Abstract
Background
The 8th edition of the tumor, node and metastasis (TNM) classification of lung cancer will be enacted in January 2017. The aim of this study was to analyze the survival differences among the three new categories of metastatic disease: intrathoracic metastasis (M1a), single extrathoracic metastasis (M1b) and multiple extrathoracic metastases (M1c) in our cohort of patients with non-small cell lung cancer (NSCLC).
Methods
This is a retrospective single-center study including NSCLC patients with metastatic disease at diagnosis. Patients were divided into three groups (M1a, M1b, M1c). Overall survival (OS) within and between these subgroups was calculated using the Kaplan-Meier method.
Results
A total of 288 patients were included (112 M1a, 28 M1b and 148 M1c). Median OS of M1c was significantly worse than M1a or M1b tumors (P<0.001). No significant differences were found among the M1a descriptors (pleural/pericardial nodules/effusion, bilateral tumor nodules or both descriptors) (P=0.722) and between M1a and M1b tumors (P=0.517). OS of patients with one metastasis in a single organ was not significantly different from OS of patients with two metastases in a single organ (P=0.180). Among M1c tumors, OS was significantly better in patients with multiple metastases in a single organ than in patients with multiple metastases in multiple organs (P=0.001).
Conclusions
Our results support the proposal to keep the M1a category unchanged in the 8th edition as well as the proposed restructuring of the M1b in the new M1b and M1c categories. However, our results raise questions about the definition of oligometastatic disease and, consequently, the criteria of M1b and M1c category.
Keywords: Non-small cell lung cancer (NSCLC), TNM classification, lung cancer staging, stage IV
Introduction
Lung cancer is the leading cause of cancer-related death worldwide (1).
Staging of lung cancer using tumor, node and metastasis (TNM) classification at the time of diagnosis is the most important predictor of survival (2). TNM classification is based on the anatomic tumor extent only and, by definition, it does not include other prognostic factors unrelated to the anatomy of the tumor (2).
Recently, the International Association for the Study of Lung Cancer (IASLC) published the proposals for the forthcoming 8th edition of the TNM classification of lung cancer (3).
One of the major changes occurred in the M component that was divided in three categories: M1a if there are lung metastases or pleural/pericardial malignant effusion or nodules; M1b if there is a single metastatic lesion in a single distant organ; M1c if there are multiple lesions in a single organ or multiple lesions in multiple organs. Furthermore, stage IV is divided into stage IVA, if the tumor is classified as M1a or M1b, and in stage IVB, if the tumor is M1c.
This new TNM edition will be enacted in January 2017 and, although it is based on a large international database, it is essential to ascertain its prognosis capacity in several institutions worldwide.
The main aim of our study was to analyze the overall survival (OS) differences among M1a, M1b and M1c in our cohort of patients with non-small cell lung cancer (NSCLC).
Methods
Patients selection
We conducted a retrospective study which included all patients diagnosed with NSCLC in our Oncologic Pulmonology Unit, between 2010 and 2014. For this study we selected patients with non-resected thoracic or extra thoracic metastasis and we excluded patients whose staging was not possible. All data were not included in the IASLC database.
Ethical statement
Given that no identifying patient information was collected for this study, no Institutional Review Board approval was required.
Evaluation of the M component
The T, the N and the M components of TNM classification were assessed by a multidisciplinary team to improve uniformity of criteria. In this study, we focused on the M component. Every metastasis location and the number of metastasis of each organ were recorded. The diagnostic procedure [computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET), bone scintigraphy or biopsy] was selected by the same multidisciplinary team and it was also recorded. The modality of treatment was chosen by the multidisciplinary team, but it was not taken into account in this study because the TNM classification is intended to predict outcome before any therapy, rather than to reflect treatment outcome (4). We divided the patients into three groups: M1a, M1b and M1c defined according to the 8th TNM edition. We analyzed the OS within and between these subgroups. We also evaluated the prognostic value of the location of the metastases. OS (months) was calculated from the date of diagnosis of lung cancer to the date of patient death or last date known to be alive.
