Abstract
Background
Formation of clots in the left atrial appendage (LAA) may lead to embolism and consecutive cerebral stroke. This risk is reduced by closure and resection. To address the efficacy of surgical LAA closure, resilience to pneumatic pressure was studied. Different surgical techniques were compared in an experimental model.
Methods
From freshly slaughtered pigs cardiopulmonary preparations were taken. The left atrium was clamped airtight and the LAA was cannulated. Via a manually operated pump pressure was applied and a digital pressure gauge was connected. Four groups (each n=12) with different epicardial closures were studied: (I) purse string; (II) single layered continuous suture; (III) double layered suture; (IV) surgical stapler. A nonparametric test was used for group comparisons of mean burst pressures (mmHg). Statistical significance was defined at P<0.05.
Results
Mean burst pressures in group 1 amounted to 97.1±13.0 mmHg, in group 2 to 105.5±13.3 mmHg, in group 3 to 124.6±14.2 mmHg and in group 4 to 136.6±12.5 mmHg. Excepting differences between groups 1 and 2 comparisons between groups were significantly different.
Conclusions
In an ex vivo model surgical staplers and double layered hand crafted sutures proved well suitable for closure of the LAA. They were significantly superior to single layered sutures in terms of resilience to pneumatic pressure. This may be relevant to durability and should be discussed with regard to clinical choice.
Keywords: Atrial fibrillation, left atrial appendage (LAA), stroke, surgical staplers, surgical suture techniques
Introduction
Atrial fibrillation is the most significant arrhythmia worldwide (1), at least 30 million people suffer from it (2). It increases the risk of stroke fivefold (3), 15–20% of strokes are associated with atrial fibrillation. Thrombus is formed in the left atrial appendage (LAA) in up to 90% of cases, which may lead to embolism (1,4). To prevent this, the LAA may be closed either surgically or interventionally (1,5). Surgical suturing has been in clinical practice for many years and is the procedure of choice in combination with open heart surgery for structural disease. The entrance to the LAA is often closed by a continuous or purse string suture. Other suture types are not common and in older studies surgical staplers have been reported to lead to frequent postoperative leakage (6). At present literature regarding systematic technical analysis of surgical LAA closure techniques is scarce (7) and no guidelines exist with regard to their specific employment.
In an attempt for systematic approach in our experimental study we examined different surgical closure techniques in terms of resistance to pressure immediately after the procedure. Results of the study may contribute to a better understanding of factors influencing the quality of left atrial appendage (LAA) closure, eventually leading to clinical preference.
Methods
An experimental model of an isolated left heart was developed, reproducibly facilitating pneumatic pressure measurements in the left atrium. As neither patients nor living animals were part of the study concept, no ethics approval was required. From commercially slaughtered pigs (nutritional purposes, weight EU—standard: 90 kg), fresh heart and lungs packages were removed. The packages were carefully inspected for anatomic integrity, immediately cooled and taken to the laboratory. The pulmonary artery and the aorta were isolated; the ligament of Botalli was severed. The pulmonary artery was cut above the pulmonary valve; the ascending aorta was cut proximal to the aortic arch. The ascending aorta was cross-clamped, as were both coronary arteries near their origins from the aorta. The heart was lifted as a whole and the left atrium was clamped at the inlets of the pulmonary veins. A cannula (Sorin Group, Milano, Italy) was inserted into the roof of the left atrium 3 to 4 cm from the origin of the LAA and secured with two purse-strings. (Prolene 4-0, Johnson & Johnson Medical Ltd., Ethicon Germany, Norderstedt, Germany). The cannula was connected to a digital manometer (GDH—200-13, Greisinger Company, Regenstauf, Germany) and to a hand operated pump. Air was pumped into the left atrium in order to apply pneumatic pressure to the left atrial walls and appendage.
