Ventilator-associated pneumonia (VAP) is a major complication of mechanical ventilation and a recognized marker of quality of care in an intensive care unit (ICU) (1). Prevention of VAP with the bundle approach seems to be effective in decreasing VAP rate (2,3), despite the absence of controlled trials.
However, VAP diagnosis is one of the most complex diagnostic issue for intensivists, as many of the diagnostic criteria are very subjective (4,5). The inaccuracy of diagnostic methods may partly explain why the reduction of VAP rates with bundles was not associated with a decrease of antimicrobial consumption. The Center for Disease Control and Prevention (CDC) and Critical Care Societies have therefore recently developed a new approach, potentially automatable and based on objective criterion, to diagnose ventilator-associated events (VAE) instead of VAP (6).
What are VAE?
The VAE surveillance definition algorithm uses three new indicators, moving from ventilator-associated conditions (VAC), to infection-related ventilator-associated complications (IVAC), to possible and probable VAP (7). VAC is the first step of VAE surveillance which attempts to identify any complication occurring in mechanically ventilated patients, whatever its origin or mechanism. To meet the VAC definition, a mechanically ventilated patient must have at least 2 days of stability or improvement on the ventilator [assessed by a stable or decreasing daily minimum positive end-expiratory pressure (PEEP) or fraction of inspired oxygen (FiO2)], followed by at least 2 days of worsening oxygenation (diagnosed by an increase of the daily minimum PEEP (≥3 cmH2O) or FiO2 (≥20%)). The second tier, IVAC or IVAC, aims at identifying the subgroup of VACs that are potentially related to infection. A VAC associated to an abnormal white blood cell count or a modified temperature, and the initiation of a new antimicrobial agent continued for at least 4 days becomes an IVAC. With evidence of purulent respiratory secretions and/or positive results of microbiological tests performed on respiratory tract specimens, an IVAC becomes a possible VAP (PVAP). The PVAP becomes probable when the positive lower respiratory tract culture meets certain quantitative or semiquantitative thresholds of pathogen growth. All these definitions are summarized in Figure 1.
Figure 1.
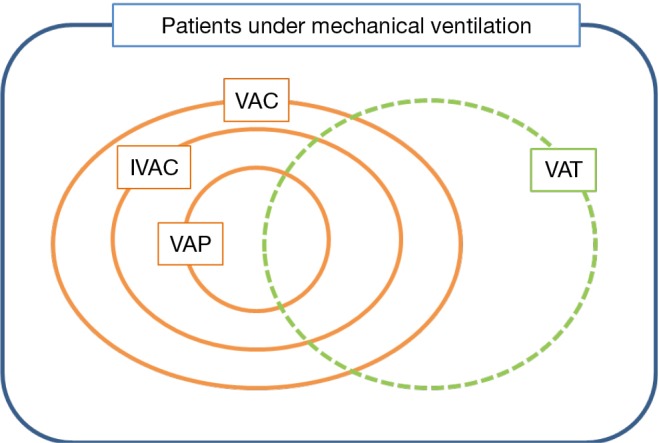
Patterns of ventilator-associated events (VAE). Ventilator-associated conditions (VAC): ≥2 calendar days of stable or decreasing daily minimum PEEP or FiO2 followed by rise in PEEP ≥3cmH2O or rise in FiO2 ≥20 points sustained for ≥2 days. Infection-related ventilator-associated complications (IVAC): VAC plus: temp <36 °C or >38 °C or WBC ≤4 or ≥12×103 cells/mm3 AND ≥1 new antibiotics continued for ≥4 days, within 2 days of VAC onset excluding first 2 days on the ventilator. Possible or probable pneumonia: IVAC plus: sputum/BAL with ≥25 neutrophils/field and ≤10 epithelial cells/field and/or positive respiratory culture, within 2 days of VAC onset excluding first 2 days on the ventilator. Ventilator-associated tracheobronchitis (VAT): purulent sputum/BAL with positive respiratory culture with or without clinical and biological signs of sepsis.
