B) at 2 weeks demonstrating initial annular ring appearance and outer nuclear layer (ONL) hyperreflectivity at location of the ring (inset a [white bracket] and b/F [white solid arrow]. C–E reveal changing area of hyperautofluorescence over time (presentation to 8 weeks) on ultra-wide-field fundus autofluorescence. At presentation, the patient had pinpoint satellite lesions of hyperautofluorescence in the temporal macula non-contiguous with the main area of involvement; these spots resolved in two weeks. F–H demonstrate improved appearance overall of the ellipsoid zone, external limiting membrane, and outer nuclear layer by 8 weeks. Initial full-field and multifocal ERG results demonstrated in I and J. K–L Initial and follow-up perimetry of the right eye demonstrating partial improvement.
Figure 1.
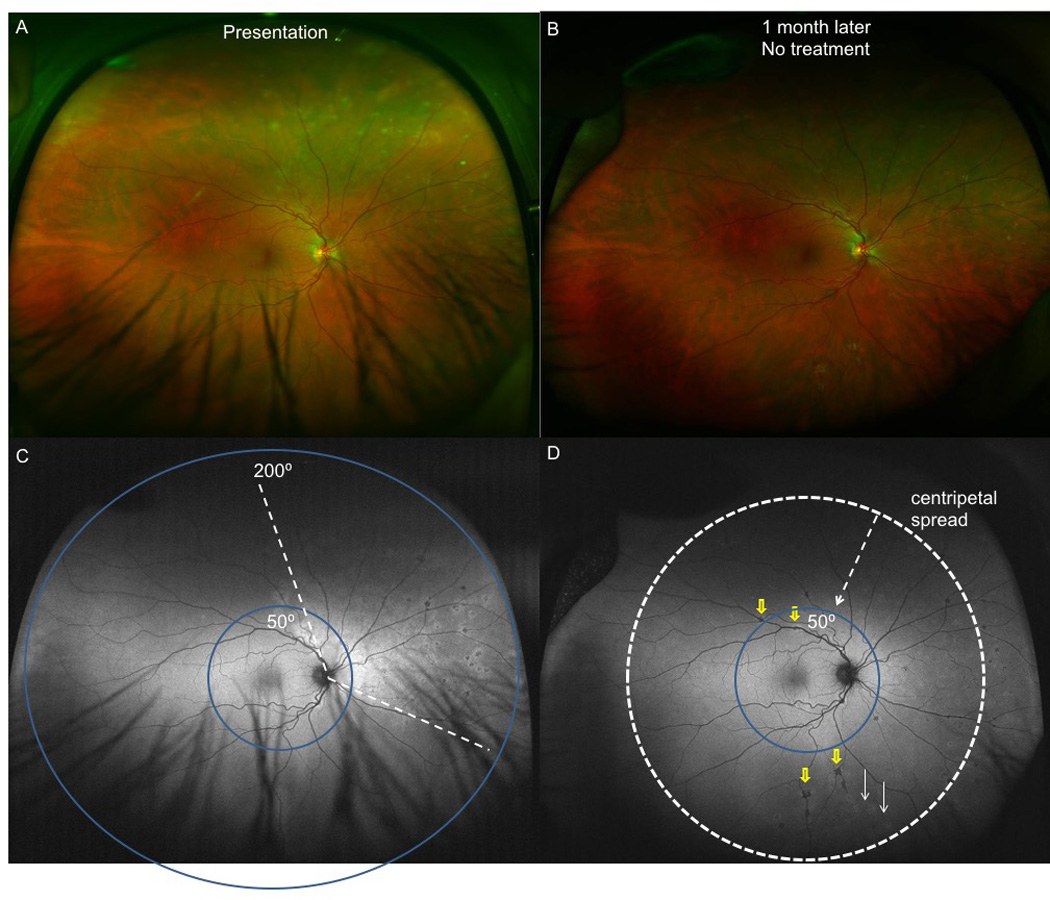
Acute zonal occult outer retinopathy with centripetal spread (with possible multifocal choroiditis) in subject10. A. Fundus image of the affected right eye revealed normal fundus appearance except for a superonasal and nasal contiguous zone of multifocal pinpoint and granular appearing RPE level lesions. B. 1 month later, the above lesions appear to progress inferiorly and posteriorly without treatment. C. Ultra-wide-field fundus autofluorescence (UWFFAF) of the right eye reveals a zone of hyperautofluorescence in the superonasal and nasal areas with concomitant multifocal hypoautofluorescent lesions. Blue circles designate the difference in field covered between lesions seen on regular wide-field 50 degree autofluorescence vs. 200 degrees covered in UWFFAF. Regular wide-field 50° FAF cannot detect hypoautofluorescent or hyperautofluoresent lesions in the peripheral retina. D. UWFFAF at 1 month demonstrates progression of both hyper- (white arrows) and hypo- (yellow arrows) autofluorescent lesions inferiorly and posteriorly, with the white dotted arrow and circle designating the type of spread as centripetal spread. Regular wide-field 50° FAF only detected 1 hypoautofluorescent lesion (yellow arrow) posteriorly.
