Abstract
Exclusive breastfeeding (EBF) provides infants with optimal nutrition, and together with appropriate antiretroviral therapy has also been shown to decrease mother-to-child transmission of HIV from 45% to less than 1%. However, rates of EBF are particularly low in South Africa, where rates of HIV are some of the highest in the world. Although perinatal depression has been identified as a potential barrier to EBF, little is known about its impact on EBF among HIV-infected women. A cohort study was conducted as part of a pilot RCT examining the effect of an Information, Motivation and Behavioral skills-based intervention promoting EBF among South African women living with HIV in their third trimester (28–42 weeks) of pregnancy. At baseline and follow-up, participants were interviewed on depression symptoms (PHQ-9), and breastfeeding intentions and behavior. Multivariate logistic regressions were conducted to determine predictors of EBF at six-weeks postpartum. A total of 68 women were enrolled and 58 women completed both baseline and follow-up assessments. Most (80.9%) of the sample reported at least some symptoms of depression prenatally. Rates of depression were lower postpartum (47.1%). In multivariate models, higher prenatal depression scores significantly predicted lower likelihood of EBF at six-weeks postpartum after adjusting for demographics, condition, and intentions (AOR=0.68, p<0.05). Postpartum depression was not a significant predictor of EBF rates (AOR=0.99, p=0.96). These findings demonstrate the negative impact of prenatal depression on breastfeeding behavior. Future interventions focused on depression are warranted to identify those at risk for sub-optimal EBF. Improving maternal psychosocial well-being could be a new frontier to improving infant and young child feeding and reducing pre/postnatal transmission.
Keywords: Prenatal depression, HIV, Perinatal depression, South Africa
Introduction
With the advancement of antiretroviral therapy (ART) and through the practice of exclusive breastfeeding (EBF), risk of mother-to-child transmission (MTCT) of human immunodeficiency virus (HIV) decreases from approximately 45% to less than 1% [1,2]. Thus, the prevention of MTCT of HIV and an AIDS-free generation is in our grasp [3]. In addition to the protective mechanism from EBF practice on decreasing MTCT of HIV, the nutritional benefits of breastmilk for infants are unparalleled [4,5]. In fact, breastfeeding provides all the nutrients an infant requires in their first 6 months of life [5,6]. The health benefits for mothers are also noteworthy, including protection against breast and ovarian cancer [7], cancer recurrence [8] and greater birth intervals [9]. Economically, breastfeeding reduces the financial burden on families who would otherwise need to procure formula.
However, only 39% of infants in developing countries are EBF to six months [6] and in South Africa rates of EBF at six months are reported at 8% from a demographic survey [10] and up to 26% in the setting of a randomized control trial [11]. Among HIV-infected women, EBF rates are similarly low despite increased resources promoting EBF within prevention of MTCT of HIV programs. Targeting barriers to breastfeeding is critical for providing appropriate support systems that recognize known factors that may derail women from achieving their infant feeding objectives.
Perinatal (prenatal and postnatal) depression rates are high globally. Ten to fifteen percent of pregnant or postpartum women experience depression in resource rich countries [12]. In limited-resource settings, such as sub-Saharan Africa, perinatal depression poses a significant burden to women with rates ranging from 10%–35% [11, 13]. Among women in South Africa, perinatal depression rates as high as 47% have been noted [14]. Women living with HIV encounter a range of obstacles that may contribute to even greater levels of depression perinatally. Although high rates of depression have been found among HIV-infected women in general [15], we know little about depression rates during the perinatal period [16]. Furthermore, most of the attention from the public health community has focused on postpartum depression [17]. Thus the impact of prenatal depression on maternal and child health is less clear, and the relationship that risk factors (social, behavioral, environment) have on pre and/or postpartum depression are lacking in the literature.
There are significant negative consequences of prenatal and postpartum depression for both mother and infant [18–22]. Prenatal depression is associated with prolonged labor, spontaneous abortion, increased maternal morbidity and mortality [23–25], and delivery of preterm and low birth weight infants [26]. Postpartum depression is linked to poor mother-infant bonding [27] as well as adverse infant outcomes, such as cognitive and developmental delays [28]. In addition, among HIV-infected women, perinatal depression has been found to contribute to poor adherence to HIV treatment [29,30], faster disease progression with lower survival rates, and risk of MTCT of HIV [16, 18]. Finally, its negative impact on breastfeeding duration and exclusivity is considerable, often resulting in early breastfeeding cessation among all breastfeeding women [31,32].