Statistical analysis
Descriptive statistics of the variables of interest were expressed as absolute and relative frequencies or mean ± standard deviation. We compared patients’ characteristics among M1a, M1b and M1c groups using Chi-square and one-way ANOVA tests.
Survival analysis was estimated using Kaplan-Meier method, and differences in OS were evaluated using the log-rank test. The level of significance was set to 0.05.
The statistical analysis was carried out using SPSS 22.0 (IBM Corp., Armonk, NY, USA).
Results
A total of 288 patients with NSCLC and metastases at diagnosis were included in this study. Patient characteristics are presented in Table 1. Mean age was 66±11 years old. Most patients were current or former smokers. The most frequent histological type was adenocarcinoma (76.7%).
Table 1. Baseline patient and disease characteristics.
| Characteristics | n | % |
|---|---|---|
| Gender | ||
| Male | 214 | 74.3 |
| Female | 74 | 25.7 |
| Age (years) | ||
| ≤70 | 183 | 63.5 |
| >70 | 105 | 36.5 |
| Smoking status | ||
| Current smoker | 123 | 42.7 |
| Former smoker | 87 | 30.2 |
| Never smoker | 78 | 27.1 |
| Performance status | ||
| ECOG 0 | 41 | 14.2 |
| ECOG 1 | 163 | 56.6 |
| ECOG 2 | 63 | 21.9 |
| ECOG 3 | 15 | 5.2 |
| ECOG 4 | 6 | 2.1 |
| Histological type | ||
| Adenocarcinoma | 221 | 76.7 |
| Squamous | 35 | 12.2 |
| Other | 32 | 11.1 |
| M category—8th TMN edition | ||
| M1a | 112 | 38.9 |
| M1b | 28 | 9.7 |
| M1c, single organ | 37 | 12.8 |
| M1c, multiple organ | 111 | 38.5 |
ECOG, Eastern Cooperative Oncology Group.
According to the definitions of the 8th TNM edition, 112 (38.9%) tumors were classified as M1a, 28 (9.7%) as M1b and 148 (51.3%) as M1c. In the group of M1c, 37 patients had multiple metastases in a single organ and 111 patients had multiple metastases in multiple organs.
The characteristics of the patients and of the tumor at baseline were similar between groups and there were no statistically significant differences (Table 2).
Table 2. Characteristics of the patients, according to the M descriptor—univariate analysis.
| Characteristics | M1a | M1b | M1c | P |
|---|---|---|---|---|
| Gender, n (%) | 0.075 | |||
| Male | 75 (67.0) | 22 (78.6) | 117 (79.1) | |
| Female | 37 (33.0) | 6 (21.4) | 31 (20.9) | |
| Age (mean ± SD) | 67±12 | 64±10 | 65±11 | 0.258 |
| Smoking status, n (%) | 0.290 | |||
| Never smoker | 36 (32.1) | 6 (21.4) | 36 (24.3) | |
| Current/former smoker | 76 (67.9) | 22 (78.6) | 112 (75.7) | |
| Performance status, n (%) | 0.325 | |||
| ECOG 0–1 | 83 (74.1) | 21 (75.0) | 98 (66.2) | |
| ECOG 2–4 | 29 (25.9) | 7 (25.0) | 50 (33.8) | |
| Histological type, n (%) | 0.682 | |||
| Adenocarcinoma | 89 (79.5) | 21 (75.0) | 111 (75.0) | |
| Other | 23 (20.5) | 7 (25.0) | 37 (25.0) |
SD, standard deviation; ECOG, Eastern Cooperative Oncology Group.
The procedure used for metastases diagnosis is shown in Table 3. Pleural/pericardial nodules and effusions were histopathologically confirmed in 50 (72.5%) patients and bilateral lung nodules in 4 (6.1%). In 35 (53%) patients the diagnosis of bilateral lung nodules was based on PET results. Extra-thoracic metastases were histopathologically confirmed in 24 (13.6%) patients.