UWFFAF Findings and visual acuity in AZOOR at presentation
In subjects who presented within three months of onset of symptoms, the affected areas exhibited a contiguous zone of hyperautofluorescence on UWFFAF (subjects 2, 3, 7, and 10, a total of 5 eyes; supplementary table 1). Subject 7, who presented one week after symptom onset, had multiple spots of hyperautofluorescence in the temporal macula along the temporal boundary of the affected diffusely hyperautofluorescent zone (Figure 5). In subject 3, who presented within one week of onset of symptoms but was also symptomatic four years earlier, the FAF showed a peripapillary wedge of hypoautofluorescence adjoined by a diffuse ring of hyperautofluorescence with pinpoint hyperautofluorescenct dots at the edges of the affected area (Figure 2). It should be noted that 1 week after symptom onset in the right eye in subject 3, the left eye subsequently became symptomatic and developed a hyperautofluorescent area in the periphery. In all other subjects, who had symptoms that lasted for 6 months or more, the typical appearance on UWFFAF was a zone of hypoautofluorescence surrounded by a ring of hyperautofluorescence. The zones of hypoautofluorescence were uniform in some of these cases, but were patchy or mottled in the remaining cases. The ring of hyperautofluorescence bordering the hypoautofluorescent zone in chronic subjects was more intensely hyperautofluorescent than the hyperautofluorescence noted in acute cases. In 14 of the 16 eyes, the peripapillary area and/or the macula were affected on UWFFAF. In four of the 10 subjects, satellite lesions non-contiguous with the main lesion were seen (subjects 3, 7, 8, 10).
Among the 16 eyes included in the study, two main patterns of spread of AZOOR were identified based on the appearance on UWFFAF: centrifugal spread (7/16 eyes or 43.8%) (Figures 2 and 3) and centripetal spread (5/16 eyes or 31.3%) (Figure 1) (Table 1 and Supplementary Table 1). The remaining 4 out of 16 eyes (25.0%) had evidence of both centrifugal and centripetal spread. Definitions of these designations of lesion spread are specified in the Methods section. In two subjects (subjects 3 and 6), spread was centripetal in one eye and both centripetal and centrifugal in their contralateral eyes.
Figure 3.
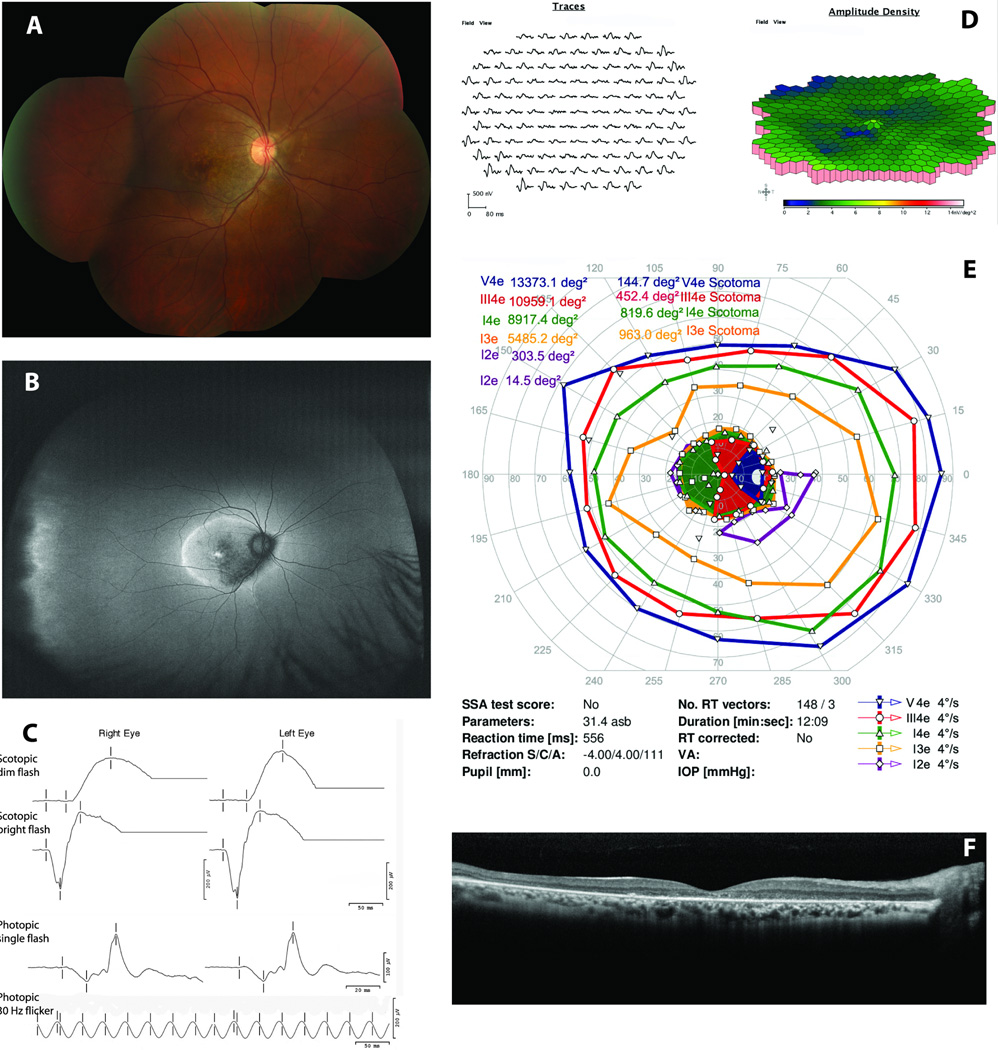
Correlation of ultra-wide-field fundus autofluorescence (UWFFAF) findings with perimetry and electroretinography (ERG) in subject 5. A. Fundus image of the right eye showing retinal pigment epithelial cell mottling and outer retinal ring surrounding the macula. B. UWFFAF demonstrates mottled macular hypoautofluorescence with ring of hyperautofluorescence surrounding the macula. Far temporal scalloped hypoautfluorescence with a posterior hyperautofluorescent border likely represents the ora serrata rather than pathological changes. C. Full-field ERG findings (see Table 4 for details). D. Multifocal ERG findings from the right eye demonstrating decreased amplitudes and timing centrally greater than in the peripheral macula. E. Kinetic perimetry reveals dense cecocentral scotoma and optical coherence tomography (F) reveals ellipsoid zone, external limiting membrane, and outer nuclear layer loss throughout the macula including the foveal area.