Despite these known risks and high prevalence, depression during the perinatal period, especially prenatally, is often under recognized and goes undiagnosed [14]. All women are expected to EBF their infants to six months postpartum, and HIV-infected women living in resource-limited settings are additionally recommended to adhere to ART [33]. Additionally, the presence of perinatal depression may also interfere with some of the beneficial aspects to breastfeeding, including mother and infant bonding as well as the nutritional benefits to the infant. Identification and appropriate treatment of risk factors (e.g., depression) that may be prohibitive to achieving EBF is critical to preventing mother to child transmission of HIV and poor maternal and infant outcomes.
Being HIV-infected, pregnant and depressed may synergistically contribute to worse maternal and infant health outcomes. The link between perinatal depression and poor ART adherence is well documented [34]. However, there exists the pressing need to identify levels of perinatal depression among HIV-infected women and its relationship on EBF- a crucial component to both optimal infant health and the prevention of vertical transmission. Thus, the primary goal of this study was to identify levels of perinatal depression among women living with HIV in South Africa and examine the relationship of perinatal depression on infant feeding outcomes.
Methods
Setting
The setting included two comparable public health clinics in the Sweetwaters and Edendale townships, each located outside Pietermaritzburg, South Africa, in the KwaZulu-Natal province. The estimated population of the area is approximately 500,000 with informal and formal housing, high population density and high rates of unemployment. HIV prevalence in KwaZulu- Natal province is estimated at 16.9% (Human Resources Research Council, 2012) and the infant mortality rate in South Africa for children under 5 is estimated is 44 per 1000 live births [35].
Study Design and Participants
A retrospective cohort study within the context of a randomized controlled trial (RCT) was conducted. The aims of the primary study were to test a small-scale Information, Motivation, Behavioral Skills (IMB) [36] model-based pilot intervention on levels of breastfeeding determinants and EBF among women living with HIV. The RCT from which these data come from had null findings on the EBF outcome so participants were collapsed across conditions. Participants were eligible if they were: (a) 18 years or older, (b) a woman infected with HIV, (c) pregnant in her third trimester, (d) currently taking ART, and (d) planning on returning to the clinic for her infant’s 6-week immunization visit. All participants provided written consent in their preferred language, isiZulu.
Measures
Demographic variables were assessed at baseline and 6-weeks postpartum. These demographic variables included education level, currently in school, employment, partner status, living with mother and age.
Depression
The Patient Health Questionnaire (PHQ-9) [37] was used to measure prenatal and postnatal depression. The PHQ-9 is a nine item screening scale that assesses 9 depressive symptoms, including anhedonia, depressed mood, insomnia, fatigue, appetite disturbance, guilt, diminished ability to think, psychomotor disruption, and suicidal ideation. The PHQ-9 uses a 4-point Likert Scale with scores ranging from 0 to 27. A score that is greater than 10 meets criteria for probable major depression diagnosis on the Diagnostic and Statistical Manual of Mental Disorder-IV (DSM-IV)[27]. The PHQ-9 has been used widely and adapted for diverse populations worldwide [38] with strong validity and reliability of the data (Cronbach alpha= 0.90)[39]. Given the ease of use, short administration time and success with adaptation, the PHQ was an appropriate scale to incorporate for use among our participant population in South Africa. Cross-cultural adaptation of the compiled instruments took place prior to implementation and details of that process have been published [40]
EBF intentions
Infant feeding intentions were assessed during pregnancy using a single item. Participants were asked, “ Do you intend to exclusively breastfeed, exclusively formula feed, or give your baby breast milk, formula and other foods or fluids for your baby’s first six weeks of life?” Responses were coded dichotomously, indicating intentions to EBF or no intentions to EBF.
EBF at six-weeks postpartum
EBF was assessed based on participant recall. Questions included, “Are you currently exclusively breastfeeding (name of infant)?” and a series of questions about feeding behaviors, to ensure that mothers who responded yes to the EBF question were actually EBF. These questions included “Have you started giving (name of infant) food or fluids in addition to giving breast milk?” and “If your choice was to exclusively breastfeed and you or your family introduced other fluids or foods in addition to your initial choice, when did this occur?”
Statistical Analyses
IMB SPSS Statistics Version 22 was used for all analyses. Women who completed both the baseline and follow-up assessments were included in these analyses. To characterize the sample, means and rates were calculated for demographic variables. Scores on the PHQ-9 were calculated to determine rates of depression prenatally and postpartum. To qualitatively describe the sample’s levels of prenatal and postpartum depression, PHQ-9 cut-offs of 5 (mild depression), 10 (moderate depression), 15 (moderately severe depression), and 20 (severe depression) were used [36].