Table 3. Procedure for metastases diagnosis.
| Metastases | Histological confirmation (%) | PET-CT (%) | Others (CT, MRI, bone scintigraphy) (%) |
|---|---|---|---|
| Pleural/pericardial nodules or effusion | 73 | 6 | 21 |
| Bilateral lung nodules | 23 | 67 | 10 |
| Extra-thoracic metastases | 14 | 54 | 32 |
PET-CT, positron emission tomography computerized tomography; CT, computerized tomography; MRI, magnetic resonance imaging.
At completion of this study, after a mean follow up of 11 months (range from 1 to 72 months), 18 (6%) patients were still alive and 270 (94%) had died.
The anatomical extent and the prognosis of M1a tumors are shown in Table 4. The OS was not significantly different between patients with bilateral lung nodules, patients with pleural/pericardial nodules or effusions and patients with both M1a descriptors (P=0.722).
Table 4. Prognostic of M1a descriptors (N=112).
| Variable | N (%) | Median OS (months) | P value |
|---|---|---|---|
| Pleural/pericardial nodules or effusion | 46 (41.1) | 11 | 0.722 |
| Bilateral lung nodules | 43 (38.4) | 11 | |
| Both M1a descriptors | 23 (20.5) | 7 |
The location of metastases of M1b group is presented in Table 5. Isolated metastases were more frequent in bone, brain and adrenals. The OS did not differ significantly among the metastatic organs (bone, brain or adrenals; P=0.897) (Figure 1).
Table 5. Metastases location in the M1b subgroup.
| Metastases location | N | % |
|---|---|---|
| Bone | 11 | 39.3 |
| Brain | 7 | 25.0 |
| Adrenals | 6 | 21.4 |
| Liver | 2 | 7.1 |
| Skin | 1 | 3.6 |
| Colon | 1 | 3.6 |
Figure 1.
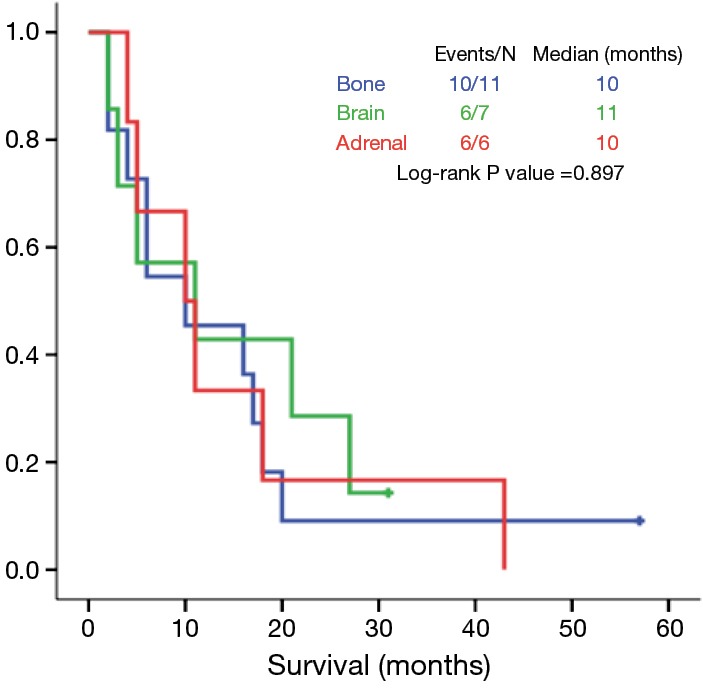
Isolated metastasis in a single organ: overall survival according to the location of metastasis.
Multiple metastases of M1c group were more frequent in bone, brain, adrenals and liver (Table 6). Among patients with multiple metastases in a single organ, patients with brain metastases had longer OS that patients with multiple bone metastases, but without statistical significance (11 vs. 4 months, P=0.445) (Figure 2).
