Abstract
Background
Pulmonary inflammatory myofibroblastic tumor (IMT) has been considered as a synonym for inflammatory pseudotumor (IPT) for a long time. Recent studies have indicated that IMT and IgG4-related IPT are distinct diseases. However, no consensus criteria have been recommended. Here we propose a set of criteria for the differential diagnosis.
Methods
Twenty-six archived IMT and IgG4-related IPT samples were examined for histological characteristics and the expression of IgG, IgG4, SMA and ALK-1. Based on our proposed criteria, we reclassified the cases into either IMT or IgG4-related IPT group and compared the clinicopathological features, laboratory findings, overall survivals (OS) and disease-free survivals between groups to validate the effectiveness and dependability of the diagnostic criteria.
Results
The average age of IgG4-related IPT group was higher than IMTs (48.82 vs. 39.22 years, P=0.031). In IMT group, tumors were characterized by bigger tumor sizes (3.47 vs. 2.22 cm, P=0.007), diffuse and total destroyed alveoli (88.89% vs. 17.65%, P=0.002), fewer lymphoid follicles (1.6/HPF vs. 3.0/HPF, P=0.045) and lower expression of IgG (74.7/HPF vs. 149.1/HPF; P<0.001). As an exclusion criterion of IgG4-related IPT, ALK-positivity was correlated with the higher cytological atypia (mean 3.7/HPF, P<0.001) and lesser lymphoid follicles (mean 1.2/HPF, P=0.021). And it’s the first study to show the liner positive correlation between the lymphocytes + plasma cells count and IgG4-positive plasma cells count in these lesions (r=0.914, P<0.001). The negative correlation between the IgG4-positive plasma cells count and the expression of ALK-1 are reported for the first time as well (rs=−0.632, P=0.001). However, despite two patients with recurrence or metastasis were divided into IMT group, only borderline values were detected in the survival analysis (OS 88.89% vs. 100%, P=0.197, DFS 77.78% vs. 100.00%; P=0.056).
Conclusions
The significant differences of clinicopathological characteristics between the IMTs and IgG4-related IPTs indicated that a combination of lymphocytes + plasma cells count, cytological atypia, IgG4 and ALK-1 staining will be helpful in differential diagnosis.
Keywords: Inflammatory myofibroblastic tumor (IMT), inflammatory pseudotumor (IPT), differential diagnosis
Introduction
Pulmonary inflammatory myofibroblastic tumor (IMT) is a distinctive mesenchymal neoplasm composed of spindle-shaped myofibroblasts accompanied by inflammatory infiltration. In the 2004 edition of World Health Organization’s classifications, IMT is considered as a synonym for inflammatory pseudotumor (IPT) which is an inflammatory reactive lesion (1). However, a part of IMT cases suffer from local recurrences and/or occasionally distant metastases which cannot be well explained by inflammatory reaction (2,3). In clinical practice, ambiguous definition brings a great challenge to the diagnosis and treatment. Despite that they represent rare types of pulmonary lesions, there is an intense discussion about whether IMT and IPT are separate entities rather than parts of a clinicopathological continuum. Gene rearrangements involving the anaplastic lymphoma kinase (ALK) have been found in IMT, providing a support for the neoplastic nature of IMT (4). In addition, some studies have reported that IgG4-positive plasma cells might play an important role in the pathogenesis in some populations of IPT (5). For this reason, though controversial, most of the recent reports have separated IMT from IgG4-related IPT (6). However, previous studies have failed to propose any consensus criteria for the differential diagnosis of IMT and IgG4-related IPT. In this study, we reviewed the patients diagnosed as pulmonary IMT or IPT in our single institution to propose a set of diagnostic criteria to distinguish between IMT and IgG4-related IPT.
Methods
Human tissue samples
Specimens were obtained from the archives of formalin-fixed, paraffin-embedded tissue blocks in the Department of Thoracic Surgery, West China Hospital, Sichuan University. The collection and use of human tissue samples were approved by the Institutional Review Board of West China Hospital, Sichuan University (NO. 2016-194). The procedures were in accordance with the Ethical Principles for Medical Research Involving Human Subjects as formulated in the World Medical Association Declaration of Helsinki (revised in 2008). In this retrospective study, 26 cases with surgically resected pulmonary IMT or IPT were enrolled from December, 2008 to August, 2015. Two patients without available sections were excluded. In our institution, IMTs and IPTs were recognized as synonyms in pathological analysis until November, 2010. Therefore, the pathological diagnoses of 8 patients prior to 2010 were ambiguous. After that, based on several previous researches and our experience, neoplasms with obvious proliferation of spindled myofibroblasts and less inflammatory cells infiltration were separated from IPTs and diagnosed as IMT. Clinical characteristics were collected from the medical records. We reviewed all pathological sections to ensure consistency with the criteria of WHO classification (1). Written informed consents were received from all enrolled patients. The patients were followed up until December, 2015, through outpatient visits and/or correspondences to family members.
Pathological review
The slides were evaluated by a pathologist (W.D.) for nuclear atypia, number of lymphoid follicles, vascular invasion, and inflammatory component. The average count of lymphocytes + plasma cells (L + P), number of lymphoid follicles, and number of mitoses in spindle cells were calculated in ten high-power fields (×400 magnification, HPF) in most mitotically active area (i.e., hotspot method) and represented as the number per HPF. Immunohistochemical (IHC) staining was performed using standard techniques. Rabbit anti-smooth muscle actin (SMA) antibodies and anti-ALK-1 antibodies were purchased from Wuhan Sanying Biotechnology, Wuhan, China. Rabbit anti-IgG4 and anti-IgG antibodies were obtained from Abcam Trading (Shanghai) Company Ltd., Shanghai, China. A few of previously known positive lung cancer tissue sections were used as positive control. Non-immune rabbit serum served as negative control. The results were assessed by two investigators who were blinded to the clinicopathological data using an Olympus B43 microscope (Olympus, Tokyo, Japan).