To determine whether prenatal and/or postpartum depression significantly predict EBF, PHQ-9 scores were used continuously given the low rates of women who met tentative depression diagnoses (PHQ-9 ≥10), particularly postpartum. To determine the predictors of EBF at 6-weeks postpartum multivariate logistic regressions were used. Demographic variables available in the dataset that had the potential to impact EBF behavior were entered into the model (i.e. education, currently in school, currently employed, age, currently has a partner, living with own mother, prenatal intentions to EBF) in addition to the variable of interest, prenatal depression as determined by PHQ-9 scores at baseline. Intervention condition was also added as a control variable. To determine the impact of postpartum depression on EBF this model was replicated with PHQ-9 scores at 6-weeks postpartum. B, standard errors, adjusted odds ratios, and confidence intervals are reported for all variables included in the multivariate logistic regressions in the tables.
Results
This study enrolled 68 women living with HIV who were pregnant. Of these, 58 women completed the 6-week postpartum follow-up assessment and are included in the current analyses (Table 1). These women were fairly well educated and a small number of women were still in school during the postpartum period (N=4, 5.9%). The majority had a boyfriend, partner, or husband postpartum (N=53, 91.4%). Approximately half of the women did not live with their mother postpartum (N=33, 56.9%). Almost two-thirds of the women were still EBF at the 6-week follow-up (N=44, 81.5%).
Table 1.
Demographic characteristics of postpartum women living with HIV
| N | % | |
|---|---|---|
| Education | ||
| Secondary School | 56 | 96.6 |
| Post School | 2 | 3.4 |
| In School Postpartum | ||
| No | 54 | 93.1 |
| Yes | 4 | 6.9 |
| Employed Postpartum | ||
| No | 52 | 89.7 |
| Yes | 6 | 10.3 |
| Had a Partner Postpartum | ||
| No | 5 | 8.6 |
| Yes | 53 | 91.4 |
| Living with Mother Postpartum | ||
| No | 33 | 56.9 |
| Yes | 25 | 43.1 |
| Exclusively Breastfeeding at 6 weeks Postpartum | ||
| No | 10 | 18.5 |
| Yes | 44 | 81.5 |
|
| ||
| M | SD | |
|
| ||
| Age (range: 18–40) | 27.59 | 6.08 |
| Prenatal Depression (range: 0–20) | 7.96 | 4.53 |
| Postpartum Depression (range: 0–18) | 5.43 | 3.92 |
Rates of Prenatal and Postpartum Depression
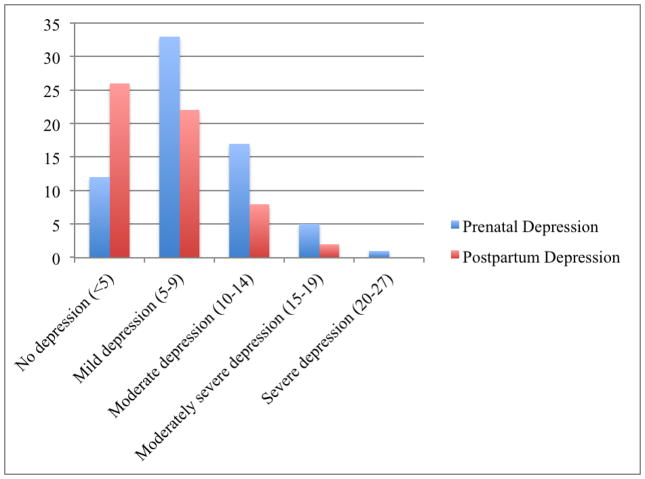
There were high rates of prenatal depression in this sample (Figure 1). Almost half of the sample was experiencing symptoms associated with mild depression (N=33, 48.5%). Additionally, 25% had scores indicative of moderate depression (N=17), 7.4% of moderately severe depression (N=5), and 1.5% of severe depression (N=1). Depression scores were lower postpartum; 38.2% experienced no depression and 32.4% had mild depression. Eight women experienced moderate depression (11.8%) and two experienced moderately severe depression (2.9%). No women were experiencing severe depression postpartum. Prenatal depression was not significantly correlated with postpartum depression (Pearson’s r = 0.23, p = 0.08). Additionally, women who did not complete the follow-up did not differ from women that completed the study on prenatal depression (t=0.12, p=0.91).
Figure 1.