VAE: strengths and weaknesses
The VAE concept has several advantages. First, the required data for surveillance are potentially automatable, and the criteria are all objective. We know VAP definition is still widely discussed, and a recent study showed that applying different diagnostic criteria to the same patient population resulted in an important variation in the incidence of VAP, i.e., from 4% to 42% (5). This new approach might overcome the inaccuracy of the VAP definition, facilitate its electronic assessment, and make inter-ICUs comparisons more relevant. Second, the showed association between VAE and antibiotic consumption (considering VAC rates and not only IVAC) could be a convincing point for the use of VAC rate as one of the process indicator of quality of care in the ICU for antimicrobial stewardship programs (8). However, the lack of standard ventilator settings and oxygenation objective in ICUs may limit the reliability of the ventilator settings for the VAC definition. In addition, patients can be diagnosed with a VAC after at least 4 days under mechanical ventilation; how to consider infections occurring earlier is not known. The chosen criteria may also be criticized, as an abnormal temperature or white blood cell count in ICU patients have a poor predictive value for pulmonary infections (9). Due to the low specificity of these data, IVAC will also concern patients with extrapulmonary infections of sufficient severity to trigger respiratory deterioration. As a result, VAP accounted for only 14.5% of the VAC episodes and 27.6% of the IVAC episodes in a study of our research group ; in addition, all IVAC episodes were not related to a nosocomial infection, although patients received antibiotics (8). Furthermore, radiological criteria are not taken into account, so that IVAC definition may include both ventilator-associated tracheobronchitis (VAT) and VAP (Figure 1). Although these two entities are associated with an increased duration of mechanical ventilation, their impact on ICU mortality is not the same; in the TAVeM study, mortality of patients with VAP was 40%, as compared to 29% for those with tracheobronchitis (10). Finally, embedding VAP in the larger definition of IVAC may lead to difficulties in the understanding of VAP pathophysiology and consequently, in improving its prevention.
How to prevent VAE?
Bundles have been developed to prevent VAP in order to improve outcomes for patients undergoing mechanical ventilation. The core constituents of most hospitals’ ventilator bundle are well recognized, although the ideal combination remains unknown. Using complex survival competing risks models, Klompas et al. studies the associations between ventilator bundle components and VAE, time under mechanical ventilation, length of ICU and hospital stay and mortality during a 4-year follow-up in one hospital. The six selected components of the ventilator bundle included head-of-bed elevation, daily interruptions of sedative infusions, daily spontaneous breathing trials, thromboembolism prophylaxis, stress ulcer prophylaxis, and oral care with chlorhexidine gluconate. None of these interventions has been proven to decrease ICU mortality in a recent meta-analysis (11). Other interesting measures could have been studied, i.e., maintaining an endotracheal tube cuff pressure >20 cmH2O, or using an orogastric tube rather than a nasogastric tube.
In their study, a significant reduction or VAE and IVAC occurred when implementing spontaneous breathing trials, but none of the other constituents of the bundle decreased the risk of possible VAP. Importantly, spontaneous breathing trials and thromboprophylaxis were also associated with a shorter length of intubation. Spontaneous breathing trial even decreased the mortality under mechanical ventilation but not in-hospital mortality. At last, sedative infusion interruptions decrease duration of mechanical ventilation and hospital stay and mortality. Although the authors made particular efforts to adjust for confounders in this observational study, they made important assumptions that should be kept in mind. First, they considered the impact of continuous performance of one component during 4 days as compared to 4 days of not doing so. Second, they adjusted on factors that they retrospectively assumed to be associated to a specific process of care. For example, they considered for statistical analysis that adjustment on the daily use of sedation and analgesia was a direct reflection of sedative infusion interruptions. Last, the authors did not provide data comparing the patients who were taken care of with the bundle with those who were not, raising the possibility that other factors—linked to their clinical characteristics, healthcare professionals, or system-level-related—could partially explain their findings.
The major pitfall of this analysis is that we are unable to demonstrate what is (are) the disease(s) responsible for VAE that benefit from these interventions. As circumstances causing VAE are multiple, and include pulmonary oedema, atelectasis, pneumothorax or non-cardiogenic oedema, we wonder how the studied measures could have been able to prevent VAE if not by preventing VAPs. In fact, are VAE really preventable? Given that VAE are related to many different causes and do not result from the same mechanisms as VAP, VAE prevention needs a broader prevention strategy. The impact of VAE on mortality and length of ICU stay is discussed, and results are controversial (12,13). Indeed, in their study, the only measure associated with a reduction of VAE (spontaneous breathing trials) is not correlated with hospital mortality; the impact of spontaneous breathing trials on the risk of VAE is probably due to a decrease in the duration of mechanical ventilation leading, by definition, to a decrease in VAE rate. It may explain why the head-of-bed elevation and thromboprophylaxis—which are not directly affecting duration of mechanical ventilation—were not effective.
