Abstract
Background
In the eighth TNM staging system proposal, lung cancer with part or complete obstructive pneumonitis/atelectasis was classified to T2 category, and dividing lines of T category were changed. We conducted this study to search prognostic effect of preoperative obstructive pneumonitis/atelectasis and its comparison with tumor size.
Methods
We collected clinical characteristics, preoperative hematological indicators, follow-up information of 1,313 lung cancer patients. Chi-square test was used to search relationship between obstruction pneumonitis/atelectasis and other factors. Kaplan-Meier (K-M) curves and cox regression methods were used for survival analysis.
Results
Preoperative obstructive pneumonitis/atelectasis indicated shorter OS (HR: 1.308; 95% CI: 1.058–1.619) and RFS (HR: 1.276; 95% CI: 1.032–1.579) as an independent factor. In comparison with tumor size, we found patients with obstructive pneumonitis/atelectasis and T1 size tumor had similar prognosis to those with T2 size but without obstructive pneumonitis/atelectasis, and OS, RFS of patients with obstructive pneumonitis/atelectasis and T2 size were significantly shorter than those with T2 tumor size but without obstructive pneumonitis/atelectasis, while similar to patients with T3 tumor size but without obstructive pneumonitis/atelectasis according to division by the eighth edition. We also found obstructive pneumonitis/atelectasis was significantly related to higher neutrophil (P<0.001), platelet (P<0.001), monocyte (P<0.001), NLR (P<0.001), PLR (P=0.002), ESR (P<0.001) and lower LMR (P<0.001).
Conclusions
Preoperative obstructive pneumonitis/atelectasis predicted poor survival independently in non-small cell lung cancer (NSCLC). And we suggested which T staging group the patients with obstructive pneumonitis/atelectasis would be divided to should depend on tumor size in the eighth TNM staging system.
Keywords: Non-small cell lung cancer (NSCLC), obstructive pneumonitis/atelectasis, tumor size, prognosis
Introduction
Lung cancer is the first leading cause among cancer-related death worldwide (1). Surgery is a curative strategy, but most lung cancer patients lose opportunity of operation because lung cancer is hard to be discovered at early stage. And as cancer cells can easily transfer to blood and lymph nodes causing metastasis, the prognosis for lung cancer is unsatisfied yet, with five-year survival rates 18.2% for non-small cell lung cancer (NSCLC) (2).
Obstructive pneumonitis and atelectasis are common complications for lung cancer patients before treatment, and most are discovered while initial diagnosis. They formed due to the blockage of tracheal bronchus by cancer tissue partially or completely, which will easily cause repeated infection of the same position or lung tissue shrink. In recent years, many studies have proved that systemic inflammation and immunology played important roles in development and progression of various cancers. Inflammatory cells interacted with cell matrix to make up tumor microenvironment, which could influence the occurrence and development of neoplasm (3,4). Several hematological markers, which could reflect the status of host inflammation, immunity, and hemostasis, have been reported to have prognostic utility in many cancers (5), such as C-reactive protein (CRP), neutrophils, platelets, lymphocytes, Glasgow prognostic score, prognostic nutrition index (PNI), neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR) and lymphocyte to monocyte ratio (LMR) (6-12). So we assumed that presence of obstructive pneumonitis or atelectasis might be associated with these inflammation indicators and predicted poor survival.
TNM staging system for lung cancer plays a critical role in determining disease degree, making clinical decisions or predicting prognosis (13). T category, which is mainly divided by tumor size, is also related to some other non-size-based factors including obstructive pneumonitis or atelectasis. In September 2015, the eighth TNM staging system proposal was published. There were some changes comparing with the seventh edition that was applied in 2009. One was that lung cancer patients associated with atelectasis or obstructive pneumonitis which extended to the hilar region either partly or completely were included in T2 category (14). Another was about the division of T category by tumor size, which became more detailed. This change emphasized the importance of tumor size for prognosis. We found that the division of T2 and T3 became 5 cm instead of 7 cm in the eighth TNM staging proposal. In other words, the prognostic value of obstructive pneumonitis/atelectasis before surgery should be similar to tumor size between 3 and 5 cm in the eighth edition. In order to search predictive effects of preoperative obstructive pneumonitis/atelectasis and the relationship between obstructive pneumonitis/atelectasis and tumor size, we conducted a retrospective for lung cancer patients receiving surgery in Shandong Provincial Hospital affiliated to Shandong University.