Levels of prenatal and postpartum depression using PHQ-9 score cut-offs
Prenatal and Postpartum Depression as Predictors of Exclusive Breastfeeding
Table 2 shows the results of a multivariate logistic regression predicting EBF at 6-weeks postpartum. Prenatal depression scores were negatively associated with EBF, such that women who were more depressed prenatally were less likely to be EBF at 6 weeks (Adj. OR= 0.68, 95% CI [0.49, 0.95], p=0.04. Living with one’s mother was positively associated with EBF (Adj. OR=51.77, 95% CI [1.32, 2034.62], p=0.02). Maternal education, currently in school, current employment, age, currently in a relationship, and prenatal intentions to EBF did not predict EBF at 6 weeks.
Table 2.
Multivariate logistic regression including prenatal depression predicting exclusive breastfeeding at 6-weeks postpartum
| Variables | B | SE | Adj. OR | 95% CI | p-value |
|---|---|---|---|---|---|
| Condition | 0.47 | 1.10 | 1.60 | [0.19, 13.90] | 0.67 |
| Education | −4.22 | 2.73 | 0.02 | [0.00, 3.08] | 0.12 |
| Currently in School | −6.89 | 3.72 | 0.001 | [0.00, 1.48] | 0.06 |
| Currently Employed | −2.38 | 2.02 | 0.09 | [0.002, 4.81] | 0.24 |
| Age | 0.10 | 0.10 | 1.11 | [0.91, 1.35] | 0.31 |
| Current Partner | −0.88 | 1.95 | 0.42 | [0.009, 18.85] | 0.65 |
| Living with Mother | 3.95 | 1.87 | 51.77 | [1.32, 2034.62] | 0.04* |
| EBF Intentions | 1.23 | 2.14 | 3.40 | [0.05, 225.96] | 0.57 |
| Prenatal Depression | −0.39 | 0.17 | 0.68 | [0.49, 0.95] | 0.02* |
p<0.05
This analysis was replicated with postpartum depression scores to determine if current depression levels were associated with EBF (Table 3). Postpartum depression was not significantly related to EBF (Adj. OR=0.99, 95% CI [0.82, 1.22], p=0.96), nor was living with one’s mother (Adj. OR=9.94, 95% CI [0.89, 111.45], p=0.06).
Table 3.
Multivariate regression including postpartum depression predicting exclusive breastfeeding at 6-weeks postpartum
| Variables | B | SE | Adj. OR | 95% CI | p-value |
|---|---|---|---|---|---|
| Condition | 0.20 | 0.96 | 1.23 | [0.19, 7.98] | 0.83 |
| Education | −2.34 | 2.18 | 0.10 | [0.001, 6.89] | 0.28 |
| Currently in School | −3.97 | 2.48 | 0.02 | [0.00, 2.44] | 0.11 |
| Currently Employed | −1.02 | 1.46 | 0.36 | [0.02, 6.27] | 0.48 |
| Age | 0.07 | 0.08 | 1.07 | [0.91, 1.25] | 0.41 |
| Current Partner | 0.67 | 1.46 | 1.96 | [0.11, 34.12] | 0.64 |
| Living with Mother | 2.30 | 1.23 | 9.94 | [0.89, 111.45] | 0.06 |
| EBF Intentions | 0.64 | 1.63 | 1.89 | [0.08, 45.83] | 0.70 |
| Postpartum Depression | −0.005 | 0.10 | 0.99 | [0.82, 1.22] | 0.96 |
Discussion
The aim of this study was to investigate the effects of perinatal depression on EBF among women living with HIV in South Africa. Previous research reported mixed findings on the effects of postpartum depression on breastfeeding with some studies showing breastfeeding decreasing risk of depression [31, 41], depression causing breastfeeding abandonment [30, 38] or no relationship between the two [42, 43]. Clear findings illustrating the relationship between prenatal depression on breastfeeding outcomes is lacking. Our results demonstrate that prenatal depression is negatively associated with EBF at six-weeks postpartum.
Rates of mild and moderate prenatal depression among our participants were very high, at 48.5% and 25%, respectively. Previous research has shown women screening positive for prenatal depression are more likely to experience postnatal depression at 6-weeks [31,44,45]. However, prenatal depression did not predict postpartum depression in our sample. At the 6-week follow up visit 32% and 11% of women were experiencing mild or moderate depression postpartum, respectively. Although these postpartum rates were lower than prenatal rates, such levels may negatively impact infant feeding [13, 46], relationships with partners, and mother and baby attachment [47]. Thus, appropriate support and monitoring for postpartum depression remains critical during this timeframe. Mother-infant attachment is also a positive outcome of EBF [48]. This attachment can positively impact mothers’ mental health and give an infant a sense of security. Thus, determinants that derail mothers from EBF (e.g., depression) and potentially hindering the positive mental health outcomes facilitated by EBF should be managed.