Statistical analysis
The Statistical Package for Social Sciences (SPSS Version 22.0, Chicago, IL, USA) was applied in statistical analysis. Scatter plots, Pearson correlation analysis, Spearman analysis were used to reveal the correlations among morphological features and IHC results. The clinicopathological differences between the two groups were evaluated by the Fisher exact test or Student’s t-test. The patients’ overall survival (OS) and disease-free survival was calculated by the Kaplan–Meier method and the differences between the groups were evaluated by the log-rank test. P<0.05 was considered statistically significant.
Results
Criteria for differential diagnosis
Based on our initial analysis of the collected data and previous literature (6,7), we proposed a set of semi-quantitative criteria for differential diagnosis. Criteria for diagnosis of IgG4-related IPT were: (I) L + P count >120/HPF; and (II) cytological atypia <3/HPF; and (III) IgG4-positive plasma cells >60/HPF or IgG4+/IgG+ plasma cells >50%. In contrast, criteria for IMT were: (I) L + P count <120/HPF; and (II) cytological atypia >3/HPF; and (III) IgG4-positive plasma cells <60/HPF and IgG4+/IgG+ plasma cells <50%. Besides that, ALK-1 staining positivity serves better as an exclusion criterion for IPT than as a diagnostic criterion for IMT since the ALK-1 negative IMTs have been reported before (2).
In this study, these diagnostic indicators show fairly good consistency and histopathological comprehensive evaluations have been performed in only two patients (7.69%) who obtained conflicting results using this set of criteria. The lesions with focal and incomplete destruction of pulmonary alveoli, lymphoid follicles more than 3/HPF and the presence of obstructive phlebitis were considered as IgG4-related IPT firstly, on the contrary, diffuse and complete destruction of alveoli and less than 3/HPF lymphoid follicles will help us to make a diagnose of IMT. For instance, although the ratio of IgG4+/IgG+ plasma cells was 50.7% in case 4 (IgG4=43.8/HPF), we still diagnosed him as IMT since the totally destroyed alveoli and the absence of lymphoid follicle and obstructive phlebitis. In case 7, all the indicators gave a support for the diagnose of IMT except the inconspicuous cell atypia (2.4/HPF). With diffuse destruction of alveoli, less than 1.0/HPF of lymphoid follicles and the absence of obstructive phlebitis, he was also diagnosed as IMT after the comprehensive evaluation.
According to the proposed criteria and following histopathological comprehensive evaluations, we reclassified the 26 cases into either IMT or IgG4-related IPT group regardless of previous diagnosis. A total of 17 cases (65.38%) were diagnosed as IgG4-related IPTs and the remaining 9 cases were included in IMT group.
Clinicopathological characteristics of all patients
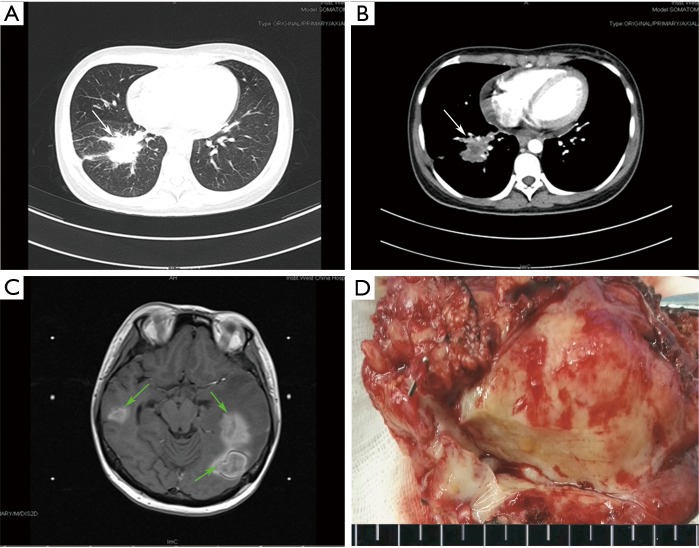
The overall clinicopathological features of all 26 patients are summarized in Table 1. There were 16 males and 10 females, with ages ranging from 23 to 66 years old (median 46 years). Twenty patients (76.92%) presented with symptoms, mainly including cough (n=12), chest pain (n=9), fever (n=5), and hemoptysis (n=5). The remaining 6 asymptomatic patients were found by presenting abnormal shadows on chest X-ray or computed tomography (CT) during routine medical examinations. The tumors seemed to locate more often in the right lung than left lung (69.23% vs. 30.77%) and more often in the peripheral pulmonary field than pulmonary hilus (92.31% vs. 7.69%; Figure 1A,B). The treatment was mostly lobectomy (17 cases), followed by wedge resection (7 cases) and sleeve resection (2 cases). All patients were considered to receive complete resections (R0) and the postoperative recoveries were uneventful. During an average follow-up time of more than 40 months (ranging from 3 to 84 months), two patients (7.69%) were finally confirmed as a local recurrence (Case 6) and an intracranial metastasis (Case 2; Figure 1C), respectively.