Methods
Setting and patient selection
We performed a retrospective study about patients who were diagnosed with lung cancer and received surgical treatment between 2006 and 2011 in Shandong Provincial Hospital. Patients would be included if they met the following criteria: (I) diagnosed with NSCLC pathologically; (II) receiving tumor resection; (III) having Computed tomography reports, X-ray reports of chest, bronchofiberscope test results or other evidence to classify patients into different groups (presence of obstructive pneumonitis/atelectasis or not); (IV) having complete serum indicators about inflammation except ESR (erythrocyte sedimentation rate) before surgery; (V) having complete follow-up data; (VI) not accompanied with other cancers. Patients who were undergoing non-cancer related inflammation or did not meet the criteria would be excluded.
Clinical and follow-up data collection
We collected following clinical characteristics about patients: age, gender, pathological TNM stage, histology, tumor location, tumor size, tumor differentiation degree, presence of obstructive pneumonitis/atelectasis, neutrophil count, lymphocyte count, platelet count, monocyte count, ESR, overall survival (OS), recurrence-free survival (RFS). And we calculated the ratio values such as NLR, PLR, LMR. Computed tomography reports and bronchofiberscope test results before surgery were used to confirm the diagnosis of obstructive pneumonitis/atelectasis.
OS referred to the time from date of surgery to death for any cause. If the patient was alive or out of touch, the endpoint of OS was the date of last follow-up. RFS was calculated from the date of surgery to recurrence. If there was no recurrence, the endpoint was the date of death or last follow-up.
Statistical analysis
There were both numerical and categorical variables in description of clinicopathological characteristics. For further analysis, we changed the former into dichotomous variables. And the cut-off value was determined by receiver operating characteristic (ROC) curve. To search the correlation between obstructive pneumonitis/atelectasis and other clinicopathological variables, we used chi-square test. When conducting survival analysis, we performed Kaplan-Meier (K-M) analysis to test if presence of obstructive pneumonitis/atelectasis before surgery was significant for prognosis, and univariate and multivariate cox regression methods were also used to explore significant markers for survival. All statistical calculations were performed by SPSS (version 20.0) software (Inc., Chicago, IL, USA), and a two-sided P≤0.05 was considered to be significant.
Ethic statement
The study was approved by Ethics Committee of Shandong Provincial Hospital in China (No. 356).
Results
Characteristics of patients
After screening, 1,177 NSCLC patients containing 342 (29.1%) females and 835 (70.9%) males were included in our study finally. The mean age of those patients was 58.5 ranging from 20 to 83, and there were 859 (73.0%) patients ≤65 years old and 318 (27.0%) patients >65 years old. Four hundred and twenty (35.7%) patients were diagnosed for accompanying with obstructive pneumonitis/atelectasis. The incidence of this complication might differ from region to region. There were 209 (17.8%) patients out of touch during our follow-up, and the mean survival were 44.4 months for OS and 39.1 months for RFS of all patients. Six hundred and twenty nine patients were diagnosed of lung adenocarcinoma, while 470 patients were considered as lung squamous cancer. As for TNM stage according to the 7th edition, there were 838 patients at I/II stage, and others were at III stage.
Survival analysis of obstructive pneumonitis/atelectasis
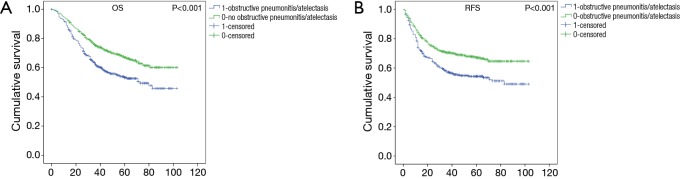
The K-M curves (Figure 1) showed that there was significant difference between the two groups (having obstructive pneumonitis/atelectasis or not) for OS (P<0.001) and RFS (P<0.001). And as seen in Table 1, presence of obstructive pneumonitis/atelectasis before surgery suggested poor OS (HR: 1.308; 95% CI: 1.058–1.619) and RFS (HR: 1.276; 95% CI: 1.032–1.579) as an independent factor.
Figure 1.
Kaplan-Meier survival curves for overall survival (OS) and recurrence-free survival (RFS) between groups of having and not having obstructive pneumonitis/atelectasis patients.
