Abstract
Bone defects arising from a variety of reasons cannot be treated effectively without bone tissue reconstruction. Autografts and allografts have been used in clinical application for some time, but they have disadvantages. With the inherent drawback in the precision and reproducibility of conventional scaffold fabrication techniques, the results of bone surgery may not be ideal. This is despite the introduction of bone tissue engineering which provides a powerful approach for bone repair. Rapid prototyping technologies have emerged as an alternative and have been widely used in bone tissue engineering, enhancing bone tissue regeneration in terms of mechanical strength, pore geometry, and bioactive factors, and overcoming some of the disadvantages of conventional technologies. This review focuses on the basic principles and characteristics of various fabrication technologies, such as stereolithography, selective laser sintering, and fused deposition modeling, and reviews the application of rapid prototyping techniques to scaffolds for bone tissue engineering. In the near future, the use of scaffolds for bone tissue engineering prepared by rapid prototyping technology might be an effective therapeutic strategy for bone defects.
Keywords: Rapid prototyping, Bone tissue engineering, Scaffolds
1. Introduction
The use of biological materials to repair injured tissue dates back to prehistory (Ratner et al., 2004). Now, tissue transplantation (Hernigou, 2014), which was pioneered by Scottish surgeon Dr. John Hunter, has gradually became a reality and has played a critical role in every field of medicine, especially orthopedics. The prevalence of bone disorders caused by trauma, deformity, degeneration, or tumors, and which require bone tissue reconstruction, is increasing yearly. The morbidity of bone fractures and tumors as well as the expenditure on bone grafts continues to rise in both Asia and Western countries (Mithal et al., 2009; Orthoworld, 2011; 2014). These factors constantly promote innovation of the materials and manufacturing processes of bone graft substitutes.
For bone tissue reconstruction, appropriate materials are imperative. As the gold standard for bone defect repair, autografts possess ideal osteogenic, osteoinductive, and osteoconductive properties. However, limited resources as well as donor site infection and pain may limit its development (Campana et al., 2014; Oryan et al., 2014). Although the osteogenic capacities of allografts and xenografts are acceptable, they have risks including potential disease transmission and immunological rejection (Giannoudis et al., 2011; Oryan et al., 2014; Polo-Corrales et al., 2014). Currently, researchers are focusing more on artificial bone substitutes.
Ideal bone graft substitutes should closely mimic the environment of natural bone. The introduction of tissue engineering provides a new approach to repair bone defects. The three components of bone tissue engineering, scaffolds, seeding cells, and bioactive factors, form an interactive network. Among them, scaffolds play an important role. Ideal scaffolds for bone tissue engineering should meet the following requirements (Lee et al., 2010; Calori et al., 2011; Bose et al., 2012; Saiz et al., 2013; Narayan, 2014; Polo-Corrales et al., 2014). (1) Inner spaces should possess interconnecting pores, including both macropores (pore size: >100 µm) and micropores (pore size: <20 µm), which are beneficial for tissue growth, substance transplantation, and vascularization. (2) Scaffolds should consist of biodegradable or bioabsorbable materials with strong mechanical properties and controllable degradation kinetics, and should be able to transfer the load to the surrounding tissue. (3) They should have good interface affinity that allows cells to adhere, proliferate, and differentiate efficiently. (4) Scaffolds should be easily prepared at various sizes and shapes. (5) They should regulate the release of biologically active factors. Conventional scaffold fabrication technologies, including solvent-casting/particulate leaching (Huang et al., 2007; Chuenjitkuntaworn et al., 2010; Thadavirul et al., 2014), gas foaming (Montjovent et al., 2005; 2007), phase separation (Ma et al., 2001), emulsion freeze drying (Sultana and Wang, 2008; 2012), and freeze drying (Tabata, 2009), have been widely employed in the biomedical area for several decades. Although they can partially meet the requirements of ideal scaffolds, they still have inherent limitations. Specifically, they are unable to control size, geometry, or spatial distribution of pores, and a random porous structure with low reproducibility does not support bone formation (Hutmacher, 2001). Organic solvents used in fabrication processes pose the risks of toxicity and carcinogenicity to cells (Lee et al., 2010). The ingrowth of tissues is not sufficient because the cells colonize at the scaffold periphery, which will act as a barrier to diffusion of oxygen and nutrients (Sachlos and Czernuszka, 2003). This phenomenon is more apparent after mineralization of the tissue on the scaffold surface (Tabata, 2009). In addition, individualized scaffolds for patients cannot be achieved by conventional scaffold fabrication technologies. Encouragingly, with the emergence of the rapid prototyping (RP) technique, it is possible to solve these problems. The biggest advantage of RP technology is that it can individualize patients’ needs to efficiently apply scaffolds during surgery. Furthermore, it is able to precisely control the inner pore structure with high reproducibility (Lee et al., 2013).
2. Search strategy
To make this review focusing on the basic principles and characteristics of various RP technologies and the application of bone tissue engineering, we searched electronic databases (PubMed, Embase, Web of Science, ScienceDirect) using the terms “rapid prototyping”, “additive manufacturing”, “solid freeform fabrication”, “bone tissue engineering”, “scaffolds”, “stereolithography”, “selective laser sintering”, “fused deposition modeling”, and “three-dimensional printing” to look for papers published in English from 1995 to 2016 that reported the application of RP technologies to bone tissue engineering.
3. Rapid prototyping technique
RP, also referred to as additive manufacturing and solid freeform fabrication, is a kind of material processing method. Assisted by imaging data and computer-aided design (CAD) models, the RP technique directly manufactures highly accurate three-dimensional (3D) physical entities layer by layer (Frame and Huntley, 2012; Narayan, 2014). Moreover, the RP technique has quickly gained popularity in bone tissue engineering for its high precision, reproducibility, and controllable pore structure (Kim et al., 2012). According to the Wohler’s Report 2015 (Wohlers Associates, Inc., 2015), the global market for 3D printing has reached 4.1 billion dollars with a compound annual growth rate of 35.2%. Most notably, medical application is one of the fastest growing areas. The term “3D printing”, which is the one most exposed to the public, refers to the RP technique.