Table 6. Metastases location in the M1c subgroup.
| Metastases location | M1c, single organ (N=37), n (%) | M1c, multiple organ (N=111), n (%) |
|---|---|---|
| Bone | 20 (54.1) | 73 (65.8) |
| Brain | 13 (35.1) | 32 (28.8) |
| Liver | 3 (8.1) | 35 (31.5) |
| Adrenals | 1 (2.7) | 48 (43.2) |
| Muscle | – | 10 (9.0) |
| Kidney | – | 8 (7.2) |
| Skin | – | 7 (6.3) |
| Spleen | – | 4 (3.6) |
| Thyroid | – | 1 (0.9) |
Figure 2.
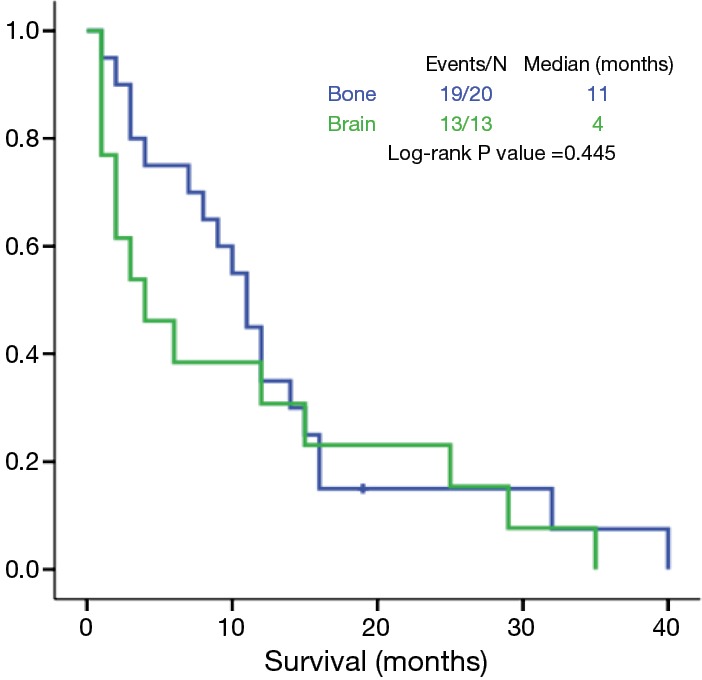
Multiple metastases in a single organ: overall survival according to the location of metastasis.
Median OS of patients with M1a, M1b and M1c tumors was 11, 10 and 4 months, respectively (P<0.001) (Figure 3).
Figure 3.
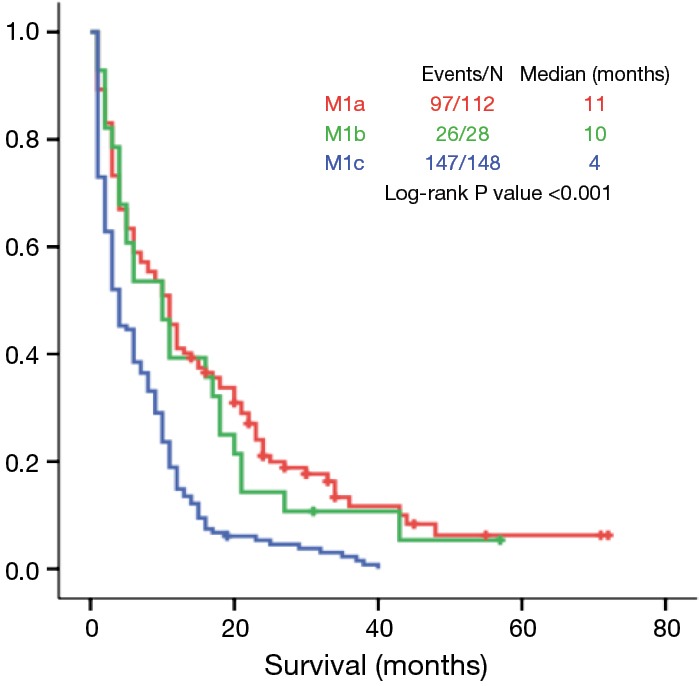
Overall survival according to different M1 categories.
The OS of M1a and M1b subgroups was not significantly different (P=0.517). OS of patients with a single metastasis in a single organ and the OS of patients with two lesions in a single organ were not significantly different (10 vs. 7 months, P=0.180) (Figure 4).
