Abstract
Herpes simplex virus type 1 (HSV-1), a neurotropic member of the alphaherpes virus family, is among the most prevalent and successful human pathogens. HSV-1 can cause serious diseases at every stage of life including fatal disseminated disease in newborns, cold sores, eye disease, and fatal encephalitis in adults. HSV-1 infection can trigger rapid immune responses, and efficient inhibition and clearance of HSV-1 infection rely on both the innate and adaptive immune responses of the host. Multiple strategies have been used to restrict host innate immune responses by HSV-1 to facilitate its infection in host cells. The adaptive immunity of the host plays an important role in inhibiting HSV-1 infections. The activation and regulation of T cells are the important aspects of the adaptive immunity. They play a crucial role in host-mediated immunity and are important for clearing HSV-1. In this review, we examine the findings on T cell immune responses during HSV-1 infection, which hold promise in the design of new vaccine candidates for HSV-1.
Keywords: Herpes simplex virus type 1, Adaptive immunity, T cells, Vaccine
1. Introduction
Herpes simplex virus type 1 (HSV-1), from the alphaherpes virus subfamily, is an enveloped, nuclear-replicating, and large double-stranded DNA virus. The genome of HSV-1 is an about 152 kb linear double-stranded GC-rich DNA sequence, and contains two unique regions called the long unique region (UL) and the short unique region (US) (Fig. 1a), which encodes at least 84 proteins (Kieff et al., 1971). The genome of HSV-1 is located within the nucleocapsid, which is surrounded by a group of tegument proteins. The nucleocapsid and tegument proteins are surrounded by a lipid envelope studded with glycoproteins which are important for binding to and entry into new susceptible cells (Egan et al., 2013). The major steps of the life cycle of HSV-1 are: entry into the host cell, viral gene expression, genome replication, virion assembly, and release of new infectious virus (Fig. 1b) (Kukhanova et al., 2014). Three classes of genes of HSV-1 are expressed in a consecutive manner, including immediate early (IE) genes, early genes, and late genes. The products of IE genes regulate the expressions of early genes and late genes (Harkness et al., 2014).
Fig. 1.

Genome information and life cycle of HSV-1
(a) Structure of the HSV-1 DNA. The unique long (UL) region is flanked by the terminal repeat (TRL) and the internal repeat (IRL). The unique short (US) region is bounded by the terminal repeat (TRS) and the internal repeat (IRS). (b) HSV-1 life cycle. 1: entry into the host cell; 2: viral gene expression; 3: genome replication; 4: virion assembly; 5: release of new infectious virus
The primary infection of HSV-1 is mainly in epithelial or mucosal cells, and then establishes a latent infection when it is transported to the sensory ganglia (Nicoll et al., 2012). During HSV-1 latent infection, the genome transcription is inhibited with the exception of a sequence encoding the latency-associated transcripts (LATs) (Wagner and Bloom, 1997; Preston, 2000; Efstathiou and Preston, 2005). The renewed lytic infection at epithelial or mucosal cells happens when there is reactivation of latent HSV-1 (Wuest and Carr, 2008).
HSV-1 infection is widespread, and its seropositivity may cover more than 70% of the world population. In developing countries, HSV-1 infection is universal, and acquired from intimate contact with family in early childhood (Whitley et al., 1988). In developed countries, some data suggest that acquisition of HSV-1 is delayed from early childhood to young adulthood (Hashido et al., 1999; Mertz et al., 2003). In the United States, 65% of people have antibodies to HSV-1, which is similar to the epidemiology in Europe (Xu et al., 2002). HSV-1 infection can cause clinical disease in various parts of the human body, such as genitalia, eye, oral, and central nervous system (CNS). The diseases associated with HSV-1 are listed in Table 1.
Table 1.
Diseases associated with HSV-1 infection
| Infected body part | Disease | Reference |
| Skin | Cutaneous herpes | Zendri et al., 2005; Faron et al., 2016 |
| Genital herpes | Nieuwenhuis et al., 2006; Khoury-Hanold et al., 2016 | |
| Ocular | Herpes simplex keratitis (HSK) | Burrel et al., 2013; Tsatsos et al., 2016 |
| Uveitis | Krichevskaia et al., 2005; van Velzen et al., 2013 | |
| Acute retinal necrosis | Mora et al., 2009; Fong et al., 2014 | |
| Orolabial | Cold sores | Richardson et al., 2013; Chi et al., 2015 |
| Oral ulcers | Sepulveda et al., 2005; Nicolatou-Galitis et al., 2006 | |
| CNS | Encephalitis | Bradshaw and Venkatesan, 2016; Eriksson et al., 2016 |
| Meningitis | Eisenstein et al., 2004; Azadfar et al., 2014 | |
| Alzheimer’s disease | Beffert et al., 1998; Itzhaki et al., 1998 |
Inhibition of viral infection and clearance of the virus from infected cells rely on the innate and adaptive immunity of the host. The host innate immune system has evolved soluble components and specialized cells to block viral infection, replication, and shedding (Medzhitov and Janeway, 2000; Kawai and Akira, 2006). During viral infection, pattern recognition receptors (PRRs) have a role in detecting the viral pathogen-associated molecular patterns (PAMPs) in infected cells. The activated PRRs, such as Toll-like receptors (TLRs) and retinoic acid inducible gene-I (RIG-I)-like receptors (RLRs), will induce interferon production and cytokine release (Akira et al., 2006). The cellular PRRs to detect HSV-1 PAMPs have been reviewed extensively (Paludan et al., 2011; Melchjorsen, 2012). The type I interferon (IFN) signal pathway is the important first line of defense for the host against HSV-1. The innate immune cells including monocytes, neutrophils, dendritic cells (DCs), macrophages, and natural killer (NK) cells also play a crucial role in inhibiting HSV-1 infection (Kodukula et al., 1999; Barr et al., 2007; Murphy et al., 2008; Zheng et al., 2008; Mott et al., 2011; 2014; Frank et al., 2012; Kim et al., 2012; Molesworth-Kenyon et al., 2012; Swiecki et al., 2013; Vogel et al., 2014; Menasria et al., 2015). During infection, HSV-1 has developed multiple mechanisms to evade innate host immune responses and attenuate host antiviral elements (Paladino and Mossman, 2009; Suazo et al., 2015; Su et al., 2016). Although the functions of host innate immunity such as type I IFN and innate immune cell activity are need to suppress HSV-1 replication and infection, the generation of both CD8+ and CD4+ T cells is ultimately required to inhibit viral infection, drive HSV-1 into latency, and repress reactivation. This review will summarize and discuss current findings of the T cell immune response during HSV-1 infection, which could facilitate the attempts of more effective vaccine development for treating HSV-1 infection.
2. Immune responses of CD8+ T cells during HSV-1 infection
Among the immune cells involved in the immunity induced by pathogen invasion, CD8+ T cells play a central role in host adaptive immunity against many intracellular pathogens and clearing the viruses from the host (Wiesel et al., 2009; Kalia et al., 2010). Following pathogen recognition in the context of major histocompatibility complex class I (MHC-I) on antigen presenting cells (APCs), the naive CD8+ T cells could be differentiated into Tc1, Tc2, or Tc17 cells (Mosmann et al., 1997; Lee et al., 2011; Zhang and Bevan, 2011). During viral infection, the immune response of CD8+ T cells was divided into three characteristic phases, which are the initial activation and expansion, a contraction phase, and the establishment and maintenance of memory (Kaech et al., 2002a). During the acute phase of viral infection in humans, the robust immune responses of CD8+ T cells have also been observed (Callan et al., 1998). At the peak of proliferation, there is up to 104-to 105-fold expansion of CD8+ T cells, which divide approximately every 6 to 8 h, and then undergo activation and differentiation after this dramatic proliferation (Murali-Krishna et al., 1998). Upon antigenic stimulation, the expressions of granzymes and perforin are up-regulated by CD8+ T cells, and then these cells become cytolytic and gain the ability to enter non-lymphoid tissues (Bachmann et al., 1999; Cerwenka et al., 1999; Kaech et al., 2002a; 2002b; Wherry et al., 2003). CD8+ T cells also secret the IFN-γ when responding to viral infection, and IFN-γ promotes presentation of antigens to CD8+ T cells through enhanced processing of viral peptides for loading into MHC-I, which promotes immune response and inhibits viral infection (Groettrup et al., 1996; Schroder et al., 2004).