Among the 7 eyes with a centrifugal pattern of AZOOR, both eyes of subject 7 showed an annular ring of hyperautofluorescence centered on the optic disc (Figure 5, right eye shown) and subject 5 showed a concentric ring of hyperautofluorescence more or less centered on the fovea but also encompassing the optic nerve (Figure 3). In subject 7, who presented acutely, UWFFAF showed uniform hyperautofluorescence in the area enclosed within the annular ring and small spots of hyperautofluorescence at the temporal border (Figure 5C). This case was similar to reported cases of a likely subset of AZOOR termed acute annular outer retinopathy (AAOR) by Gass.4 This subject presented to us one week after the onset of visual symptoms which began 1 week after a flu-like illness. Laboratory testing showed IgG but no IgM antibodies in the serum against cytomegalovirus and Epstein-Barr virus, and no antibodies were found against herpes simplex, varicella zoster, hepatitis B, hepatitis C and human immunodeficiency viruses.
One patient (Subject 8) had bilateral AZOOR with centrifugal + centripetal spread in both eyes (Figure 4, left eye shown). Ophthalmoscopy in this patient revealed chorioretinal atrophy inferiorly in the macula and bone spicules in the periphery. UWFFAF showed a zone of hypoautofluorescence in the macula with an outer border of hyperautofluorescence (centrifugal spread) and a zone of nasal hypoautofluorescence with an inner border of hyperautofluorescence in the peripheral retina extending in a circumferential manner (centripetal spread) (Figure 4B). In this case, the peripheral involvement would have been missed on a 50° field FAF centered on the fovea. The UWFFAF was able to reveal the full extent of the AZOOR lesions in 11 of 16 eyes (68.8%) which may have been missed if a 50° field FAF centered on the fovea had been performed instead. While mean logMAR converted BCVA was slightly higher (worse) in eyes with centrifugal spread (0.3) than eyes with centripetal spread (0.1) on UWFFAF, this was not statistically significant (Table 1), likely due to the small sample size.
Figure 4.
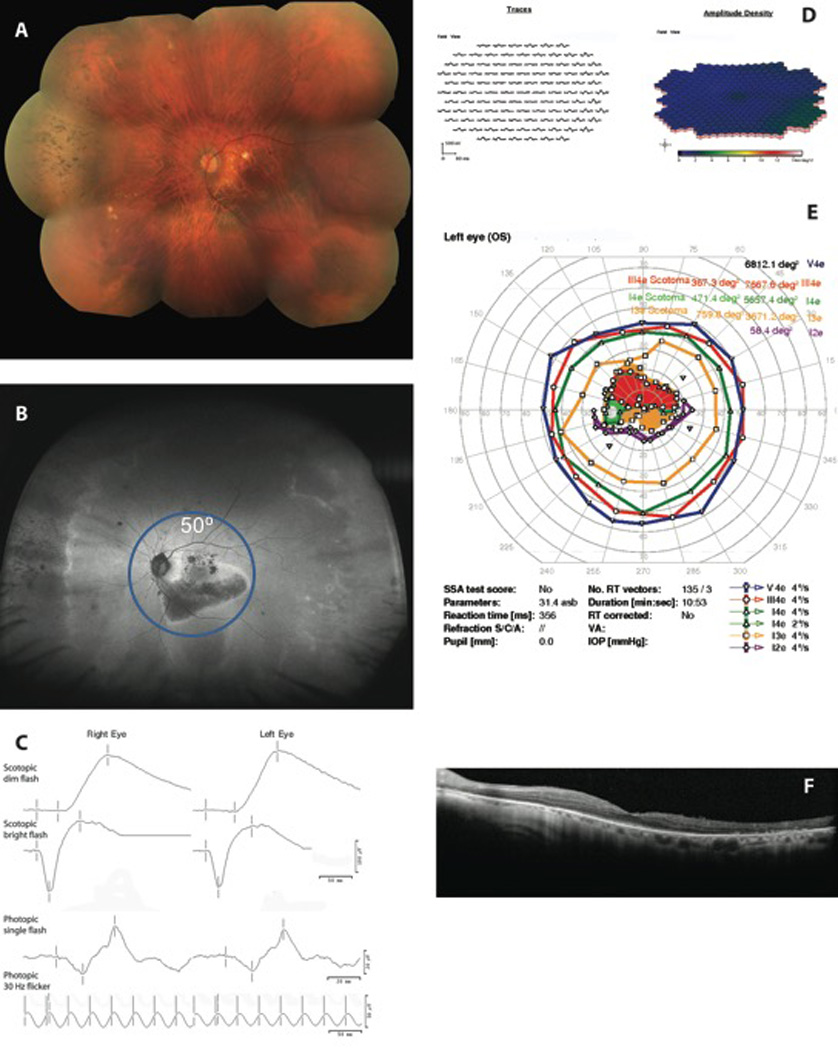
Centripetal and centrifugal spread demonstrated by ultra-wide-field fundus autofluorescence (UWFFAF) findings correspond to perimetry, electroretinography (ERG), and optical coherence tomography (OCT) findings in subject 8. A. Fundus image of the left eye reveals bone spicules in the nasal periphery and retinal pigment epithelial mottling in the macula B. UWFFAF of the left eye revealing hypoautofluorescence with a hyperautofluorescent border in the macula and the nasal periphery; there is also a temporal area of hyperautofluorescence. C. Full-field ERG from both eyes. D. Multifocal ERG results from the left eye show diffuse attenuation in the macula; E. Perimetry shows a dense cecocentral scotoma and peripheral isopter constriction and OCT (F) shows diffuse ellipsoid zone, external limiting membrane, and outer nuclear layer loss in the macula.