Table 1. Univariate and multivariate analysis of prognostic factors for OS and RFS.
| Characteristics | OS | RFS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | ||||||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | ||||
| Age | |||||||||||
| ≤ge | Reference | Reference | Reference | Reference | |||||||
| >65 | 1.477 (1.207–1.807) | <0.001 | 1.823 (1.480–2.245) | <0.001 | 1.477 (1.207–1.807) | <0.001 | 1.802 (1.464–2.219) | <0.001 | |||
| Gender | |||||||||||
| Female | Reference | Reference | Reference | Reference | |||||||
| Male | 1.410 (1.127–1.763) | 0.003 | 1.063 (0.782–1.443) | 0.697 | 1.407 (1.125–1.760) | 0.003 | 1.047 (0.773–1.420) | 0.766 | |||
| Histological subtype | |||||||||||
| Adenocarcinoma | Reference | Reference | Reference | Reference | |||||||
| Squamous carcinoma | 1.277 (1.047–1.558) | 0.016 | 0.900 (0.704–1.136) | 0.359 | 1.280 (1.049–1.561) | 0.015 | 0.885 (0.697–1.124) | 0.316 | |||
| Others | 1.252 (0.858–1.827) | 0.243 | 1.699 (1.037–2.783) | 0.035 | 1.285 (0.881–1.875) | 0.193 | 1.843 (1.113–3.053) | 0.018 | |||
| Smoking index | |||||||||||
| ≤100 | Reference | Reference | Reference | Reference | |||||||
| >100 | 1.513 (1.232–1.859) | <0.001 | 1.192 (0.895–1.601) | 0.225 | 1.524 (1.241–1.873) | <0.001 | 1.207 (0.905–1.609) | 0.200 | |||
| Obstructive pneumonitis/atelectasis | |||||||||||
| No | Reference | Reference | Reference | Reference | |||||||
| Yes | 1.605 (1.324–1.946) | <0.001 | 1.308 (1.058–1.619) | 0.013 | 1.560 (1.287–1.890) | <0.001 | 1.276 (1.032–1.579) | 0.025 | |||
| Pathological TNM stage | |||||||||||
| I/II | Reference | Reference | Reference | Reference | |||||||
| III | 3.152 (2.600–3.821) | <0.001 | 3.115 (2.546–3.811) | <0.001 | 3.032 (2.502–3.675) | <0.001 | 2.975 (2.434–3.637) | <0.001 | |||
| Differentiation degree | |||||||||||
| Well | Reference | Reference | Reference | Reference | |||||||
| Moderate | 3.280 (2.055–5.236) | <0.001 | 2.397 (1.481–3.879) | <0.001 | 3.533 (2.213–5.639) | <0.001 | 2.625 (1.622–4.248) | <0.001 | |||
| Poor | 3.807 (2.358–6.145) | <0.001 | 2.503 (1.532–4.089) | <0.001 | 3.991 (2.472–6.443) | <0.001 | 2.655 (1.625–4.340) | <0.001 | |||
P≤0.05 was considered to be significant. OS, overall survival; RFS, recurrence-free survival; HR, hazard ratio; CI, confidence interval.
Results of subgroup analysis were listed in Table 2. NSCLC patients were stratified into various groups by age, gender, stage, histological subtype and differential degree. Results showed that presence of obstructive pneumonitis/atelectasis was associated with poorer prognosis significantly for patients in younger group (OS: HR =1.361, 95% CI: 1.045–1.772, P=0.022; RFS: HR =1.310, 95% CI: 1.004–1.708, P=0.047), female group (OS: HR =1.651, 95% CI: 1.058–2.576, P=0.027; RFS: HR =1.656, 95% CI: 1.065–2.573, P=0.025), I/II stage group (OS: HR =1.520, 95% CI: 1.127–2.049, P=0.006; RFS: HR =1.438, 95% CI: 1.066–1.939, P=0.017), adenocarcinoma group (OS: HR =1.458, 95% CI: 1.061–2.003, P=0.020; RFS: HR =1.431, 95% CI: 1.041–1.967, P=0.027), well (OS: HR =4.719, 95% CI: 1.113–20.010, P=0.035; RFS: HR =4.750, 95% CI: 1.191–18.949, P=0.027), moderately differential degree groups (OS: HR =1.337, 95% CI: 1.000–1.788, P=0.04; RFS: HR =1.338, 95% CI: 1.002–1.788, P=0.049) and N0 stage group (OS: HR =1.511, 95% CI: 1.026–2.2248, P=0.037; RFS: HR =1.630, 95% CI: 1.155–2.300, P=0.005).
