Abstract
Background
Minimally invasive aortic valve replacement (MAVR) has demonstrated a benefit with respect to increased patient satisfaction due to minimised pain and earlier recovery. Sutureless valves may benefit MAVR and conventional aortic valve replacement (AVR) by reducing operative times and blood transfusion requirements. The Perceval valve (Sorin, Salluggia, Italy) is a self-expanding prosthesis made from bovine pericardium mounted in a nitinol stent, designed to simplify the implantation of an aortic valve. This meta-analysis evaluates the clinical, haemodynamic, and survival outcomes of the Perceval sutureless valve.
Methods
An electronic search of 4 databases was performed from January 2000 to December 2016. Primary outcomes included mortality and stroke. Secondary outcomes included minimally invasive access, paravalvular leak, overall long-term survival, postoperative echocardiographic findings, and functional class improvement.
Results
After the application of inclusion and exclusion criteria, 14 of 66 relevant articles were selected for assessment. Of these 14 studies, a total number of 2,505 patients were included. The current evidence on the Perceval valve for aortic valve disease is limited to observational studies only. Minimally invasive surgery was performed in 976 patients, of which 336 were via the right anterior thoracotomy approach. The Perceval M and L sutureless valves were the most frequently used, 782 and 770 respectively. The incidence of major adverse events included 30-day mortality (0 to 4.9%), cerebrovascular accident (0 to 3%), permanent pacemaker insertion (0 to 17%), moderate to severe paravalvular leak (0 to 8.6%), and re-operation (0 to 4.8%). Post-operative mean aortic valve gradient ranged from 9 to 15.9 mmHg and post-operative New York Heart Association (NYHA) Class I or II ranged from 82% to 96%. The 1-year survival ranged from 86% to 100%; and 5-year survival was 71.3% to 85.5% in two studies.
Conclusions
The Perceval valve is associated with excellent post-operative results in MAVR and in conventional AVR. Larger randomised controlled studies are required to evaluate the long-term efficacy of the prosthesis.
Keywords: Perceval, sutureless, minimally invasive, meta-analysis, aortic valve replacement
Introduction
Aortic stenosis (AS) is the most common valvular disorder, resulting in decreased life expectancy in symptomatic individuals (1,2).
Surgical aortic valve replacement (AVR) has long been the definitive therapy in treating symptomatic AS. Transcatheter aortic valve implantation (TAVI) has become an accepted alternative to surgery in treating high-risk or non-operative individuals with severe AS (3). The emergence of TAVI has led to renewed interest in developing artificial valves not requiring sutures to secure the valve in position. Rapid deployment employing a radial force to secure the valve within the annulus has significantly reduced implantation time.
In the 1960s, Magovern pioneered a sutureless heart valve made from titanium using a ball-in-cage design; and achieved a reduction in cardiopulmonary bypass time (CPB) (4). Implantation of this novel valve continued into the 1980s. However, the potential for paravalvular leak and embolisation limited it’s further development (4,5). The TAVI technology has been adopted in sutureless valves, which can be deployed under direct vision via conventional or minimally invasive approaches without the need of a catheter. Minimally invasive AVR (MAVR) has demonstrated similar results to conventional AVR in regards to clinical outcomes, with the added benefit of improved patient satisfaction (6).
Currently, MAVR is limited to select cardiac centers. They can require specialised equipment and surgeons may experience a steep learning curve (7). MAVR is attributed to longer aortic cross-clamp (ACC) and CPB time, which inherently links to a higher risk of adverse events, especially amongst a high-risk surgical group (8). Therefore, the use of sutureless valves may be advantageous in the setting of MAVR by potentially reducing the operating time.
At present the Perceval (Sorin, Saluggia, Italy, CE approved 2011, FDA approved 2016) is the only true sutureless valve available for the implantation. It’s direct competitor, the Intuity Elite (Edwards Lifesciences, Irvine, US, CE approved 2014, FDA approved 2016) is a rapid deployment aortic valve prosthesis requires 3 sutures to secure the valve to the annulus. Both valves are bioprosthetic and are anchored to the aortic annulus with an expandable metal stent frame.
The Perceval features an inflow ring designed to sit at the level of the annulus and an outflow ring, which sits at the level of the sinotubular junction. The outward flexion of the winged struts, connects the two rings and occupies the sinuses of Valsalva (Figure 1). Perceval aortic valve prosthesis is currently available in four sizes: S (19–21 mm), M (22–23 mm), L (24–25 mm), and XL (27 mm).
Figure 1.
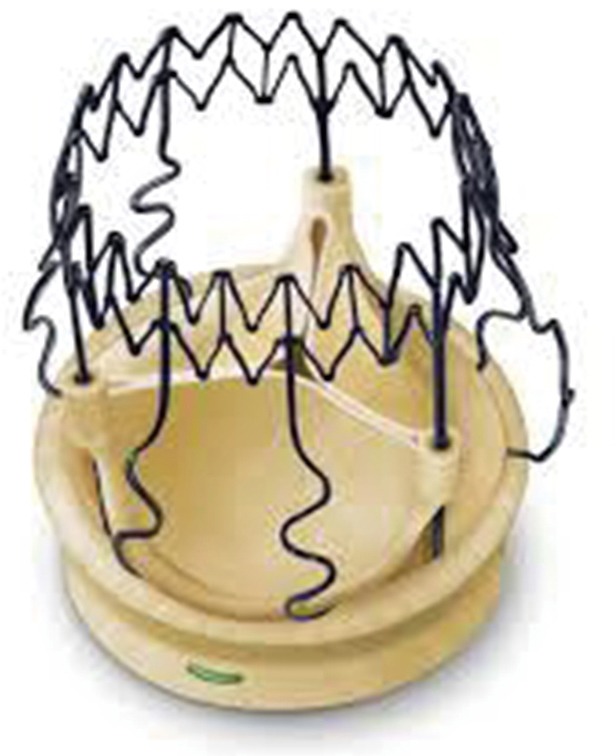
Sorin Perceval S sutureless valve (Sorin, Saluggia, Italy).
As the worldwide use of the Perceval valve continues to rise, there are no comprehensive reviews, assessing the efficacy and safety of the Perceval valve. Therefore we have performed this meta-analysis to assess the safety, clinical, and survival benefits of Perceval valve.
Methods
Search strategy
An electronic database search of four databases (MEDLINE, EMBASE, PubMed, Cochrane) was performed starting from January 2000 to December 2016. To attain the maximal search yield, we used the following free text “Perceval” or “sutureless” and “aortic valve replacement”. We identified further relevant studies after reviewing the reference lists of all retrieved articles and the Sorin Perceval website (http://www.livanova.sorin.com/products/cardiac-surgery/aortic/perceval).
Outcomes
The primary end points included procedural success rate, 30-day mortality, cerebrovascular accident, permanent pacemaker insertion, paravalvular leak (mild, moderate and severe), re-operation, and the length of hospital and intensive care stay. The secondary end points included echocardiographic findings including mean post-operative gradients (mmHg), mean long-term gradients, effective orifice area (EOA), New York Heart Association (NYHA) functional class improvement from baseline, and survival at 1-, 2-year, and longer. Additional outcomes included the procedural approach (minimally invasive vs. conventional sternotomy), valve sizes used, and ACC and CPB times.