As it is frequent and convenient to evaluate, VAE may be an important process indicator in ICU. However, given the heterogeneity of its causes, a decrease of VAE rate associated with a multicomponent intervention should be cautiously interpreted. The study by Klompas et al. does not conclude as to whether any bundle component should be abandoned, but prompts the need to revisit bundle components and re-examine the efficacy of old practices, to create an improved bundle in the future.
Acknowledgements
None.
Provenance: This is an invited Editorial commissioned by the Section Editor Zhiheng Xu (State Key Laboratory of Respiratory Disease, Guangzhou Institute of Respiratory Disease, Department of Intensive Care, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Bouadma L, Wolff M, Lucet JC. Ventilator-associated pneumonia and its prevention. Curr Opin Infect Dis 2012;25:395-404. 10.1097/QCO.0b013e328355a835 [DOI] [PubMed] [Google Scholar]
- 2.Bouadma L, Deslandes E, Lolom I, et al. Long-term impact of a multifaceted prevention program on ventilator-associated pneumonia in a medical intensive care unit. Clin Infect Dis 2010;51:1115-22. 10.1086/656737 [DOI] [PubMed] [Google Scholar]
- 3.Khan R, Al-Dorzi HM, Al-Attas K, et al. The impact of implementing multifaceted interventions on the prevention of ventilator-associated pneumonia. Am J Infect Control 2016;44:320-6. 10.1016/j.ajic.2015.09.025 [DOI] [PubMed] [Google Scholar]
- 4.Klompas M. Does this patient have ventilator-associated pneumonia? JAMA 2007;297:1583-93. 10.1001/jama.297.14.1583 [DOI] [PubMed] [Google Scholar]
- 5.Ego A, Preiser JC, Vincent JL. Impact of diagnostic criteria on the incidence of ventilator-associated pneumonia. Chest 2015;147:347-55. 10.1378/chest.14-0610 [DOI] [PubMed] [Google Scholar]
- 6.Center for Disease Control and Prevention. National Healthcare Safety Network (NHSN). Surveillance for Ventilator-associated Events. Available online: http://www.cdc.gov/nhsn/acute-care-hospital/vae/
- 7.Magill SS, Klompas M, Balk R, et al. Developing a new, national approach to surveillance for ventilator-associated events: executive summary. Clin Infect Dis 2013;57:1742-6. 10.1093/cid/cit577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouadma L, Sonneville R, Garrouste-Orgeas M, et al. Ventilator-Associated Events: Prevalence, Outcome, and Relationship With Ventilator-Associated Pneumonia. Crit Care Med 2015;43:1798-806. 10.1097/CCM.0000000000001091 [DOI] [PubMed] [Google Scholar]
- 9.Edriss H, Whiting J, Nugent K. The Frequency of White Blood Cell and Temperature Events During Mechanical Ventilation and Their Association With Ventilator-Associated Events. J Intensive Care Med 2015. [Epub ahead of print]. 10.1177/0885066615605036 [DOI] [PubMed] [Google Scholar]
- 10.Martin-Loeches I, Povoa P, Rodríguez A, et al. Incidence and prognosis of ventilator-associated tracheobronchitis (TAVeM): a multicentre, prospective, observational study. Lancet Respir Med 2015;3:859-68. 10.1016/S2213-2600(15)00326-4 [DOI] [PubMed] [Google Scholar]
- 11.Roquilly A, Marret E, Abraham E, et al. Pneumonia prevention to decrease mortality in intensive care unit: a systematic review and meta-analysis. Clin Infect Dis 2015;60:64-75. 10.1093/cid/ciu740 [DOI] [PubMed] [Google Scholar]
- 12.Boyer AF, Schoenberg N, Babcock H, et al. A prospective evaluation of ventilator-associated conditions and infection-related ventilator-associated conditions. Chest 2015;147:68-81. 10.1378/chest.14-0544 [DOI] [PubMed] [Google Scholar]
- 13.Hayashi Y, Morisawa K, Klompas M, et al. Toward improved surveillance: the impact of ventilator-associated complications on length of stay and antibiotic use in patients in intensive care units. Clin Infect Dis 2013;56:471-7. 10.1093/cid/cis926 [DOI] [PubMed] [Google Scholar]