Previous studies indicated that CD8+ T cells might play a crucial role in restricting HSV-1 infection (Marrack and Kappler, 1987; Simmons, 1989). In line with the classic paradigm that HSV-1 infection is controlled by CD8+ T cells, MHC-I-restricted CD8+ T cells can be recovered from lymph nodes draining into herpetic lesions after HSV-1 infection on Day 4 (Nash et al., 1980). CD8+ T cells also have an ability to shut down HSV-1 infection in the trigeminal ganglia (TG) and prevent neurologic damage, and the response of CD8+ T cells could be divided into acute and latent phases (Simmons and Tscharke, 1992; Liu et al., 2000). In the mouse model, the infiltration peaks of CD8+ T cells in mouse ganglia occurred on Day 12, and a great number of CD8+ T cells persisted in the ganglia for up to 90 d. Using a murine flank scarification model, CD8+ T cells were observed to be involved in inhibiting HSV-1 replication in the draining ganglia (Simmons et al., 1992). In terms of effector molecules that are associated with CD8+ T cells mediating immunity, alternatively it was reported that IFN-γ not only might exert an antiviral effect and block the replication of numerous viruses in vitro, but also has an antiviral activity in controlling HSV-1 infection in primary human neurons and astrocytes (Li et al., 2011; 2012). Furthermore, another possible effector molecule is granzyme A (GrA), and the animals deficient in GrA show the decreased viral clearance from infection sites after peripheral HSV-1 inoculation (Pereira et al., 2001).
The CD8+ T cells might interact with many APCs during HSV-1 infection, such as DCs and ganglionic cells. The type and condition of APC used to characterize CD8+ T cell responses are also critically important for the inhibition of HSV-1 infection. Compared with B cells, the fibroblasts are more susceptible to HSV-1-mediated down-regulation of human leukocyte antigen class I (HLA-I) and they were observed to poorly re-stimulate memory CD8+ T cell responses (Yasukawa and Zarling, 1984; Kohl, 1991; Tigges et al., 1996).
Tissue-resident memory T cells (TRM cells) are a subtype of memory lymphocytes and reside in nonlymphoid tissues in humans and mice (Schenkel and Masopust, 2014). CD8+ TRM cells, a novel class of CD8+ memory T cells, have been well characterized (Krzysiek et al., 2013). During HSV-1 infection, virus-specific CD8+ TRM cells are created in both ganglia and mucosa (Khanna et al., 2003; Gebhardt et al., 2009; Ariotti et al., 2014). CD8+ TRM cells exist in non-lymphoid tissue compartments for long periods, and now these cells are also found in brain, kidney, joints, and other non-barrier tissues, which can trigger protective innate and adaptive immunity (Schenkel et al., 2014). CD8+ TRM cells could express the effector molecules IFN-γ and granzyme B (GrB), and more these cells are not replenished from the circulating CD8+ T cell pool (Mackay et al., 2012). The roles of CD8+ TRM cells are listed in Fig. 2.
Fig. 2.
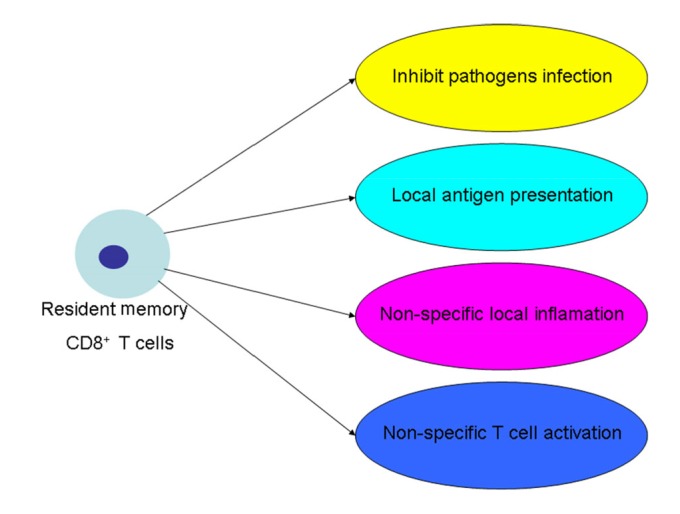
Function of CD8+ TRM cells
3. Immune response of CD4+ T cells during HSV-1 infection
In the host adaptive immune system, CD4+ T cells are another important branch, which not only have the ability to regulate an effective immune response to pathogens, but also have a role in control of host survival. Naive CD4+ T cells could differentiate into Th1, Th2, Th17, and induced regulatory T (iTreg) cells through interaction with antigen-MHC complex, and CD4+ T cell differentiation depends on the cytokines of the microenvironment (Luckheeram et al., 2012; Zheng, 2013). CD4+ T cells have ability to restrict and eliminate acute viral infection, and depend on the distinct phenotypes with their respective cytokine profiles. CD4+ T cells also modulate the functions of adaptive immunity and the innate immune cells (Sant and McMichael, 2012). Th1 cells have been regarded as critical for immunity to intracellular microorganisms and Th2 cells for immunity to many extracellular pathogens, including helminthes (Mosmann and Coffman, 1989; Paul and Seder, 1994). Treg cells function is needed to limit the extent of virus-induced inflammatory lesions, which implies that expanding and activating Tregs could be therapeutically valuable (Sehrawat and Rouse, 2011). During HSV-1 infection, the expression of IL-17 was up-regulated mainly through the Th17 cells in host cornea. Th1 cells are also responsible for orchestrating herpes stromal keratitis (HSK) during viral infection (Suryawanshi et al., 2011).
In the HSV-1 corneal infection model, HSV-1 replication is largely eliminated by 4–6 day post infection (dpi), and the CD4+ T cells that mediate HSK infiltrate the cornea around 7 dpi (Lepisto et al., 2006; Yun et al., 2014). Previous study has shown that the CD4+ T cells are important for preventing genital disease in a mice model during HSV-1 infection (Kuklin et al., 1998). When the mice are inoculated intravaginally (i.vag.) with HSV-1, CD4+ T cells can accumulate and persist within the spinal cords and dorsal root ganglia, which suggested that CD4+ T cells have the ability to clear virus from both neural and genital sites after HSV-1 primary infection (Johnson et al., 2008). It had been demonstrated that the CD4+CD25+ Tregs participate in T cell immune response to HSV-1 (Suvas et al., 2003). Following i.vag. inoculation of HSV-1, the number of CD4+ T cells from iliac lymph nodes, spinal cords, or dorsal root ganglia generally peaked around 6 or 8 dpi, and these cells increased expression of the activation marker CD25, CD44, or CD69 (Johnson et al., 2008). CD4+ T cells are expanded in the draining lymph nodes (DLNs) and re-stimulated in the infected cornea to regulate the destructive inflammatory disease after HSV-1 corneal infection (Buela and Hendricks, 2015). Recruitment of memory CD4+ T cells after infection of HSV-1 is also involved in the development of Th1 cells (Sin et al., 2000). DCs are resident in the DLNs and account for the expanding of naive HSV-specific CD4+ T cells in DLNs and the re-stimulating of the CD4+ T effector cells that infiltrate the cornea to mediate HSK (Hendricks et al., 1992). CD4+ T cells contribute to protection against HSV-1 in mice and ablation of CD4+ T cells increased susceptibility in naive animals (Manickan et al., 1995a; 1995b). In the mice model, CD4+ T cells are sufficient and have a ability to inhibit and clear HSV-1 from both neural and genital sites after primary infection (Johnson et al., 2008).
4. Infiltration of CD8+ and CD4+ T cells into central nervous system during HSV-1 infection
Despite the fact that the CNS of a host generally has the restrictive nature of the blood-brain barrier (BBB), CD8+ and CD4+ T cells can infiltrate into CNS during a variety of disease states and viral infection (Stohlman et al., 1998; Marten et al., 2003). When animals are exposed to glucocorticoids, the CNSs of them are shown to be vulnerable, which could enhance the susceptibility to viral infection. In the mouse model, psychological stress can induce the production of glucocorticoid, which enhances the infection of HSV-1 in mice and causes the development of HSV-1 encephalitis (HSE) (Nair et al., 2007). In a mouse model of HSE, the HSV-1-specific T cells infiltrated into CNS, and the brain of the mouse had an increase in CD8+ and CD4+ T cells during HSV-1 infection (Anglen et al., 2003). Multifocal brain demyelination (MBD) has been reported in susceptible mouse strains upon lip inoculation with HSV-1 and immunosuppression prevents the development of such lesions (Kastrukoff et al., 1987; 1993). CD8+ T cells appear to be involved in the focal lesions of the brain and the depletion of such cells prevents lesion development (Hudson and Streilein, 1994). Due to the limited evidence, it still remains unclear whether CD8+ T cells are responsible for limitation of HSV-1 replication and spreading within the CNS prior to an infection, or the delayed entrance of CD8+ T cells could result in pathology (Anglen et al., 2003).