Perimetric Findings in AZOOR
All 14 eyes that had perimetry done showed visual field changes that corresponded well to areas of UWFFAF abnormalities (Supplementary Table 1) except for subject 2 in which the extent of visual field loss appeared larger than the extent of UWFFAF changes likely due to the optic nerve itself being inflamed upon presentation in addition to the peripapillary outer retina. For instance, in subject 7, the C-shaped (Figure 5C) or reverse C-shaped areas of hyperautofluorescence on UWFFAF were analogous to the reverse C-shaped (Figure 5K) or C-shaped scotomas seen on perimetry. The visual field changes were variable depending on presentation and UWFFAF findings, consisting of enlarged blind spots, superior altitudinal scotoma, inferior arcuate defects mimicking glaucoma, cecocentral scotomas, and peripheral isopter constrictions (Supplementary Table 1).
Optical Coherence Tomographic Findings in AZOOR
All 16 eyes had macular OCTs done, and if the macula was involved on UWFFAF, this corresponded to areas of EZ and external limiting membrane (ELM) loss (Supplementary Table 1) in acutely presenting cases. During acute presentation in subject 7, there was parafoveal hyperreflectivity of the ONL in the transition area between the affected and unaffected retina (in the area of the yellowish/grayish outer retinal ring on fundus examination) which appears to resolve over time once this ring is no longer visible on fundus imaging or fundus examination (Figure 5F,G). The other subjects, who presented 6 months or more from the onset of symptoms, had varying degrees of EZ and ELM loss in the macula also accompanied by ONL loss with or without underlying RPE atrophy. In subjects in whom RPE atrophy had occurred, there was always overlying EZ, ELM, and ONL loss. Choroidal atrophy was not common, occurring in only two subjects, one of whom was elderly at the age of 76 (subject 6), and another was age 59, but a moderate myope (subject 8).
ERG Findings in AZOOR
Eleven eyes of 6 subjects underwent both ffERG and mfERG, whereas one subject (subject 3, both eyes) underwent only ffERG, and one subject (subject 2, left eye only) underwent only mfERG. On ffERG, the rod-driven responses (dark-adapted 0.01 stimulus) were both subnormal in amplitude and prolonged in timing in 7 of 13 eyes (53.8%) tested (Supplementary Table 2). The amplitude of the a wave in response to the dark-adapted 3.0 stimulus was subnormal in 9 of 13 eyes (69.2%). Cone-driven responses were subnormal in amplitude, prolonged in timing or both in 8 of 13 eyes (61.5%). The photopic 30 Hz flicker was subnormal in amplitude, prolonged in timing or both in 9 of 13 eyes (69.2%). Not surprisingly, the 5 eyes that had purely centrifugal spread had less abnormalities on their ffERG compared to eyes that had centripetal spread or centripetal + centrifugal spread on UWFFAF.
In the 11 eyes tested successfully, mfERG demonstrated reduced amplitudes and prolonged implicit times (Table 3). The specific areas that showed subnormal responses on mfERG corresponded well with the macular areas that showed changes on UWFFAF and outer retinal/RPE changes on OCT. There was good correlation between location of mfERG abnormalities and perimetric findings. In the eyes with purely centrifugal spread, mfERG was more likely to affect only part of the tested field (6 of 6 eyes) compared to those with other patterns of spread, which were more likely to have diffuse involvement on the mfERG rather than partial involvement (1 of 5 eyes had partial involvement) (p=0.006).
Table 3.
Multi-focal electroretinographic findings in patients with AZOOR
| Subject No. |
Affected eye |
P1 amplitudes | P1 implicit times |
|---|---|---|---|
| 1 | OD& | Diffusely reduced | Prolonged outside the fovea |
| OS& | Reduced in nasal macular field | Prolonged in the nasal macular field | |
| 2 | OS* | Reduced in the temporal macular field | Prolonged in the temporal macular field |
| 4 | OD* | Reduced in the inferotemporal macular field | Prolonged in the inferotemporal macular field |
| 5 | OD* | Reduced mainly in the central macula | Prolonged mainly in the central macula |
| OS* | Reduced mainly in the central macula | Prolonged mainly in the central macula | |
| 6 | OD& | Diffusely reduced | Diffusely prolonged |
| OS1 | -- | -- | |
| 7 | OD* | Reduced in the temporal macular field involving fixation |
Prolonged in the temporal macular field involving fixation |
| OS* | Reduced in the temporal macular field sparing fixation |
Prolonged in the temporal macular field involving fixation sparing fixation |
|
| 8 | OD1 | Diffusely reduced | Diffusely prolonged |
| OS1 | Diffusely reduced | Diffusely prolonged |
--not done due to poor patient tolerance;
centripetal spread;
centrifugal+centripetal;
centrifugal spread
Utility of UWFFAF in monitoring the progression of AZOOR
Nine of 10 subjects (14 eyes) had some degree of follow-up ranging from 1 to 29 months with repeat UWFFAF imaging at last follow up. In 4 of these subjects (subjects 2, 3, 4 and 7), there was an improvement in symptoms and/or UWFFAF findings (Table 4). Three of 4 of these patients were given oral prednisone alone and 1 subject (subject 7) was given oral prednisone plus an oral anti-viral agent. Subject 4 had a clear improvement in symptoms while on prednisone which seemed to recur upon steroid taper, but was later able to taper off steroids completely without recurrence in symptoms. Because subject 4 did not present acutely with symptoms but rather with worsening of baseline photopsias, his UWFFAF findings were chronic to begin with (hypoautfluorescent lesion with hyperautofluorescent border), and did not change upon treatment even though there was symptomatic improvement. Subject 10 was treated with oral prednisone at 1 month follow up due to worsening of symptoms and UWFFAF findings and after a negative infectious work-up, but was then lost to follow up. Subject 7 (whose right eye is demonstrated in Figure 5), presented acutely after a viral prodrome. While the total area of hyperautofluorescence appeared to enlarge initially between his first UWFFAF image and his 2-week UWWFAF image, for the first week he had not yet received treatment and was later started on valacyclovir for 1 week followed by initiation of oral prednisone 1 mg/kg by his treating physician. Treatment with the combined regimen appeared to lessen symptoms, decrease the overall area of hyperautofluorescence from the 2-week visit, as well as improve foveal EZ reflectivity on OCT (Figure 5 F–H), although with residual parafoveal defects. Subjects who were not treated had no change in UWFFAF findings (except for the above mentioned subject 10 who worsened on UWFFAF and by symptoms in the 1 month prior to treatment initiation) and appeared to be stable symptomatically except for subject 1, who seemed to have worsening scotomas in the right eye despite a stable UWFFAF, and treatment of his cystoid macular edema with dorzolamide and bromfenac. Overall, stability in UWFFAF findings appeared to correspond to stability in OCT findings and stability in symptoms. On the other hand, improvements in UWFFAF appeared to correspond to improvements in OCT (Figure 2 and Figure 5) and improvement in symptoms (Table 4). In 3 eyes of 2 different patients (subjects 2 and 3), there was return to a normal fundus autofluorescence in once-hyperautofluorescent areas on follow-up.