Table 1. Clinical characteristics of patients.
| Case | Group† | Age (y)/sex | Symptoms | Site | Size (cm)# | Treatment |
|---|---|---|---|---|---|---|
| 1* | IMT | 44/F | Chest pain | LLL, P | 2.5 | Lobectomy |
| 2 | IMT | 24/M | Cough, fever | RLL, P | 5.2 | Lobectomy |
| 3⬥* | IMT | 40/M | − | LLL, P | 2.0 | wedge resection |
| 4* | IMT | 23/M | Cough, hemoptysis | RML, P | 2.0 | Lobectomy |
| 5* | IMT | 48/M | Cough, chest pain | RML, P | 3.5 | Lobectomy |
| 6* | IMT | 66/F | Cough, hemoptysis | RUL, H | 6.3 | sleeve resection |
| 7* | IMT | 47/M | Cough, hemoptysis | RUL, H | 3.0 | sleeve resection |
| 8⬥ | IMT | 49/M | − | RUL, P | 3.0 | wedge resection |
| 9* | IMT | 44/M | Cough | RUL, P | 3.7 | Lobectomy |
| 10 | IgG4-related IPT | 35/F | Cough, chest pain | RLL, P | 2.5 | Lobectomy |
| 11⬥ | IgG4-related IPT | 63/F | − | LUL, P | 1.0 | Wedge resection |
| 12 | IgG4-related IPT | 55/F | Chest pain | LUL, P | 2.5 | Lobectomy |
| 13⬥ | IgG4-related IPT | 43/M | Cough, fever | RUL, P | 2.0 | Lobectomy |
| 14⬥ | IgG4-related IPT | 46/F | − | RLL, P | 1.0 | wedge resection |
| 15 | IgG4-related IPT | 48/M | Chest pain | LLL, P | 2.0 | wedge resection |
| 16 | IgG4-related IPT | 56/F | Cough, fever | RUL, P | 3.5 | Lobectomy |
| 17 | IgG4-related IPT | 34/F | Cough, hemoptysis | RUL, P | 2.5 | Lobectomy |
| 18 | IgG4-related IPT | 62/M | Chest pain | LLL, P | 1.8 | Lobectomy |
| 19⬥ | IgG4-related IPT | 52/M | Chest pain | RLL, P | 2.8 | wedge resection |
| 20 | IgG4-related IPT | 41/M | − | RLL, P | 1.0 | Lobectomy |
| 21 | IgG4-related IPT | 46/F | Chest pain | LLL, P | 3.0 | Lobectomy |
| 22⬥ | IgG4-related IPT | 46/M | Hemoptysis | RLL, P | 2.0 | Lobectomy |
| 23 | IgG4-related IPT | 61/F | Cough, fever | RUL, P | 2.5 | Lobectomy |
| 24 | IgG4-related IPT | 33/M | Chest pain, fever | RLL, P | 2.2 | wedge resection |
| 25 | IgG4-related IPT | 38/M | − | RLL, P | 3.0 | Lobectomy |
| 26 | IgG4-related IPT | 39/M | Cough | LUL, P | 2.5 | Lobectomy |
†, The grouping was based on the new criteria for differential diagnosis proposed in our study; #, nodule sizes were measured from pathological specimens or from described by operational records; ⬥, the patients received CT guided biopsy of the lesions; *, the patients were confirmed as ALK-1 positive; M, male; F, female; RLL, right lower lobe; RUL, right upper lobe; RML, right middle lobe; LLL, left lower lobe; LUL, left upper lobe; P, peripheral pulmonary fields; H, hilar parts of the lungs; IMT, inflammatory myofibroblastic tumor; IgG4-related IPT, IgG4-related inflammatory pseudotumor (IPT).
Figure 1.
Computed tomography (CT) and macroscopic imaging data of the patient with intracranial metastasis (Case 2) (A,B). CT examinations of the patient before the resection. A solid tumor (Marked by the white arrows) in the right lower lobe of the lung with deep lobulated sign, pleural indentation and markedly inhomogeneous enhancement showed on CT imaging; (C) 5 months after surgery, magnetic resonance imaging (MRI) showed a few of subcortical nodules with obvious peripheral edema (Marked by the green arrows) which were considered as intracranial metastasis; (D) a macroscopic image of the lobectomy specimen showed that the tumor was circumscribed, and whitish-yellow in color on the cut-surface.
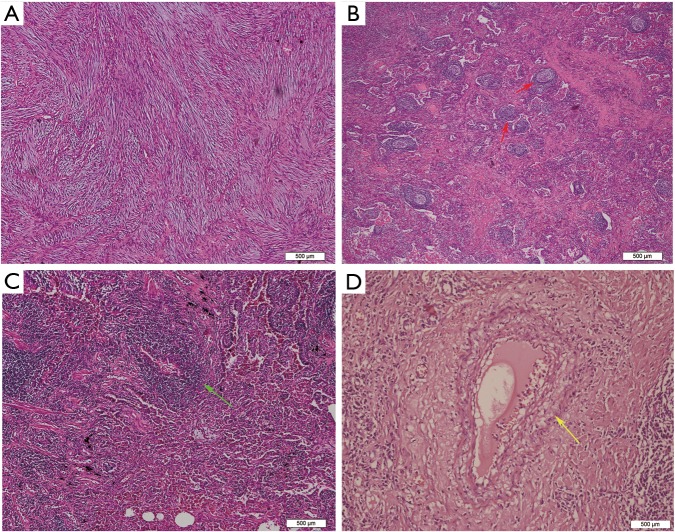
All the tumor size ranges from 1.0 to 6.3 cm in diameter (mean 2.65±1.17 cm), with a white to tan fleshy cut surface as previous reported (Figure 1D) (1,8,9). Microscopic examination showed that alveoli had been totally or partially destroyed by the lesion which composed of spindle-shaped tumor cells in fascicles or storiform architecture accompanied by various degrees of inflammatory infiltrations (Figure 2A,B,C). In all cases, the tumor cells had oval nuclei, inconspicuous nucleoli and a lightly eosinophilic cytoplasm. The spindle tumor cells had relatively low cellular atypia (0–5.0/HPF) and no mitotic activity. Some lymphoid follicles with germinal center and obliterate phlebitis (Figure 2D) were found in tumor tissues as well.
Figure 2.
Hematoxylin and eosin staining in IMT and IgG4-related IPT. (A) Original alveolar structures were totally destroyed by intersecting fascicles of spindle tumor cells in IMTs; (B) tumor cells were admixed with numerous lymphocytes, plasma cells and lymphoid follicles (red arrows) in IgG4-related IPTs; (C) a large number of inflammatory cells were infiltrative and involves the small bronchi (green arrow) in IPTs; (D) inflammatory infiltration of vessel walls or obstructive phlebitis (yellow arrow) were only observed in the tumors diagnosed as IgG4-related IPT.