Prenatal depression
Negative effects of perinatal depression on ART adherence have been well documented. In turn, maternal and infant health are negatively impacted from greater HIV disease progression and, higher rates of MTCT of HIV [17,33,48]. In our sample, prenatal depression was both high and predictive of poor EBF rates at six-weeks postpartum. Consequently, prenatal depression among HIV-infected women threatens their ability to successfully adhere to WHO recommendations (EBF to six-months and adhere to ART) and as such thwart the realization of an AIDS-free generation.
Places to Intervene
These data highlight the opportunity for targeted intervention. In South Africa, where HIV prevalence among reproductive-aged women is the highest in the world and levels of perinatal depression have been reported in almost half of all women [14] providing support systems that effectively target the complex, multi-factorial landscape (e.g., HIV and perinatal depression) inhibiting EBF and medication adherence and thus negatively impacting maternal and child health are necessary. A recent HIV diagnosis, an unplanned pregnancy, previous experience breastfeeding, awareness of cultural norms surrounding breastfeeding, disclosure among family or partner may all be pathways that impact incidence of perinatal depression and its relationship on EBF and perhaps medication adherence.
In addition, future interventions should take into account the positive effects of living with one’s mother prenatally being predictive of EBF behavior. Although the confidence interval is wide for the impact of living with one’s mother on EBF making the precise estimate of impact uncertain, the lower bound of the interval indicates a 32% increase in likelihood in EBF at 6 weeks. This highlights the importance of establishing strong social support systems during pregnancy, in particular from family members like one’s mother whose own experience may act as a model. Furthermore, determining the most influential determinants that both support or inhibit EBF is critical to enhancing its practice.
Modifiable risk factors (e.g., HIV related challenges, depression) that concurrently compromise a mother’s ability to EBF need to be addressed in interventions aiming to support women perinatally. Our findings demonstrating a strong link between prenatal depression decreasing a women’s likelihood to EBF at six-weeks, despite infant feeding intentions, highlight the critical need to increase attention on mental health support within prevention of MTCT of HIV programs. Identifying women with even mild prenatal depression is important to achieve better maternal and infant health outcomes [50].
Limitations
Although our study has several strengths, it is not without limitations. Given our study was a pilot designed to test an IMB model-based intervention promoting EBF it was underpowered for definitive analyses. The small sample size may have also contributed to the wide confidence intervals, leading to uncertainty regarding the magnitude of the impact of living with one’s mother on EBF. Furthermore, we did not account for many potentially impactful variables of perinatal depression, including previous depression diagnosis, history of violence, poverty, health systems supports, or health status (i.e. HIV RNA viral load or CD4 T-cell count). In addition, the potential impact of hormones (e.g., cortisol, thyroid, or gonadal steroid- estradiol) on pre and postpartum depression are noteworthy and may be a confounding factor. Thus, we cannot determine if prenatal depression alone predicts EBF or if one or more of these other factors that has been shown to influence depression in the literature is also the factor that is driving the relationship between prenatal depression and EBF. However, we feel that this study represents a first step in determining the role that depression plays in EBF among women living with HIV and future research should control for these potential confounding factors.
Conclusions
Perinatal depression among our participant population was high. Screening women for depression during pregnancy can assist in diagnosis and early treatment that can have positive impacts on women postpartum, including enhancing EBF. Furthermore, mental health support delivered during a relatively less intense time than the postpartum period may facilitate better outcomes. Thus, effective treatment delivered prenatally may better equip women for the challenges they’ll soon face, in addition to addressing the present considerations contributing to their individual situation. In particular, women living with HIV face known challenges to practice EBF to 6 months despite the high consequences when EBF isn’t achieved. Furthermore, medication adherence is negatively impacted by prenatal depression further contributing to poor maternal and infant health outcomes.
Future Implications
The consequences of poor mental health may result in poor infant feeding practice and medication adherence, both critical components for HIV-free infant survival. Furthermore, EBF facilitates additional, positive, mental health outcomes that are important to recognize, including mother-infant bonding and attachment. As such, positive outcomes attributed to EBF may contribute to reducing postpartum depression symptoms [21]. Thus, maximizing a mother’s success to effectively EBF by identifying and managing perinatal depression is an important step to improved maternal and infant health.
Acknowledgments
Funding: ELT was supported by Grant Number T32NR007081 from the National Institute of Nursing Research and F31MH099990 from National Institute of Mental Health. SLY was supported by Grant number K01 MH098902 from the National Institute of Mental Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest: Drs. Tuthill, Pellowski, Young and Butler declare no conflict of interest.
Ethical Considerations
The University of Connecticut Institutional Review Board and the Human Sciences Research Council Ethics Review Board approved the study protocol.