The microglia, mstrocytes, perivascular macrophages, DCs, and endothelial cells of the brain have a function in presenting antigen to T cells in CNS (Aloisi, 1999; 2001; Aloisi et al., 2000). During HSV-1 infection, the dendritic-like cells and macrophage-like cells increase the expression of H-2Kb, which indicates a potential role of these cells to prime of HSV-1-specific CD8+ T cells in host CNS (Nair et al., 2007).
5. Exhaustion of CD8+ T cells during HSV-1 infection
The CD8+ T cells can be exhausted during chronic viral infection. These exhausted CD8+ T cells exhibit lost memory potential and poor effector function (Zajac et al., 1998; Wherry, 2011; Schietinger and Greenberg, 2014). The first report on CD8+ T cells exhaustion was found in mice when infected with lymphocytic choriomeningitis virus (LCMV), and virus-specific CD8+ T cells become less functional, as the infection persists, and may gradually lose their effector function, including proliferation, cytotoxicity, and cytokine production (Zajac et al., 1998). In addition, the exhaustion of CD8+ T cell function has been demonstrated in several human chronic virus infections, including hepatitis C virus (HCV), hepatitis B virus (HBV), and human immunodeficiency virus (HIV). The inhibitory receptors are expressed in exhausted CD8+ T cells, such as programmed cell death protein 1 (PD-1), T-cell immunoglobulin domain and mucin domain-3 (Tim-3), cluster of differentiation 244 (CD244), B-and T-lymphocyte attenuator (BTLA), cytotoxic T-lymphocyte associated protein 4 (CTLA-4), CD160, and killer cell lectin-like receptor subfamily G member 1 (KLRG1) (Bengsch et al., 2010; Wherry, 2011).
HSV-1 can reactivate from the TG, where there is a sizable pool of virus-specific CD8+ T cells. This phenomenon may depend on exhausting CD8+ T cells (Hoshino et al., 2007; Chentoufi et al., 2010). PD-1 is the most common inhibitory marker expressed in exhausted CD8+ T cells (Barber et al., 2006; Petrovas et al., 2007; Fourcade et al., 2010; Sakuishi et al., 2010). Besides the expression of PD-1, the high expressions of Tim-3 and CTLA-4 were also detected on the HSV-1-specific CD8+ T cells from LAT+ TG, which helped to discriminate between exhaustion and activation (Srivastava et al., 2016). During HSV-1 latency infection, LAT influences the CD8+ T cell levels and exhaustion. LAT+ HSV-1 can present more viral agent to CD8+ T cells, and this leads to increase in the expression of PD-1/Tim-3 and CD8+ T cell exhaustion. There are three subsets of exhausted CD8+ T cells in the TG of mice latently infected by HSV-1, the first one expressing PD-1, the second expressing Tim-3, and the third expressing both PD-1 and Tim-3 (Allen et al., 2011). The GrB of CD8+ T cells plays a major role in cytotoxic lytic granule-mediated apoptosis of cells. HSV-1 LAT not only restricts the GrB-induced cell apoptosis, but also inhibits the GrB-induced cleavage of caspase-3 (Jiang et al., 2011).
6. Vaccine for HSV-1
HSV-1 has the ability to build a primary and latent infection in human bodies, and causes serious diseases in human beings (Laing et al., 2012). It will benefit public health worldwide by reducing HSV-1 infection in human beings, and the efficient and effective pathway to control viral infectious disease is to inject vaccines. However, many of people are infected by HSV-1 during childhood, and the most of infected persons never undergo recurrent herpetic disease (Khanna et al., 2004). Therefore, the efficient and effective approach to prevent the infection of HSV-1 is prophylactic vaccination. The method to produce a therapeutic vaccine is targeting the viral proteins during HSV-1 latent infection, which proves more efficacious in restricting recurrent disease in human beings.
The advantages and disadvantages of experimental vaccine formats, which are mixture of viral proteins, peptides, attenuated replication-competent viruses, and replication-defective viruses, are reviewed (Stanberry et al., 2000). Many vaccine types including prophylactic and therapeutic containing viral DNA, glycoproteins, or replication-defective virus have been built in the past ten years. Nowadays, the approaches to develop vaccines are focused on the mechanism of viral evasion of the host immune system, and combination with the use of new and more specific adjuvants (Coleman and Shukla, 2013).
Researchers are interested in exploiting T cells to develop the vaccines, because the T cells of the host have specific and long-term immunologic memory and the ability to clear the virus in vitro and in animals (Laing et al., 2012). It is known that T cells have taken part in protective immunity after vaccination and correlate with viral reactivation in animal manipulation (Noisakran and Carr, 1999; Liu et al., 2000; Sheridan et al., 2009). Some studies suggest that T cell epitope specificities vary with different clinical presentations of HSV-1, and the epitope discovery has facilitated investigation of HSV-1-specific T cells in the search for a natural immune response (Chentoufi et al., 2008). T cells respond to a complex and serious pathogen with HSV-1, which has been decoded with a linked set of cellular and molecular tools to reveal novel candidate vaccine antigens (Jing et al., 2012). During HSV-1 cornea infection in mouse models, it has been shown that the CD8+ T cells might have no influence on the immunopathology in HSV-1. However, CD8+ T cells are protective at peripheral sites of infection and in the TG of the host, so a vaccine targeting CD8+ T cells might be particularly efficient and effective (Khanna et al., 2004).
Based on the protective immune response of T cells, it is important to develop a vaccine that elevates T cells. The best protection of the host during viral infection is to induce stronger cell-mediated immunity as well as better humoral responses, because viruses have used clever immune evasion strategies to inhibit the neutralizing antibody response (Coleman and Shukla, 2013). Nowadays, there has no specific vaccine or immunization strategy for HSV-1, but fascinating recent work comparing HSV-1-seropositive persons with and without histories of symptomatic orolabial herpes raises the possibility that humoral response patterns to specific proteins, or T-cell response patterns to defined epitopes within HSV-1 glycoproteins, may correlate with clinical severity, offering a set of criteria for rational down-selection of vaccine candidates (Chentoufi et al., 2008; Dasgupta et al., 2012).
7. Conclusions
In summary, HSV-1 is one of the persistent pathogens of human beings, and can cause a variety of diseases, such as cold sores, cutaneous herpes, genital herpes, and encephalitis. HSV-1 can establish a latent infection in TG of host, while CD4+ and CD8+ T cells can control the reactivation of the HSV-1 by surrounding latently infected neurons. The interactions between the host immunity and HSV-1 are very complicated. There is a battle between the host and HSV-1, and construction of a potent HSV-1-specific T cell immunity relies on multifaceted DCs priming of T cells as well as both CD4+ and CD8+ T cell cooperation at several stages and anatomic sites. Thus, favored vaccines elicit complex T cell responses, and likely also B cell responses, to generate long-lasting virus-neutralizing antibodies. Now the approaches to design the new vaccine against HSV-1 are by attracting and retaining TRM cells in peripheral tissue locations. In any case, development of a useful HSV-1 vaccine needs to overcome the challenges posed by HSV-1 to traditional vaccine strategies.