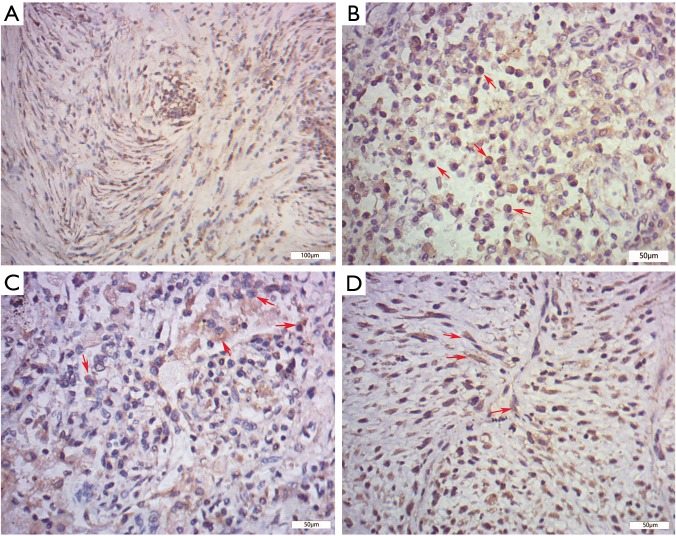
IHC, SMA was diffusely or focally positive in tumor cells in eighteen cases and statistically there was no significant difference in SMA immunoreactivity between the IPT and IMT (IMT 88.89% vs. IPT 58.82%, P=0.190, Figure 3A). The number of IgG-positive plasma cells per HPF ranged from 34.8 to 187.8 in 26 cases. And the overall mean of the IgG-positive plasma cells was 123.3 per HPF. Cells stained for IgG4 were detected in 24 of 26 cases (92.31%), ranging from 0 to 132.4/HPF. We found that the IgG4 expressions were more significant in the regions with obvious inflammatory cells infiltration, especially in the lymphoid follicles areas. And both IgG and IgG4 expressions were patchy and cytoplasmic (Figure 3B,C). Instead of nuclear or membrane pattern, seven cases (26.92%) had a cytoplasmic pattern of staining for ALK-1 (Figure 3D), including the patient (Case 6) who was finally confirmed to recur after the resection.
Figure 3.
Immunohistochemical (IHC) staining of IMT and IgG4-related IPT. (A) Smooth muscle actin (SMA) was focally positive in spindle-shaped cells in inflammatory myofibroblastic tumors (IMT); (B) in IgG4-related IPTs, IgG expressions were patchy and cytoplasmic, arrows, IgG-positive plasma cells; (C) IgG4 immunostain showed numerous IgG4-positive plasma cells which marked by red arrows in the lesions of IgG4-related IPTs; (D) spindle-shaped tumor cells presented diffuse and strong reactivity for ALK-1 immunostain in the IMT, red arrows, ALK-1-positive spindle cells.
Difference of clinicopathological characteristics of IMT and IgG4-related IPT groups
Table 2 shows the main differences of clinicopathological characteristics between IMT and IgG4-related IPT groups. The average age of IgG4-related IPT group was significantly higher than IMTs (48.82 vs. 39.22 years, P=0.031), indicating younger people seemed to be more susceptible to the IMTs. Compared with IMT group which comprised by 7 men and 2 women, IgG4-related IPT group included more female patients, despite the result was not statistically significant (9 men and 8 women; P=0.399). Besides that, no statistical differences were detected in symptoms, smoking status and lesion distributions between the two groups.
Table 2. Comparison of the characteristics between IMT and IPT groups.
| Variable | IMT group (n=9) | IgG4-related IPT group (n=17) | P value |
|---|---|---|---|
| Age (mean) | 39.22 | 48.82 | 0.031 |
| Sex (M/F) | 7/2 | 9/8 | 0.399 |
| Symptoms (S/A)† | 7/2 | 13/4 | 0.997 |
| Smoking history (yes/never) | 4/5 | 6/11 | 0.692 |
| Tumor size (cm) | 3.47 | 2.22 | 0.007 |
| Destroyed alveolars | 9 (100%) | 15 (88.24%) | 0.002 |
| Totally | 8 (88.89%) | 3 (17.65%) | |
| Partially | 1 (11.11%) | 12 (70.59%) | |
| Cytological atypia | 2.4–5.0/HPF⬥ (mean 3.5/HPF) | 0.0–2.9/HPF (mean 1.4/HPF) | - |
| Obstructive phlebitis | 0 | 7 (41.18%) | 0.058 |
| Lymphoid follicles | 0.0–3.0/HPF (mean 1.6/HPF) | 1.0–7.1/HPF (mean 3.0/HPF) | 0.045 |
| SMA | 8 (88.89%) | 10 (58.82%) | 0.190 |
| IgG+ plasma cells | 34.8-127.0/HPF (mean 74.7/HPF) | 107.3-187.8/HPF (mean 149.1/HPF) | <0.0001 |
| IgG4+/IgG+ plasma cells# | 0–50.70% (mean 22.58%) | 52.86–81.63% (mean 68.28%) | - |
| ALK-1# | 7 (77.78%) | 0 | - |
| OS (%) | 88.89% | 100% | 0.197 |
| DFS (%) | 7 (77.78%) | 17 (100%) | 0.056 |
†, S and A represent symptomatic and asymptomatic patients, respectively; ⬥, HPF is short for high-power field; #, due to the fact that cytological atypia, IgG4 and ALK-1 were regard as diagnostic criteria, P values were not calculated.