Compliance with Ethical Standards
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent Informed consent was obtained for all individual participants included in the study.
Conflicts of Interest The authors have no conflicts of interest to declare.
References
- 1.Coovadia HM, Rollins NC, Bland RM, Little K, Coutsoudis A, Bennish ML, Newell ML. Mother-to-child transmission of HIV-1 infection during exclusive breastfeeding in the first 6 months of life: An intervention cohort study. Lancet. 2007;369(9567):1107–1116. doi: 10.1016/S0140-6736(07)60283-9. [DOI] [PubMed] [Google Scholar]
- 2.Kuhn L, Skinkala M, Kankasa C, et al. High uptake of exclusive breastfeeding and reduced early post-natal HIV transmission. PLoS One. 2007;2(12) doi: 10.1371/journal.pone.0001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.UNICEF. [Accessed January 26, 2016];Achieving an AIDS-free Generation. 2013 Retrieved from: http://www.unicef.org/aids/files/VisionPaper_Interactive_ENG.pdf.
- 4.American Academy of Pediatrics. [Accessed January 26, 2016];Breastfeeding Initiatives. Retrieved from http://www2.aap.org/breastfeeding/
- 5.Centres for Disease Control and Prevention. [Accessed January 26, 2016];The Surgeon General’s Call to Action to Support Breastfeeding. 2012 Retrieved from: http://www.cdc.gov/breastfeeding/promotion/calltoaction.htm.
- 6.UNICEF. [accessed on January 26, 2016];2015 http://www.unicef.org/nutrition/index_24824.html.
- 7.Babita, Kumar N, Singh M, Malik J, Kalhan M. Breastfeeding reduces breast cancer risk: a case-control study in north India. Int Jour Prev Med. 2014;5(6):791–5. [PMC free article] [PubMed] [Google Scholar]
- 8.Kwan M, Bernard P, Kroenke C, Factor R, Habel L, Weltzien E, Castillo A, Gunderson E, Maxfield K, Stijleman I, Langholz B, Quesenberry C, Kushi L, Sweeney C, Caan B. Breastfeeding, PAM50 tumor subtype, and breast cancer prognosis and survival. Journal of National Cancer Inst. 2015;4(28):107. doi: 10.1093/jnci/djv087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chowdhury R, Sinha B, Sankar MJ, et al. Breastfeeding and maternal health outcomes: a systematic review and meta-analysis. Acta Paediatrica. 2015;104:96–113. doi: 10.1111/apa.13102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Department of Health South Africa. Demographic and Health Survey 2003. Department of Health; Pretoria: 2007. [Google Scholar]
- 11.Tomlinson M, Cooper P, Stein A, Swartz L, Molteno C. Post-partum depression and infant growth in a South African peri-urban settlement. Child: Care, Health and Development. 2006;32:81–86. doi: 10.1111/j.1365-2214.2006.00598.x. [DOI] [PubMed] [Google Scholar]
- 12.CDC. Mental health among women of reproductive age. Available at: http://www.cdc.gov/reproductivehealth/depression/pdfs/mental_health_women_repo_age.pdf. Retrieved: January 26, 2016.
- 13.Antelman g, Kaaya S, Wei R, Mbwambo J, Msamanga G, Fawzi W. Depressive symptoms increase risk of HIV disease progression and mortality among women in Tanzania. Journal of acquired immune deficiency syndromes. 2007;44:470–477. doi: 10.1097/QAI.0b013e31802f1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaaya S, Garcia ME, Li N, et al. Association of maternal depression and infant nutritional status among women living with HIV in Tanzania. Maternal and Child Health. 2014 doi: 10.1111/mcn.12154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rochat TJ, Tomlinson M, Barnighausen T, Newell ML, Stein A. The prevalence and clinical presentation of antenatal depression in rural South Africa. J of Aff Disorders. 2011;135:362–373. doi: 10.1016/j.jad.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basu S, Chwastiak LA, Bruce RD. Clinical management of depression and anxiety in HIV-infected adults. AIDS. 2005;19(18):2057–67. doi: 10.1097/01.aids.0000182518.84407.32. [DOI] [PubMed] [Google Scholar]
- 17.Lusskin S, Pundiak TM, Habib SM. Perinatal depression: hiding in plain sight. Can Journal of Psychiatry. 2007;52(8):479–488. doi: 10.1177/070674370705200802. [DOI] [PubMed] [Google Scholar]