Table 4.
Ultra-wide-field fundus autofluorescence findings during the follow-up of AZOOR
| Subject No. | Eye | Treatment | Follow-up Duration (months) |
Symptoms | Initial BCVA |
Follow -up BCVA |
Ultra-wide-field fundus autofluorescence |
|---|---|---|---|---|---|---|---|
| Centrifugal spread | |||||||
| 2 | OS | Oral prednisone | 20 | Improved | 20/30 | 20/20 | Improved; normal fundus autofluorescence; no recurrence off prednisone |
| 4 | OD | Oral prednisone | 8 | Improved, recurred during steroid taper × 1 |
20/20 | 20/15 | Stable |
| 7 | OD | Valacyclovir; oral prednisone |
8 | Improved | 20/20 | 20/15 | Improved; decreased area of hyper-AF; resolved temporal hyper-AF spots; no recurrence off prednisone |
| OS | Valacyclovir; oral prednisone |
8 | Improved | 20/20 | 20/15 | Improved; overall reduced area of hyper-AF; resolved temporaly hyper-AF spots; no recurrence off prednisone |
|
| 9 | OD | None | 29 | Stable | 20/25 | 20/20 | Stable |
| Centripetal spread | |||||||
| 1 | OD | Dorzolamide; bromfenac |
24 | Worse scotomas |
20/40 | 20/40 | Stable |
| OS | None | 24 | Stable | 20/25 | 20/30 | Stable | |
| 3 | OS | Oral prednisone | 20 | Improved | 20/20 | 20/20 | Improved; normal fundus autolfuorescence; no recurrence off prednisone |
| 6 | OD | None | 15 | Stable | 20/40 | 20/50 | Stable |
| 10 | OD | None | 1 | Increased photopsias |
20/20 | 20/20 | Worse; additional hyper-AF and hypo-AF lesions extending closer to the macula from superior and inferior periphery |
| Centrifugal + centripetal spread | |||||||
| 3 | OD | Oral prednisone | 20 | Improved | 20/70 | 20/25 | Improved; zones of hyper-AF resolved to normal fundus autofluorescence; zone of hypo- AF remains stable; no recurrence off prednisone |
| 6 | OS | None | 15 | Stable | 20/40 | 20/30 | Stable |
Hyper-AF: hyper-autofluorescence; hypo-AF: hypo-autofluorescence; BCVA: best corrected visual acuity
Discussion
Our study demonstrated UWFFAF abnormalities in all AZOOR subjects and, in addition, demonstrated a high degree of correlation between UWFFAF findings and perimetry and either ffERG or mfERG depending on the zone of retina involved. We also demonstrated good correlation between UWFFAF macular abnormalities (which can also be demonstrated by standard or 50° FAF) and SD-OCT findings. Acute findings appear to differ from chronic findings in AZOOR: acute disease presents with diffuse hyperautofluorescence on UWFFAF that corresponds to EZ and ELM disruption (without ONL or RPE disruption) on OCT, both of which can improve or resolve over time; chronic findings are comprised of either mottled or diffuse hypoautofluorescence with a hyperautofluorescent border, and correspond to EZ, ELM, and ONL disruption often with some degree of underlying RPE atrophy. Contiguous zones of involvement were classified into distinct patterns, centrifugal (spreading outward from the optic nerve) versus centripetal (spreading inward from the ora serrata), or both, and was unlikely to involve the mid-peripheral retina by itself. One plausible explanation for these contiguous zones of involvement of the retina in AZOOR is cell-to-cell spread of an infectious agent involving the photoreceptors followed by a delayed immune response as proposed by Gass.1 According to Gass’s hypothesis, AZOOR occurs in these contiguous zones, which we have described as centrifugal versus centripetal spread, because the inciting agent may gain access to the neurosensory retina through areas where it is not as well isolated from the systemic circulation by the RPE (at the optic nerve and the ora serrata).28 In patients with centripetal + centrifugal spread it is possible that the putative etiologic agent gains access to the neurosensory retina both at the optic disc and at the ora serrata since the central and peripheral zones of involvement can exist simultaneously without an overlap between the two zones. Centripetal and centrifugal patterns of distribution have also been recently reported by Tan and colleagues34. Whether or not the pattern of spread influences the visual acuity outcome is unknown since this study was not sufficiently powered to establish a significant difference, although we did find a trend towards worse visual acuity in subjects with centrifugal spread.