Footnotes
Project supported by the Wuhan Institute of Virology (WIV) “One-Three-Five” Strategic Programs, China
Compliance with ethics guidelines: Jie ZHANG, Huan LIU, and Bin WEI declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. (Available from: http://dx.doi.org/10.1016/j.cell.2006.02.015) [DOI] [PubMed] [Google Scholar]
- 2.Allen SJ, Hamrah P, Gate D, et al. The role of LAT in increased CD8+ T cell exhaustion in trigeminal ganglia of mice latently infected with herpes simplex virus 1. J Virol. 2011;85(9):4184–4197. doi: 10.1128/JVI.02290-10. (Available from: http://dx.doi.org/10.1128/JVI.02290-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aloisi F. The role of microglia and astrocytes in CNS immune surveillance and immunopathology. In: Matsas R, Tsacopoulos M, editors. The Functional Roles of Glial Cells in Health and Disease. New York: Springer Science+Business Media; 1999. pp. 123–133. (Available from: http://dx.doi.org/10.1007/978-1-4615-4685-6_10) [DOI] [PubMed] [Google Scholar]
- 4.Aloisi F. Immune function of microglia. Glia. 2001;36(2):165–179. doi: 10.1002/glia.1106. (Available from: http://dx.doi.org/10.1002/glia.1106) [DOI] [PubMed] [Google Scholar]
- 5.Aloisi F, Ria F, Adorini L. Regulation of T-cell responses by CNS antigen-presenting cells: different roles for microglia and astrocytes. Immunol Today. 2000;21(3):141–147. doi: 10.1016/s0167-5699(99)01512-1. (Available from: http://dx.doi.org/10.1016/S0167-5699(99)01512-1) [DOI] [PubMed] [Google Scholar]
- 6.Anglen CS, Truckenmiller ME, Schell TD, et al. The dual role of CD8+ T lymphocytes in the development of stress-induced herpes simplex encephalitis. J Neuroimmunol. 2003;140(1-2):13–27. doi: 10.1016/s0165-5728(03)00159-0. (Available from: http://dx.doi.org/10.1016/S0165-5728(03)00159-0) [DOI] [PubMed] [Google Scholar]
- 7.Ariotti S, Hogenbirk MA, Dijkgraaf FE, et al. T cell memory. Skin-resident memory CD8+ T cells trigger a state of tissue-wide pathogen alert. Science. 2014;346(6205):101–105. doi: 10.1126/science.1254803. (Available from: http://dx.doi.org/10.1126/science.1254803) [DOI] [PubMed] [Google Scholar]
- 8.Azadfar S, Cheraghali F, Moradi A, et al. Herpes simplex virus meningitis in children in south east of Caspian sea, Iran. Jundishapur J Microbiol. 2014;7(1):e8599. doi: 10.5812/jjm.8599. (Available from: http://dx.doi.org/10.5812/jjm.8599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bachmann MF, Barner M, Viola A, et al. Distinct kinetics of cytokine production and cytolysis in effector and memory T cells after viral infection. Eur J Immunol. 1999;29(1):291–299. doi: 10.1002/(SICI)1521-4141(199901)29:01<291::AID-IMMU291>3.0.CO;2-K. (Available from: http://dx.doi.org/10.1002/(SICI)1521-4141(199901)29:01<291::AID-IMMU291>3.0.CO;2-K) [DOI] [PubMed] [Google Scholar]
- 10.Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682–687. doi: 10.1038/nature04444. (Available from: http://dx.doi.org/10.1038/nature04444) [DOI] [PubMed] [Google Scholar]
- 11.Barr DP, Belz GT, Reading PC, et al. A role for plasmacytoid dendritic cells in the rapid IL-18-dependent activation of NK cells following HSV-1 infection. Eur J Immunol. 2007;37(5):1334–1342. doi: 10.1002/eji.200636362. (Available from: http://dx.doi.org/10.1002/eji.200636362) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beffert U, Bertrand P, Champagne D, et al. HSV-1 in brain and risk of Alzheimer’s disease. Lancet. 1998;351(9112):1330–1331. doi: 10.1016/S0140-6736(05)79057-7. (Available from: http://dx.doi.org/10.1016/S0140-6736(05)79057-7) [DOI] [PubMed] [Google Scholar]
- 13.Bengsch B, Seigel B, Ruhl M, et al. Coexpression of PD-1, 2B4, CD160 and KLRG1 on exhausted HCV-specific CD8+ T cells is linked to antigen recognition and T cell differentiation. PLoS Pathog. 2010;6(6):e1000947. doi: 10.1371/journal.ppat.1000947. (Available from: http://dx.doi.org/10.1371/journal.ppat.1000947) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bradshaw MJ, Venkatesan A. Herpes simplex virus-1 encephalitis in adults: pathophysiology, diagnosis, and management. Neurotherapeutics. 2016;13(3):493–508. doi: 10.1007/s13311-016-0433-7. (Available from: http://dx.doi.org/10.1007/s13311-016-0433-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buela KA, Hendricks RL. Cornea-infiltrating and lymph node dendritic cells contribute to CD4+ T cell expansion after herpes simplex virus-1 ocular infection. J Immunol. 2015;194(1):379–387. doi: 10.4049/jimmunol.1402326. (Available from: http://dx.doi.org/10.4049/jimmunol.1402326) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burrel S, Boutolleau D, Azar G, et al. Phenotypic and genotypic characterization of acyclovir-resistant corneal HSV-1 isolates from immunocompetent patients with recurrent herpetic keratitis. J Clin Virol. 2013;58(1):321–324. doi: 10.1016/j.jcv.2013.05.001. (Available from: http://dx.doi.org/10.1016/j.jcv.2013.05.001) [DOI] [PubMed] [Google Scholar]
- 17.Callan MF, Tan L, Annels N, et al. Direct visualization of antigen-specific CD8+ T cells during the primary immune response to epstein-barr virus in vivo. J Exp Med. 1998;187(9):1395–1402. doi: 10.1084/jem.187.9.1395. (Available from: http://dx.doi.org/10.1084/jem.187.9.1395) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cerwenka A, Morgan TM, Dutton RW. Naive, effector, and memory CD8 T cells in protection against pulmonary influenza virus infection: homing properties rather than initial frequencies are crucial. J Immunol. 1999;163(10):5535–5543. doi: 10.4049/jimmunol.163.10.5535. [DOI] [PubMed] [Google Scholar]
- 19.Chentoufi AA, Binder NR, Berka N, et al. Asymptomatic human CD4+ cytotoxic T-cell epitopes identified from herpes simplex virus glycoprotein B. J Virol. 2008;82(23):11792–11802. doi: 10.1128/JVI.00692-08. (Available from: http://dx.doi.org/10.1128/JVI.00692-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chentoufi AA, Dasgupta G, Christensen ND, et al. A novel HLA (HLA-A*0201) transgenic rabbit model for preclinical evaluation of human CD8+ T cell epitope-based vaccines against ocular herpes. J Immunol. 2010;184(5):2561–2571. doi: 10.4049/jimmunol.0902322. (Available from: http://dx.doi.org/10.4049/jimmunol.0902322) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chi CC, Wang SH, Delamere FM, et al. Interventions for prevention of herpes simplex labialis (cold sores on the lips) Cochrane Database Syst Rev. 2015;(8):CD010095. doi: 10.1002/14651858.CD010095.pub2. (Available from: http://dx.doi.org/10.1002/14651858.CD010095.pub2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coleman JL, Shukla D. Recent advances in vaccine development for herpes simplex virus types I and II. Hum Vaccin Immunother. 2013;9(4):729–735. doi: 10.4161/hv.23289. (Available from: http://dx.doi.org/10.4161/hv.23289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dasgupta G, Chentoufi AA, Kalantari M, et al. Immunodominant “asymptomatic” herpes simplex virus 1 and 2 protein antigens identified by probing whole-orfome microarrays with serum antibodies from seropositive asymptomatic versus symptomatic individuals. J Virol. 2012;86(8):4358–4369. doi: 10.1128/JVI.07107-11. (Available from: http://dx.doi.org/10.1128/JVI.07107-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Efstathiou S, Preston CM. Towards an understanding of the molecular basis of herpes simplex virus latency. Virus Res. 2005;111(2):108–119. doi: 10.1016/j.virusres.2005.04.017. (Available from: http://dx.doi.org/10.1016/j.virusres.2005.04.017) [DOI] [PubMed] [Google Scholar]