- 18.Aaron E, Bonacquisti A, Geller PA, Polansky M. Perinatal depression and anxiety in women with and without human immunodeficiency virus infection. Women’s Health Issues. 2015;25(5):579–585. doi: 10.1016/j.whi.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Kapetanovic S, Dass-Brailsford P, Nora D, Talisman N. Mental health of HIV-seropositive women during pregnancy and postpartum period: A comprehensive literature review. AIDS Behav. 2014;18(6):1152–1173. doi: 10.1007/s10461-014-0728-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sidebottom A, Hellerstedt W, Harrison P, Hennrikus D. An examination of prenatal and postpartum depressive symptoms among women served by urban community health centers. Arch Womens Mental Health. 2014;17:27–40. doi: 10.1007/s00737-013-0378-3. [DOI] [PubMed] [Google Scholar]
- 21.Gregory EF, Butz AM, Ghazarian SR, Gross SM, Johnson SB. Are unmet breastfeeding expectations associated with maternal depressive symptoms. Acad Pediatr. 2015 May-Jun;(3):319–25. doi: 10.1016/j.acap.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Schwarze C, Hellhammer D, Stroehle V, Lieb K, Mobascher A. Lack of breastfeeding: A potential risk factor in the multifactorial genesis of borderline personality disorder and impaired maternal bonding. Journal of Personality Disorder. 2014;29(5):610–626. doi: 10.1521/pedi_2014_28_160. [DOI] [PubMed] [Google Scholar]
- 23.Diego M, Field T, Hernandez-Reif M, Schanberg S, Kuhn C, Gonalez-Quintero V. Prenatal depression restricts fetal growth. Early Human Dev. 2009;85(1):65–70. doi: 10.1016/j.earlhumdev.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grigoriadis S, VonderPorten EH, Mamisashvili L, et al. The impact of maternal depression during pregnancy on perinatal outcomes: A systematic review and meta-analysis. J Clin Psychiatry. 2013;74(4):321–341. doi: 10.4088/JCP.12r07968. [DOI] [PubMed] [Google Scholar]
- 25.Weobong B, Asbroek AH, Soremekun A, et al. Assocation of antenatal depression with adverse consequences for the mother and newborn in rural Ghana: Findings from the DON population-based cohort study. PLOSone. 2014;9(12):1–16. doi: 10.1371/journal.pone.0116333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung TK, Lau TK, Yip AS, Chiu HF, Lee DT. Antepartum depressive symptomatology is associated with adverse obstetric and neonatal outcomes. Pschosomatic Med. 2001;63(5):830–4. doi: 10.1097/00006842-200109000-00017. [DOI] [PubMed] [Google Scholar]
- 27.Dubber S, Reck C, Muller M, Gawlik S. Postpartum bonding: the role of perinatal depression, anxiety and maternal-fetal bonding during pregnancy. Arch Womens Mental Health. 2015;18(2):187–95. doi: 10.1007/s00737-014-0445-4. [DOI] [PubMed] [Google Scholar]
- 28.Muzic M, Borovska S. Perinatal depression: implications for child mental health. Mental Health Fam Med. 2010;7(4):239–247. [PMC free article] [PubMed] [Google Scholar]
- 29.Sheth SS, Coleman J, Cannon T, et al. Association between depression and nonadherence to antiretroviral therapy in pregnant women with perinatally acquired HIV. AIDS Care. 2015;27(3):350–354. doi: 10.1080/09540121.2014.998610. [DOI] [PubMed] [Google Scholar]
- 30.Nachega JB, Morroni C, Zuniga JM, Sherer R, Beyrer C, Solomon S, Schechter M, Rockstroh J. HIV-related stigma, isolation, discrimination, and serostatus disclosure: a global survey of 2035 HIV- infected adults. J Int Assoc Physicians AIDS Care. 2012;11(3):172–8. doi: 10.1177/1545109712436723. [DOI] [PubMed] [Google Scholar]
- 31.Dias CC, Figueiredo B. Breastfeeding and depression: A systematic review of the literature. J Affective Disorders. 2015;171:142–154. doi: 10.1016/j.jad.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 32.Sowa N, Cholera R, Pence BW, Gaynes BN. Perinatal depression in HIV-infected African women: A systematic review. J of Clin Psych. 2015;76(10):1385–96. doi: 10.4088/JCP.14r09186. [DOI] [PubMed] [Google Scholar]
- 33.WHO. [Accessed January 26, 2016];Guidelines on HIV and infant feeding. 2010 Available at: http://whqlibdoc.who.int/publications/2010/9789241599535_eng.pdf.