Although this study maintained a relatively strict clinical definition for AZOOR, the trizonal pattern described by Mrejen and colleagues to be pathognomonic for AZOOR35 was not present uniformly in our cohort. They astutely describe the trizonal pattern of AZOOR on FAF to include a normal zone of autofluorescence (zone 1), a speckled hyperautofluorescent zone (zone 2), and a hypoautofluorescent zone (zone 3) thought to be associated with choroidal atrophy. Analogous zones were also described by this group on SD-OCT. While zones 1 and 2 were seen in all of our AZOOR patients, acutely presenting patients did not have zone 3, nor was choroidal atrophy a prevalent finding in our entire cohort outside of age-related or myopic thinning. Chronically symptomatic patients in our cohort did meet trizonal criteria on autofluorescence but did not meet trizonal criteria on SD-OCT for the latter reason. Indeed, Mrejen and colleagues recognize that 3 patients in their large series did not show a trizonal pattern but rather, showed diffuse hyperautofluorescence,35 similar to the presentation of our acutely presenting patients. We propose that acute presentation of AZOOR to a referral clinic is rare given its often insidious nature and tendency for initial misdiagnosis, and that acutely presenting AZOOR patients will have diffuse hyperautofluorescence rather than a trizonal appearance unless they have an acute on chronic presentation. Also, unlike the above-mentioned study, our investigators did not perform indocyanine green angiography on our patients. Furthermore, while progression is a hallmark of AZOOR, this was seen variably in our cohort. This is likely due to the fact that acutely presenting patients were usually treated, and this was potentially associated with regression, whereas the acutely presenting patients who were not treated right away did in fact show initial progression. Our chronically presenting patients, on the other hand, had such long-standing disease that they did not show progression during the relatively short follow-up period, likely due to stabilization of disease prior to presentation to our practice.
The exact mechanism of hyperautofluorescence in acutely affected areas of the retina is not entirely clear. It is possible for instance, that this is due to photoreceptor outer segment inflammation resulting in loss of photopigment, and a subsequent relative increase in emission of autofluorescence from the underlying RPE. The subjects who demonstrated return to normal autofluorescence appear to support this hypothesis rather than a primary RPE process for hyperautofluorescence, especially given the relatively low intensity hyperautofluorescnece this acute process produces. Alternatively, increased production of autofluorophores from inflamed photoreceptor outer segments or infiltrating leukocytes could potentially contribute to the above finding. Finally, we cannot rule out that the increased lipofuscin accumulation in abnormal RPE is not progressing towards normal autofluorescence as it transitions towards atrophy prior to exhibiting hypoautofluorescence. Regarding the mechanism of hyperautofluorescence at the border of hypoautofluorescent lesions in chronic patients, this is likely due to increased production and/or decreased clearance of lipofuscin by the RPE36 given its persistent nature and increased intensity of hyperautofluorescence compared to acute patients. Hypoautofluorescent areas in our chronic patients were generally thought to be due to RPE atrophy, although the RPE layer was at times not as thin as expected on OCT for the degree of hypoautofluorescence, perhaps due to compensatory pigment hyperplasia. It is not clear if the RPE involvement in AZOOR is part of the primary pathology or if it occurs in a secondary manner following involvement of the outer neurosensory retina.
It is also not clear whether or not the hyperautofluorescent border surrounding the hypoautofluorescent zone indicates the presence of disease activity in AZOOR. In fact, the development of a hyperautofluorescent border may be a secondary sign of disease resolution and stabilization, as demonstrated in subject 7. In a retrospective study carried out by Fujiwara et al involving 19 eyes of 11 patients, FAF abnormalities were seen in 17 of the 19 eyes.14 In that study, there was progression of the affected area on FAF after a mean follow-up of 69.7 months. While our study utilized UWFFAF instead of standard FAF, and we had a shorter mean follow-up of 12.7 months, in this time period, we did not see progression in patients who presented with chronic symptoms. We did see temporary worsening in two patients who presented acutely, but this was reversed with treatment, lending some degree of credence to a possible benefit of treatment over observation in acutely presenting patients. UWFFAF appeared to be useful in capturing more extensive involvement than standard or even regular wide-field FAF in 69% of eyes. UWFFAF also demonstrated imaging improvements in acute patients all of whom were treated with oral prednisone, which correlated to SD-OCT improvements and symptomatic improvement. The long-term progression or stability of these patients compared to untreated patients remains to be determined. It should also be noted that whether the acute UWFFAF lesions (also seen on SD-OCT) would have improved spontaneously without treatment remains a possibility that could not be established in this study.
The study subject who presented with an AAOR type AZOOR deserves special attention. AAOR was first reported by Gass and Stern in 1995 in a 23-year-old male who had a sudden onset of progressive scotoma in one eye.4 In that patient, the annular ring was located in the superotemporal quadrant of the affected eye. The ring subsided within 4–5 weeks after the onset of symptoms, and within a year after presentation, the patient developed depigmentation of the RPE within the affected area followed by pigment migration into the retina. Subsequently, Fekrat et al reported 4 patients with AAOR two of whom later developed fundus changes that varied from mild RPE atrophy to marked atrophy with subretinal fibrosis.5 In our patient with AAOR, there was OCT ONL hyperreflectivity precisely at the location of this annular ring, which resolved on its own prior to initiation of treatment (2 weeks after presentation but 3 weeks after symptom onset). We believe that the appearance of the ring on examination is due to this change in the photoreceptor nuclei, but it is unclear whether this represents a different process than other types of AZOOR, or if it is missed in other AZOOR cases based on timing of presentation.
The strengths of this study include the relative rigor with which an AZOOR diagnosis was defined, the use of UWFFAF and OCT in all cases at both presentation and follow-up, and the high proportion of patients who had concomitant perimetry and ERG for comparison. The weaknesses of this study include its size, follow-up period, and retrospective nature, including slightly differing scan protocols and an inconsistent set of multi-modal imaging ordered by the various contributing clinicians. Despite the above weaknesses, cross-sectional information could be drawn at the initial time point on the correlation of UWFFAF findings to other imaging modalities, including functional studies such as ERG and perimetry. While ERG changes seen in this cohort were not specific to AZOOR as a disease entity, they were important in establishing functional correlates to UWFFAF findings (along with visual fields). Furthermore, follow-up UWFFAF imaging in AZOOR patients provided important information in terms of regression or stability of lesions, and corresponded well to SD-OCT findings at follow-up. Therefore, we can conclude that UWFFAF, especially in conjunction with SD-OCT, both of which have the benefit of being quicker and more easily obtained than perimetry and ERG, is a useful imaging tool in the initial evaluation and monitoring of AZOOR patients.