Although the characteristics of gross specimens were quite similar in two groups, the tumor sizes in IMT group were much bigger than IgG4-related IPTs (3.47 vs. 2.22 cm, P=0.007). Compared with IgG4-related IPT group, diffuse and total destroyed alveoli were presented in most of IMT lesions (17.65% vs. 88.89%, P=0.002). On the contrary, most of tumors in IgG4-related IPT group were featured with a variety of inflammatory cell infiltration and thickened alveoli septum, however parts of alveolar structures still maintained relatively integrity. And the mean number of lymphoid follicles with germinal center in IMTs was only about half as many as we found in IgG4-related IPT group (1.6/HPF vs. 3.0/HPF, P=0.045). Considered as one of the characteristics of the inflammatory diseases, the obstructive phlebitis (7 cases, P=0.058) were merely detected in IgG4-related IPT group as previous literatures (5-7).
In further analysis, the number of IgG-positive plasma cells per HPF ranged from 34.8 to 127.0 in IMT group. In IgG4-related IPTs, it ranged from 107.3 to 187.8 per HPF, with a mean of 149.1/HPF. Although the ranges overlapped, the mean IgG-positive cells in IMT group was only half of that in IgG4-related IPTs (74.7/HPF; P<0.001). And the mean ratio of IgG4+/IgG+ plasma cells in IMT and IgG4-related IPT group were 22.58% and 68.28% (range: 0% to 50.70% vs. 52.86% to 81.63%), respectively. In addition, ALK-1 positive cases occupied a large proportion in IMT group (7 of 9 cases, 77.78%). And we also found that the ALK-1 expression was correlated with the higher cytological atypia (mean 3.7/HPF, P<0.001) and lesser lymphoid follicles (mean 1.2/HPF, P=0.021), indicating more aggressive biological behaviors than the most of ALK-1 negative tumors. Nonetheless, ALK-1 positivity could not be detected in all the tumors with obvious cytological atypia and it was noteworthy that both IgG4 and ALK-1 were negative in the only died case.
Follow-up outcome
According to the follow-up information, 2 of 26 selected patients (7.69%) were confirmed as recurrence or metastasis within one year after the operations. It’s worth pointing out that both of the patients with disease progression were diagnosed as IMT according to our standards. In case 6, CT showed a soft tissue shadow appeared on bronchial stump when she came back for a check 7 months after operation. The new lesion was removed by an additional thoracotomy and finally confirmed as a local recurrence. At the end of follow-up, she remains well without any sign of recurrence or metastasis. The other patient, case 2, involved a 24-year-old male with serious headache five months after the surgery. Magnetic resonance imaging showed a few subcortical nodules with obvious peripheral edema which were considered as intracranial metastasis. Although gamma knife radiosurgeries were performed, his condition deteriorated progressively. Unfortunately, the patient was deceased after 2 months of hospitalization due to disease progression.
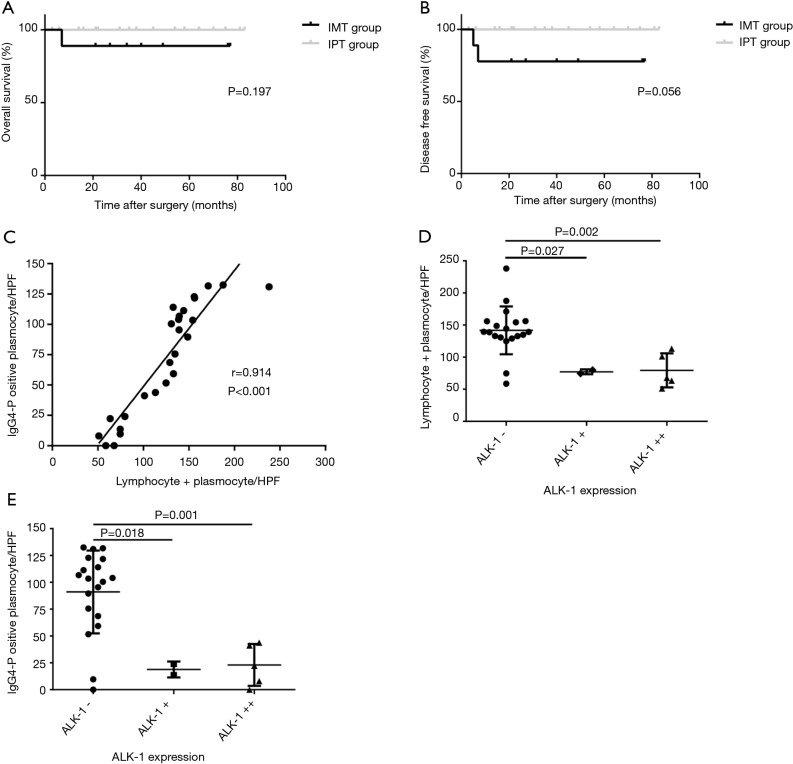
Despite both of patients with disease progression were in the IMT group based on the new diagnostic criteria, however there was no significant difference in the OS rate between the two groups (88.89% vs. 100%, P=0.197, Figure 4A), and only a threshold level was detected in disease-free survival rate (77.78% vs. 100%, P=0.056, Figure 4B).
Figure 4.
The results of statistical analysis based on the clinicopathological and immunohistochemical (IHC) findings. (A) Overall survival (OS) between the IMT and IgG4-related IPT group were found no statistically significant difference (P=0.197); (B) a borderline value was found in disease-free survival (DFS) between the two group (P=0.056); (C) Pearson correlation analysis showed that the lymphocytes + plasmocytes level had a possible linear positive correlation with the number of IgG4-positive plasma cells (r=0.914, P<0.001); (D) the significant differences were found in the average number of L + P between the ALK-negative and weakly or strongly positive lesions respectively (P=0.027 and P=0.002). And a negative correlation was found between the ALK-1 expression and L + P count as well (rs=−0.663, P<0.001); (E) similar results were also found between the ALK-1 expression and the number of IgG4-positive plasmocyte (P=0.018, P=0.001, rs=−0.632 and P=0.001). ALK-1 weakly focally positive reaction was represented by ALK-1 + in the graph.