Selection criteria
All patients undergoing implantation of the Perceval valve were eligible for this meta-analysis. The patient selection criteria for Perceval valve implantation varied with each institution. Only observational studies were included in this review. Case reports, case series with less than thirty patients, recent abstracts, expert opinions and editorial reports were excluded.
Data analysis and critical appraisal
Two reviewers (KS, SL) separately appraised the selected studies using a standardised data table. The relevant data extracted from the articles’ text, tables, and figures was tabulated. Any discrepancies were resolved by discussion. The quality of scoring of observational studies used in meta-analyses can be controversial; therefore each article was analysed in accordance with the critical review checklist from the Dutch Cochrane Centre, as suggested by the MOOSE group (9).
Quality appraisal included: (I) a clear definition of study population; (II) a clear definition of outcomes and outcome assessment; (III) independent assessment of outcome parameters; (IV) sufficient duration period for follow-up; (V) no selective loss during follow-up; and (VI) identification of important confounders and prognostic factors.
Intervention
Despite some variation, similar steps were performed at different centers. The surgical approach was via a right anterior thoracotomy, mini-sternotomy or full sternotomy. In general, a transverse aortotomy was made 1.5 to 2 cm above the sinotubular junction. The aortic valve leaflets were excised and the annulus was either semi-debrided or fully debrided. Three guiding sutures, commonly 4-0 monofilament, were passed at the nadir of the aortic annulus. An appropriately sized prosthesis was collapsed onto the dual holder. Each guiding thread is passed through eyelets on the prosthesis inflow ring, to allow for accurate seating of the prosthetic valve onto the debrided annulus. A deflated post-dilation catheter was placed across the prosthesis and the balloon was inflated at 4 atmospheres for 30 s, as per the manufacturer’s recommendations. Warm sterile saline at 37 degree Celsius was poured within the aortic root to allow for fixing of the nitinol stent and ensure optimal valve sealing. Following closure of the aortotomy, a transesophageal echocardiography was performed to assess the correct implantation of the prosthesis and exclude the presence of a paravalvular leak.
Results
Quality of the studies
After removal of duplicated studies, the titles and abstracts of 66 publications were identified as described in the search strategy. An initial review of these abstracts identified 24 potentially relevant articles. After applying the inclusion and exclusion criteria, 14 articles were reviewed (10-23) (See Figure 2 PRISMA diagram). A total of 2,505 patients were represented in this study, of which 2,205 patients had undergone AVR using the Perceval valve. Quality assessment using the MOOSE criteria (9) checklist is summarised in Table S1.
Figure 2.
PRISMA diagram.
Quality of evidence
All 14 studies were observational, which included 7 prospective (11,14,16,19-22) and 7 retrospective studies (10,12,13,15,17,18,23). There were no randomized controlled trials. The mean follow-up time was 6 to 8 months for 3 studies (15,18,19), 10 to 16 months for 5 studies (10,11,13,21,22), and 18 to 24 months for 3 studies (12,20,23), as seen in Table 1.
Table 1. Study characteristics.
| Study | Year | Institution | Country | Study Period | Study type | Sample size | Mean follow-up time (months) |
|---|---|---|---|---|---|---|---|
| Santarpino | 2013 | Klinikum Nürnberg, Nuremberg | Germany | 2010–2012 | OS, R | 78 | 13.5±2.4 |
| Zannis | 2014 | L’Institut Mutualiste Montsouris, Paris | France | 2007–2011 | OS, P | 143 | 13.4±11.6 |
| Villa | 2015 | Fondazione Poliambulanza, Brescia | Italy | 2007–2013 | OS, R | 276 | 18.0±15.6 |
| L’Institut Mutualiste Montsouris, Paris | France | ||||||
| Medizinische Hochschule, Hannover | Germany | ||||||
| Centre Hospitalier Universitaire de Nancy, Universite de Lorraine, Vandœuvre-les-Nancy | France | ||||||
| Rubino | 2014 | A.O.U. Policlinico-Vittorio Emanuele, University of Catania, Catania | Italy | 2007–2013 | OS, R | 314 | 10.7 |
| Klinikum Nürnberg | Germany | ||||||
| University Hospital Gasthuisberg, Leuven | Belgium | ||||||
| Karolinska Institute, Karolinska University Hospital, Stockholm | Sweden | ||||||
| Oulu University Hospital, Oulu | Finland | ||||||
| Muneretto | 2014 | University of Brescia Medical School, Brescia | Italy | 2010–2013 | OS, P | 163 | NR |
| Miceli | 2014 | Fondazione Toscana G. Monasterio, Massa | Italy | 2010–2013 | OS, R | 281 | 8.0 |
| Klinikum Nürnberg, Nuremberg | Germany | OS, P | 30 | NR | |||
| Meuris | 2015 | Hannover Medical School, Hannover | Germany | 2007–2008 | OS, R | 215 | NR |
| Universitaire Ziekenhuizen Gasthuisberg, Leuven | Belgium | ||||||
| L’Institut Mutualiste Montsouris, Paris | France | ||||||
| Mazine | 2015 | Montreal Heart Institute, Universite de Montreal, Montreal, Quebec | Canada | 2011–2015 | |||
| Southlake Regional Health Center, McMaster University, Newmarket, Ontario | |||||||
| Hamilton Health Sciences, McMaster University, Hamilton, Ontario | |||||||
| Trillium Health Center, Mississauga, Ontario | |||||||
| New Brunswick Heart Center, Saint John, New Brunswick | |||||||
| Institut Universitaire de Cardiologie de Qu ebec/Hôpital Laval, Quebec | |||||||
| Gilmanov | 2013 | G. Pasquinucci Heart Hospital, Massa | Italy | 2011–2013 | OS, R | 137 | 6.0 (3.0–12.0) |
| Folliguet | 2012 | Medizinsche Hochschule Hannover, Hannover | Germany | 2007–2011 | OS, P | 211 | 6.0 (1.0-18.0) |
| L’Institut Mutualiste Montsouris, Paris | Italy | OS, P | 262 | 23.5±14.4 | |||
| Fischlein | 2015 | Klinikum Nürnberg, Paracelsus Medical University Nuremberg | Germany | 2010–2015 | OS, P | 32 | 15.8 |
| Fleming | 2011 | Katholieke Universiteit Leuven, Leuven | Belgium | 2007–2009 | OS, R | 243 | 14.5 |
| Shrestha | 2014 | Hannover Medical School, Hannover | Germany | 2007–2013 | |||
| L’Institut Mutualiste Montsouris, Paris | France | ||||||
| Klinikum Nuremberg | Germany | ||||||
| U.Z. Gasthuisberg, Leuven | Belgium | ||||||
| Inselspital, Bern | Switzerland | ||||||
| Ruhr University of Bochum, Bochum | Germany | ||||||
| Shrestha | 2013 | Hannover Medical School, Hannover | Germany | 2007–2012 | OS, P | 120 | 22.7±17.5 |
OS, observational study; P, prospective; R, retrospective; NR, not recorded.