A wide variety of technologies are used in RP, which are similar in terms of the main procedures, including the creation and slicing of a virtual computer model followed by the layer-by-layer fabrication process. This process usually involves five steps (Narayan, 2014). First, a CAD model is created or captured from a physical entity by a digital method. Second, the CAD model is converted into an STL file for virtual slicing. Third, the STL file is sliced digitally into cross-sectional layers, also known as “pre-processing”. Fourth, the RP apparatus creates one layer of the prototype at a time, and then the workstation raises or lowers to the next layer and continues printing until the entire process is complete. Finally, “post-processing”, such as hardening and surface treatment, can be applied depending on the manufacturing technique and purpose.
3.1. Stereolithography
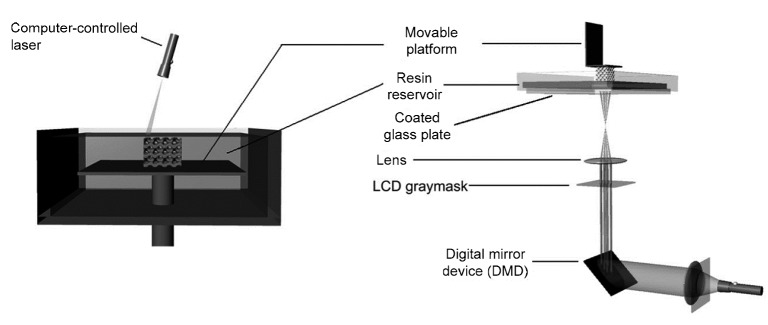
Stereolithography (SLA) is regarded as the first commercial RP technique and can be divided into two main types: bottom-up and top-down (Melchels et al., 2010). The basic principles of this technology are as follows (Figs. 1 and 2). With an aqueous photocurable polymer as the main raw material, SLA uses an ultraviolet laser beam to irradiate predetermined sites on the material surface, followed by solidification of these areas for photopolymerization, while the uncured peripheral region remains liquid. After solidifying one layer, the lifting table moves to the next layer, and the built layer is recoated with new liquid resin. By repeating this process, the final 3D entity is fabricated layer by layer. Then, the excess resin is drained and washed off.
Fig. 1.
Schematic diagram of SLA (Lee et al., 2010)
Fig. 2.
Schematic diagram of two types of SLA
Left: bottom-up approrch. Right: top-down approach. Reprinted from Melchels et al. (2010), Copyright 2010, with permission from Elsevier
The key advantage of SLA is extremely high feature resolution. Owing to the emergence of microstereolithography (MSTL), its precision can reach 20 µm (Melchels et al., 2010), which enables SLA to create more complex structures as scaffolds. Lee et al. (2011) reported the fabrication of highly porous, fully interconnected scaffolds (Fig. 3) for bone tissue engineering with the MSTL technique and a mixture of polypropylene fumarate (PPF) and bone morphogenetic protein (BMP)-2-loaded polylactide-co-glycolide (PLGA) microspheres. In vitro studies showed gradual release of growth factors and excellent differentiation of MC3T3-E1 pre-osteoblasts. Owen et al. (2016) used MSTL combined with emulsion templating to produce polymerized high internal phase emulsion (PolyHIPE) scaffolds consisting of two acrylate monomers and isobornyl acrylate. The scaffolds supported osteogenic differentiation of cells. MSTL allowed the individual fibers to be thin enough for cell ingrowth and plasma penetration.
Fig. 3.
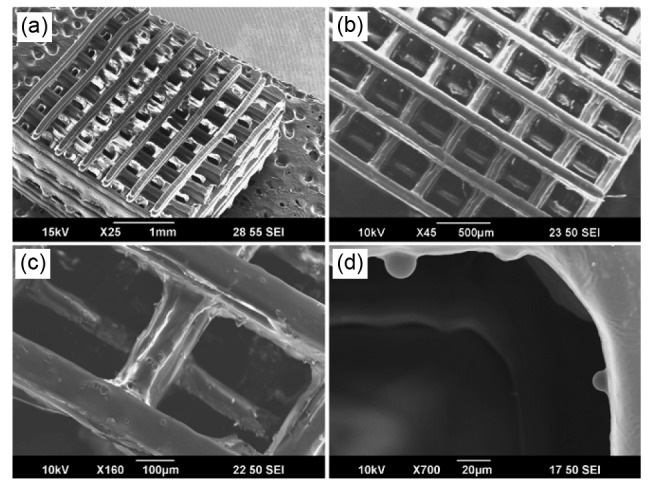
SEM image of high-precision 3D PPF-PLGA scaffold
(a–c) BMP-2-loaded 3D scaffold; (d) Microspheres embedded in the scaffold. Reprinted from Lee et al. (2011), Copyright 2011, with permission from Elsevier
However, the main disadvantage of SLA is the scarcity of available biocompatible resin for the SLA process. The materials used in SLA are mainly based on PPF, trimethylene carbonate, or gelatin (Lee et al., 2010). With continuous exploration and development, researchers are increasing the types of photocurable polymers and attempting to apply multiple materials (Arcaute et al., 2010). In addition, encapsulation of cells during the process has proved feasible (Chan et al., 2010; Lin et al., 2013). Although this method has not been used in bone tissue engineering, there is a great potential to use this technology in the future.
Given the weak mechanical strength of photopolymerization, researchers have tried to mix certain minerals with raw materials. Ronca et al. (2012) fabricated a composite scaffold using a mixture of poly-D L-lactide and nano-hydroxyapatite (nanoHA) with SLA. The presence of HA conferred higher structural strength and better biocompatibility.