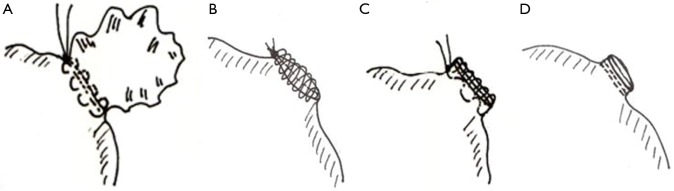
Four groups—12 experiments each—were defined (Figure 1). All closures were from an epicardial access and performed using Prolene 4-0. In group 1 the atrial appendage was closed using a continuous purse string suture. Stitches were placed loosely into the muscle at the base of the LAA and a surgical knot was tied. In group 2 the LAA was amputated leaving a rim of 3 to 4 mm and the entrance was closed employing a continuous straight suture. In group 3, the atrial appendage was sealed through a double layered suture. One row of continuous U-shaped stitches was placed across the appendage at its base. After reaching the far end, the appendage was amputated with a rim again of 3 to 4 mm. The stitches were returned as a continuous running spiral suture around the free edges and tied at the starting end. In group 4, the atrial appendage was closed by a modern stapler (ENDO GIATM, Universal RoticulatorTM 45 mm, staple height 2 mm × 3 mm, Covidien Germany GmbH, Neustadt, Germany) employing two lines of staples. The appendage was amputated. All surgical procedures were exclusively performed by two experienced cardiovascular surgeons.
Figure 1.
Surgical closure techniques of the left atrial appendage (LAA). (A) Purse string suture; (B) continuous straight line; (C) double layered suture; (D) stapler.
After each closing procedure pneumatic pressure was applied. Air provided easy, precise measurements and was well suitable to the model. The entire preparation was submerged in water to check for air leaks. The measuring process was performed under water too, so that air leaks were easily observed. Burst pressure was defined as the measured pressure at which sudden leakage of the appendage occurred. For all groups, mean burst pressures and standard deviations were calculated. Mean burst pressures of each group were compared using the nonparametric Mann-Whitney U test. Statistical significance was set at P<0.05. For statistical analysis the Graph Pad Prism 6 software (La Jolla, CA, USA) was employed.
Results
Table 1 and Figure 2 give an overview of the results obtained. When bursting, seams in group 1 (epicardial purse string) leaked centrally where the left atrium was pulled together by the purse string suture. In group 2 (straight running suture) leaks were typically found towards the middle parts of the continuous suture. Mean burst pressures of groups 1 and 2 did not differ significantly (P=0.86). The double layered suture in group 3 remained intact; however leakage occurred from the puncture channels of the basal U-type suture layer. Despite maximum pneumatic pressure, stapled seams in group 4 overall remained intact. Leakage occurred typically at the basic layer closest to the atrium. Differences between groups were significant (P<0.05) except comparison of groups 1 and 2 (Figure 2).
Table 1. Epicardial closure techniques and mean burst pressures:
| Burst pressures, n=12 (mmHg) | Purse string suture | Single layered suture | Double layered suture | Stapler |
|---|---|---|---|---|
| Minimum | 84.2 | 91.7 | 104.2 | 109.7 |
| Median | 92.6 | 100.3 | 122.1 | 138.7 |
| Maximum | 120.9 | 131.0 | 151.7 | 153.8 |
| Mean | 97.1 | 105.5 | 124.6 | 136.6 |
| Standard deviation (±) | 13.0 | 13.3 | 14.2 | 12.5 |
Group 1, purse string suture; group 2, single layered suture; group 3, single layered suture; group 4, stapler.
Figure 2.
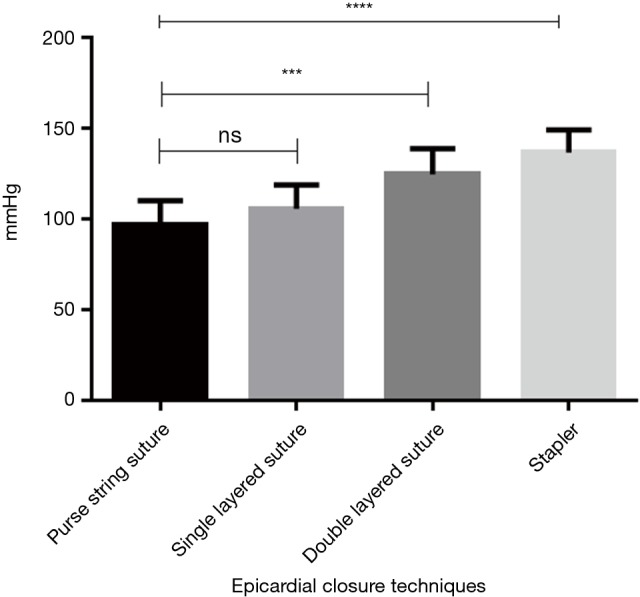
Mean burst pressures—groups 1 to 4. Differences between groups were significant excepting comparison of groups 1 and 2. Group comparisons: 1 vs. 2: P=0.086; 1 vs. 3: P=0.0001; 1 vs. 4: P<0.0001; 2 vs. 3: P=0.0036; 2 vs. 4: P<0.0001; 3 vs. 4: P=0.033.