One study compared conventional AVR, sutureless valve implantation and TAVI (14). Shrestha et al. (22) compared patients receiving conventional aortic valves to patients receiving the Perceval valve. The study by Villa (12) chose to stratify patient groups to the size of the valve implanted. Patients with a “S” size valve were compared to patients implanted with a “L” sized valve. Three studies (13,15,19) compared the minimally invasive to full sternotomy surgical approach. All studies originated from specialised tertiary referral centers. Eleven centers reported results in greater than 100 patients (range, 120–314) (11-23). Seven studies presented multi-center data (12,13,15-17,19,23).
Seven studies published explicit inclusion criteria (13,15,16,18,19,21,22). The definition of patients not suitable for the Perceval valve varied between institutions. For example, Flameng et al. (21) chose to include only those patients with an aortic annulus between 19 to 23 mm; whereas Meuris et al. (16) excluded patients with intraoperative annulus diameters greater than 23 mm. The operative technique was defined in ten studies (10,12-16,18).
Overall, 61.5% of patients were female. The mean age was 78.7 years old (range, 76.6–80.4 years old). The mean body surface area was 1.78 m2 (range, 1.6–1.85 m2). The mean of patients with AS was 57.9% (range, 56.1–76.7%). The reported mean left ventricular ejection fraction was 56.9%. Hypertension and hyperlipidemia were reported in the majority of patients, 77.6 and 63.4% respectively. Additional comorbidities included chronic lung disease (18.7%), diabetes (28.1%), coronary artery disease (22.6%), peripheral vascular disease (18.7%), atrial fibrillation (15.9%) and chronic renal impairment (12.8%). The mean NYHA Class III or IV reported was 70.9% (range, 47.7–100%). European System for Cardiac Operative Risk Evaluation (EuroSCORE) was inconsistently reported; logistic versus additive, hence a mean could not be calculated (see Table S2).
Median sternotomy approach was used most frequently for valve implantation (weighted mean 75.2%; range, 15.3–100%). The Perceval valve size “M” was used in 46.4% of patients (range, 32–78.1%). The weighted mean ACC and CPB times for an isolated AVR were 39.7 minutes (range, 17–59.3 minutes) and 64.2 minutes (range, 35–92.3 minutes) respectively. Additional cardiac procedures were performed in 42.6% of patients (range, 9.7–100%) (see Table 2).
Table 2. Operative characteristics.
| Study | Incision | Aortic crossclamp (min) | Cardiopulmonary bypass time (min) | Valve by size | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RT | Mini | Stern | Redo | Add Pro | AVR | Combined | AVR | Combined | S | M | L | XL | ||||
| Santarpino | 3.8 | 80.7 | 15.3 | 15.3 | NR | 38.3±12.6 | NR | 68.2±19.4 | NR | 7.7 | 42.3 | 47.4 | 2.6 | |||
| Zannis | NR | NR | 100.0 | NR | 33.5 | NR | 32.0±14.9 | NR | 44.7±18.6 | 100.0 | NR | NR | NR | |||
| Villaa | NR | 12.8 | 87.2 | NR | 31.9 | NR | 40.0±17.1 | NR | 61.6±25.5 | 100.0 | NR | NR | NR | |||
| Villab | NR | 20.5 | 79.5 | NR | 34.5 | NR | 38.6±17.4 | NR | 60.9±27.2 | NR | 66.3 | 33.6 | NR | |||
| Rubino | 2.8 | 41.7 | 55.4 | 7.6 | 29.9 | 39.0±15.0 | 43.0±20.0 | 66.0±23.0 | 73.0±28.0 | 11.8 | 43.0 | 40.0 | 5.0 | |||
| Muneretto | NR | 18.8 | 81.1 | NR | NR | 30.9±13.6 | NR | 47.0±18.5 | NR | 16.9 | 62.3 | 26.4 | NR | |||
| Miceli | 58.3 | 41.6& | NR | NR | NR | 55.0 (47.0–65.0); 37.0 (30.0–46.0)& | 48.0 (37.0–60.0) | 74.0 (87.0–107.0); 72.0 (58.0–89.0)& | 81.0 (68.0–98.0) | 7.5/4.8& | 20.3; 14.2& | 30.6; 19.6& | 3.2& | |||
| Meuris | NR | NR | 100.0 | 10.0 | 46.7 | 29.3±8.0 (23.0-55.0) | 45.4±15.4 (21.0–79.0) | 46.4±6.7 (34.0–60.0) | 73.4±21.8 (41.0–130.0) | 36.7 | 63.3 | NR | NR | |||
| Mazine | 10.7 | 8.8 | 80.4 | 8.8 | 52.5 | 40.5±11.6 | 69.6±28.8 | 56.6±16.6 | 88.7±38.4 | 21.4 | 32.0 | 42.3 | 4.2 | |||
| Gilmanov | 100.0 | NR | NR | NR | NR | 59.3±19.5 | NR | 92.3±27.4 | NR | 13.9 | 32.8 | 53.2 | – | |||
| Folliguet** | NR | 21.6 | 78.3# | NR | 23.1 | 33.6±9.5; 33.5±14.9# | 44.2±13.4 | 65.7±21.4; 51.1±24.0# | 67.6±23.9 | 15.4 | 53.8 | 30.7 | NR | |||
| Fischlein | NR | 100.0 | NR | 1.4 | 9.7 | 35.0±11.0 | 38.0±12.0 | NR | NR | 8.3 | 33.8 | 46.2 | 11.7 | |||
| Fleming | NR | 3.3 | 96.8 | NR | 50.0 | 17.0 (12.0–34.0) | 22.0 (17.0–51.0) | 35.0 (24.0–54.0) | 62.0 (40.0–120.0) | 21.9 | 78.1 | NR | NR | |||
| Shrestha | NR | 5.8 | 94.2 | NR | 100.0 | NR | 50.7±22.8 | NR | 78.9±32.3 | 14.0 | 53.0 | 33.0 | NR | |||
| Shrestha* | NR | 72.0 | 28.0 | NR | NR | 30.1±9.0 | NR | 58.7±20.9 | NR | 24.0 | 76.0 | NR | NR | |||
| Weighted mean | 32.4 | 36.6 | 75.2 | 7.2 | 42.6 | 39.7 | 45.6 | 64.2 | 66.5 | 22.7 | 46.4 | 40.3 | 6.1 | |||
| Max | 100.0 | 100.0 | 100.0 | 15.3 | 100.0 | 59.3 | 69.6 | 92.3 | 88.7 | 100.0 | 78.1 | 53.2 | 11.7 | |||
| Mean | 2.8 | 3.3 | 15.3 | 0 | 9.7 | 17.0 | 22.0 | 35.0 | 44.7 | 7.7 | 32.0 | 26.4 | 2.6 | |||
Values are % unless indicated. Study by Villa is represented by “a” and “b” as Villa chose to compare cohorts by valve type (S vs. M, L, XL). The study by Miceli chose to compare cohorts by incision hence those values with & represent cohort approached via ministernotomy. Folliguet** chose to compare cohorts aortic cross clamp and cardiopulmonary bypass times for isolated AVR hence # values represent patients undergoing surgery via sternotomy. *, represents the study by Shrestha 2013. RT, right anterior thoracotomy; Mini, ministernotomy; Stern, sternotomy; Redo, redosternotomy; Add pro, additional cardiac procedure; AVR, aortic valve replacement; S, M, L, XL, Perceval S valve types; NR, not reported.