- 25.Egan KP, Wu S, Wigdahl B, et al. Immunological control of herpes simplex virus infections. J Neurovirol. 2013;19(4):328–345. doi: 10.1007/s13365-013-0189-3. (Available from: http://dx.doi.org/10.1007/s13365-013-0189-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eisenstein LE, Calio AJ, Cunha BA. Herpes simplex (HSV-1) aseptic meningitis. Heart Lung J Acute Critical Care. 2004;33(3):196–197. doi: 10.1016/j.hrtlng.2002.12.002. (Available from: http://dx.doi.org/10.1016/j.hrtlng.2002.12.002) [DOI] [PubMed] [Google Scholar]
- 27.Eriksson CE, Studahl M, Bergstrom T. Acute and prolonged complement activation in the central nervous system during herpes simplex encephalitis. J Neuroimmunol. 2016;295-296:130–138. doi: 10.1016/j.jneuroim.2016.04.013. (Available from: http://dx.doi.org/10.1016/j.jneuroim.2016.04.013) [DOI] [PubMed] [Google Scholar]
- 28.Faron ML, Ledeboer NA, Patel A, et al. Multicenter evaluation of meridian bioscience HSV 1&2 molecular assay for detection of herpes simplex virus 1 and 2 from clinical cutaneous and mucocutaneous specimens. J Clin Microbiol. 2016;54(8):2008–2013. doi: 10.1128/JCM.00483-16. (Available from: http://dx.doi.org/10.1128/JCM.00483-16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fong CY, Aye AM, Peyman M, et al. Neonatal herpes simplex virus type-1 central nervous system disease with acute retinal necrosis. Pediatr Infect Dis J. 2014;33(4):424–426. doi: 10.1097/INF.0000000000000137. (Available from: http://dx.doi.org/10.1097/INF.0000000000000137) [DOI] [PubMed] [Google Scholar]
- 30.Fourcade J, Sun Z, Benallaoua M, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207(10):2175–2186. doi: 10.1084/jem.20100637. (Available from: http://dx.doi.org/10.1084/jem.20100637) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frank GM, Buela KA, Maker DM, et al. Early responding dendritic cells direct the local NK response to control herpes simplex virus 1 infection within the cornea. J Immunol. 2012;188(3):1350–1359. doi: 10.4049/jimmunol.1101968. (Available from: http://dx.doi.org/10.4049/jimmunol.1101968) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gebhardt T, Wakim LM, Eidsmo L, et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10(5):524–530. doi: 10.1038/ni.1718. (Available from: http://dx.doi.org/10.1038/ni.1718) [DOI] [PubMed] [Google Scholar]
- 33.Groettrup M, Kraft R, Kostka S, et al. A third interferon-γ-induced subunit exchange in the 20S proteasome. Eur J Immunol. 1996;26(4):863–869. doi: 10.1002/eji.1830260421. (Available from: http://dx.doi.org/10.1002/eji.1830260421) [DOI] [PubMed] [Google Scholar]
- 34.Harkness JM, Kader M, Deluca NA. Transcription of the herpes simplex virus 1 genome during productive and quiescent infection of neuronal and nonneuronal cells. J Virol. 2014;88(12):6847–6861. doi: 10.1128/JVI.00516-14. (Available from: http://dx.doi.org/10.1128/JVI.00516-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hashido M, Kawana T, Matsunaga Y, et al. Changes in prevalence of herpes simplex virus type 1 and 2 antibodies from 1973 to 1993 in the rural districts of Japan. Microbiol Immunol. 1999;43(2):177–180. doi: 10.1111/j.1348-0421.1999.tb02390.x. (Available from: http://dx.doi.org/10.1111/j.1348-0421.1999.tb02390.x) [DOI] [PubMed] [Google Scholar]
- 36.Hendricks RL, Janowicz M, Tumpey TM. Critical role of corneal langerhans cells in the CD4-but not CD8-mediated immunopathology in herpes simplex virus-1-infected mouse corneas. J Immunol. 1992;148(8):2522–2529. [PubMed] [Google Scholar]
- 37.Hoshino Y, Pesnicak L, Cohen JI, et al. Rates of reactivation of latent herpes simplex virus from mouse trigeminal ganglia ex vivo correlate directly with viral load and inversely with number of infiltrating CD8+ T cells. J Virol. 2007;81(15):8157–8164. doi: 10.1128/JVI.00474-07. (Available from: http://dx.doi.org/10.1128/JVI.00474-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hudson SJ, Streilein JW. Functional cytotoxic T cells are associated with focal lesions in the brains of SJL mice with experimental herpes simplex encephalitis. J Immunol. 1994;152(11):5540–5547. [PubMed] [Google Scholar]
- 39.Itzhaki RF, Lin WR, Wilcock GK, et al. HSV-1 and risk of Alzheimer’s disease. Lancet. 1998;352(9123):238. doi: 10.1016/S0140-6736(05)77844-2. (Available from: http://dx.doi.org/10.1016/S0140-6736(05)77844-2) [DOI] [PubMed] [Google Scholar]
- 40.Jiang X, Chentoufi AA, Hsiang C, et al. The herpes simplex virus type 1 latency-associated transcript can protect neuron-derived C1300 and Neuro2A cells from granzyme B-induced apoptosis and CD8 T-cell killing. J Virol. 2011;85(5):2325–2332. doi: 10.1128/JVI.01791-10. (Available from: http://dx.doi.org/10.1128/JVI.01791-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jing L, Haas J, Chong TM, et al. Cross-presentation and genome-wide screening reveal candidate T cells antigens for a herpes simplex virus type 1 vaccine. J Clin Invest. 2012;122(2):654–673. doi: 10.1172/JCI60556. (Available from: http://dx.doi.org/10.1172/JCI60556) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson AJ, Chu CF, Milligan GN. Effector CD4+ T-cell involvement in clearance of infectious herpes simplex virus type 1 from sensory ganglia and spinal cords. J Virol. 2008;82(19):9678–9688. doi: 10.1128/JVI.01159-08. (Available from: http://dx.doi.org/10.1128/JVI.01159-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2(4):251–262. doi: 10.1038/nri778. (Available from: http://dx.doi.org/10.1038/nri778) [DOI] [PubMed] [Google Scholar]
- 44.Kaech SM, Hemby S, Kersh E, et al. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111(6):837–851. doi: 10.1016/s0092-8674(02)01139-x. (Available from: http://dx.doi.org/10.1016/S0092-8674(02)01139-X) [DOI] [PubMed] [Google Scholar]
- 45.Kalia V, Sarkar S, Ahmed R. CD8 T-cell memory differentiation during acute and chronic viral infections. In: Zanetti M, Schoenberger SP, editors. Memory T Cells. New York: Springer Science+Business Media; 2010. pp. 79–95. (Available from: http://dx.doi.org/10.1007/978-1-4419-6451-9_7) [DOI] [PubMed] [Google Scholar]
- 46.Kastrukoff LF, Lau AS, Kim SU. Multifocal CNS demyelination following peripheral inoculation with herpes simplex virus type 1. Ann Neurol. 1987;22(1):52–59. doi: 10.1002/ana.410220113. (Available from: http://dx.doi.org/10.1002/ana.410220113) [DOI] [PubMed] [Google Scholar]
- 47.Kastrukoff LF, Lau AS, Leung GY, et al. Contrasting effects of immunosuppression on herpes simplex virus type I (HSV I) induced central nervous system (CNS) demyelination in mice. J Neurol Sci. 1993;117(1-2):148–158. doi: 10.1016/0022-510X(93)90167-W. (Available from: http://dx.doi.org/10.1016/0022-510X(93)90167-W) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7(2):131–137. doi: 10.1038/ni1303. (Available from: http://dx.doi.org/10.1038/ni1303) [DOI] [PubMed] [Google Scholar]
- 49.Khanna KM, Bonneau RH, Kinchington PR, et al. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity. 2003;18(5):593–603. doi: 10.1016/s1074-7613(03)00112-2. (Available from: http://dx.doi.org/10.1016/S1074-7613(03)00112-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khanna KM, Lepisto AJ, Decman V, et al. Immune control of herpes simplex virus during latency. Curr Opin Immunol. 2004;16(4):463–469. doi: 10.1016/j.coi.2004.05.003. (Available from: http://dx.doi.org/10.1016/j.coi.2004.05.003) [DOI] [PubMed] [Google Scholar]
- 51.Khoury-Hanold W, Yordy B, Kong P, et al. Viral spread to enteric neurons links genital HSV-1 infection to toxic megacolon and lethality. Cell Host Microbe. 2016;19(6):788–799. doi: 10.1016/j.chom.2016.05.008. (Available from: http://dx.doi.org/10.1016/j.chom.2016.05.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kieff ED, Bachenheimer SL, Roizman B. Size, composition, and structure of the deoxyribonucleic acid of herpes simplex virus subtypes 1 and 2. J Virol. 1971;8(2):125–132. doi: 10.1128/jvi.8.2.125-132.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim M, Osborne NR, Zeng W, et al. Herpes simplex virus antigens directly activate NK cells via TLR2, thus facilitating their presentation to CD4 T lymphocytes. J Immunol. 2012;188(9):4158–4170. doi: 10.4049/jimmunol.1103450. (Available from: http://dx.doi.org/10.4049/jimmunol.1103450) [DOI] [PubMed] [Google Scholar]