Table 2. Subgroup analysis of prognostic effect of obstructive pneumonitis/atelectasis for OS and RFS.
| Characteristics | Number of patients | OS | RFS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |||||
| Age | ||||||||||||
| ≤ge | 859 | 1.786 (1.412–2.258) | <0.001 | 1.361 (1.045–1.772) | 0.022 | 1.733 (1.370–2.191) | <0.001 | 1.310 (1.004–1.708) | 0.047 | |||
| >65 | 318 | 1.376 (0.977–1.938) | 0.068 | 1.184 (0.813–1.725) | 0.379 | 1.351 (0.960–1.903) | 0.085 | 1.196 (0.823–1.737) | 0.348 | |||
| Gender | ||||||||||||
| Female | 342 | 1.924 (1.269–2.917) | 0.002 | 1.651 (1.058–2.576) | 0.027 | 1.951 (1.287–2.958) | 0.002 | 1.656 (1.065–2.573) | 0.025 | |||
| Male | 835 | 1.450 (1.164–1.805) | 0.001 | 1.219 (0.956–1.555) | 0.110 | 1.393 (1.199–1.734) | 0.003 | 1.176 (0.922–1.500) | 0.191 | |||
| Stage | ||||||||||||
| I/II | 838 | 1.560 (1.189–2.046) | 0.001 | 1.520 (1.127–2.049) | 0.006 | 1.513 (1.154–1.985) | 0.003 | 1.438 (1.066–1.939) | 0.017 | |||
| III | 339 | 1.196 (0.908–1.575) | 0.203 | 1.150 (0.849–1.559) | 0.367 | 1.175 (0.892–1.548) | 0.251 | 1.171 (0.864–1.588) | 0.309 | |||
| Histological subtype | ||||||||||||
| Squamous carcinoma | 470 | 1.379 (1.023–1.858) | 0.035 | 1.233 (0.908–1.675) | 0.180 | 1.311 (0.973–1.767) | 0.076 | 1.194 (0.879–1.623) | 0.256 | |||
| Adenocarcinoma | 629 | 1.991 (1.461–2.713) | <0.001 | 1.458 (1.061–2.003) | 0.020 | 1.972 (1.448–2.688) | <0.001 | 1.431 (1.041–1.967) | 0.027 | |||
| Differentiation degree | ||||||||||||
| Well | 147 | 3.671 (1.306–10.318) | 0.014 | 4.719 (1.113–20.010) | 0.035 | 3.697 (1.315–10.389) | 0.013 | 4.750 (1.191–18.949) | 0.027 | |||
| Moderate | 595 | 1.509 (1.168–1.950) | 0.002 | 1.337 (1.000–1.788) | 0.04 | 1.455 (1.127–1.880) | 0.004 | 1.338 (1.002–1.788) | 0.049 | |||
| Poor | 341 | 1.290 (0.925–1.798) | 0.134 | 1.254 (0.878–1.791) | 0.213 | 1.216 (0.872–1.695) | 0.249 | 1.164 (0.815–1.664) | 0.403 | |||
| N stage | ||||||||||||
| N0 | 656 | 1.631 (1.155–2.302) | 0.005 | 1.511 (1.026–2.224) | 0.037 | 1.486 (1.009–2.187) | 0.045 | 1.630 (1.155–2.300) | 0.005 | |||
| N1 | 244 | 0.913 (0.629–1.326) | 0.633 | 0.953 (0.624–1.457) | 0.825 | 0.805 (0.555–1.168) | 0.253 | 0.825 (0.537–1.268) | 0.381 | |||
| N2 | 266 | 1.414 (1.040–1.922) | 0.027 | 1.306 (0.933–1.829) | 0.120 | 1.443 (1.061–1.962) | 0.019 | 1.426 (1.021–1.991) | 0.038 | |||
P≤0.05 was considered to be significant. OS, overall survival; RFS, recurrence-free survival; HR, hazard ratio; CI, confidence interval.
Univariate and multivariate analysis of prognostic factors
Firstly, we used univariate analysis to search significant factors for OS and RFS. Then age, gender, histological subtype, smoking index, presence of obstructive pneumonitis/atelectasis, pathological TNM stage and differential degree met the criteria to be included in multivariate analysis. As presented in Table 1, age, presence of obstructive pneumonitis/atelectasis, pathological TNM stage and differential degree were confirmed to be independent prognostic indicators for NSCLC patients.
Comparison about prognostic effects between obstructive pneumonitis/atelectasis and tumor size
According to the seventh edition of TNM staging system, we selected four groups of patients: (I) patients with preoperative obstructive pneumonitis/atelectasis and tumor size ≤3 cm (T1 tumor size in 7th edition with obstructive pneumonitis/atelectasis, T1O7); (II) patients having obstructive pneumonitis/atelectasis and tumor size between 3 and 7 cm before surgery (T2 tumor size in 7th edition with obstructive pneumonitis/atelectasis, T2O7); (III) patients without preoperative obstructive pneumonitis/atelectasis and tumor size ≤3 cm (T1 tumor size in 7th edition without obstructive pneumonitis/atelectasis, T1NO7); (IV) patients without preoperative obstructive pneumonitis/atelectasis and tumor size between 3 and 7 cm (T2 tumor size in 7th edition without obstructive pneumonitis/atelectasis, T2NO7). Figure 2 showed K-M curves of T1O7 and T2O7 groups comparing with the other two groups for OS and RFS respectively, and there was no statistically significant difference between T1O7 group and T2NO7 group for OS and RFS (OS: P=0.709; RFS: P=0.726). The result was same for T2O7 group and T2NO7 group (OS: P=0.194; RFS: P=0.347). The curves also revealed that patients in T1O7 group had negative prognosis comparing with those in T1NO7 group (OS: P<0.001; RFS: P<0.001).