Assessment of safety
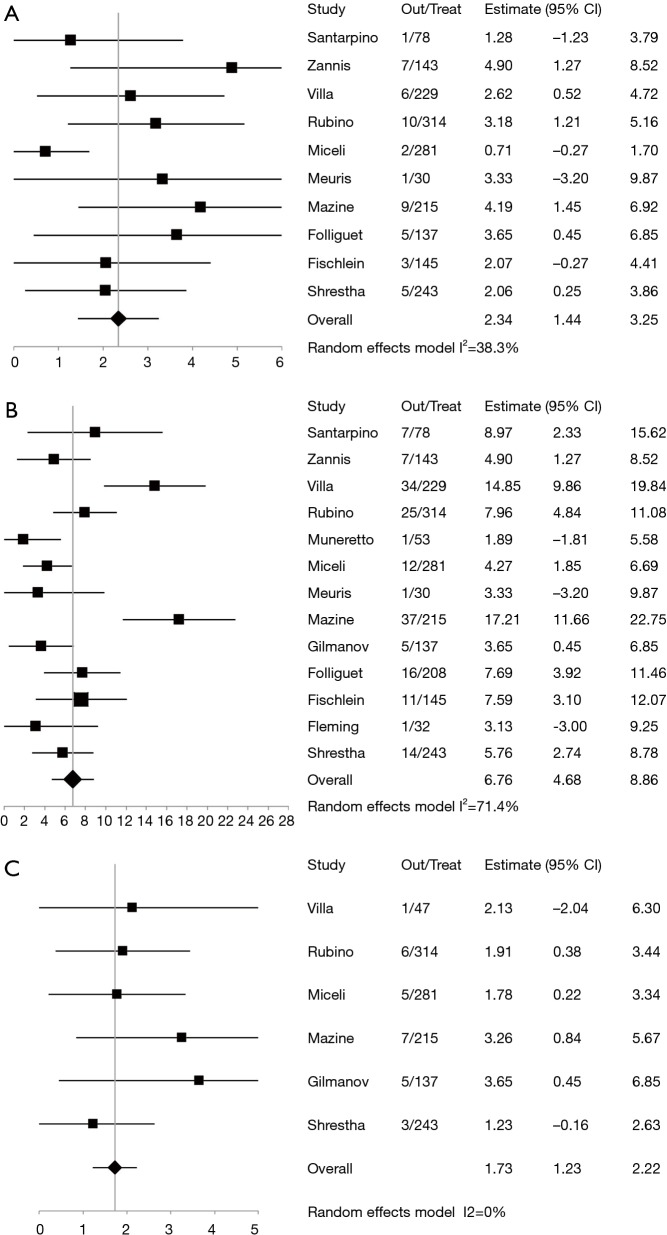
The 30-day mortality rate was 2.3% (pooled weighted mean, 95% CI, 1.44–3.25%) (Figure 3A). The pooled rate of pacemaker implantation 6.76% (95% CI, 4.68–8.86%) (Figure 3B) and the pooled rate of cerebrovascular accidents was 1.73% (95% CI, 1.23–2.22%) (Figure 3C). Table 3 provides a summary of additional perioperative outcomes including atrial fibrillation, pericardial tamponade, myocardial infarction, reoperation, explantation of the sutureless valve, exploration for bleeding, infection, length of intensive care unit stay and hospital stay.
Figure 3.
Patients undergoing perceval sutureless valve insertion, forest plots indicate: (A) 30-day mortality; (B) pacemaker insertion; (C) cerebrovascular accidents. Out, outcomes; Treat; treatment.
Table 3. Procedural outcomes.
| Study | 30-d mortality | PPM | CVA | AF | Tamp | MI | Re-op | Explant | Re-exp | Infection | Con | ICU stay (d) | Hospital stay (d) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Santarpino | 1.2 | 8.9 | 0 | NR | NR | 0 | NR | NR | NR | NR | NR | NR | 12.3±4.5 |
| Zannis | 4.9 | 4.9 | NR | NR | NR | NR | 4.1 | NR | NR | NR | NR | NR | NR |
| Villaa | 0 | 0 | 2.1 | 13.6 | NR | NR | NR | NR | NR | NR | NR | NR | 7.48±2.7 |
| Villab | 2.6 | 15.0 | 0 | 2.3 | NR | NR | NR | NR | NR | NR | NR | NR | 12±29.9 |
| Rubino | 3.2 | 8.0 | 1.9 | NR | NR | NR | 1.0 | NR | 2.5 | NR | 0.6 | 3.2±3.4 | 13.4±6.5 |
| Muneretto | 0 | 2.0 | 0 | 58.5 | 5.4 | NR | NR | NR | 7.5 | NR | NR | 1.07±0.4 | NR |
| Miceli | 0.7 | 4.2 | 1.8 | NR | NR | NR | 0.3 | NR | 2.8 | NR | 1.4 | 1.0 (1.0–2.0) | 8.0 (6.0–10.0) |
| Meuris | 3.3 | 3.3 | NR | NR | 3.3 | NR | 0 | NR | 10.0 | 6.7 | NR | NR | NR |
| Mazine | 4.0 | 17.0 | 3.0 | 41.0 | NR | NR | 0 | NR | 5.0 | 0 | NR | 3.7±3.9 | 11.4±7.6 |
| Gilmanov | 0 | 3.6 | 2.2 | 27.0 | NR | 0.7 | 0.7 | NR | 5.1 | NR | NR | 1.6±1.8 | 7.1±3.1 |
| Folliguet | 2.4 | 7.0 | NR | NR | NR | NR | 3.2 | NR | NR | NR | NR | NR | NR |
| Fischlein | 2.1 | 7.6 | NR | NR | NR | NR | NR | NR | NR | NR | 2.0 | NR | 11.6±4.9 |
| Fleming | 0 | 3.1 | 0 | NR | NR | NR | NR | NR | 3.1 | NR | NR | 2.0 | 15.0 (4.0–30.0) |
| Shrestha | 2.1 | 5.9 | 1.3 | NR | NR | 0.8 | 2.1 | 2.1 | 3.8 | 0.4 | NR | NR | NR |
| Shrestha* | 0 | NR | NR | NR | NR | NR | NR | NR | 4.0 | NR | NR | 1.8±1.8 | 14.1±7.5 |
| Weighted mean | 2.21 | 7.9 | 1.5 | 24.6 | 4.6 | 0.6 | 1.4 | NA | 3.9 | 0.6 | 1.2 | 2.3 | 11.0 |
| Max | 4.9 | 17.0 | 3.0 | 58.5 | 5.4 | 0.8 | 4.1 | NA | 10.0 | 6.7 | 1.4 | 3.2 | 13.4 |
| Min | 0 | 0 | 0 | 2.3 | 3.3 | 0 | 0 | NA | 2.5 | 0 | 0.6 | 1.0 | 7.1 |
Values are %, unless otherwise indicated. Study by Villa is represented by “a” and “b” as Villa chose to compare cohorts by valve (S vs. M, L, XL). *, represents the study by Shrestha 2013. PPM, permanent pacemaker insertion; CVA, cerebrovascular accident; AF, new onset atrial fibrillation; Tamp, cardiac tamponade; MI, myocardial infarction; Re-op, reoperation; Explant, explantation; Conv, conversion to sternotomy; ICU, intensive care unit; NR, not reported; NA, not applicable.