Taking another perspective, researchers consider SLA as an indirect fabrication method. Brie et al. (2013) used SLA to directly fabricate an HA-resin structure from image data with a mixture of HA powder and a photosensitive resin, and then removed the resin by heating to obtain the final 3D porous HA scaffold. Furthermore, they implanted the scaffolds into bone defect sites of the skull of eight patients. During the 12-month follow-up, no major complications were observed, and X-rays showed perfect continuity between the implant and skull. Du et al. (2014) employed MSTL as an indirect RP technique to manufacture negative molds with acrylic resin. After impregnation with β-tricalcium phosphate (β-TCP) ceramic slurry and sintering, the scaffold was complete. It possessed not only the correct external shape but also an ideal internal structure for bone tissue ingrowth, which was confirmed by perfusion culture and in vitro tests.
3.2. Selective laser sintering
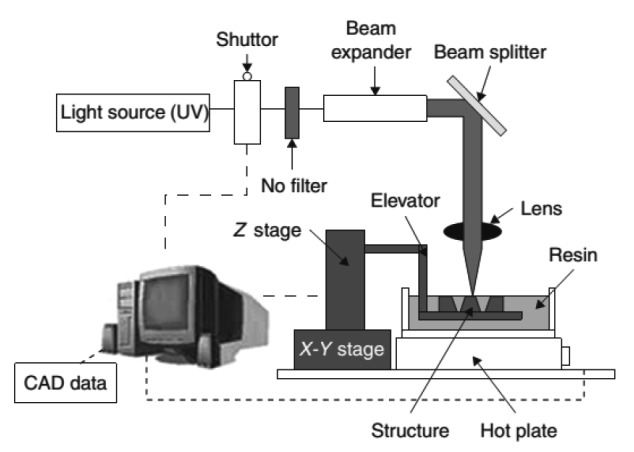
Selective laser sintering (SLS) is also a laser-based system, while its materials come in the form of powders. The basic principles of this technology are as follows (Fig. 4). SLS uses a CO2 laser beam to heat powder particles to glass transition temperature, which is near their melting point, sintering the material to directly form a solid model without entering the melting phase. Along with the workstation moving down layer by layer, new powder is spread on the sintered object with a roller. The process repeats until the 3D part is complete.
Fig. 4.
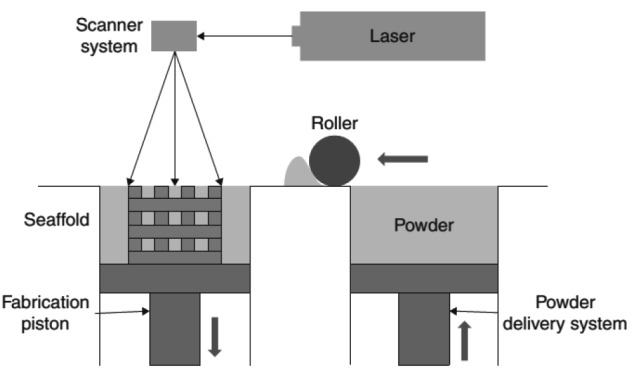
Schematic diagram of SLS
Reprinted from Williams et al. (2005), Copyright 2005, with permission from Elsevier
Because unbound solid particles support any sintered cantilever structure, SLS does not need temporary support structures, which simplifies post-processing. In addition, material sources for SLS are comparatively wide ranging. For bone tissue engineering, scaffolds can be built through this technique with polycaprolactone (PCL) (Williams et al., 2005), poly-L-lactic acid (PLLA) (Tan et al., 2005), as well as inorganic ceramic (Duan et al., 2010) and their composites (Wiria et al., 2007; Simpson et al., 2008).
The bases of selective laser melting and electron beam melting are similar to those of SLS, except that an intense energy device is used to heat the powder above its melting point, forming a highly dense structure. However, the former two are only suitable for processing a single metal.
The outstanding advantage of SLS lies in its ability to directly fabricate metallic implants that can be applied to repair load-bearing bone. The resolution of features of this technique is determined by the powder size, diameter of the laser beam, and heat transfer in the powder bed (Chia and Wu, 2015). Heat conduction during the process may cause undesirable fusion of peripheral powder particles, which is called the “growth effect”, limiting the resolution of the final construct. SLS can also be used to produce polyetheretherketone (PEEK) scaffolds that are widely applied in orthopedic surgery. Roskies et al. (2016) used SLS to fabricate customized porous PEEK scaffolds for seeding mesenchymal stem cells (MSCs) to enhance osteodifferentiation.
Recent research concerning SLS for bone tissue engineering has focused on composite biomaterials. For example, studies have demonstrated the potential of polyvinyl alcohol HA (Wiria et al., 2008) or PCL/HA (Eosoly et al., 2010; Ye et al., 2010; Xia et al., 2013) biocomposites to improve hydrophilicity and mechanical strength. Likewise, a mixture of Ca-P/poly(hydroxybutyrate-co-hydroxyvalerate) (Duan and Wang, 2010; Duan et al., 2011) or carbonated HA/PLLA (Zhou et al., 2008; Duan et al., 2011) may enhance the adhesion, proliferation, and differentiation of cells. Feng et al. (2014) fabricated highly interconnected porous scaffolds by SLS with β-TCP doping of zinc oxide powder. They found excellent mechanical and biological properties of these scaffolds by evaluating fracture toughness, compressive strength, osteoinduction, and osteoconduction.
3.3. Aerosol jet printing
During aerosol jet printing, liquid ink droplets are replaced by a focused aerosol stream. A composite suspension is atomized into dense aerosol droplets (usually 1–5 µm in diameter) and then transported to the deposition head via a carrier gas (N2). In the calibration function of sheath gas, a high-speed co-axial aerosol stream is sprayed onto the substrate layer by layer to create the 3D structure.
Materials, including ceramics, metals, and polymers in the form of a solid or nanoparticles, can be used for aerosol jet printing. Liu and Webster (2011) attempted to use this method to fabricate 3D titanium (Ti)/PLGA nanocomposite scaffolds. In vitro cytocompatibility tests demonstrated enhancement of the permeability of this kind of scaffold. Furthermore, because aerosol jet printing is a low-temperature process, it is a good candidate for biomanufacturing. The low kinetic energy of droplets does not harm cells. However, there is little research on applying aerosol jet printing to bone tissue engineering.