- 54.Kodukula P, Liu T, Rooijen NV, et al. Macrophage control of herpes simplex virus type 1 replication in the peripheral nervous system. J Immunol. 1999;162(5):2895–2905. [PubMed] [Google Scholar]
- 55.Kohl S. Role of antibody-dependent cellular cytotoxicity in defense against herpes simplex virus infections. Rev Infect Dis. 1991;13(1):108–114. doi: 10.1093/clinids/13.1.108. (Available from: http://dx.doi.org/10.1093/clinids/13.1.108) [DOI] [PubMed] [Google Scholar]
- 56.Krichevskaia GI, Andzhelov VO, Katargina LA, et al. Reactivation of persistent herpes virus infection as a factor of endogenous uveitis in children. Vestn Oftalmol. 2005;121(2):22–24. (in Russian) [PubMed] [Google Scholar]
- 57.Krzysiek R, de Goër de Herve MG, Yang H, et al. Tissue competence imprinting and tissue residency of CD8 T cells. Front Immunol. 2013;4:283. doi: 10.3389/fimmu.2013.00283. (Available from: http://dx.doi.org/10.3389/fimmu.2013.00283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kukhanova MK, Korovina AN, Kochetkov SN. Human herpes simplex virus: life cycle and development of inhibitors. Biochemistry (Mosc) 2014;79(13):1635–1652. doi: 10.1134/S0006297914130124. (Available from: http://dx.doi.org/10.1134/S0006297914130124) [DOI] [PubMed] [Google Scholar]
- 59.Kuklin NA, Daheshia M, Chun S, et al. Role of mucosal immunity in herpes simplex virus infection. J Immunol. 1998;160(12):5998–6003. [PubMed] [Google Scholar]
- 60.Laing KJ, Dong L, Sidney J, et al. Immunology in the clinic review series; focus on host responses: T cell responses to herpes simplex viruses. Clin Exp Immunol. 2012;167(1):47–58. doi: 10.1111/j.1365-2249.2011.04502.x. (Available from: http://dx.doi.org/10.1111/j.1365-2249.2011.04502.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee YJ, Jameson SC, Hogquist KA. Alternative memory in the CD8 T cell lineage. Trends Immunol. 2011;32(2):50–56. doi: 10.1016/j.it.2010.12.004. (Available from: http://dx.doi.org/10.1016/j.it.2010.12.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lepisto AJ, Frank GM, Xu M, et al. CD8 T cells mediate transient herpes stromal keratitis in CD4-deficient mice. Invest Ophthalmol Vis Sci. 2006;47(8):3400–3409. doi: 10.1167/iovs.05-0898. (Available from: http://dx.doi.org/10.1167/iovs.05-0898) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li J, Hu S, Zhou L, et al. Interferon lambda inhibits herpes simplex virus type I infection of human astrocytes and neurons. Glia. 2011;59(1):58–67. doi: 10.1002/glia.21076. (Available from: http://dx.doi.org/10.1002/glia.21076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li J, Ye L, Wang X, et al. Induction of interferon-λ contributes to Toll-like receptor 3-mediated herpes simplex virus type 1 inhibition in astrocytes. J Neurosci Res. 2012;90(2):399–406. doi: 10.1002/jnr.22758. (Available from: http://dx.doi.org/10.1002/jnr.22758) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu T, Khanna KM, Chen X, et al. CD8+ T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J Exp Med. 2000;191(9):1459–1466. doi: 10.1084/jem.191.9.1459. (Available from: http://dx.doi.org/10.1084/jem.191.9.1459) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Luckheeram RV, Zhou R, Verma AD, et al. CD4+ T cells: differentiation and functions. Clin Dev Immunol. 2012;2012:925135. doi: 10.1155/2012/925135. (Available from: http://dx.doi.org/10.1155/2012/925135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mackay LK, Stock AT, Ma JZ, et al. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc Natl Acad Sci USA. 2012;109(18):7037–7042. doi: 10.1073/pnas.1202288109. (Available from: http://dx.doi.org/10.1073/pnas.1202288109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Manickan E, Rouse RJ, Yu Z, et al. Genetic immunization against herpes simplex virus. Protection is mediated by CD4+ T lymphocytes. J Immunol. 1995;155(1):259–265. [PubMed] [Google Scholar]
- 69.Manickan E, Francotte M, Kuklin N, et al. Vaccination with recombinant vaccinia viruses expressing ICP27 induces protective immunity against herpes simplex virus through CD4+ Th1+ T cells. J Virol. 1995;69(8):4711–4716. doi: 10.1128/jvi.69.8.4711-4716.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marrack P, Kappler J. The T cell receptor. Science. 1987;238(4830):1073–1079. doi: 10.1126/science.3317824. (Available from: http://dx.doi.org/10.1126/science.3317824) [DOI] [PubMed] [Google Scholar]
- 71.Marten NW, Stohlman SA, Zhou J, et al. Kinetics of virus-specific CD8+-T-cell expansion and trafficking following central nervous system infection. J Virol. 2003;77(4):2775–2778. doi: 10.1128/JVI.77.4.2775-2778.2003. (Available from: http://dx.doi.org/10.1128/JVI.77.4.2775-2778.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Medzhitov R, Janeway CJr. Innate immunity. N Engl J Med. 2000;343(5):338–344. doi: 10.1056/NEJM200008033430506. (Available from: http://dx.doi.org/10.1056/NEJM200008033430506) [DOI] [PubMed] [Google Scholar]
- 73.Melchjorsen J. Sensing herpes: more than Toll. Rev Med Virol. 2012;22(2):106–121. doi: 10.1002/rmv.716. (Available from: http://dx.doi.org/10.1002/rmv.716) [DOI] [PubMed] [Google Scholar]
- 74.Menasria R, Canivet C, Piret J, et al. Infiltration pattern of blood monocytes into the central nervous system during experimental herpes simplex virus encephalitis. PLoS ONE. 2015;10(12):e0145773. doi: 10.1371/journal.pone.0145773. (Available from: http://dx.doi.org/10.1371/journal.pone.0145773) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mertz GJ, Rosenthal SL, Stanberry LR. Is herpes simplex virus type 1 (HSV-1) now more common than HSV-2 in first episodes of genital herpes? Sex Transm Dis. 2003;30(10):801–802. doi: 10.1097/01.OLQ.0000093080.55201.D1. (Available from: http://dx.doi.org/10.1097/01.OLQ.0000093080.55201.D1) [DOI] [PubMed] [Google Scholar]
- 76.Molesworth-Kenyon SJ, Popham N, Milam A, et al. Resident corneal cells communicate with neutrophils leading to the production of IP-10 during the primary inflammatory response to HSV-1 infection. Int J Inflam. 2012;2012:810359. doi: 10.1155/2012/810359. (Available from: http://dx.doi.org/10.1155/2012/810359) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mora P, Guex-Crosier Y, Kamberi E, et al. Acute retinal necrosis in primary herpes simplex virus type I infection. Pediatr Infect Dis J. 2009;28(2):163–164. doi: 10.1097/INF.0b013e318186258f. (Available from: http://dx.doi.org/10.1097/INF.0b013e318186258f) [DOI] [PubMed] [Google Scholar]
- 78.Mosmann TR, Coffman RL. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7(1):145–173. doi: 10.1146/annurev.iy.07.040189.001045. (Available from: http://dx.doi.org/10.1146/annurev.iy.07.040189.001045) [DOI] [PubMed] [Google Scholar]
- 79.Mosmann TR, Li L, Sad S. Functions of CD8 T-cell subsets secreting different cytokine patterns. Semin Immunol. 1997;9(2):87–92. doi: 10.1006/smim.1997.0065. (Available from: http://dx.doi.org/10.1006/smim.1997.0065) [DOI] [PubMed] [Google Scholar]