Haemodynamic outcomes
Prior to MAVR, the pooled baseline mean gradient was 32.9 mmHg (95% CI, 24.4–41.5 mmHg) and the post-operative pooled mean gradient improved to 8.02 mmHg (95% CI, 5.12–8.00 mmHg). The weight mean pre-operative EOA was 0.75 cm2 (range, 0.7–0.8 cm2), which improved to 1.51 cm2 (range, 1.4–1.6 cm2). The pooled post-operative indexed EOA was 0.85 cm2/m2 (range, 0.80–0.90 cm2/m2). Patient prosthesis mismatch (PPM) was defined and reported in only one study (12). The pooled mean of moderate to severe paravalvular leak was 1.9% (95% CI, 0.97–3.7%). Table 4 shows a summary of haemodynamic measurements including follow-up gradients.
Table 4. Echocardiography measurements and clinical data following Perceval S implantation.
| Study | Preop Echo (mean gradient) | Pre op EOA (cm2) | Post op Echo (mean gradient) | Mean gradient | Post op EOA (cm2) | EOAi (cm2/m2) | Mild PVL (%) | Mod to Sev PVL (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 6 months | 1 year | 2 years | ||||||||
| Santarpino | 49.5±15.8 | NR | 11.6±5.1 | NR | NR | NR | NR | NR | NR | 0 |
| Zannis | 38.8±17.0 | 0.8±0.2 | 9.0±3.4 | NR | NR | NR | 1.6±0.3 | NR | NR | 2.1 |
| Villaa | 43.1±20.0 | NR | 11.3±3.6 | NR | NR | NR | NR | 0.8±0.2 | 4.3 | NR |
| Villab | 40.2±15.1 | NR | 10.3±4.4 | NR | NR | NR | NR | 0.8±0.2 | 4.3 | NR |
| Rubino | 51.9±18.3 | NR | 14.6±6.0 | NR | NR | NR | NR | NR | 11.9 | 0.6 |
| Muneretto | 50.5±15.3 | 0.7±0.3 | 10.8±6.8 | NR | NR | NR | NR | NR | NR | NR |
| Miceli | NR | NR | 13.0±4.0 | NR | NR | NR | NR | NR | 1.4 | NR |
| Meuris | NR | NR | NR | NR | 9.9±4.6 | 8.0±4.1 | 1.6±0.4 | 0.9±0.2 | 0 | NR |
| Mazine | 47.3±18.9 | NR | 13.3±6.4 | NR | NR | NR | 1.6±0.4 | 0.9±0.2 | 9.3 | NR |
| Gilmanov | NR | NR | 11.0 | 11 | 10 | NR | NR | NR | 1.5 | NR |
| Folliguet | 48.6±18.6 | 0.7±0.2 | 10.4±4.3 | 8.9±3.2 | NR | NR | 1.4 ±0.4 | 0.85±0.23 | 2.4 | 8.7 |
| Fischlein | NR | NR | NR | 12.8±4.9 | 12.5±4.5 | 11.8±4.7 | NR | NR | 0 | 0 |
| Flameng | NR | NR | 11.0 (5.0–28.0) | 10.0 (6.0–28.0) | 9.0 (3.0–21.0) | NR | 1.5 (0.8–2.2) | NR | 15.6 | NR |
| Shrestha | 40.6 ±16.6 | 0.8±0.2 | 10.1±4.7 | 8.9±4.2 | 8.9±50.4.6 | 9.0±3.4 | 1.5±0.4 | 0.8±0.2 | 0.4 | NR |
| Shrestha* | NR | 0.7±0.2 | 13.6±5.4 | NR | NR | NR | 1.5±0.3 | NR | 8.0 | NR |
| Weighted mean | 45.6 | 0.75 | 12.1 | 10.1 | 10.1 | 9.9 | 1.5 | 0.8 | 4.7 | 5.4 |
| Max | 51.9 | 0.8 | 14.6 | 12.8 | 12.5 | 11.8 | 1.6 | 0.9 | 15.6 | 8.7 |
| Min | 38.8 | 0.7 | 9 | 8.9 | 8.9 | 8 | 1.4 | 0.8 | 0 | 0 |
Values are mmHg unless indicated. Study by Villa is represented by “a” and “b” as Villa chose to compare cohorts solely by valve type (S vs. M, L, XL). *, represents the study by Shrestha 2013. Echo, echocardiography; EOA, effective orifice area; EOAi, indexed effective orifice area; NR, not reported.
Long-term outcomes
Post-operative NYHA Class improvement of one or two classes occurred in 87.8% of patients (95% CI, 81.0–92.5%). Survival at 1-year was a pooled weighted mean of 90.4% (95% CI, 87.2–93.7%). Loss to follow-up, reoperation, and endocarditis were inconsistently reported. Survival beyond 1-year was reported variably in the included studies, with the longest period of survival, 5-year reported in two studies (11,16) for a weighted mean of 83% (range, 71.3–85.5%) (see Table 5).
Table 5. NYHA functional class and survival data in patients post Perceval S insertion.
| Study | Post-op NYHA class I II, (1 year) | Loss to follow-up 1 year | Re-operation | Endocarditis | 1-year survival | 2-year survival | 3-year survival | 4-year survival | 5-year survival |
|---|---|---|---|---|---|---|---|---|---|
| Santarpino | NR | 0 | NR | NR | NR | NR | NR | NR | NR |
| Zannis | 94.0 | 0 | 0.7 | 0.7 | 100 | NR | NR | NR | 85.5 |
| Villaa | NR | NR | NR | NR | NR | NR | 84.0 | NR | NR |
| Villab | NR | NR | NR | NR | NR | NR | 77.0 | NR | NR |
| Rubino | NR | NR | NR | NR | 90.5 | 87.0 | NR | NR | NR |
| Muneretto | NR | NR | NR | NR | 90.6 | NR | NR | NR | NR |
| Miceli | NR | 0 | 0.4 | 0.4 | 90.0 | NR | NR | NR | NR |
| Meuris | 95.6 | NR | NR | 6.7 | NR | NR | NR | NR | 71.3 |
| Mazine | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Gilmanov | 92.0 | NR | 0.7 | 0.7 | NR | NR | NR | NR | NR |
| Folliguet | 82.0 | NR | 0.9 | 0.5 | 87.1 | 82.4 | 82.0 | 69.4 | NR |
| Fischlein | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Fleming | 96.0 | NR | 3.1 | 3.1 | 90.6 | NR | NR | NR | NR |
| Shrestha | 91.9 | 13.4 | NR | NR | NR | 86.4 | NR | NR | NR |
| Shrestha* | NR | 6.1 | 4.0 | 6.0 | 86.8 | NR | 60.9 | NR | NR |
| Weighted mean | 90.0 | 4.1 | 0.7 | 0.8 | 86.8 | 85.6 | 72.4 | NA | 83.0 |
| Max | 96.0 | 13.4 | 4.0 | 6.7 | 100.0 | 86.4 | 84.0 | NA | 85.5 |
| Min | 82.0 | 0 | 0.7 | 0.5 | 87.1 | 82.4 | 60.9 | NA | 71.3 |
Values are % unless indicated. Study by Villa is represented by “a” and “b” as Villa chose to compare cohorts solely by valve type (S vs. M, L, XL). *, represents the study by Shrestha 2013. NR, not reported.