3.4. Fused deposition modeling
Fused deposition modeling (FDM) is the first RP technique based on extruding a molten polymer. It is a mature technology with broad application, low cost, and rapid processes. Thermoplastic materials in the form of a filament are used as feedstock, and a pinch roller or screw feed mechanism is used to push the filament into a liquefier, followed by extruding fused materials onto the x-y-z platform through a computer-controlled nozzle. Because of the relatively low temperature, the fused filaments solidify and deposit layer by layer to complete the 3D model. Precise temperature control is essential for this procedure to achieve the desired accuracy. The architecture of the scaffold is determined by the nozzle diameter, deposition speed, space between filaments in the same layer, layer thickness, and deposition angle. Theoretically, polymer viscosity and nozzle size dominate the resolution of FDM, which can be relatively high provided there is no extrusion clogging (Narayan, 2014).
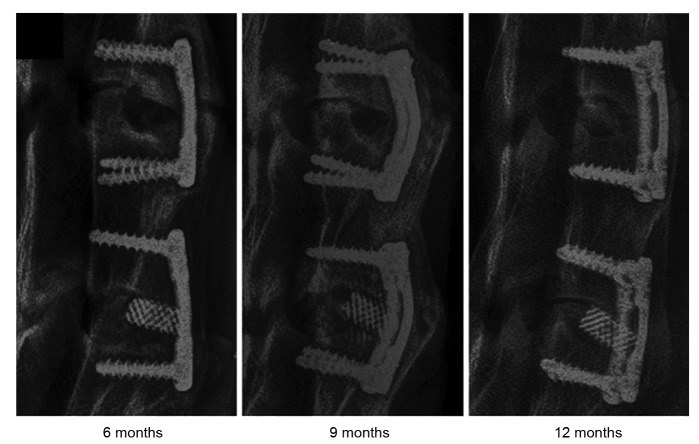
The process of FDM is simple and flexible. In addition, it does not require an organic solvent. Regardless of the early studies on collagen, ceramics, PLGA (Yen et al., 2009), and PCL (Zein et al., 2002), or the combinations of metals and polymers in recent years, researchers continue to broaden the range of materials for FDM, such as composite materials and nanomaterials. Li et al. (2014) made a comparison between a Ti cage and PCL-TCP scaffold prepared by FDM as a spinal fusion cage (Fig. 5). In vivo experiments showed that despite inferior fusion performance of the PCL-TCP scaffold at 6 months, it had a fusion rate similar to the Ti cage at 12 months. Moreover, the PCL-TCP scaffold resulted in better bone ingrowth and distribution. Jensen et al. (2014) fabricated a PCL scaffold with a nanoporous structure by the combination of FDM and thermal-induced phase separation. Excellent osteoconduction and osteointegration were found in their study. Xu et al. (2014) manufactured PCL-nanoHA and PCL scaffolds by FDM with commercial artificial bones as the control group. The PCL-nanoHA scaffold was superior in terms of mechanical properties, cell biocompatibility, and in vivo behavior in a long load-bearing goat femur bone segmental defect model. It possessed good biomechanical properties that reduced the stress-shielding effect. Idaszek et al. (2015) fabricated a ternary PCL-based scaffold consisting of PCL, TCP, and PLGA through FDM, and its mechanical characteristics, degradation kinetics, and surface properties were evaluated in vitro. As a result, the introduction of PLGA improved the degradation rate and surface roughness.
Fig. 5.
X-ray examination of harvested specimens at different time points
Top: PCL-TCP scaffold. Bottom: Ti cages. Reprinted from Li et al. (2014), Copyright 2014, with permission from Elsevier
FDM allows porous scaffolds to form a mesh-like structure that consists of either hollow or solid filaments. Filaments of one layer are usually deposited at a certain angle (0°, 60°, 90°, or 120°) or irregularly, through which a more stable mechanically lay-down pattern is obtained in all directions. To explore the biomechanical properties at various deposition angles, Korpela et al. (2013) designed and fabricated PCL/bioactive glass composite scaffolds using five different geometries to test their compressive moduli. Kim et al. (2012) also used FDM to fabricate D L-PLGA/β-TCP scaffolds at deposition angles of 0° and 90° or 0°, 90°, 45°, and −45°. The results showed no significant differences in scaffold degradation or bone regeneration.
The major deficiencies of FDM are the following. (1) Thermoplastic materials narrow the range of possible materials. (2) The build time of FDM is comparatively long. (3) Heating processes hamper the incorporation of biomolecules into scaffolds. (4) A smooth surface, which is not beneficial for cell adhesion, needs further modification or coating. (5) It is difficult to establish microporosity that promotes neovascularization and cell ingrowth.