Correlation analysis
In order to further investigate the relationships among these indexes and confirm the validity of new criteria, correlation analysis were performed. In the scatter plot (Figure 4C), a positive correlation trend was detected, indicating a possible link between the lymphocytes + plasma cells count and the IgG4-positive plasma cells count. Confirmed by Pearson correlation analysis, the lymphocytes + plasma cells level had a linear positive correlation with the number of IgG4-positive plasma cells (r=0.914, P<0.001). Figure 4D revealed the significant differences in the average number of L + P between the ALK-negative tumors and weakly or strongly positive lesions respectively. The average number of L + P in ALK-positive lesions, either weakly or strongly positive lesions, was only about half that of the ALK-negative tumors (77.2 vs. 141.7/HPF, P=0.027 and 79.4 vs. 141.7/HPF, P=0.002). A negative correlation was found between the ALK-1 expression and L + P count as well (rs=−0.663, P<0.001). In addition, the similar results were also found between the ALK-1 expression and the number of IgG4-positive plasma cell. On average, there are 91.0 IgG4-positive plasma cell per HPF in ALK-negative tumors, however only 18.9 and 23.0/HPF IgG4-positive plasma cells were found in weakly and strongly positive lesions, respectively (Figure 4E, P=0.018, P=0.001). And a significant negative correlation between the IgG4-positive plasma cell counts and the expressions of ALK-1 was confirmed by Spearman analysis (rs=−0.632, P=0.001).
Discussion
For a long time, IMT is widely recognized as a subgroup of the broad category of IPT which is a nonmalignant lesion and could be cured by resection or anti-inflammatory drugs. However, people have gradually realized that IMT should be regarded as a neoplasm of intermediate biologic potential since the tendency of local recurrence and distant metastasis. Despite the role of ALK-rearrangements in the pathogenesis is not quite clear yet, these chromosomal abnormalities provide evidence for the viewpoint that IMT is a true neoplasm rather than an inflammatory lesion. Compared with IMT, IPT looks more like an inflammatory disorder which is histologically characterized by an obvious infiltrative inflammation (10). Recent studies have reported increased IgG4-positive plasma cells in parts of the IPTs, especially in the plasma cell-rich lesions, indicating IgG4-positive plasma cells might be involved in the pathogenesis of pulmonary IPT. And the discoveries of chromosome rearrangement and IgG4-positive plasma cell infiltration not only support the concept that IMT and IgG4-related IPT represent two separated diseases with totally different etiologies and prognosis, but also provide a promising method to distinguish these two diseases.
IgG4-related disease is a relative newly recognized condition characterized by abundant IgG4-positive plasma cells infiltration (11-13). Despite some previous studies have tried to establish the diagnostic criteria, there is no consensus criteria for IgG4-related pulmonary IPT yet. It has been reported that the L + P count and the IgG4-positive plasma cells count were adopted as the diagnostic criteria for IgG4-related sclerosing pancreatitis (7,11,14). And we evaluated whether or not they could apply in differentiating the IgG4-related pulmonary IPTs and IMTs. In current study, a significantly linear positive correlation between the L + P level and IgG4-positive plasma cells count were confirmed by Pearson analysis, indicating a good consistency among these indicators. These findings may reflect the observation that the tumor tissues from IgG4-relative pulmonary IPTs show diffuse infiltrates of abundant inflammatory cells and IgG4-positive plasma cells, in contrast to the fewer and focal aggregate of inflammatory cells and IgG4-positive plasma cells in most IMTs. Hereby, we believe that the L + P count and the degree of IgG4-positive cell infiltration should be incorporated into current diagnostic criteria. In addition, the ratio of IgG4+/IgG+ plasma cells, ignoring the variances of the plasma cells, is a relatively stable and important indicator for the diagnosis of IgG4-related diseases as well (5).
In previous literatures, many cut-off values for diagnosis have been proposed ranging from 10 to more than 60 IgG4-positive plasma cells /HPF (6,9,14). The ratio of IgG4+/IgG+ plasma cell has ranged from 10% to 50% as well, although a ratio of 40% or 50% is currently favored as more supportive of the diagnosis. In this study, seeking to avoid the missed diagnosis for IMTs, IgG4-positive cells >60/HPF and IgG4+/IgG+plasma cells >50% were taken as a diagnostic criterion for IgG4-related IPTs. Besides, based on previous literatures and the discovery of the linear positive correlation between IgG4-positive plasma cells count and lymphocytes + plasma cells level, L + P count more than 120/HPF was proposed as a criterion for diagnosis. Although the IgG4-positive plasma cells count and the ratio of IgG4+/IgG+ plasma cell are generally lower in IMT group, overlap can occur. For that reason, making diagnosis only based on L + P level and IgG4-positive plasma cells count is not reliable enough. Therefore, combination with other histological results and expression of ALK-1 is quite necessary, especially for patients with contradictory results using different indicators (15).