Discussion
As life expectancy improves, the prevalence of severe AS increases. Subsequently, patients are of higher risk due to their age and other comorbidities. In the past several years, TAVI has emerged as an established alternative to conventional AVR, particularly in the high-risk surgical cohort. TAVI has shown that rapid deployment and anchoring of the aortic valve without sutures, is feasible and safe. The advantage of the Perceval valve is that rapid deployment is achieved under direct visualisation in a semi-debrided annulus. The procedure can be performed using a minimally invasive approach, potentially reducing morbidity and mortality. This meta-analysis of the Perceval valve has demonstrated excellent initial results, which are comparable to conventional AVR.
In a cohort consisting of near octogenarians (mean age 78.5 years old), we found that the early postoperative outcomes are promising, as the 30-day mortality rate was 2.34% and the cerebrovascular event rate of 1.37%. This is in the context of a concomitant procedure rate of 42.5%. This result is similar to another recently published meta-analysis which demonstrated an acceptable early mortality rate of 2.1% and stroke rate of 1.4%, when compared to conventional AVR (24).
Interestingly, the pacemaker rate was 6.76% and is higher than in conventional AVR (3.6%) (25). One theory is that the radial force applied during deployment of the sutureless valve may be a precipitant for a higher pacemaker implantation rate (26) Pacemaker rates following after TAVI have ranged between 9% to 42% (27,28) and have been associated with the continued expansion of the valve following implantation. This has resulted in conduction blocks occurring days after TAVI (29). However recently, there is evidence to suggest that the depth of placement of the Perceval has a correlation with a higher pacemaker implantation rate (30). Yanagawa et al. have shown a reduction in pacemaker implantation from 28% to 0% by the placement of guiding sutures millimeters higher than the manufacturer recommendation resulting in a slightly shallower valve position with respect to the aortic annulus (31).
Additional adverse events related to valve implantation are very low. There was one reported case of endocarditis in the perioperative phase after the sutureless valve was explanted (23). Overall rates of reoperation (1.43%) and early infection (<1%) rates are low, further supporting the safe early outcomes.
Long ACC and CPB times are associated with increased perioperative mortality (8). Our analysis has shown that the Perceval valve has significantly reduced the ACC and CPB times when compared to conventional AVR. The weighted mean ACC and CPB times for isolated AVR was 39.7 and 64.2 minutes respectively, which were less than the established conventional AVR times of 76 and 106 minutes respectively (15).
Overall, there were 1,022 patients that underwent Perceval valve implantation via median sternotomy, representing a weighted mean of 75.2%. Several studies undertook a primarily minimally invasive approach (15,18) with no concomitant cardiac surgical procedure. Currently, minimally invasive approaches have been associated with longer ACC and CPB time, with no concise evidence to suggest reduced mortality and improved survival rates (32,33).
Even with the use of a rapid deployment valve, studies using the right thoracotomy approach were found to have longer ACC and CPB times, compared to the median sternotomy approach (see Table 2). This could be explained by difficult access, inability in debriding the aortic annulus effectively, patient selection and operator experience in minimally invasive surgery.
Early haemodynamic values have been promising with weighted mean valve gradient of 12.1 mmHg and post-operative EOA of 1.51 cm2. Several studies have shown further improvement at 1 and 2 years with weighted mean gradients of 10.1 and 9.9 mmHg respectively (see Table 5). Moderate to severe paravalvular leak was noted to be a pooled mean of 1.9%. Several authors advocate the need for proper annular decalcification or potential replacement with a conventional aortic valve at the time of implantation if there is significant paravalvular leak (19,20). In one study, severe paravalvular leak was not corrected at the time of surgery, and in those patients the leak remained stable with no further intervention at a later date (13). Though the early results with the Perceval valve suggest excellent haemodynamic results, demonstrating stable mean gradients up to at least 2 years, the long-term follow-up data was limited in most instances and not statistically comparable to other sutureless valves such as the Intuity (Edwards LifeSciences, Irvine, Calif, USA) or conventional valves such as the Mosaic (Medtronic, Inc., Minneapolis, Minn, USA).
This study is limited by several factors. The majority of the studies reviewed in this meta-analysis have only reported early survival and haemodynamics outcomes. The available long-term data is limited to a very small cohort of patients. Although the early haemodynamic data was encouraging, the data cannot be stratified to a particular valve type or size. The studies presented were observational and in many instances, they involve the same specialised centers; thereby causing a sample selection and publication bias. Additional limiting factors to consider were the proportion of isolated AVRs to concomitant procedures, bias introduced by surgical approach and the experience of the surgeon. Further randomised controlled trials at specialised centers with extensive experience in implanting Perceval valves are required to validate the data in this meta-analysis (34). The Perceval Sutureless Implant vs. Standard Aortic Valve Replacement (PERSIST-AVR) trial (http:livanova.sorin.com), will be the first such large international multicenter trial to adequately compare the efficacy and performance of the Perceval valve to conventional AVR.
Conclusions
This systematic review and meta-analysis has demonstrated that early clinical and haemodynamic performance of the Perceval valve is satisfactory and comparable to that of conventional AVR. However, the long-term durability and haemodynamic data for the Perceval valve is somewhat limited. Large-scale randomised studies are recommended to accurately assess long-term durability and complications associated with the Perceval valve.
Acknowledgements
None.
Table S1. Quality assessment of included studies.
| Study | Study population clearly defined? | Outcomes clearly defined? | Outcomes assessed? | Follow up duration sufficient? | Large sample size? | Loss during follow-up? | Prognostic factors and confounders identified? |
|---|---|---|---|---|---|---|---|
| Santarpino | Yes | No | Yes | No | Yes | No | Yes |
| Zannis | Yes | No | Yes | Yes | Yes | No | No |
| Villa | Yes | Yes | Yes | Yes | Yes | NR | Yes |
| Rubino | Yes | Yes | Yes | Yes | Yes | NR | Yes |
| Muneretto | Yes | Yes | Yes | Yes | Yes | NR | Yes |
| Miceli | Yes | No | Yes | Yes | Yes | No | Yes |
| Meuris | Yes | No | Yes | Yes | No | NR | No |
| Mazine | Yes | No | Yes | No | Yes | NR | Yes |
| Gilmanov | Yes | No | Yes | No | Yes | NR | Yes |
| Folliguet | Yes | Yes | Yes | Yes | Yes | NR | Yes |
| Fischlein | Yes | No | Yes | No | Yes | NR | Yes |
| Fleming | Yes | No | Yes | Yes | No | NR | Yes |
| Shrestha | Yes | No | Yes | Yes | Yes | Yes | No |
| Shrestha* | Yes | No | Yes | Yes | Yes | Yes | Yes |
*, represents the study by Shrestha 2013. NR, not recorded.