Figure 2.
Kaplan-Meier survival curves of obstructive pneumonitis/atelectasis and tumor size according to the seventh TNM staging system for overall survival (OS) and recurrence-free survival (RFS). (A,B) OS and RFS curves for patients in T1O7, T1NO7 and T2NO7 groups; (C,D) OS and RFS curves for patients in T2O7, T1NO7 and T2NO7 groups.
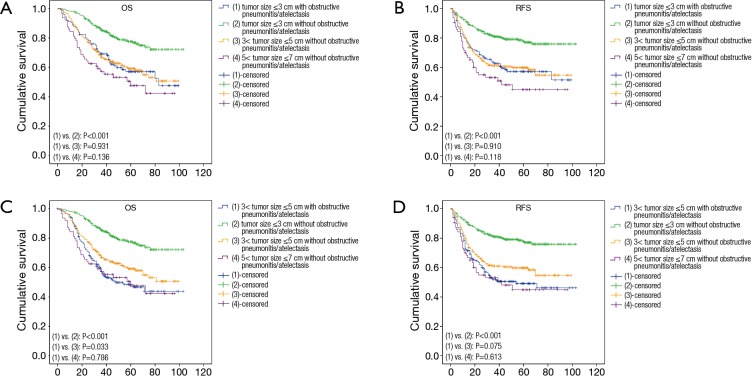
And according to the eighth edition, we selected five groups of patients: (I) patients with preoperative obstructive pneumonitis/atelectasis and tumor size ≤3 cm (T1 size in 8th edition with obstructive pneumonitis/atelectasis, T1O8); (II) patients having obstructive pneumonitis/atelectasis and tumor size between 3 and 5 cm before surgery (T2 size in 8th edition with obstructive pneumonitis/atelectasis, T2O8); (III) patients without preoperative obstructive pneumonitis/atelectasis and tumor size ≤3 cm (T1 size in 8th edition without obstructive pneumonitis/atelectasis, T1NO8); (IV) patients without preoperative obstructive pneumonitis/atelectasis and tumor size between 3 and 5 cm (T2 size in 8th edition without obstructive pneumonitis/atelectasis, T2NO8); (V) patients without preoperative obstructive pneumonitis/atelectasis and tumor size between 5 and 7 cm (T3 size in 8th edition without obstructive pneumonitis/atelectasis, T3NO8). Figure 3 showed K-M curves of T1O8 and T2O8 groups comparing with the other three groups for OS and RFS respectively, and there was no statistically significant difference about prognosis of patients in T1O8 group and those in T2NO8 and T3NO8 groups (for the T2NO8 group OS: P=0.931; RFS: P=0.910; for T3NO8 group OS: P=0.136; RFS: P=0.118). But for patients in T2O8 group, their OS was significantly shorter than those in T2NO8 group (P=0.033), but similar with patients in T3NO8 group (P=0.786).
Figure 3.
Kaplan-Meier survival curves of obstructive pneumonitis/atelectasis and tumor size according to the eighth TNM staging system for overall survival (OS) and recurrence-free survival (RFS). (A,B) OS and RFS curves for patients in T1O8, T1NO8, T2NO8 and T3NO8 groups; (C,D) OS and RFS curves for patients in T2O8, T1NO8, T2NO8 and T3NO8 groups.
Factors associated with obstructive pneumonitis/atelectasis
The cut-off values of each inflammation maker by ROC curves were as follows: neutrophil 4.5; lymphocyte 1.5; platelet 189.5; monocyte 0.5; NLR 2.475; PLR 169.8; LMR 3.685; ESR 10.5. Table 3 showed that presence of obstructive pneumonitis/atelectasis was significantly related to higher neutrophil (P<0.001), platelet (P=0.012), monocyte (P<0.001), NLR (P<0.001), PLR (P=0.002), ESR (P<0.001) and lower LMR (P<0.001). But the difference of lymphocyte number between having and not having obstructive pneumonitis/atelectasis groups was not significant (P=0.469).