Discussion
In order to close the LAA hand crafted surgical closure techniques are regularly applied during open heart surgery (8), even though according to previous publications only 40% of LAA remain permanently sealed (1,9). The mechanisms of failure over time have not been identified in detail. Closures of the LAA, which were properly effective and without flow initially, may loosen due to insufficient mechanical strength, but other causes such as permanently moving myocardium, may be of influence.
Evidence regarding systematic analysis of different surgical closures is poor and has not been able to provide practical guidelines. Overall published clinical results of LAA surgical closure techniques are far from ideal (1,10). Not only the rate of reopenings over time, but also the initial rate of incompletely closed LAAs is high (11).
An insufficiently closed or reopened atrial appendage presents a serious problem. Forty one percent of unsuccessfully closed LAA developed an atrial thrombus (12-14). In a study of Kanderian (6) ligature of the LAA was performed in 72 patients. In 24% of patients, the LAA were incompletely closed in an echocardiographic control. After a follow-up of 44±19 months 24% of patients had suffered a stroke as compared to 2% in the fully closed LAA. If atrial appendages were not permanently and completely occluded, patients did not benefit from the surgical procedure. This was even more the case, if patients did not receive oral anticoagulation postoperatively.
Kato (11) reported that LAA closure combined with other cardiac surgery reduced the incidence of early cerebrovascular incidents. This group did not address the issue of suture quality. Some authors reported good results with surgical staplers (15-18). Ohtsuka (19) reported on his experience with 32 thoracoscopic LAA resections using a stapler. No complications were observed.
In clinical routine during open heart surgery continuous sutures, purse strings or—less frequently staplers are in use (8). In order to prevent potentially thrombogenic stumps it is important to position the line of surgical closure precisely at the border zone of the LAA entrance. To test for potentially increased stability in our study we included a double layered suture. In comparison initial burst pressures of single layered sutures were significantly inferior to double layered sutures and stapled closures. In our experience voluminous and broad based appendages are technically difficult to close completely with a purse string. Visually closure by continuous suture led to better adaptation of LAA entrances. This observation did not lead to a significant increase in burst pressures though.
In combination of a U-shaped basal seam followed by a spiral running second layer the double layered suture technique produces a very well adapted seam. To the authors’ knowledge in LAA closure literature does not provide specific comparative studies regarding application of this technique.
In our study the highest initial burst pressures were achieved by stapler closure. Very good adaptation of the base of the LAA was observed as a rule. We used modern staplers, which were not available in previous studies. They are characterized by two staple lines at staple heights of 2 and 3 mm resulting in a strong adaptation of the tissue.
All closure techniques tested were strong enough to easily match physiological pressures in the left atrium. Interpretation of these findings is difficult, as reported clinical failures may be the result of other, probably combined factors. However, lower burst pressures may predispose to secondary leak occurrence. Very clearly in our study single layered sutures, which are being utilized in clinical practice, were less resistant to pressure compared to other closures tested and their value is questioned.
There are several limitations of the study. One is the fact, that we examined exclusively static initial pressures in our model. Furthermore the choice of closure methods represents an arbitrary selection, excluding some surgical and all interventional devices. Long-term closure properties are likely to be influenced by the moving heart, but performance in living tissue could not be addressed.