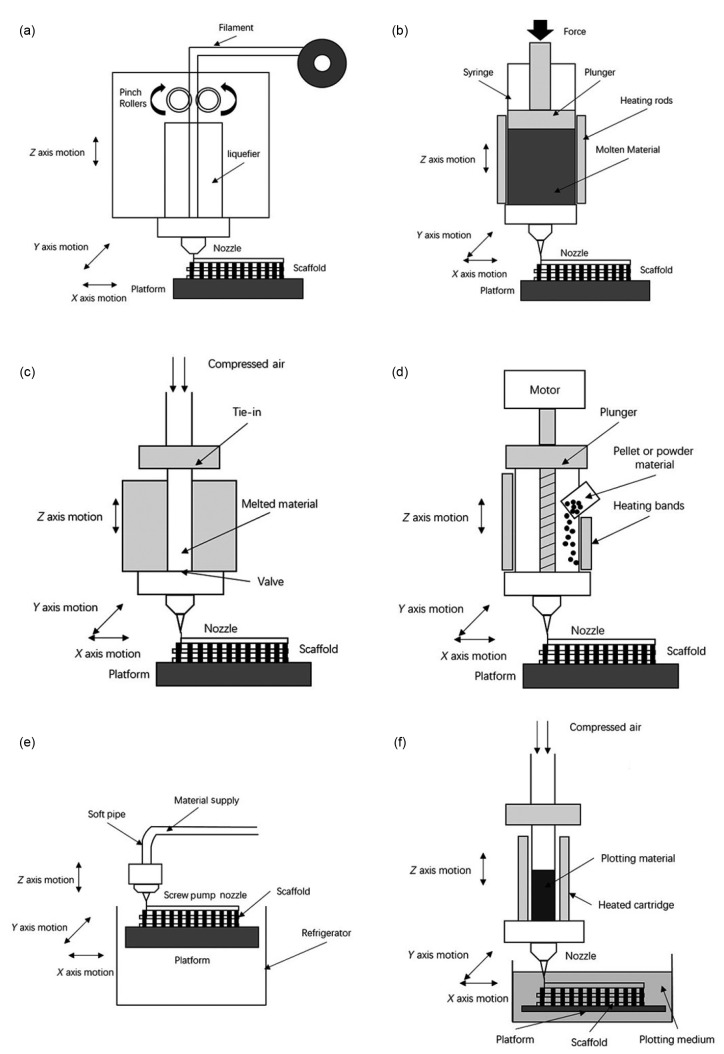
To overcome such problems, previous studies (Greulich et al., 1995; Landers and Mülhaupt, 2000; Xiong et al., 2002; Wang et al., 2004; Woodfield et al., 2004; Narayan, 2014) developed a series of nozzle-based RP techniques based on FDM (Fig. 6). 3D fiber deposition, precise extrusion manufacturing (PEM), precision extrusion deposition (PED), and multi-head deposition system (MHDS) tried changing pressure-driven methods (Woodfield et al., 2004), shapes of materials (Wang et al., 2004) and extrusion methods (Kim et al., 2010), and they have been employed to optimize micropore formation. Such methods have improved the efficiency and reproducibility of manufacturing scaffolds with better biocompatibility for bone tissue engineering.
Fig. 6.
Schematic diagrams of some nozzle-based systems
(a) FDM; (b) 3D fiber deposition; (c) PEM; (d) PED; (e) LDM; (f) 3D bioplotting. The figure is referenced from Narayan (2014)
Li et al. (2007a; 2007b) evaluated the biocompatible properties of Ti and Ti alloy-biphasic calcium phosphate (BCP) composite scaffolds prepared by 3D fiber deposition. The integration of BCP dramatically enhanced cell adhesiveness and biocompatibility. de Santis et al. (2011; 2015a; 2015b) preformed a series of studies and fabricated nanocomposite magnetic PCL scaffolds (the ratio of PCL and Fe3O4 was 90:10 (w/w)) and novel PCL/FeHA scaffolds by 3D fiber deposition, which confirmed the feasibility of this technology for bone tissue engineering and the advantages of magnetized scaffolds. Xiong et al. (2001) developed a PEM system and fabricated a porous PLLA scaffold using this technique. Morphologies of this scaffold are suitable for vascularization and bone tissue regeneration, and it possesses the appropriate mechanical properties. Shor et al. (2007; 2009) used PED to manufacture PCL and PCL-HA scaffolds, and verified the structural integrity, controllable pore size, and good biocompatibility by in vitro and in vivo studies. Kim and Cho (2009) introduced a novel blended scaffold fabrication technique through MHDS and fabricated PCL-PLGA composite scaffolds. These scaffolds had a fully interconnected structure, porosity of approximately 69.6%, and excellent cytocompatibility with MC3R3-E1 cells. Kim et al. (2014) also used MDHS to prepare PCL/PLGA scaffolds combined with a heparin-dopamine conjugate for controlled release of BMP-2 to improve osteoblast activity.
Low-temperature deposition modeling (LDM), robocasting/direct-write assembly, and 3D bioplotting remove the processes of heating and liquefying, and can add thermally sensitive biocomponents or cells into materials.
Xu et al. (2010) used LDM technology to fabricate a pearl/PLGA composite scaffold. Scanning electron microscopy, WST-1 assay, and alkaline phosphatase activity assay were used to determine the adhesion, proliferation, and differentiation of MSCs on the scaffold. The new scaffold exhibited better biocompatibility and osteoconductivity than a TCP/PLGA scaffold. Wang et al. (2012) also used LDM to manufacture a PLGA/β-TCP scaffold and coated it with collagen I to enhance bioactivity. Houmard et al. (2013) prepared HA/β-TCP composite scaffolds at various ratios (pure HA/β-TCP: 60/40 and 20/80 (w/w)) by the robocasting technique. Dorj et al. (2013) used this method to manufacture a nano-PCL/HA scaffold, and integrated modified carbon nanotubes into the scaffold to improve mechanical strength. 3D bioplotting has the capacity to prepare scaffolds using the widest range of materials, but its mechanical strength is weak. Schuurman et al. (2011) developed a hybrid bioplotting approach to fabricate scaffolds with a solid biodegradable material and cell-laden hydrogels as its material, resulting in significant improvement of the mechanical properties. Using multiple hydrogels, this method can also build a structure containing multiple cell types and bioactive factors to facilitate cell colonization (Melchels et al., 2012).
3.5. 3D printing
3D printing (3DP) has similar principles to SLS without using a CO2 laser beam. The basic principles of this technology are as follows (Fig. 7). A print head sprays a binder onto a specific location on each layer, and then the strip heater dries the binder. Along with the workstation lowering down layer by layer, a new layer of powder is spread on each layer. The cycle of drop-spread-print-heat is repeated until the structure is complete. Then, extra powder should be removed, and the entire part needs further reinforcement. Packing density, flowability, and wettability of the powder, layer thickness, drop volume, and saturation of the binder may influence the quality of the final product (Bose et al., 2013).