Figure 4.

Overall survival of patients with a single metastasis versus patients with two metastases in a single organ.
Regarding the analysis of the M1c group, there were significant differences between patients with multiple lesions in a single organ and those with multiple lesions in multiple organs (9 vs. 3 months, P=0.001) (Figure 5). However, the OS of patients with multiple lesions in a single organ and of patients with a single lesion in a single organ was not statistically different (9 vs. 10 months, P=0.297) (Figure 6).
Figure 5.
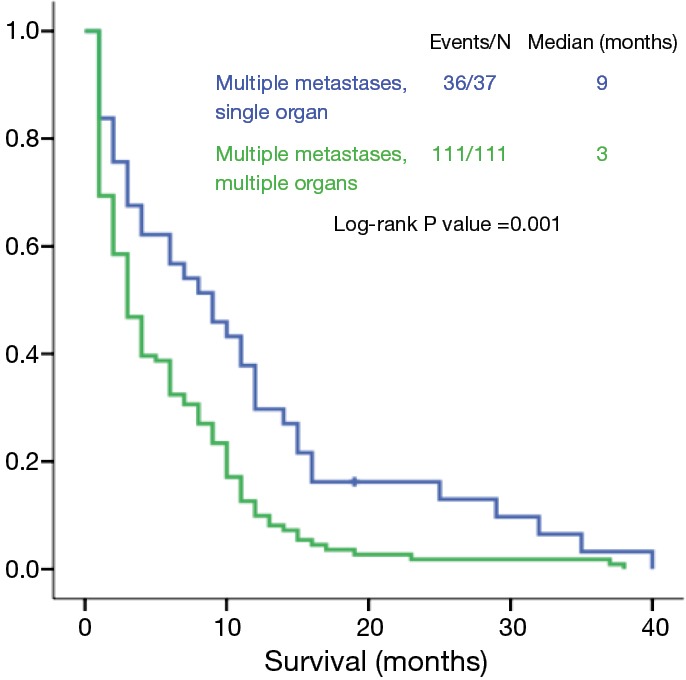
Overall survival of patients with M1c tumors: multiple metastases in a single organ versus in multiple organs.
Figure 6.
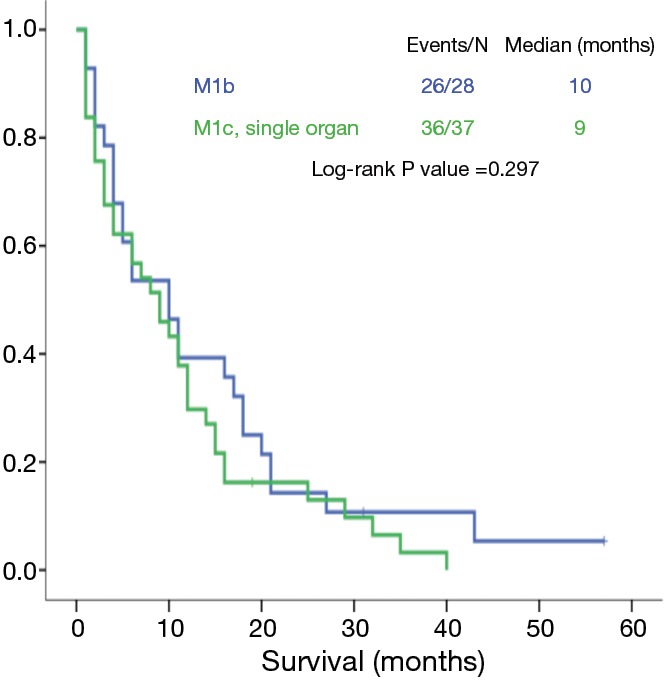
Overall survival of patients with M1b tumors versus patients with multiple metastases in a single organ.
Discussion
One of the main changes of the forthcoming 8th edition of the TNM classification of lung cancer was in the M component (5). External validation of these proposals was performed against the North American Surveillance, Epidemiology, and End Results Registries (SEER) database (3). The current study evaluated the M component according to the 8th TNM edition, using the registry of a European Oncologic Pulmonology Unit.