It may be concluded, that the ex vivo model designed reproducibly measured post-procedural resilience to pressure in LAA closures. With employment of either surgical staplers or hand crafted double layered sutures very stable closures can be achieved. In comparison of single and double layered sutures, double layered sutures should be preferred.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Salzberg SP, Tolboom H. Management of the left atrial appendage. Multimed Man Cardiothorac Surg 2011;2011:mmcts. [DOI] [PubMed]
- 2.Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 2014;129:837-47. 10.1161/CIRCULATIONAHA.113.005119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramlawi B, Abu Saleh WK, Edgerton J. The Left Atrial Appendage: Target for Stroke Reduction in Atrial Fibrillation. Methodist Debakey Cardiovasc J 2015;11:100-3. 10.14797/mdcj-11-2-100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.John Camm A, Colombo A, Corbucci G, et al. Left atrial appendage closure: a new technique for clinical practice. Heart Rhythm 2014;11:514-21. 10.1016/j.hrthm.2013.11.030 [DOI] [PubMed] [Google Scholar]
- 5.De Maat GE, Benussi S, Hummel YM, et al. Surgical Left Atrial Appendage Exclusion Does Not Impair Left Atrial Contraction Function: A Pilot Study. Biomed Res Int 2015;2015:318901. 10.1155/2015/318901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanderian AS, Gillinov AM, Petterson GB, et al. Success of surgical left atrial appendage closure: assessment by transesophageal echocardiography. J Am Coll Cardiol 2008;52:924-9. 10.1016/j.jacc.2008.03.067 [DOI] [PubMed] [Google Scholar]
- 7.Damiano RJ., Jr What is the best way to surgically eliminate the left atrial appendage? J Am Coll Cardiol 2008;52:930-1. 10.1016/j.jacc.2008.06.007 [DOI] [PubMed] [Google Scholar]
- 8.Tiwari KK, Gasbarri T, Bevilacqua S, et al. Right-Sided Minithoracotomy as a Surgical Approach for the Concomitant Treatment of Atrial Fibrillation. Res Cardiovasc Med 2016;5:e31374. 10.5812/cardiovascmed.31374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henry L, Ad N. The surgical treatment for atrial fibrillation: ablation technology and surgical approaches. Rambam Maimonides Med J 2013;4:e0021. 10.5041/RMMJ.10121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schneider B, Stollberger C, Sievers HH. Surgical closure of the left atrial appendage - a beneficial procedure? Cardiology 2005;104:127-32. 10.1159/000087632 [DOI] [PubMed] [Google Scholar]
- 11.Kato TS, Iwamura T, Endo D, et al. Left Atrial Appendage Closure Reduces the Incidence of Postoperative Cerebrovascular Accident in Patients Undergoing Cardiac Surgery. Circ J 2015;79:2591-7. 10.1253/circj.CJ-15-0524 [DOI] [PubMed] [Google Scholar]
- 12.Price MJ, Valderrábano M. Left atrial appendage closure to prevent stroke in patients with atrial fibrillation. Circulation 2014;130:202-12. 10.1161/CIRCULATIONAHA.114.009060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bando K, Hashimoto K. Closure of the Left Atrial Appendage During Cardiac Surgery--Why, When and How? Circ J 2015;79:2541-3. 10.1253/circj.CJ-15-1168 [DOI] [PubMed] [Google Scholar]
- 14.Stergiopoulos K, Seifert F, Brown DL. Thrombus formation after successful stapler exclusion of the left atrial appendage. J Am Coll Cardiol 2010;55:379. 10.1016/j.jacc.2008.11.070 [DOI] [PubMed] [Google Scholar]
- 15.Zakkar M, Kanagasabay R. Ligation of the left atrial appendage with surgical stapler. Ann R Coll Surg Engl 2013;95:380. 10.1308/rcsann.2013.95.5.380a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wudel JH, Chaudhuri P, Hiller JJ. Video-assisted epicardial ablation and left atrial appendage exclusion for atrial fibrillation: extended follow-up. Ann Thorac Surg 2008;85:34-8. 10.1016/j.athoracsur.2007.08.014 [DOI] [PubMed] [Google Scholar]
- 17.DiSesa VJ, Tam S, Cohn LH. Ligation of the left atrial appendage using an automatic surgical stapler. Ann Thorac Surg 1988;46:652-3. 10.1016/S0003-4975(10)64728-5 [DOI] [PubMed] [Google Scholar]
- 18.Landymore R, Kinley CE. Staple closure of the left atrial appendage. Can J Surg 1984;27:144-5. [PubMed] [Google Scholar]
- 19.Ohtsuka T, Ninomiya M, Nonaka T, et al. Thoracoscopic stand-alone left atrial appendectomy for thromboembolism prevention in nonvalvular atrial fibrillation. J Am Coll Cardiol 2013;62:103-7. 10.1016/j.jacc.2013.01.017 [DOI] [PubMed] [Google Scholar]