Table 3. Clinicopathological and inflammation factors associated with obstructive pneumonitis/atelectasis.
| Factors | Number of patients | No obstructive pneumonitis/atelectasis (%) | Obstructive pneumonitis/atelectasis (%) | P |
|---|---|---|---|---|
| Age | 0.047 | |||
| ≤65 | 859 | 538 (62.6) | 321 (37.4) | |
| >65 | 318 | 219 (68.9) | 99 (31.1) | |
| Gender | <0.001 | |||
| Female | 342 | 267 (78.1) | 75 (21.9) | |
| Male | 835 | 490 (58.7) | 345 (41.3) | |
| Histological subtype | <0.001 | |||
| Adenocarcinoma | 629 | 515 (81.9) | 114 (18.1) | |
| Squamous carcinoma | 470 | 198 (42.1) | 272 (57.9) | |
| Others | 78 | 44 (56.4) | 34 (43.6) | |
| Pathological TNM stage | <0.001 | |||
| I/II | 838 | 576 (68.7) | 262 (31.3) | |
| III | 339 | 181 (53.4) | 158 (46.6) | |
| Neutrophil | <0.001 | |||
| ≤4.5 | 749 | 525 (70.1) | 224 (29.9) | |
| >4.5 | 428 | 232 (54.2) | 196 (45.8) | |
| Lymphocyte | 0.469 | |||
| >1.5 | 839 | 545 (65.0) | 294 (35.0) | |
| ≤1.5 | 338 | 212 (62.7) | 126 (37.3) | |
| Platelet | 0.012 | |||
| ≤189.5 | 282 | 199 (70.6) | 83 (29.4) | |
| >189.5 | 895 | 558 (62.3) | 337 (70.6) | |
| Monocyte | <0.001 | |||
| ≤0.5 | 702 | 502 (71.5) | 200 (28.5) | |
| >0.5 | 475 | 255 (53.7) | 220 (46.3) | |
| NLR | <0.001 | |||
| ≤2.475 | 713 | 489 (68.6) | 224 (31.4) | |
| >2.475 | 464 | 268 (57.8) | 196 (42.4) | |
| PLR | 0.002 | |||
| ≤169.8 | 914 | 609 (66.6) | 305 (33.4) | |
| >169.8 | 263 | 148 (56.3) | 115 (43.7) | |
| LMR | <0.001 | |||
| >3.685 | 644 | 463 (71.9) | 181 (29.1) | |
| ≤3.685 | 533 | 294 (55.2) | 239 (44.8) | |
| ESR | <0.001 | |||
| ≤10.5 | 162 | 123 (75.9) | 39 (24.1) | |
| >10.5 | 280 | 147 (52.5) | 133 (47.5) |
SCLC, small cell lung cancer; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; LMR, lymphocyte to monocyte ratio; ESR, erythrocyte sedimentation rate. P≤0.05 was considered to be significant.
Discussion
The TNM staging system was first established in 1973 by The American Joint Committee on Cancer (AJCC) and the Union Internationale Contre le Cancer (13). In September 2015, the proposal of the eighth version was published. There were some slight changes comparing to the seventh edition which was applied in 2009, but presence of obstructive pneumonitis/atelectasis is still one of the non-size based descriptors for T category. Ou et al.’s study once confirmed that visceral pleura invasion, hilar atelectasis, or obstructive pneumonitis with tumor size >3 cm were poor prognostic factors for survival, but they predicted favorable prognosis when tumor size ≤3 cm (15). Besides, Dediu and Bulbul et al.’s articles showed the positive prognostic value of obstructive pneumonitis/atelectasis in patients with advanced lung cancer (16,17). They thought the favorable effect of atelectasis might owe to the decreased intratumoral blood flow and a specific growth pattern.
In order to figure out the controversial issue, we conducted a retrospective study based on 1,177 NSCLC patients receiving surgery treatment in Shandong Provincial Hospital affiliated to Shandong University between 2006 and 2011. However, our study suggested that presence of obstructive pneumonitis/atelectasis before surgery predicted shorter OS and RFS as an independent factor. In subgroup analysis, we found there was no significant difference between having preoperative obstructive pneumonitis/atelectasis or not for prognosis of patients in stage III, while survival differed significantly for patients in I/II stage. As to patients with cancer cells differentiating well or moderately, a significant result was also observed.