Fig. 7.
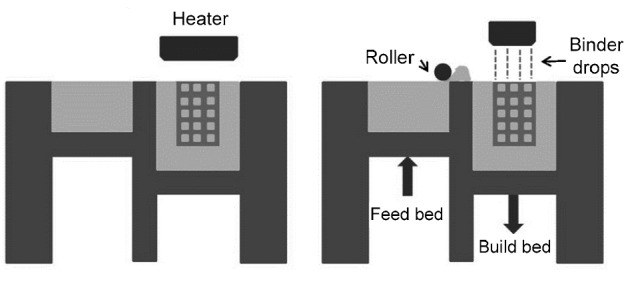
Schematic diagram of 3DP (Bose et al., 2013)
Unlike the FDM technique, 3DP does not require extra support structures. However, for the porous structures needed in bone tissue engineering, removal of unbound powder is a challenge (Bose et al., 2013). Theoretically, a wide range of biomaterials, including ceramics, metal, polymers, and composite materials can be used for 3DP processes. Furthermore, because the processes are performed at room temperature, heat-sensitive biologically active factors can be added during printing (Chia and Wu, 2015). However, the final products usually have weak biomechanical strength, which require high-temperature sintering to obtain structures suitable for bone tissue engineering (Seitz et al., 2005; Lu et al., 2012; Tarafder et al., 2013; Tarafder and Bose, 2014; Qian et al., 2015). Exploration of better binders is currently underway (Inzana et al., 2014).
Tarafder and Bose (2014) used 3DP technology to fabricate a TCP scaffold and coated it with alendronate (AD) after sintering. In vivo experiments demonstrated that slow release of AD promoted early healing of bone defects. Qian et al. (2015) evaluated the feasibility of scaffolds consisting of Ti and HA prepared by 3DP technology. The pore size was 50–150 µm, and the compressive strength was (184.3±27.1) MPa, which are suitable for bone tissue engineering. 3DP technology has also been applied to indirect methods (Bose et al., 2013; Lee et al., 2013; Chia and Wu, 2015).
4. Conclusions and future directions
Recent years have seen far-reaching improvements in bone regenerative medicine. Along with the development of various biomaterials and manufacturing technologies, researchers have a deeper understanding of bone tissue engineering. RP technologies have overcome some of the shortcomings of conventional scaffold fabrication strategies. Various RP techniques have distinctive features and advantages, but they all have some deficiencies and problems (Table 1). For SLA, the main challenges are limited material types and poor biomechanical strength of scaffolds. The strategy to overcome these shortcomings is development of new photocurable resins or combining materials. Some new technologies, such as digital light processing (DLP), have promising prospects, although DLP has not yet been used in bone tissue engineering. Both SLS and 3DP result in weak structures. The emphasis of present research is how to improve strength without sacrificing precision. Although accuracy is high, the more precise these structures are, the harder it is to remove the extra powder inside. Therefore, post-processing also needs to be optimized. The applications of nozzle-based RP technologies, such as FDM and 3D bioplotting, are becoming greater because of their rapid development and broad variety.
Table 1.
Comparison of main RP technologies
| RP technology | Materials | Advantages | Disadvantages |
| SLA | PEG, PEGDA, PPF, PCL, PDLLA | Fast speed, high resolution, easy to remove support materials | Limited range of photosensitive resin and polymers |
| SLS | Polymers, ceramics (PCL, HA, TCP) | Wide range of materials, good mechanical strength, relatively high precision, high porosity | Materials in powder form, difficult to remove trapped materials |
| FDM | Thermoplastic polymers and their composites | Easy operation, low cost, various lay-down patterns | Materials in filament form, low speed, high temperature, smooth surface |
| 3DF | Thermoplastic polymers and their composites, hydrogels | Easy operation, low cost, materials in pellet form, reduced preparation time | High temperature |
| PED | PCL, PCL-HA | Materials in pellet form | High temperature |
| PEM | PLLA, PLLA-TCP | Materials in grain form | High temperature |
| LDM | PLLA, PLGA, collagen, gelatin, chitosan, alginate | Materials in grain form, retain bioactive agents, can incorporate biomolecules | Need solvent and freeze drying process |
| Robocasting | Ceramics, organic inks | Wide range of materials, multi-material is possible | Difficult operation |
| 3D bioplotting | TCP, PCL, PLGA, PLLA, soft tissue | Remarkably wide range of materials, biomolecules can be used | Low mechanical strength, smooth surface, slow speed, difficult operation |
| 3DP | Cermics, polymers, metals | Fast speed, low cost, wide range of materials | Need post-processing, powdery surface |
RP: rapid prototyping; SLA: stereolithography; SLS: selective laser sintering; FDM: fused deposition modeling; 3DF: 3D fiber deposition; PED: precision extrusion deposition; PEM: precise extrusion manufacturing; LDM: low-temperature deposition modeling; 3DP: 3D printing; PEG: polyethyleneglycol; PEGDA: poly(ethylene glycol) diacrylate; PPF: polypropylene fumarate; PCL: polycaprolactone; PDLLA: poly-D , L-lactatide; HA: hydroxyapatite; TCP: tricalcium phosphate; PLLA: poly-L-lactatide; PLGA: polylactide-co-glycolide
There remain some difficulties and areas to explore. (1) Although RP technologies can theoretically manufacture subtle regulated scaffolds with uniformly distributed pores, there are few reports on comparisons or research of RP technologies and traditional technologies in terms of osteogenic potential. In addition, most research remains at the experimental stage, which needs long testing periods before clinical application. (2) Natural bones are not homogenous structures. The design of scaffolds also requires modification. (3) Biologically active factors can only be incorporated into scaffolds during post-processing because of the harsh processes of most RP technologies. (4) Treatment of mass bone defects is still a challenge in bone tissue reconstruction because of unsatisfactory bone graft substitutes.