Table S2. Baseline characteristics.
| Study | Age | Female (%) | BSA (m2) | AS | AR | LVEF | CAD | HTN | Chol | DM | COPD | Pulm HTN | CVA | AF | CRI | PVD | Prev surg | Logistic EuroSCORE | EuroSCORE II | NYHA class (III or IV) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Santarpino | 77.1±5.3 | 44.0 | 1.8±0.2 | NR | NR | NR | NR | 83.3 | NR | 24.3 | NR | NR | NR | NR | 12.8 | NR | NR | 11±7.5 | NR | NR |
| Zannis | 79.4±5.9 | 55.0 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 12.04±10.7 | NR | 58.0 |
| Villaa | 80.1±4.3 | 100.0 | 1.6±0.2 | NR | NR | NR | NR | 76.7 | NR | 31.6 | 5.3 | NR | 2.1 | 8.9 | NR | 18.9 | 4.3 | 11.4±6.2 | NR | 71.1 |
| Villab | 79.7±5.4 | 63.8 | 1.8±0.2 | NR | NR | NR | NR | 73.7 | NR | 33.8 | 4.3 | NR | 4.4 | 12.5 | NR | 20.2 | 3.1 | 12.6±9.6 | NR | 71.2 |
| Rubino | 79.9±5.0 | 60.2 | NR | 56.1 | 0.3 | NR | NR | NR | NR | 29.9 | 17.8 | NR | NR | NR | NR | 23.9 | 7.6 | NR | 9.0±7.6 | 80.6 |
| Muneretto | 79.0±4.0 | 69.8 | NR | NR | NR | 54.2±11.3 | 22.6 | 86.6 | 29.1 | 20.1 | 15.1 | 47.2 | NR | 15.1 | 18.2 | 15.1 | 0 | 16.0±11.7 | NR | 88.7 |
| Miceli | 76.8±6.0 | 61.9 | NR | NR | NR | 56.8±8.5 | NR | 84.7 | NR | 22.1 | 16 | 13.2 | NR | NR | 3.9 | 11.7 | 1.8 | 8.0 (5.0–12.0) | NR | 47.7 |
| Meuris | 80.4±4.3 | 73.3 | 1.8±0.2 | 76.7 | NR | 63.0±11.0 | 14.0 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 13.2±7.3 | NR | 100.0 |
| Mazine | 78.9±5.9 | 54.0 | NR | NR | NR | NR | 49.0 | 83.0 | 72.0 | 35.0 | 13.0 | NR | NR | 22.0 | 20.0 | 13.0 | 9.0 | NR | 7.2±8.4 | 56.0 |
| Gilmanov | 76.6±7.1 | 65.7 | NR | NR | NR | 58.5± 8.5 | 21.9 | 82.5 | 66.4 | 26.2 | 13.9 | 13.9 | 2.9 | 18.2 | 12.4 | 18.2 | NR | 10.0±7.0 | NR | 54.0 |
| Folliguet | 79.0±5.3 | 60.9 | NR | NR | NR | NR | 23.1 | 68.0 | NR | 28 | 15 | NR | NR | NR | NR | NR | NR | 8.75±5.3 | NR | 98.5 |
| Fischlein | 77.8±4.7 | 55.1 | NR | NR | NR | 57.9±9.0 | NR | 69.6 | NR | 23.4 | NR | 23.4 | NR | NR | 17.9 | 14.5 | NR | 9.9%±5.9% | NR | NR |
| Fleming | 78.0 (75.0–87.0) | 65.0 | 1.7 (1.3–2.0) | NR | NR | NR | NR | 59.0 | 50.0 | 22.0 | 12.5 | NR | 9.3 | 3.0 | 12.5 | NR | NR | 10.0 (6.2–34.7) | NR | 100.0 |
| Shrestha | 79.7±5.1 | 60.0 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 12.1% | NR | 75.2 |
| Shrestha* | 79.8±4.5 | 94.0 | 1.7±0.18 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 20.4±10.7 | NR | 93.9 |
| Weighted mean | 78.7 | 61.5 | 1.8 | 57.9 | NA | 56.9 | 22.6 | 77.6 | 63.4 | 28.1 | 13.4 | 18.7 | 4.0 | 15.9 | 12.8 | 17.2 | 5.0 | NA | NA | 70.9 |
| Max | 80.4 | 100.0 | 1.8 | 76.7 | NA | 58.5 | 49.0 | 86.6 | 72.0 | 35.0 | 17.8 | 23.4 | 9.3 | 22.0 | 20.0 | 23.9 | 9.0 | NA | NA | 100.0 |
| Min | 76.6 | 44.0 | 1.6 | 56.1 | NA | 54.2 | 14.0 | 59.0 | 29.1 | 20.1 | 4.3 | 13.9 | 2.1 | 3.0 | 3.9 | 11.7 | 0 | NA | NA | 47.7 |
Values are %, unless otherwise indicated. Study by Villa is represented by “a” and “b” as Villa chose to compare cohorts by valve types (S vs. M, L, XL). *, represents study by Shrestha 2013. Rubino, and Mazine used Euroscore II, with the remaining group of authors utilising Logistic Euroscore. BSA, Body surface area; AS, aortic stenosis; AR, aortic regurgitation; LVEF, leI ventricular ejection fraction; CAD, coronary artery disease; HTN, hypertension; Chol, hyperlipidemia; DM, diabetes mellitus; COPD, chronic obstructive pulmonary disease; Pulm HTN, pulmonary hypertension; CVA, cerebral vascular accident; CRI, chronic renal impairment; PVD, peripheral vascular disease; Prev Surg, prior cardiac surgery; Logistic EuroSCORE, European System for Cardiac Operative Risk Evaluation; EuroSCORE II, additive EuroSCORE, NYHA, New York Heart Association Class III or IV; NR, not reported; NA, not applicable.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003;24:1231-43. 10.1016/S0195-668X(03)00201-X [DOI] [PubMed] [Google Scholar]
- 2.Carabello BA, Paulus WJ. Aortic stenosis. Lancet 2009;373:956-66. 10.1016/S0140-6736(09)60211-7 [DOI] [PubMed] [Google Scholar]
- 3.Zahn R, Gerckens U, Grube E, et al. Transcatheter aortic valve implantation: first results from a multi-centre real-world registry. Eur Heart J 2011;32:198-204. 10.1093/eurheartj/ehq339 [DOI] [PubMed] [Google Scholar]
- 4.Magovern GJ, Cromie HW. Sutureless prosthetic heart valves. J Thorac Cardiovasc Surg 1963;46:726-36. [PubMed] [Google Scholar]
- 5.Gott VL, Alejo DE, Cameron DE. Mechanical heart valves: 50 years of evolution. Ann Thorac Surg 2003;76:S2230-9. 10.1016/j.athoracsur.2003.09.002 [DOI] [PubMed] [Google Scholar]
- 6.Brown ML, McKellar SH, Sundt TM, et al. Ministernotomy versus conventional sternotomy for aortic valve replacement: a systematic review and meta-analysis. J Thorac Cardiovasc Surg 2009;137:670-679.e5. 10.1016/j.jtcvs.2008.08.010 [DOI] [PubMed] [Google Scholar]
- 7.Di Eusanio M, Phan K. Sutureless aortic valve replacement. Ann Cardiothorac Surg 2015;4:123-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ranucci M, Frigiola A, Menicanti L, et al. Aortic cross-clamp time, new prostheses, and outcome in aortic valve replacement. J Heart Valve Dis 2012;21:732-9. [PubMed] [Google Scholar]
- 9.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008-12. 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 10.Santarpino G, Pfeiffer S, Pollari F, et al. Left ventricular mass regression after sutureless implantation of the Perceval S aortic valve bioprosthesis: preliminary results. Interact Cardiovasc Thorac Surg 2014;18:38-42. 10.1093/icvts/ivt362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zannis K, Joffre J, Czitrom D, et al. Aortic valve replacement with the perceval S bioprosthesis: single-center experience in 143 patients. J Heart Valve Dis 2014;23:795-802. [PubMed] [Google Scholar]