What’s more, we searched the relationship between obstructive pneumonitis/atelectasis and tumor size for survival. In the eighth TNM staging proposal for lung cancer, the tumor size to divide T2 and T3 became 5 cm, not 7 cm comparing with the seventh edition. And lung cancer patients with obstructive pneumonitis/atelectasis partially or completely are included in T2 category. It seemed that they thought the prognostic value of obstructive pneumonitis/atelectasis before surgery was similar to the factor of tumor size between 3 and 5 cm in the eighth edition. In order to find out the problem, we divided the patients into different groups according to the seventh and the eighth edition respectively, which was mentioned in the part of results. According to the division by the seventh edition, K-M curves (Figure 2) indicated that there were no significant differences comparing T1O7 group, T2O7 group with T2NO7 group. When divided according to the eighth edition, the prognosis of patients in T1O8 group was similar to those in T2NO8 group, but the survival of patients in T2O8 group were significantly shorter than those in T2NO8 group, while similar with patients in T3NO8 group. So we suggested that which T staging group the patients with obstructive pneumonitis/atelectasis should be divided to should depend on the tumor size in the eighth TNM staging system.
However, the mechanism of preoperative obstructive pneumonitis/atelectasis’s negative effect on survival was not sure yet. Miyamoto et al.’s study on clinical investigation of obstructive pneumonia with lung cancer indicated that the majority had neutrophilia and high CRP (18). An authoritative study once mentioned inflammation was a critical hallmark of cancer, which could affect occurrence and development of neoplasm (19). And recently, some inflammation makers included neutrophil, CRP, lymphocyte and other specific values were reported to be associated with prognosis of lung cancer patients. So a research to search the relationship between them was conducted. We found that presence of obstructive pneumonitis/atelectasis was significantly relative to higher neutrophil, platelet, monocyte, NLR, PLR, ESR and lower LMR. The biological reason behind prognostic effect of preoperative obstructive pneumonitis/atelectasis might owe to high level of neutrophil, platelet and monocyte. Some studies have suggested that a large amount of neutrophils might have negative effect on tumor growth by influencing cytolytic activity of lymphocyte or natural killer cells and inhibit proliferation of T-cells (20). Elevated platelet count was also confirmed to be a negative factor for prognosis of lung cancer patients due to releasing some platelet-derived cytokines related to tumor angiogenesis regulatory, such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), platelet derived growth factor (PDGF) (21). Evidence also showed that tumor-related macrophages which derived from circulating monocytes were related to poor survival in various cancers. Macrophages could secrete TNF-α, VEGF, epidermal growth factor, promoting tumor angiogenesis and tumor growth (22-24). However, underlying infection within the obstructed space might also contribute to the result. Although we have excluded the patients who were undergoing non-cancer related inflammation, some bias might exist, so further researches should be conducted.
In conclusion, presence of obstructive pneumonitis/atelectasis before surgery in lung cancer patients predicted poor OS and RFS independently. This was particularly obvious for patients in early stage group, younger group, female group, adenocarcinoma group and group of tumor cells differentiating well or moderately. There was no significant difference for patients in advanced stage. And in comparison the predictive effects of preoperative obstructive pneumonitis/atelectasis with tumor size, we found that the prognosis of patients with obstructive pneumonitis/atelectasis and T1 tumor size was similar to patients with T2 tumor size but without obstructive pneumonitis/atelectasis, while the survival of patients with obstructive pneumonitis/atelectasis and T2 tumor size was significantly shorter than patients with T2 tumor size but without obstructive pneumonitis/atelectasis, and similar to patients with T3 tumor size but without obstructive pneumonitis/atelectasis according to division by the eighth edition. Our results also showed that presence of preoperative obstructive pneumonitis/atelectasis was associated with higher neutrophil, platelet, monocyte, NLR, PLR, ESR and lower LMR, which might play a role in its negative effect for survival.
Acknowledgements
Funding: This work was supported by Provincial science and technology development plan of Shandong (2015GSF118063), Shandong Provincial Natural Science foundation (ZR2014HQ028, ZR2014HQ073).