For further development, RP-based 3D biochemical printing technology and nanotechnology will be key in overcoming the “development bottleneck”. Ultimately, it is of great significance to choose proper biomaterials, preparation processes, and scaffold design. Bone tissue engineering will encounter challenges in the innovation of materials and techniques, optimization of scaffolds, treatment of interfaces, and incorporation of biologically active factors.
Tissue engineering is an interdisciplinary field with improvements achieved by balanced development of various subjects, which is well illustrated by the symbol of clinical transplantation, the chimera composed of a lion’s head, goat body, and snake tail.
Footnotes
Project supported by the Science and Technology Commission of Shanghai Municipality (No. 15JC1491003), China
Compliance with ethics guidelines: Bo YUAN, Sheng-yuan ZHOU, and Xiong-sheng CHEN declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Arcaute K, Mann B, Wicker R. Stereolithography of spatially controlled multi-material bioactive poly(ethylene glycol) scaffolds. Acta Biomater. 2010;6(3):1047–1054. doi: 10.1016/j.actbio.2009.08.017. (Available from: http://dx.doi.org/10.1016/j.actbio.2009.08.017) [DOI] [PubMed] [Google Scholar]
- 2.Bose S, Roy M, Bandyopadhyay A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012;30(10):546–554. doi: 10.1016/j.tibtech.2012.07.005. (Available from: http://dx.doi.org/10.1016/j.tibtech.2012.07.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bose S, Vahabzadeh S, Bandyopadhyay A. Bone tissue engineering using 3D printing. Mater Today. 2013;16(12):496–504. (Available from: http://dx.doi.org/10.1016/j.mattod.2013.11.017) [Google Scholar]
- 4.Brie J, Chartier T, Chaput C, et al. A new custom made bioceramic implant for the repair of large and complex craniofacial bone defects. J Cranio Maxill Surg. 2013;41(5):403–407. doi: 10.1016/j.jcms.2012.11.005. (Available from: http://dx.doi.org/10.1016/j.jcms.2012.11.005) [DOI] [PubMed] [Google Scholar]
- 5.Calori GM, Mazza E, Colombo M, et al. The use of bone-graft substitutes in large bone defects: any specific needs? Injury. 2011;42(Suppl. 2):S56–S63. doi: 10.1016/j.injury.2011.06.011. (Available from: http://dx.doi.org/10.1016/j.injury.2011.06.011) [DOI] [PubMed] [Google Scholar]
- 6.Campana V, Milano G, Pagano E, et al. Bone substitutes in orthopaedic surgery: from basic science to clinical practice. J Mater Sci Mater Med. 2014;25(10):2445–2461. doi: 10.1007/s10856-014-5240-2. (Available from: http://dx.doi.org/10.1007/s10856-014-5240-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan V, Zorlutuna P, Jeong JH, et al. Three-dimensional photopatterning of hydrogels using stereolithography for long-term cell encapsulation. Lab Chip. 2010;10(16):2062–2070. doi: 10.1039/c004285d. (Available from: http://dx.doi.org/10.1039/c004285d) [DOI] [PubMed] [Google Scholar]
- 8.Chia HN, Wu BM. Recent advances in 3D printing of biomaterials. J Biol Eng. 2015;9(1):1–14. doi: 10.1186/s13036-015-0001-4. (Available from: http://dx.doi.org/10.1186/s13036-015-0001-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chuenjitkuntaworn B, Inrung W, Damrongsri D, et al. Polycaprolactone/hydroxyapatite composite scaffolds: preparation, characterization, and in vitro and in vivo biological responses of human primary bone cells. J Biomed Mater Res. 2010;94A(1):241–251. doi: 10.1002/jbm.a.32657. (Available from: http://dx.doi.org/10.1002/jbm.a.32657) [DOI] [PubMed] [Google Scholar]
- 10.de Santis R, Gloria A, Russo T, et al. A basic approach toward the development of nanocomposite magnetic scaffolds for advanced bone tissue engineering. J Appl Polym Sci. 2011;122(6):3599–3605. (Available from: http://dx.doi.org/10.1002/app.34771) [Google Scholar]
- 11.de Santis R, Amora U, Russo T, et al. 3D fibre deposition and stereolithography techniques for the design of multifunctional nanocomposite magnetic scaffolds. J Mater Sci Mater Med. 2015;26:250. doi: 10.1007/s10856-015-5582-4. (Available from: http://dx.doi.org/10.1007/s10856-015-5582-4) [DOI] [PubMed] [Google Scholar]
- 12.de Santis R, Russo A, Gloria A, et al. Towards the design of 3D fiber-deposited poly(ε-caprolactone)/iron-doped hydroxyapatite nanocomposite magnetic scaffolds for bone regeneration. J Biomed Nanotechnol. 2015;11(7):1236–1246. doi: 10.1166/jbn.2015.2065. (Available from: http://dx.doi.org/10.1166/jbn.2015.2065) [DOI] [PubMed] [Google Scholar]
- 13.Dorj B, Won JE, Kim JH, et al. Robocasting nanocomposite scaffolds of poly(caprolactone)/hydroxyapatite incorporating modified carbon nanotubes for hard tissue reconstruction. J Biomed Mater Res A. 2013;101A(6):1670–1681. doi: 10.1002/jbm.a.34470. (Available from: http://dx.doi.org/10.1002/jbm.a.34470) [DOI] [PubMed] [Google Scholar]
- 14.Du D, Asaoka T, Ushida T, et al. Fabrication and perfusion culture of anatomically shaped artificial bone using sterolithography. Biofabrication. 2014;6(4):045002. doi: 10.1088/1758-5082/6/4/045002. (Available from: http://dx.doi.org/10.1088/1758-5082/6/4/045002) [DOI] [PubMed] [Google Scholar]