- 80.Mott KR, Gate D, Zandian M, et al. Macrophage IL-12p70 signaling prevents HSV-1-induced CNS autoimmunity triggered by autoaggressive CD4+ Tregs. Invest Ophthalmol Vis Sci. 2011;52(5):2321–2333. doi: 10.1167/iovs.10-6536. (Available from: http://dx.doi.org/10.1167/iovs.10-6536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mott KR, Allen SJ, Zandian M, et al. CD8α dendritic cells drive establishment of HSV-1 latency. PLoS ONE. 2014;9(4):e93444. doi: 10.1371/journal.pone.0093444. (Available from: http://dx.doi.org/10.1371/journal.pone.0093444) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Murali-Krishna K, Altman JD, Suresh M, et al. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8(2):177–187. doi: 10.1016/s1074-7613(00)80470-7. (Available from: http://dx.doi.org/10.1016/S1074-7613(00)80470-7) [DOI] [PubMed] [Google Scholar]
- 83.Murphy EA, Davis JM, Brown AS, et al. Effect of IL-6 deficiency on susceptibility to HSV-1 respiratory infection and intrinsic macrophage antiviral resistance. J Interferon Cytokine Res. 2008;28(10):589–595. doi: 10.1089/jir.2007.0103. (Available from: http://dx.doi.org/10.1089/jir.2007.0103) [DOI] [PubMed] [Google Scholar]
- 84.Nair A, Hunzeker J, Bonneau RH. Modulation of microglia and CD8+ T cell activation during the development of stress-induced herpes simplex virus type-1 encephalitis. Brain Behav Immun. 2007;21(6):791–806. doi: 10.1016/j.bbi.2007.01.005. (Available from: http://dx.doi.org/10.1016/j.bbi.2007.01.005) [DOI] [PubMed] [Google Scholar]
- 85.Nash AA, Quartey-Papafio R, Wildy P. Cell-mediated immunity in herpes simplex virus-infected mice: functional analysis of lymph node cells during periods of acute and latent infection, with reference to cytotoxic and memory cells. J Gen Virol. 1980;49(2):309–317. doi: 10.1099/0022-1317-49-2-309. (Available from: http://dx.doi.org/10.1099/0022-1317-49-2-309) [DOI] [PubMed] [Google Scholar]
- 86.Nicolatou-Galitis O, Athanassiadou P, Kouloulias V, et al. Herpes simplex virus-1 (HSV-1) infection in radiation-induced oral mucositis. Support Care Cancer. 2006;14(7):753–762. doi: 10.1007/s00520-005-0006-5. (Available from: http://dx.doi.org/10.1007/s00520-005-0006-5) [DOI] [PubMed] [Google Scholar]
- 87.Nicoll MP, Proenca JT, Efstathiou S. The molecular basis of herpes simplex virus latency. FEMS Microbiol Rev. 2012;36(3):684–705. doi: 10.1111/j.1574-6976.2011.00320.x. (Available from: http://dx.doi.org/10.1111/j.1574-6976.2011.00320.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nieuwenhuis RF, van Doornum GJ, Mulder PG, et al. Importance of herpes simplex virus type-1 (HSV-1) in primary genital herpes. Acta Derm Venereol. 2006;86(2):129–134. doi: 10.2340/00015555-0029. (Available from: http://dx.doi.org/10.2340/00015555-0029) [DOI] [PubMed] [Google Scholar]
- 89.Noisakran S, Carr DJ. Lymphocytes delay kinetics of HSV-1 reactivation from in vitro explants of latent infected trigeminal ganglia. J Neuroimmunol. 1999;95(1-2):126–135. doi: 10.1016/s0165-5728(99)00008-9. (Available from: http://dx.doi.org/10.1016/S0165-5728(99)00008-9) [DOI] [PubMed] [Google Scholar]
- 90.Paladino P, Mossman KL. Mechanisms employed by herpes simplex virus 1 to inhibit the interferon response. J Interferon Cytokine Res. 2009;29(9):599–607. doi: 10.1089/jir.2009.0074. (Available from: http://dx.doi.org/10.1089/jir.2009.0074) [DOI] [PubMed] [Google Scholar]
- 91.Paludan SR, Bowie AG, Horan KA, et al. Recognition of herpesviruses by the innate immune system. Nat Rev Immunol. 2011;11(2):143–154. doi: 10.1038/nri2937. (Available from: http://dx.doi.org/10.1038/nri2937) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Paul WE, Seder RA. Lymphocyte responses and cytokines. Cell. 1994;76(2):241–251. doi: 10.1016/0092-8674(94)90332-8. (Available from: http://dx.doi.org/10.1016/0092-8674(94)90332-8) [DOI] [PubMed] [Google Scholar]
- 93.Pereira RA, Scalzo A, Simmons A. Cutting edge: a NK complex-linked locus governs acute versus latent herpes simplex virus infection of neurons. J Immunol. 2001;166(10):5869–5873. doi: 10.4049/jimmunol.166.10.5869. (Available from: http://dx.doi.org/10.4049/jimmunol.166.10.5869) [DOI] [PubMed] [Google Scholar]
- 94.Petrovas C, Price DA, Mattapallil J, et al. SIV-specific CD8+ T cells express high levels of PD1 and cytokines but have impaired proliferative capacity in acute and chronic SIVmac251 infection. Blood. 2007;110(3):928–936. doi: 10.1182/blood-2007-01-069112. (Available from: http://dx.doi.org/10.1182/blood-2007-01-069112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Preston CM. Repression of viral transcription during herpes simplex virus latency. J Gen Virol. 2000;81:1–19. doi: 10.1099/0022-1317-81-1-1. (Available from: http://dx.doi.org/10.1099/0022-1317-81-1-1) [DOI] [PubMed] [Google Scholar]
- 96.Richardson VN, Davis SA, Gustafson CJ, et al. Patterns of disease and treatment of cold sores. J Dermatolog Treat. 2013;24(6):439–443. doi: 10.3109/09546634.2013.789476. (Available from: http://dx.doi.org/10.3109/09546634.2013.789476) [DOI] [PubMed] [Google Scholar]
- 97.Sakuishi K, Apetoh L, Sullivan JM, et al. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207(10):2187–2194. doi: 10.1084/jem.20100643. (Available from: http://dx.doi.org/10.1084/jem.20100643) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sant AJ, McMichael A. Revealing the role of CD4+ T cells in viral immunity. J Exp Med. 2012;209(8):1391–1395. doi: 10.1084/jem.20121517. (Available from: http://dx.doi.org/10.1084/jem.20121517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schenkel JM, Masopust D. Tissue-resident memory T cells. Immunity. 2014;41(6):886–897. doi: 10.1016/j.immuni.2014.12.007. (Available from: http://dx.doi.org/10.1016/j.immuni.2014.12.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schenkel JM, Fraser KA, Beura LK, et al. T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science. 2014;346(6205):98–101. doi: 10.1126/science.1254536. (Available from: http://dx.doi.org/10.1126/science.1254536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schietinger A, Greenberg PD. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol. 2014;35(2):51–60. doi: 10.1016/j.it.2013.10.001. (Available from: http://dx.doi.org/10.1016/j.it.2013.10.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schroder K, Hertzog PJ, Ravasi T, et al. Interferon-γ: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75(2):163–189. doi: 10.1189/jlb.0603252. (Available from: http://dx.doi.org/10.1189/jlb.0603252) [DOI] [PubMed] [Google Scholar]
- 103.Sehrawat S, Rouse BT. Tregs and infections: on the potential value of modifying their function. J Leukoc Biol. 2011;90(6):1079–1087. doi: 10.1189/jlb.0611271. (Available from: http://dx.doi.org/10.1189/jlb.0611271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sepulveda E, Brethauer U, Rojas J, et al. Oral ulcers in children under chemotherapy: clinical characteristics and their relation with herpes simplex virus type 1 and Candida albicans . Med Oral Patol Oral Cir Bucal. 2005;10(Suppl. 1):E1–E8. [PubMed] [Google Scholar]
- 105.Sheridan BS, Cherpes TL, Urban J, et al. Reevaluating the CD8 T-cell response to herpes simplex virus type 1: involvement of CD8 T cells reactive to subdominant epitopes. J Virol. 2009;83(5):2237–2245. doi: 10.1128/JVI.01699-08. (Available from: http://dx.doi.org/10.1128/JVI.01699-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Simmons A. H-2-linked genes influence the severity of herpes simplex virus infection of the peripheral nervous system. J Exp Med. 1989;169(4):1503–1507. doi: 10.1084/jem.169.4.1503. (Available from: http://dx.doi.org/10.1084/jem.169.4.1503) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Simmons A, Tscharke DC. Anti-CD8 impairs clearance of herpes simplex virus from the nervous system: implications for the fate of virally infected neurons. J Exp Med. 1992;175(5):1337–1344. doi: 10.1084/jem.175.5.1337. (Available from: http://dx.doi.org/10.1084/jem.175.5.1337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Simmons A, Tscharke D, Speck P. The role of immune mechanisms in control of herpes simplex virus infection of the peripheral nervous system. Curr Top Microbiol Immunol. 1992;179:31–56. doi: 10.1007/978-3-642-77247-4_3. [DOI] [PubMed] [Google Scholar]