Regarding the M1a category, we found no significant survival differences between patients with bilateral lung nodules, patients with pleural/pericardial nodules or effusion and patients with both of these anatomic tumor characteristics. In some series, pleural and pericardial dissemination was associated with a worse prognosis compared to bilateral lung nodules (5,6). However, in those series most cases of bilateral lung nodules were not histopathologically confirmed and therefore could be benign. Likewise, in our study, only 73% of pleural/pericardial nodules/effusions and 23% of cases with bilateral nodules were histopathologically confirmed but more than half bilateral nodules were PET positive, unlike the other series. Several studies showed that PET had better sensitivity, specificity and accuracy than classic imaging in cancer staging (7). Consequently, the degree of certainty about the malignant nature of bilateral lung nodules in our study is higher. This can explain the similarity in OS between patients with pleural/pericardial dissemination and patients with bilateral nodules. In conclusion, our results support the use of M1a category for patients with any or both of these descriptors.
For the first time, the eighth TNM edition recognizes the oligometastatic disease and assigns it to a separate category—M1b. This category was proposed to be used in patients with a single lesion in a single organ. However, there is not yet a unique definition of oligometastatic disease worldwide. The European Society for Medical Oncology states that oligometastases are defined as at maximum five metastatic lesions in the body (8). However, some studies found that patients with 1 or 2 lesions had significantly better survival than those with 3 to 5 metastatic lesions (9). In our study, the OS of patients with two lesions in a single organ was not statistically inferior to OS of patients with a single lesion in a single organ (P=0.180). This result may be due to a small number of cases. Nevertheless, if no statistical difference is confirmed in larger studies, patients with at maximum two lesions in a single organ should be considered for inclusion in the M1b category. In our study the prognosis of a single metastatic lesion was not significantly different depending on the affected organ. The prognostic value of the location of oligometastases in previous studies was not unanimous. The systematic review of Ashworth et al. suggested that the presence of adrenal metastases tends to confer a poorer OS (10). Griffioen et al. found that brain oligometastases are associated with a more favorable outcome (11). Sheu et al. reported that no disease site was found to predict worse OS (12). These different results can be explained by the disparities in study design, in the population characteristics and in the treatment of choice of oligometastases. Prospective, larger studies are needed to clarify this subject.
Regarding the M1c category, our study found that the prognosis of its subgroups (single organ vs. multiple organs) was significantly different. Patients with multiple metastases in a single organ had significantly better OS than patients with multiple metastases in multiple organs (9 vs. 3 months, P=0.001) and an OS similar to M1b patients. One of the possible explanations for this result is that in our study 70% of patients with multiple metastases in a single organ had 5 or less metastases. According to some authors, this could be considered oligometastases and could be associated to a better prognosis (8,9). This finding of our study raises the question whether patients with few metastases in a single organ should be included in M1c category.
The 8th TNM edition divides the stage IV into two: stage IVA which includes M1a and M1b and stage IVB which includes all M1c tumors. Our study supports that M1a and M1b should be staged in the same category, as there are no significant differences in their OS. However, as we reported above, the stage IVB included patients with significant different OS. Nevertheless, in our opinion, although the division of stage IVB into two would probably improve the prognostic accuracy, it would not add significant impact to clinical practice and to treatment choice.
Limitations
We recognize the following limitations in our study: first, it is a retrospective study. Although the bias is reduced as there was no missing data and the staging was done by a multidisciplinary team, some discrepancies between our results and those of the forthcoming TNM classification might be attributed to the nature of our study. Second, it is a single-institute study with a limited number of patients and therefore it cannot outweigh the results of the IASLC database. Furthermore, there was a disproportion of patients between some subgroups, especially a few number of M1b cases, which requires a careful interpretation of the results. Finally, in most cases, distant metastases were not biopsied and the assumption of a malignant nature of lesions was based in imaging procedures (PET, bone scintigraphy, CT or MRI). Therefore, false positive cases were not absolutely ruled out.