Rearrangements involving the ALK gene locus on 2p23 have been reported in 50% to 70% IMTs according to the literatures (8,10,16-18). The rearrangements cause ALK protein overexpression and can be detected by immunohistochemistry and fluorescence in situ hybridization (FISH) (8). In current study, although it is not detected in all of IMTs, ALK-positive cases are closely associated with more aggressive behaviors in histology, such as higher cytological atypia (mean 3.7/HPF, P<0.001) and lesser lymphoid follicles (mean 1.2/HPF, P=0.021). Besides that, the negative correlations with L + P count and IgG4-positive plasma cells count, which were found for the first time, indicated that it’s unadvisable to confuse ALK-positive lesions with inflammatory process or benign IPTs. Combined with a pattern of more aggressive behavior, the chromosomal abnormality lead to the conception that IMT is a true neoplasm (16,19,20). Hereby, we believe that all ALK-positive patients should be classified in low-grade malignant IMTs regardless inflammatory infiltration. Certainly, ALK-negative tumors do not mean benign lesions since ALK-negative IMTs are not rare according to present evidences (8,21). Although Coffin et al. reported same results obtained in 89% of cases using these two different methods, FISH should be used to demonstrate the negative results of IHC, especially in patients without obvious inflammatory infiltration (16). Because of the tendency of recurrence and metastasis and lack of the effective and reliable identification method, we suggest that ALK-negative patients below the criteria of IPT ought to be considered as IMT. It must be emphasized that ALK-1 can only be served as an exclusion criterion of IPT rather than a diagnostic criterion of IMT since less than half of the IMT patients cannot be detected ALK-positive staining at present using IHC or FISH method. Previously, Coffin reported that all 6 metastatic IMTs were nonreactive for ALK-1 in their study. In current study, we did not detect any ALK1-positive tumor cell in the section of the dead patient as well. We suspect that metastasis may be more frequent in ALK1-negative IMTs. Thus all the suspected ALK1-negative cases should be paid more attention when treatment and follow-up. When suspicious stump-positivity, recurrence or metastasis is found, reoperation, local radiotherapy and (or) chemotherapy should be performed. Because recurrence and metastasis are usually identified within the first year following surgeries, recommended follow-up interval is every 3–6 months during the initial 1–2 years, and annually thereafter.
Additionally, we found some other histological features, such as cytological atypia, destroyed alveoli, lymphoid follicles and obstructive phlebitis, are quite different when comparing the two groups. Cell atypia, as one of the most important characteristics of malignant tumor, were included in our diagnostic criteria. As previously noted, IMT typically lacks the obstructive phlebitis and abundant lymphoid aggregates of IgG4-related diseases (6,9). Confirmed by our study, obstructive phlebitis and more lymphoid follicles (>3.0/HPF) only be found in IgG4-related IPTs. It suggested that obstructive phlebitis and more lymphoid follicles (>3.0/HPF) are potential auxiliary diagnostic indicators of IPTs, assisting in assessment of tumors close to the borderline value. Unfortunately, no specific histological characteristic is found. Even the obstructive phlebitis which was only detected in IgG4-related IPT group showed no significant difference from the IMTs (P=0.058). It means that distinguishing IMTs and IgG4-related IPTs only through histology is insufficient, above-mentioned three indicators (L + P count, IgG4-positive plasma cell count and expression of ALK-1) should be adopted as an essential part of the differential diagnosis. The histological features, such as destroyed alveoli, lymphoid follicles and obstructive phlebitis, can be considered as additional indicators, especially when we get a borderline value or contradictory result through different indicators.
Although a few standards have been proposed in previous literatures, as far as we know, this set of criteria for differential diagnosis described in our study is the first standard based on a relatively complete and long-term follow-up data. Previous works on differential diagnosis of IMTs and IPTs are mainly based on case reports or literature reviews and the conclusions are quite different. Although Saab and colleagues suggested that IgG4-positive plasma cells count and IgG4+/IgG+ plasma cell ratio were generally lower in IMTs than in IgG4-related diseases, no recommended borderline value for differential diagnosis was proposed (7). Bhagat and associates proposed a similar set of criteria, including IgG4-positive plasma cells count, the ratio of IgG4+/IgG+ plasma cells and ALK-1 expression, however, the small sample size of the study and incomplete follow-up data reduced the reliability of the result. In comparison with published reports, a larger sample size, a relative complete follow-up data and longer follow-up period make it possible to propose a promising set of differential diagnostic criteria. Our criteria seems to be reliable and stand up to practical test since both of the patients with disease progression in current study are divided into correct group. Moreover, the linear positive correlation between L + P count and IgG4-positive plasma cell count and the negative correlation between IgG4-positive plasma cells and ALK-1 expression found for the first time provide additional evidence for establishing the current diagnostic criteria.
Unfortunately, only a borderline value was found in the survival analysis of the new criteria. However, there are indeed significant differences between the two groups in histology. It may be explained by the limited sample sizes, a low recurrence or metastasis rate and the borderline values which still need revise. Establishing a clinical database and tumor tissue bank across the globe may help us to collect patients suffer from this rare tumor and obtain reliable statistical conclusions.
In summary, the present study provides evidence that the pulmonary IMT and the IgG4-related IPT are two separate diseases. Pulmonary IMT, at least partially caused by ALK gene rearrangement, is a low-grade malignant neoplasm, having a propensity for local recurrence and metastasis. When diagnosis and research, these easily confused diseases should be distinguished from each other carefully. In clinical practice, lymphocytes + plasma cells count >120/HPF, cytological atypia < 3/HPF and IgG4-positive plasma cells >60/HPF or IgG4/IgG+ plasma cells >50% help us to diagnose the IgG4-related IPT. In contrast, criteria for IMT are: L + P count <120/HPF; cytological atypia >3/HPF; and IgG4-positive plasma cells <60/HPF and IgG4+/IgG+ plasma cells <50%. Besides that, the expression of ALK-1 in spindle cells helps to distinguish IMTs from IgG4-related IPTs as well. Future researches based upon larger sample sizes are needed to revise our criteria and to explore the pathogenesis of these two different diseases.
Acknowledgements
We gratefully acknowledge the valuable cooperation of Dr. Weiya Wang and Dr. Min Feng (Department of Pathology, West China Hospital, Sichuan University) in providing technical guidance. We must express special thanks to Dr. Zongbing You (Tulane Cancer Center and Louisiana Cancer Research Consortium, Tulane University) for giving valuable suggestions that have helped to improve the quality of the manuscript.
Funding: This work was supported by the Key Science and Technology Program of Sichuan Province, China (Grant number 2014SZ0148 to Dr. Lunxu Liu).