- 109.Sin J, Kim JJ, Pachuk C, et al. DNA vaccines encoding interleukin-8 and rantes enhance antigen-specific Th1-type CD4+ T-cell-mediated protective immunity against herpes simplex virus type 2 in vivo. J Virol. 2000;74(23):11173–11180. doi: 10.1128/jvi.74.23.11173-11180.2000. (Available from: http://dx.doi.org/10.1128/JVI.74.23.11173-11180.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Srivastava R, Dervillez X, Khan AA, et al. The herpes simplex virus latency-associated transcript gene is associated with a broader repertoire of virus-specific exhausted CD8+ T cells retained within the trigeminal ganglia of latently infected HLA transgenic rabbits. J Virol. 2016;90(8):3913–3928. doi: 10.1128/JVI.02450-15. (Available from: http://dx.doi.org/10.1128/JVI.02450-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stanberry LR, Cunningham AL, Mindel A, et al. Prospects for control of herpes simplex virus disease through immunization. Clin Infect Dis. 2000;30(3):549–566. doi: 10.1086/313687. (Available from: http://dx.doi.org/10.1086/313687) [DOI] [PubMed] [Google Scholar]
- 112.Stohlman SA, Bergmann CC, Lin MT, et al. CTL effector function within the central nervous system requires CD4+ T cells. J Immunol. 1998;160(6):2896–2904. [PubMed] [Google Scholar]
- 113.Su C, Zhan G, Zheng C. Evasion of host antiviral innate immunity by HSV-1, an update. Virol J. 2016;13:38. doi: 10.1186/s12985-016-0495-5. (Available from: http://dx.doi.org/10.1186/s12985-016-0495-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Suazo PA, Ibanez FJ, Retamal-Diaz AR, et al. Evasion of early antiviral responses by herpes simplex viruses. Mediators Inflamm. 2015;2015:593757. doi: 10.1155/2015/593757. (Available from: http://dx.doi.org/10.1155/2015/593757) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Suryawanshi A, Veiga-Parga T, Rajasagi NK, et al. Role of IL-17 and Th17 cells in herpes simplex virus-induced corneal immunopathology. J Immunol. 2011;187(4):1919–1930. doi: 10.4049/jimmunol.1100736. (Available from: http://dx.doi.org/10.4049/jimmunol.1100736) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Suvas S, Kumaraguru U, Pack CD, et al. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J Exp Med. 2003;198(6):889–901. doi: 10.1084/jem.20030171. (Available from: http://dx.doi.org/10.1084/jem.20030171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Swiecki M, Wang Y, Gilfillan S, et al. Plasmacytoid dendritic cells contribute to systemic but not local antiviral responses to HSV infections. PLoS Pathog. 2013;9(10):e1003728. doi: 10.1371/journal.ppat.1003728. (Available from: http://dx.doi.org/10.1371/journal.ppat.1003728) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tigges MA, Leng S, Johnson DC, et al. Human herpes simplex virus (HSV)-specific CD8+ CTL clones recognize HSV-2-infected fibroblasts after treatment with IFN-γ or when virion host shutoff functions are disabled. J Immunol. 1996;156(10):3901–3910. [PubMed] [Google Scholar]
- 119.Tsatsos M, MacGregor C, Athanasiadis I, et al. Herpes simplex virus keratitis: an update of the pathogenesis and current treatment with oral and topical antiviral agents. Clin Exp Ophthalmol. 2016;44(9):824–837. doi: 10.1111/ceo.12785. (Available from: http://dx.doi.org/10.1111/ceo.12785) [DOI] [PubMed] [Google Scholar]
- 120.van Velzen M, Missotten T, van Loenen FB, et al. Acyclovir-resistant herpes simplex virus type 1 in intra-ocular fluid samples of herpetic uveitis patients. J Clin Virol. 2013;57(3):215–221. doi: 10.1016/j.jcv.2013.03.014. (Available from: http://dx.doi.org/10.1016/j.jcv.2013.03.014) [DOI] [PubMed] [Google Scholar]
- 121.Vogel K, Thomann S, Vogel B, et al. Both plasmacytoid dendritic cells and monocytes stimulate natural killer cells early during human herpes simplex virus type 1 infections. Immunology. 2014;143(4):588–600. doi: 10.1111/imm.12337. (Available from: http://dx.doi.org/10.1111/imm.12337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wagner EK, Bloom DC. Experimental investigation of herpes simplex virus latency. Clin Microbiol Rev. 1997;10(3):419–443. doi: 10.1128/cmr.10.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12(6):492–499. doi: 10.1038/ni.2035. (Available from: http://dx.doi.org/10.1038/ni.2035) [DOI] [PubMed] [Google Scholar]
- 124.Wherry EJ, Teichgraber V, Becker TC, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4(3):225–234. doi: 10.1038/ni889. (Available from: http://dx.doi.org/10.1038/ni889) [DOI] [PubMed] [Google Scholar]
- 125.Whitley RJ, Corey L, Arvin A, et al. Changing presentation of herpes simplex virus infection in neonates. J Infect Dis. 1988;158(1):109–116. doi: 10.1093/infdis/158.1.109. (Available from: http://dx.doi.org/10.1093/infdis/158.1.109) [DOI] [PubMed] [Google Scholar]
- 126.Wiesel M, Walton S, Richter K, et al. Virus-specific CD8 T cells: activation, differentiation and memory formation. APMIS. 2009;117(5-6):356–381. doi: 10.1111/j.1600-0463.2009.02459.x. (Available from: http://dx.doi.org/10.1111/j.1600-0463.2009.02459.x) [DOI] [PubMed] [Google Scholar]
- 127.Wuest TR, Carr DJ. The role of chemokines during herpes simplex virus-1 infection. Front Biosci. 2008;13:4862–4872. doi: 10.2741/3045. (Available from: http://dx.doi.org/10.2741/3045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xu F, Schillinger JA, Sternberg MR, et al. Seroprevalence and coinfection with herpes simplex virus type 1 and type 2 in the united states, 1988-1994. J Infect Dis. 2002;185(8):1019–1024. doi: 10.1086/340041. (Available from: http://dx.doi.org/10.1086/340041) [DOI] [PubMed] [Google Scholar]
- 129.Yasukawa M, Zarling JM. Human cytotoxic T cell clones directed against herpes simplex virus-infected cells. I. Lysis restricted by HLA class II MB and DR antigens. J Immunol. 1984;133(1):422–427. [PubMed] [Google Scholar]
- 130.Yun H, Rowe AM, Lathrop KL, et al. Reversible nerve damage and corneal pathology in murine herpes simplex stromal keratitis. J Virol. 2014;88(14):7870–7880. doi: 10.1128/JVI.01146-14. (Available from: http://dx.doi.org/10.1128/JVI.01146-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zajac AJ, Blattman JN, Murali-Krishna K, et al. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188(12):2205–2213. doi: 10.1084/jem.188.12.2205. (Available from: http://dx.doi.org/10.1084/jem.188.12.2205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zendri E, Venturi C, Ricci R, et al. Primary cutaneous plasmacytoma: a role for a triggering stimulus Clin . Exp Dermatol. 2005;30(3):229–231. doi: 10.1111/j.1365-2230.2004.01692.x. (Available from: http://dx.doi.org/10.1111/j.1365-2230.2004.01692.x) [DOI] [PubMed] [Google Scholar]
- 133.Zhang N, Bevan MJ. CD8+ T cells: foot soldiers of the immune system. Immunity. 2011;35(2):161–168. doi: 10.1016/j.immuni.2011.07.010. (Available from: http://dx.doi.org/10.1016/j.immuni.2011.07.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zheng M, Fields MA, Liu Y, et al. Neutrophils protect the retina of the injected eye from infection after anterior chamber inoculation of HSV-1 in BALB/c mice. Invest Ophthalmol Vis Sci. 2008;49(9):4018–4025. doi: 10.1167/iovs.08-1914. (Available from: http://dx.doi.org/10.1167/iovs.08-1914) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zheng SG. Regulatory T cells vs Th17: differentiation of Th17 versus Treg, are the mutually exclusive Am . J Clin Exp Immunol. 2013;2(1):94–106. [PMC free article] [PubMed] [Google Scholar]