In conclusion, this study contributes to ascertain the M component of the 8th edition of the TNM classification of lung cancer. Our results support the proposal to keep the M1a category unchanged in the 8th edition as well as the proposed restructuring of the M1b in the new M1b and M1c categories. However, our results raise questions about the number of metastases that should define the “oligometastatic disease”, and consequently the M1b and M1c category.
Acknowledgements
None.
Ethical Statement: Given that no identifying patient information was collected for this study, no Institutional Review Board approval was required.
Footnotes
Conflicts of Interest: Dr. Barroso reports personal fees from AstraZeneca, Boehringer Ingelheim, Lda, Eli Lilly and Company, Pierre Fabre Portugal, Roche Farmacêutica Química, Lda, Merck and Bristol-Myers Squibb, outside the submitted work. Dr. Antunes has received consulting fees from Roche Farmacêutica Química, Lda. The other authors have no conflicts of interest to declare.
References
- 1.Alberg AJ, Brock MV, Ford JG, et al. Epidemiology of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e1S-29S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rami-Porta R, Asamura H, Goldstraw P. Predicting the prognosis of lung cancer: the evolution of tumor, node and metastasis in the molecular age-challenges and opportunities. Transl Lung Cancer Res 2015;4:415-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. 10.1016/j.jtho.2015.09.009 [DOI] [PubMed] [Google Scholar]
- 4.Sánchez de Cos Escuín J, Abal Arca J, Melchor Íñiguez R, et al. Tumor, node and metastasis classification of lung cancer--M1a versus M1b--analysis of M descriptors and other prognostic factors. Lung Cancer 2014;84:182-9. 10.1016/j.lungcan.2014.02.006 [DOI] [PubMed] [Google Scholar]
- 5.Eberhardt WE, Mitchell A, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revision of the M Descriptors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2015;10:1515-22. [DOI] [PubMed] [Google Scholar]
- 6.Ou SH, Zell JA. Validation study of the proposed IASLC staging revisions of the T4 and M non-small cell lung cancer descriptors using data from 23,583 patients in the California Cancer Registry. J Thorac Oncol 2008;3:216-27. 10.1097/JTO.0b013e318164545d [DOI] [PubMed] [Google Scholar]
- 7.Schrevens L, Lorent N, Dooms C, et al. The role of PET scan in diagnosis, staging, and management of non-small cell lung cancer. Oncologist 2004;9:633-43. 10.1634/theoncologist.9-6-633 [DOI] [PubMed] [Google Scholar]
- 8.Reck M, Popat S, Reinmuth N, et al. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25 Suppl 3:iii27-39. 10.1093/annonc/mdu199 [DOI] [PubMed] [Google Scholar]
- 9.Salama JK, Chmura SJ, Mehta N, et al. An initial report of a radiation dose-escalation trial in patients with one to five sites of metastatic disease. Clin Cancer Res 2008;14:5255-9. 10.1158/1078-0432.CCR-08-0358 [DOI] [PubMed] [Google Scholar]
- 10.Ashworth A, Rodrigues G, Boldt G, et al. Is there an oligometastatic state in non-small cell lung cancer? A systematic review of the literature. Lung Cancer 2013;82:197-203. 10.1016/j.lungcan.2013.07.026 [DOI] [PubMed] [Google Scholar]
- 11.Griffioen GH, Toguri D, Dahele M, et al. Radical treatment of synchronous oligometastatic non-small cell lung carcinoma (NSCLC): patient outcomes and prognostic factors. Lung Cancer 2013;82:95-102. 10.1016/j.lungcan.2013.07.023 [DOI] [PubMed] [Google Scholar]
- 12.Sheu T, Heymach JV, Swisher SG, et al. Propensity score-matched analysis of comprehensive local therapy for oligometastatic non-small cell lung cancer that did not progress after front-line chemotherapy. Int J Radiat Oncol Biol Phys 2014;90:850-7. 10.1016/j.ijrobp.2014.07.012 [DOI] [PubMed] [Google Scholar]