Ethical Statement: The collection and use of human tissue samples were approved by the Institutional Review Board of West China Hospital, Sichuan University (NO. 2016-194). The procedures were in accordance with the Ethical Principles for Medical Research Involving Human Subjects as formulated in the World Medical Association Declaration of Helsinki (revised in 2008).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Travis WD, Brambilla E, Muller-Hermelink HK, et al. Pathology and Genetics of Tumours of the Lung, Pleura,Thymus and Heart. IARC Press, International Agency for Research on Cancer. 2004. [Google Scholar]
- 2.Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol 2007;31:509-20. 10.1097/01.pas.0000213393.57322.c7 [DOI] [PubMed] [Google Scholar]
- 3.Chavez C, Hoffman MA. Complete remission of ALK-negative plasma cell granuloma (inflammatory myofibroblastic tumor) of the lung induced by celecoxib: A case report and review of the literature. Oncol Lett 2013;5:1672-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griffin CA, Hawkins AL, Dvorak C, et al. Recurrent involvement of 2p23 in inflammatory myofibroblastic tumors. Cancer Res 1999;59:2776-80. [PubMed] [Google Scholar]
- 5.Zen Y, Kitagawa S, Minato H, et al. IgG4-positive plasma cells in inflammatory pseudotumor (plasma cell granuloma) of the lung. Hum Pathol 2005;36:710-7. 10.1016/j.humpath.2005.05.011 [DOI] [PubMed] [Google Scholar]
- 6.Bhagat P, Bal A, Das A, et al. Pulmonary inflammatory myofibroblastic tumor and IgG4-related inflammatory pseudotumor: a diagnostic dilemma. Virchows Arch 2013;463:743-7. 10.1007/s00428-013-1493-2 [DOI] [PubMed] [Google Scholar]
- 7.Saab ST, Hornick JL, Fletcher CD, et al. IgG4 plasma cells in inflammatory myofibroblastic tumor: inflammatory marker or pathogenic link? Mod Pathol 2011;24:606-12. 10.1038/modpathol.2010.226 [DOI] [PubMed] [Google Scholar]
- 8.Gleason BC, Hornick JL. Inflammatory myofibroblastic tumours: where are we now? J Clin Pathol 2008;61:428-37. 10.1136/jcp.2007.049387 [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto H, Yamaguchi H, Aishima S, et al. Inflammatory myofibroblastic tumor versus IgG4-related sclerosing disease and inflammatory pseudotumor: a comparative clinicopathologic study. Am J Surg Pathol 2009;33:1330-40. 10.1097/PAS.0b013e3181a5a207 [DOI] [PubMed] [Google Scholar]
- 10.Antonescu CR, Suurmeijer AJ, Zhang L, et al. Molecular characterization of inflammatory myofibroblastic tumors with frequent ALK and ROS1 gene fusions and rare novel RET rearrangement. Am J Surg Pathol 2015;39:957-67. 10.1097/PAS.0000000000000404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim KP, Kim MH, Song MH, et al. Autoimmune chronic pancreatitis. Am J Gastroenterol 2004;99:1605-16. 10.1111/j.1572-0241.2004.30336.x [DOI] [PubMed] [Google Scholar]
- 12.Kamisawa T, Funata N, Hayashi Y, et al. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol 2003;38:982-4. 10.1007/s00535-003-1175-y [DOI] [PubMed] [Google Scholar]
- 13.Kamisawa T, Okamoto A. Autoimmune pancreatitis: proposal of IgG4-related sclerosing disease. J Gastroenterol 2006;41:613-25. 10.1007/s00535-006-1862-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyabe K, Notohara K, Nakazawa T, et al. Comparison study of immunohistochemical staining for the diagnosis of type 1 autoimmune pancreatitis. J Gastroenterol 2015;50:455-66. 10.1007/s00535-014-0980-9 [DOI] [PubMed] [Google Scholar]
- 15.Kanagaraju V, Rai D, Alluri RV, et al. An inflammatory pseudotumor in the thoracic epidural space presenting with progressive paraplegia: a histopathological diagnosis with clinical and radiological uncertainty. Case report with literature review. Eur Spine J 2016;25 Suppl 1:75-9. 10.1007/s00586-015-4106-8 [DOI] [PubMed] [Google Scholar]
- 16.Coffin CM, Patel A, Perkins S, et al. ALK1 and p80 expression and chromosomal rearrangements involving 2p23 in inflammatory myofibroblastic tumor. Mod Pathol 2001;14:569-76. 10.1038/modpathol.3880352 [DOI] [PubMed] [Google Scholar]
- 17.Borak S, Siegal GP, Reddy V, et al. Metastatic inflammatory myofibroblastic tumor identified by EUS-FNA in mediastinal lymph nodes with ancillary FISH studies for ALK rearrangement. Diagn Cytopathol 2012;40 Suppl 2:E118-25. 10.1002/dc.21663 [DOI] [PubMed] [Google Scholar]
- 18.Sokai A, Enaka M, Sokai R, et al. Pulmonary inflammatory myofibroblastic tumor harboring EML4-ALK fusion gene. Jpn J Clin Oncol 2014;44:93-6. 10.1093/jjco/hyt173 [DOI] [PubMed] [Google Scholar]
- 19.Lawrence B, Perez-Atayde A, Hibbard MK, et al. TPM3-ALK and TPM4-ALK oncogenes in inflammatory myofibroblastic tumors. Am J Pathol 2000;157:377-84. 10.1016/S0002-9440(10)64550-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bridge JA, Kanamori M, Ma Z, et al. Fusion of the ALK gene to the clathrin heavy chain gene, CLTC, in inflammatory myofibroblastic tumor. Am J Pathol 2001;159:411-5. 10.1016/S0002-9440(10)61711-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panagiotopoulos N, Patrini D, Gvinianidze L, et al. Inflammatory myofibroblastic tumour of the lung: a reactive lesion or a true neoplasm? J Thorac Dis 2015;7:908-11. [DOI] [PMC free article] [PubMed] [Google Scholar]