- 34.Stringer EM, Meltzer-Brody S, Kasaro M, et al. Depression, pregnancy, and HIV: the case to strengthen mental health services for pregnant and post-partum women in sub-Saharan Africa. Lancet Psychiatry. 2014;1(2):159–162. doi: 10.1016/S2215-0366(14)70273-1. [DOI] [PubMed] [Google Scholar]
- 35.UNICEF/WHO/World Bank/UN. [Accessed February 24, 2016];Levels and trends in child mortality 2014. Retrieved from http://www.unicef.org/media/files/Levels_and_Trends_in_Child_Mortality_2014.pdf.
- 36.Fisher JD, Fisher WA. Changing AIDS risk behavior. Psychological Bulletin. 1992;111:455–474. doi: 10.1037/0033-2909.111.3.455. [DOI] [PubMed] [Google Scholar]
- 37.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhong Q, Gelaye B, Rondon M, et al. Comparative performance of Patient Health Questionnaire-9 and Edinburgh Postnatal Depression Scale for screening antepartum depression. J of Affective Disorders. 2014;162(20):1–7. doi: 10.1016/j.jad.2014.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiong N, Fritzsche K, Wei J, et al. Validation of patient health questionnaire (PHQ) for major depression in Chinese outpatients with multiple somatic symptoms: A multicenter cross-sectional study. J of Aff Disorders. 2015;174:636–643. doi: 10.1016/j.jad.2014.12.042. org/10.1016/j.jad2014.12.042. [DOI] [PubMed] [Google Scholar]
- 40.Tuthill EL, Butler LM, McGrath JM, et al. Cross-cultural adaptation of instruments assessing breastfeeding determinants: a multi-step approach. International Breastfeeding Journal. 2014;9(1):16. doi: 10.1186/1746-4358-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yusuff AS, Tang L, Binns CW, Lee AH. Breastfeeding and postnatal depression: A prospective cohort study in Sabah, Malaysia. J of Human Lactation. 2015:1–5. doi: 10.1177/0890334415620788. [DOI] [PubMed] [Google Scholar]
- 42.Machado MC, Assis KF, Oliveira FC, et al. Determinants of the exclusive breastfeeding abandonment: psychosocial factors. Rev Saude Publica. 2014;48(6):985–994. doi: 10.1590/S0034-8910.2014048005340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pope C, Mazmanian D, Bedard M, Sharma V. Breastfeeding and postpartum depression: Assessing the influence of breastfeeding intention and other risk factors. J Affect Disorder. 2016;19(200):45–50. doi: 10.1016/j.jad.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 44.Lau Y, Wong D, Chan K. The utility of screening for perinatal depression in the second trimester among Chinese: a three-wave prospective longitudinal study. Archives of Women’s Health. 2010;13:153–164. doi: 10.1007/s00737-009-0134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rahman A, Creed F. Outcome of prenatal depression and risk factors associated with persistence in the first postnatal year: prospective study from Rawalpindi, Pakistan. J of Aff Disorders. 2007;100:115–121. doi: 10.1016/j.jad.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bascom EM, Napolitano MA. Breastfeeding duration and primary reasons for breastfeeding cessation among women with postpartum depressive symptoms. J of Human Lactation. 2015:1–10. doi: 10.1177/0890334415619908. [DOI] [PubMed] [Google Scholar]
- 47.Dennis CL, Brown S. Psychosocial interventions fro the treatment of perinatal depression. Best Practice & Research Clinical Obstetrics and Gynaecology. 2014;28:97–111. doi: 10.1016/j.bpobgyn.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 48.Tharner A, Luijk M, Raat H, Ijzendoorn M, Bakermanse-Kranenburg M, Moll H, Jaddoe V, Hofman A, Verhulst F, Tiemeier H. Breastfeeding and its relation to maternal sensitivity and infant attachment. Journal of Dev Behav Pediatr. 2012;33:396–404. doi: 10.1097/DBP.0b013e318257fac3. [DOI] [PubMed] [Google Scholar]
- 49.Sheth S, Coleman J, Cannon T, Milio L, Keller J, Anderson J, Argani C. Association between depression and nonadherence to antiretroviral therapy in pregnant women with perinatally acquired HIV. AIDS Care. 2015;27(3):350–35. doi: 10.1080/09540121.2014.998610. [DOI] [PubMed] [Google Scholar]
- 50.Kingston K, Austin MP, Hegadoren K, et al. Study protocol for a randomized, controlled, superiority trial comparing the clinical and cost-effectiveness of integrated online mental health assessment- referral-care in pregnancy to usual prenatal care on prenatal and postnatal mental health and infant health and development: the Integrated Maternal Psychosocial Assessment to Care Trial (IMPACT) TRIALS. 2014;15:72–92. doi: 10.1186/1745-6215-15-72. [DOI] [PMC free article] [PubMed] [Google Scholar]