Supplementary Material
Summary statement.
There is a strong correlation between lesions seen on ultra-wide-field fundus autofluorescence, perimetric, optical coherence tomography, and electroretinographic changes in acute zonal occult outer retinopathy. Ultra-wide-field fundus autofluorescence can be useful in the initial evaluation, including detection of the full extent of lesions, and monitoring of progression.
Acknowledgments
Supported by National Institutes of Health grants K08EY021186 (MEP) and K08EY022948 (PL), Career Development Awards from Research to Prevent Blindness (MEP, PL), and Foundation for Fighting Blindness grant CD-NMT-0914-0659-OHSU. Casey Eye Institute research is supported by an unrestricted grant from Research to Prevent Blindness and core grant P30 EY010572 from the National Institutes of Health.
MEP is a consultant for Sucampo Pharmaceuticals and for ISIS Pharmaceuticals and receives support for clinical trials from AGTC and Sanofi. AS and PL are investigators in research projects funded by AbbVie, Bristol-Myers-Squibb and Genentech. AS is an investigator in a research project sponsored by the Mallinckrodt Foundation.
Footnotes
Presented in part as a poster at the Association for Research in Vision and Ophthalmology Annual Meeting May 3–7, 2015, in Denver, Colorado.
Conflict of interest: PY does not have any financial/conflicting interests to disclose.
References
- 1.Gass JD. Acute zonal occult outer retinopathy. Donders Lecture: The Netherlands Ophthalmological Society, Maastricht, Holland, June 19, 1992. J Clin Neuroophthalmol. 1993;13:79–97. [PubMed] [Google Scholar]
- 2.Monson DM, Smith JR. Acute zonal occult outer retinopathy. Surv Ophthalmol. 2011;56:23–35. doi: 10.1016/j.survophthal.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Yang P, Vitale AT. Acute Zonal Occult Outer Retinopathy. In: Foster CS, Vitale AT, editors. Diagnosis and Treatment of Uveitis. New Delhi, India: Jaypee Brothers Medical Publishers Ltd; 2013. pp. 1116–1129. [Google Scholar]
- 4.Gass JD, Stern C. Acute annular outer retinopathy as a variant of acute zonal occult outer retinopathy. Am J Ophthalmol. 1995;119:330–334. doi: 10.1016/s0002-9394(14)71176-6. [DOI] [PubMed] [Google Scholar]
- 5.Fekrat S, Wilkinson CP, Chang B, et al. Acute annular outer retinopathy: report of four cases. Am J Ophthalmol. 2000;130:636–644. doi: 10.1016/s0002-9394(00)00560-2. [DOI] [PubMed] [Google Scholar]
- 6.Mitamura Y, Ito H, Nakamura Y, et al. Acute annular outer retinopathy. Clin Experiment Ophthalmol. 2005;33:545–548. doi: 10.1111/j.1442-9071.2005.01047.x. [DOI] [PubMed] [Google Scholar]
- 7.Hoang QV, Gallego-Pinazo R, Yannuzzi LA. Long-term follow-up of acute zonal occult outer retinopathy. Retina. 2013;33:1325–1327. doi: 10.1097/IAE.0b013e318286cc57. [DOI] [PubMed] [Google Scholar]
- 8.Kuniyoshi K, Sakuramoto H, Nakao Y, et al. Two types of acute zonal occult outer retinopathy differentiated by dark- and light-adapted perimetry. Jpn J Ophthalmol. 2014;58:177–187. doi: 10.1007/s10384-013-0297-x. [DOI] [PubMed] [Google Scholar]
- 9.Yasuda K, Shimura M, Noro M, et al. Clinical course of acute retinal zonal occult outer retinopathy in visual field and multifocal electroretinogram. Br J Ophthalmol. 1999;83:1089–1090. doi: 10.1136/bjo.83.9.1088b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimada Y, Horiguchi M, Yamada H, et al. Case of acute zonal occult outer retinopathy with altitudinal hemianopsia. Br J Ophthalmol. 2003;87:1300. doi: 10.1136/bjo.87.10.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arai M, Nao-i N, Sawada A, Hayashida T. Multifocal electroretinogram indicates visual field loss in acute zonal occult outer retinopathy. Am J Ophthalmol. 1998;126:466–469. doi: 10.1016/s0002-9394(98)00117-2. [DOI] [PubMed] [Google Scholar]
- 12.Al-Yousuf N, Parks S, Dhillon B, Keating D. Acute zonal occult outer retinopathy. Br J Ophthalmol. 2000;84:118–119. doi: 10.1136/bjo.84.1.117b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Francis PJ, Marinescu A, Fitzke FW, et al. Acute zonal occult outer retinopathy: towards a set of diagnostic criteria. Br J Ophthalmol. 2005;89:70–73. doi: 10.1136/bjo.2004.042416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujiwara T, Imamura Y, Giovinazzo VJ, Spaide RF. Fundus autofluorescence and optical coherence tomographic findings in acute zonal occult outer retinopathy. Retina. 2010;30:1206–1216. doi: 10.1097/IAE.0b013e3181e097f0. [DOI] [PubMed] [Google Scholar]
- 15.Makino S, Tampo H. Changes in optical coherence tomography findings in acute zonal occult outer retinopathy. Case Rep Ophthalmol. 2013;4:99–104. doi: 10.1159/000355108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butler NJ, Smith JR. Imaging in the diagnosis and management of acute zonal occult outer retinopathy. Int Ophthalmol Clin. 2012;52:257–261. doi: 10.1097/IIO.0b013e318265d3fd. [DOI] [PubMed] [Google Scholar]