- 12.Villa E, Messina A, Laborde F, et al. Challenge for perceval: aortic valve replacement with small sutureless valves--a multicenter study. Ann Thorac Surg 2015;99:1248-54. 10.1016/j.athoracsur.2014.09.090 [DOI] [PubMed] [Google Scholar]
- 13.Rubino AS, Santarpino G, De Praetere H, et al. Early and intermediate outcome after aortic valve replacement with a sutureless bioprosthesis: Results of a multicenter study. J Thorac Cardiovasc Surg 2014;148:865-71; discussion 871. 10.1016/j.jtcvs.2014.03.052 [DOI] [PubMed] [Google Scholar]
- 14.Muneretto C, Bisleri G, Moggi A, et al. Treating the patients in the 'grey-zone' with aortic valve disease: a comparison among conventional surgery, sutureless valves and transcatheter aortic valve replacement. Interact Cardiovasc Thorac Surg 2015;20:90-5. 10.1093/icvts/ivu340 [DOI] [PubMed] [Google Scholar]
- 15.Miceli A, Santarpino G, Pfeiffer S, et al. Minimally invasive aortic valve replacement with Perceval S sutureless valve: early outcomes and one-year survival from two European centers. J Thorac Cardiovasc Surg 2014;148:2838-43. 10.1016/j.jtcvs.2014.02.085 [DOI] [PubMed] [Google Scholar]
- 16.Meuris B, Flameng WJ, Laborde F, et al. Five-year results of the pilot trial of a sutureless valve. J Thorac Cardiovasc Surg. 2015;150:84-8. 10.1016/j.jtcvs.2015.03.040 [DOI] [PubMed] [Google Scholar]
- 17.Mazine A, Teoh K, Bouhout I, et al. Sutureless aortic valve replacement: a Canadian multicentre study. Can J Cardiol 2015;31:63-8. 10.1016/j.cjca.2014.10.030 [DOI] [PubMed] [Google Scholar]
- 18.Gilmanov D, Miceli A, Bevilacqua S, et al. Sutureless implantation of the perceval s aortic valve prosthesis through right anterior minithoracotomy. Ann Thorac Surg 2013;96:2101-8. 10.1016/j.athoracsur.2013.07.007 [DOI] [PubMed] [Google Scholar]
- 19.Folliguet TA, Laborde F, Zannis K, et al. Sutureless perceval aortic valve replacement: results of two European centers. Ann Thorac Surg 2012;93:1483-8. 10.1016/j.athoracsur.2012.01.071 [DOI] [PubMed] [Google Scholar]
- 20.Fischlein T, Pfeiffer S, Pollari F, et al. Sutureless Valve Implantation via Mini J-Sternotomy: A Single Center Experience with 2 Years Mean Follow-up. Thorac Cardiovasc Surg 2015;63:467-71. 10.1055/s-0035-1554043 [DOI] [PubMed] [Google Scholar]
- 21.Flameng W, Herregods MC, Hermans H, et al. Effect of sutureless implantation of the Perceval S aortic valve bioprosthesis on intraoperative and early postoperative outcomes. J Thorac Cardiovasc Surg 2011;142:1453-7. 10.1016/j.jtcvs.2011.02.021 [DOI] [PubMed] [Google Scholar]
- 22.Shrestha M, Maeding I, Höffler K, et al. Aortic valve replacement in geriatric patients with small aortic roots: are sutureless valves the future? Interact Cardiovasc Thorac Surg 2013;17:778-82; discussion 782. 10.1093/icvts/ivt291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shrestha M, Folliguet TA, Pfeiffer S, et al. Aortic valve replacement and concomitant procedures with the Perceval valve: results of European trials. Ann Thorac Surg 2014;98:1294-300. 10.1016/j.athoracsur.2014.05.033 [DOI] [PubMed] [Google Scholar]
- 24.Phan K, Tsai YC, Niranjan N, et al. Sutureless aortic valve replacement: a systematic review and meta-analysis. Ann Cardiothorac Surg 2015;4:100-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187-98. 10.1056/NEJMoa1103510 [DOI] [PubMed] [Google Scholar]
- 26.Bouhout I, Mazine A, Rivard L, et al. Conduction Disorders After Sutureless Aortic Valve Replacement. Ann Thorac Surg 2016. [Epub ahead of print]. 10.1016/j.athoracsur.2016.07.044 [DOI] [PubMed] [Google Scholar]
- 27.Roten L, Wenaweser P, Delacrétaz E, et al. Incidence and predictors of atrioventricular conduction impairment after transcatheter aortic valve implantation. Am J Cardiol 2010;106:1473-80. 10.1016/j.amjcard.2010.07.012 [DOI] [PubMed] [Google Scholar]
- 28.Khawaja MZ, Rajani R, Cook A, et al. Permanent pacemaker insertion after CoreValve transcatheter aortic valve implantation: incidence and contributing factors (the UK CoreValve Collaborative). Circulation 2011;123:951-60. 10.1161/CIRCULATIONAHA.109.927152 [DOI] [PubMed] [Google Scholar]
- 29.Bagur R, Rodés-Cabau J, Gurvitch R, et al. Need for permanent pacemaker as a complication of transcatheter aortic valve implantation and surgical aortic valve replacement in elderly patients with severe aortic stenosis and similar baseline electrocardiographic findings. JACC Cardiovasc Interv 2012;5:540-51. 10.1016/j.jcin.2012.03.004 [DOI] [PubMed] [Google Scholar]
- 30.Miceli A, Lio A, Glauber M. Size, position, and timing: A mixture of success. J Thorac Cardiovasc Surg 2016;152:633-4. 10.1016/j.jtcvs.2016.03.034 [DOI] [PubMed] [Google Scholar]
- 31.Yanagawa B, Cruz J, Boisvert L, et al. A simple modification to lower incidence of heart block with sutureless valve implantation. J Thorac Cardiovasc Surg 2016;152:630-2. 10.1016/j.jtcvs.2016.02.034 [DOI] [PubMed] [Google Scholar]
- 32.Murtuza B, Pepper JR, Stanbridge RD, et al. Minimal access aortic valve replacement: is it worth it? Ann Thorac Surg 2008;85:1121-31. 10.1016/j.athoracsur.2007.09.038 [DOI] [PubMed] [Google Scholar]
- 33.Brown ML, McKellar SH, Sundt TM, et al. Ministernotomy versus conventional sternotomy for aortic valve replacement: a systematic review and meta-analysis. J Thorac Cardiovasc Surg 2009;137:670-679.e5. 10.1016/j.jtcvs.2008.08.010 [DOI] [PubMed] [Google Scholar]
- 34.Leon MB, Smith CR, Mack MJ, et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med 2016;374:1609-20. 10.1056/NEJMoa1514616 [DOI] [PubMed] [Google Scholar]