Ethical Statement: The study was approved by Ethics Committee of Shandong Provincial Hospital in China (No. 356).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- 2.DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 2014;64:252-71. 10.3322/caac.21235 [DOI] [PubMed] [Google Scholar]
- 3.Candido J, Hagemann T. Cancer-related inflammation. J Clin Immunol 2013;33 Suppl 1:S79-84. 10.1007/s10875-012-9847-0 [DOI] [PubMed] [Google Scholar]
- 4.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011;331:1565-70. 10.1126/science.1203486 [DOI] [PubMed] [Google Scholar]
- 5.Crusz SM, Balkwill FR. Inflammation and cancer: advances and new agents. Nat Rev Clin Oncol 2015;12:584-96. 10.1038/nrclinonc.2015.105 [DOI] [PubMed] [Google Scholar]
- 6.Tomita M, Shimizu T, Hara M, et al. Preoperative leukocytosis, anemia and thrombocytosis are associated with poor survival in non-small cell lung cancer. Anticancer Res 2009;29:2687-90. [PubMed] [Google Scholar]
- 7.Laird BJ, Kaasa S, McMillan DC, et al. Prognostic factors in patients with advanced cancer: a comparison of clinicopathological factors and the development of an inflammation-based prognostic system. Clin Cancer Res 2013;19:5456-64. 10.1158/1078-0432.CCR-13-1066 [DOI] [PubMed] [Google Scholar]
- 8.Cedrés S, Torrejon D, Martínez A, et al. Neutrophil to lymphocyte ratio (NLR) as an indicator of poor prognosis in stage IV non-small cell lung cancer. Clin Transl Oncol 2012;14:864-9. 10.1007/s12094-012-0872-5 [DOI] [PubMed] [Google Scholar]
- 9.Proctor MJ, Morrison DS, Talwar D, et al. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer 2011;47:2633-41. 10.1016/j.ejca.2011.03.028 [DOI] [PubMed] [Google Scholar]
- 10.Mori S, Usami N, Fukumoto K, et al. The Significance of the Prognostic Nutritional Index in Patients with Completely Resected Non-Small Cell Lung Cancer. PLoS One 2015;10:e0136897. 10.1371/journal.pone.0136897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shrotriya S, Walsh D, Bennani-Baiti N, et al. C-Reactive Protein Is an Important Biomarker for Prognosis Tumor Recurrence and Treatment Response in Adult Solid Tumors: A Systematic Review. PLoS One 2015;10:e0143080. 10.1371/journal.pone.0143080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Templeton AJ, Ace O, McNamara MG, et al. Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2014;23:1204-12. 10.1158/1055-9965.EPI-14-0146 [DOI] [PubMed] [Google Scholar]
- 13.Mountain CF, Carr DT, Anderson WA. A system for the clinical staging of lung cancer. Am J Roentgenol Radium Ther Nucl Med 1974;120:130-8. 10.2214/ajr.120.1.130 [DOI] [PubMed] [Google Scholar]
- 14.Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. 10.1016/j.jtho.2015.09.009 [DOI] [PubMed] [Google Scholar]
- 15.Ou SH, Zell JA, Ziogas A, et al. Prognostic significance of the non-size-based AJCC T2 descriptors: visceral pleura invasion, hilar atelectasis, or obstructive pneumonitis in stage IB non-small cell lung cancer is dependent on tumor size. Chest 2008;133:662-9. 10.1378/chest.07-1306 [DOI] [PubMed] [Google Scholar]
- 16.Bulbul Y, Eris B, Orem A, et al. Pulmonary atelectasis and survival in advanced non-small cell lung carcinoma. Ups J Med Sci 2010;115:176-80. 10.3109/03009731003695624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dediu M, Crisan E, Radut M, et al. The favorable prognostic significance of atelectasis in patients with advanced non-small cell lung cancer: results of a prospective observational study. Lung Cancer 2009;63:271-6. 10.1016/j.lungcan.2008.05.007 [DOI] [PubMed] [Google Scholar]
- 18.Miyamoto J, Koga H, Kohno S, et al. Clinical investigation of obstructive pneumonia with lung cancer. Kansenshogaku Zasshi 1994;68:728-33. 10.11150/kansenshogakuzasshi1970.68.728 [DOI] [PubMed] [Google Scholar]
- 19.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57-70. 10.1016/S0092-8674(00)81683-9 [DOI] [PubMed] [Google Scholar]
- 20.Pillay J, Kamp VM, van Hoffen E, et al. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. J Clin Invest 2012;122:327-36. 10.1172/JCI57990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peterson JE, Zurakowski D, Italiano JE, Jr, et al. VEGF, PF4 and PDGF are elevated in platelets of colorectal cancer patients. Angiogenesis 2012;15:265-73. 10.1007/s10456-012-9259-z [DOI] [PubMed] [Google Scholar]
- 22.Leek RD, Harris AL. Tumor-associated macrophages in breast cancer. J Mammary Gland Biol Neoplasia 2002;7:177-89. 10.1023/A:1020304003704 [DOI] [PubMed] [Google Scholar]
- 23.Mantovani A, Schioppa T, Porta C, et al. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev 2006;25:315-22. 10.1007/s10555-006-9001-7 [DOI] [PubMed] [Google Scholar]
- 24.Xiong M, Elson G, Legarda D, et al. Production of vascular endothelial growth factor by murine macrophages: regulation by hypoxia, lactate, and the inducible nitric oxide synthase pathway. Am J Pathol 1998;153:587-98. 10.1016/S0002-9440(10)65601-5 [DOI] [PMC free article] [PubMed] [Google Scholar]