- 17.Takai Y, Ishiko S, Kagokawa H, et al. Morphological study of acute zonal occult outer retinopathy (AZOOR) by multiplanar optical coherence tomography. Acta Ophthalmol. 2009;87:408–418. doi: 10.1111/j.1755-3768.2008.01269.x. [DOI] [PubMed] [Google Scholar]
- 18.Wakazono T, Ooto S, Hangai M, Yoshimura N. Photoreceptor outer segment abnormalities and retinal sensitivity in acute zonal occult outer retinopathy. Retina. 2013;33:642–648. doi: 10.1097/IAE.0b013e3182671104. [DOI] [PubMed] [Google Scholar]
- 19.Spaide RF, Koizumi H, Freund KB. Photoreceptor outer segment abnormalities as a cause of blind spot enlargement in acute zonal occult outer retinopathy-complex diseases. Am J Ophthalmol. 2008;146:111–120. doi: 10.1016/j.ajo.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 20.Cohen SY, Jampol LM. Choroidal neovascularization in peripapillary acute zonal occult outer retinopathy. Retin Cases Brief Rep. 2007;1:220–222. doi: 10.1097/01.iae.0000243036.15712.a6. [DOI] [PubMed] [Google Scholar]
- 21.Fine HF, Spaide RF, Ryan EH, Jr, et al. Acute zonal occult outer retinopathy in patients with multiple evanescent white dot syndrome. Arch Ophthalmol. 2009;127:66–70. doi: 10.1001/archophthalmol.2008.530. [DOI] [PubMed] [Google Scholar]
- 22.Spaide RF. Collateral damage in acute zonal occult outer retinopathy. Am J Ophthalmol. 2004;138:887–889. doi: 10.1016/j.ajo.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Zibrandtsen N, Munch IC, Klemp K, et al. Photoreceptor atrophy in acute zonal occult outer retinopathy. Acta Ophthalmol. 2008;86:913–916. doi: 10.1111/j.1600-0420.2007.01140.x. [DOI] [PubMed] [Google Scholar]
- 24.Heussen FM, Vasconcelos-Santos DV, Pappuru RR, et al. Ultra-wide-field green-light (532-nm) autofluorescence imaging in chronic Vogt-Koyanagi-Harada disease. Ophthalmic Surg Lasers Imaging. 2011;42:272–277. doi: 10.3928/15428877-20110505-01. [DOI] [PubMed] [Google Scholar]
- 25.Witmer MT, Parlitsis G, Patel S, Kiss S. Comparison of ultra-widefield fluorescein angiography with the Heidelberg Spectralis((R)) noncontact ultra-widefield module versus the Optos((R)) Optomap((R)) Clin Ophthalmol. 2013;7:389–394. doi: 10.2147/OPTH.S41731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bellmann C, Rubin GS, Kabanarou SA, et al. Fundus autofluorescence imaging compared with different confocal scanning laser ophthalmoscopes. Br J Ophthalmol. 2003;87:1381–1386. doi: 10.1136/bjo.87.11.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Staurenghi G, Viola F, Mainster MA, et al. Scanning laser ophthalmoscopy and angiography with a wide-field contact lens system. Arch Ophthalmol. 2005;123:244–252. doi: 10.1001/archopht.123.2.244. [DOI] [PubMed] [Google Scholar]
- 28.Gass JD, Agarwal A, Scott IU. Acute zonal occult outer retinopathy: a long-term follow-up study. Am J Ophthalmol. 2002;134:329–339. doi: 10.1016/s0002-9394(02)01640-9. [DOI] [PubMed] [Google Scholar]
- 29.Marmor MF, Fulton AB, Holder GE, et al. ISCEV Standard for full-field clinical electroretinography (2008 update) Doc Ophthalmol. 2009;118:69–77. doi: 10.1007/s10633-008-9155-4. [DOI] [PubMed] [Google Scholar]
- 30.Weleber RG. The effect of age on human cone and rod ganzfeld electroretinograms. Invest Ophthalmol Vis Sci. 1981;20:392–399. [PubMed] [Google Scholar]
- 31.Weleber RG, Gupta N, Trzupek KM, et al. Electroretinographic and clinicopathologic correlations of retinal dysfunction in infantile neuronal ceroid lipofuscinosis (infantile Batten disease) Mol Genet Metab. 2004;83:128–137. doi: 10.1016/j.ymgme.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 32.Hood DC, Bach M, Brigell M, et al. ISCEV standard for clinical multifocal electroretinography (mfERG) (2011 edition) Doc Ophthalmol. 2012;124:1–13. doi: 10.1007/s10633-011-9296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang P, Chiang PW, Weleber RG, Pennesi ME. Autosomal Dominant Retinal Dystrophy With Electronegative Waveform Associated With a Novel RAX2 Mutation. JAMA Ophthalmol. 2015;133:653–661. doi: 10.1001/jamaophthalmol.2015.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan AC, Sherman J, Yannuzzi LA. ACUTE ZONAL OCCULT OUTER RETINOPATHY AFFECTING THE PERIPHERAL RETINA WITH CENTRIPETAL PROGRESSION. Retin Cases Brief Rep. 2016 doi: 10.1097/ICB.0000000000000312. [DOI] [PubMed] [Google Scholar]
- 35.Mrejen S, Khan S, Gallego-Pinazo R, et al. Acute zonal occult outer retinopathy: a classification based on multimodal imaging. JAMA Ophthalmol. 2014;132:1089–1098. doi: 10.1001/jamaophthalmol.2014.1683. [DOI] [PubMed] [Google Scholar]
- 36.Schmitz-Valckenberg S, Holz FG, Bird AC, Spaide RF. Fundus autofluorescence imaging: review and perspectives. Retina. 2008;28:385–409. doi: 10.1097/IAE.0b013e318164a907. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.