- 15.Duan B, Wang M. Customized Ca-P/PHBV nanocomposite scaffolds for bone tissue engineering: design, fabrication, surface modification and sustained release of growth factor. J R Soc Interface. 2010;5(Suppl. 5):S615–S629. doi: 10.1098/rsif.2010.0127.focus. (Available from: http://dx.doi.org/10.1098/rsif.2010.0127.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duan B, Wang M, Zhou WY, et al. Three-dimensional nanocomposite scaffolds fabricated via selective laser sintering for bone tissue engineering. Acta Biomater. 2010;6(12):4495–4505. doi: 10.1016/j.actbio.2010.06.024. (Available from: http://dx.doi.org/10.1016/j.actbio.2010.06.024) [DOI] [PubMed] [Google Scholar]
- 17.Duan B, Cheung WL, Wang M. Optimized fabrication of Ca-P/PHBV nanocomposite scaffolds via selective laser sintering for bone tissue engineering. Biofabrication. 2011;3(1):015001. doi: 10.1088/1758-5082/3/1/015001. (Available from: http://dx.doi.org/10.1088/1758-5082/3/1/015001) [DOI] [PubMed] [Google Scholar]
- 18.Eosoly S, Brabazon D, Lohfeld S, et al. Selective laser sintering of hydroxyapatite/poly-epsilon-caprolactone scaffolds. Acta Biomater. 2010;6(7):2511–2517. doi: 10.1016/j.actbio.2009.07.018. (Available from: http://dx.doi.org/10.1016/j.actbio.2009.07.018) [DOI] [PubMed] [Google Scholar]
- 19.Feng P, Wei P, Shuai C, et al. Characterization of mechanical and biological properties of 3-D scaffolds reinforced with zinc oxide for bone tissue engineering. PLoS ONE. 2014;9(1):e87755. doi: 10.1371/journal.pone.0087755. (Available from: http://dx.doi.org/10.1371/journal.pone.0087755) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frame M, Huntley JS. Rapid prototyping in orthopaedic surgery: a user’s guide. Sci World J. 2012;2012:1–7. doi: 10.1100/2012/838575. (Available from: http://dx.doi.org/10.1100/2012/838575) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giannoudis PV, Chris Arts JJ, Schmidmaier G, et al. What should be the characteristics of the ideal bone graft substitute? Injury. 2011;42(Suppl. 2):S1–S2. doi: 10.1016/j.injury.2011.06.001. (Available from: http://dx.doi.org/10.1016/j.injury.2011.06.001) [DOI] [PubMed] [Google Scholar]
- 22.Greulich M, Greul M, Pintat T. Fast, functional prototypes via multiphase jet solidification. Rapid Prototyping J. 1995;1(1):20–25. (Available from: http://dx.doi.org/10.1108/13552549510146649) [Google Scholar]
- 23.Hernigou P. Bone transplantation and tissue engineering, part I. Mythology, miracles and fantasy: from Chimera to the Miracle of the Black Leg of Saints Cosmas and Damian and the cock of John Hunter. Int Orthop. 2014;38(12):2631–2638. doi: 10.1007/s00264-014-2511-y. (Available from: http://dx.doi.org/10.1007/s00264-014-2511-y) [DOI] [PubMed] [Google Scholar]
- 24.Houmard M, Fu Q, Genet M, et al. On the structural, mechanical, and biodegradation properties of HA/β-TCP robocast scaffolds. J Biomed Mater Res B Appl Biomater. 2013;101(7):1233–1242. doi: 10.1002/jbm.b.32935. (Available from: http://dx.doi.org/10.1002/jbm.b.32935) [DOI] [PubMed] [Google Scholar]
- 25.Huang J, Lin YW, Fu XW, et al. Development of nano-sized hydroxyapatite reinforced composites for tissue engineering scaffolds. J Mater Sci Mater Med. 2007;18(11):2151–2157. doi: 10.1007/s10856-007-3201-8. (Available from: http://dx.doi.org/10.1007/s10856-007-3201-8) [DOI] [PubMed] [Google Scholar]
- 26.Hutmacher DW. Scaffold design and fabrication technologies for engineering tissues–state of the art and future perspectives. J Biomater Sci Polym Ed. 2001;12(1):107–124. doi: 10.1163/156856201744489. (Available from: http://dx.doi.org/10.1163/156856201744489) [DOI] [PubMed] [Google Scholar]
- 27.Idaszek J, Bruinink A, Swieszkowski W. Ternary composite scaffolds with tailorable degradation rate and highly improved colonization by human bone marrow stromal cells. J Biomed Mater Res A. 2015;103(7):2394–2404. doi: 10.1002/jbm.a.35377. (Available from: http://dx.doi.org/10.1002/jbm.a.35377) [DOI] [PubMed] [Google Scholar]
- 28.Inzana JA, Olvera D, Fuller SM, et al. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials. 2014;35(13):4026–4034. doi: 10.1016/j.biomaterials.2014.01.064. (Available from: http://dx.doi.org/10.1016/j.biomaterials.2014.01.064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jensen J, Rolfing JH, Le DQ, et al. Surface-modified functionalized polycaprolactone scaffolds for bone repair: in vitro and in vivo experiments. J Biomed Mater Res A. 2014;102(9):2993–3003. doi: 10.1002/jbm.a.34970. (Available from: http://dx.doi.org/10.1002/jbm.a.34970) [DOI] [PubMed] [Google Scholar]
- 30.Kim J, McBride S, Tellis B, et al. Rapid-prototyped PLGA/β-TCP/hydroxyapatite nanocomposite scaffolds in a rabbit femoral defect model. Biofabrication. 2012;4(2):025003. doi: 10.1088/1758-5082/4/2/025003. (Available from: http://dx.doi.org/10.1088/1758-5082/4/2/025003) [DOI] [PubMed] [Google Scholar]
- 31.Kim JY, Cho D. Blended PCL/PLGA scaffold fabrication using multi-head deposition system. Microelectron Eng. 2009;86(4-6):1447–1450. (Available from: http://dx.doi.org/10.1016/j.mee.2008.11.026) [Google Scholar]
- 32.Kim JY, Lee T, Cho D, et al. Solid free-form fabrication-based PCL/HA scaffolds fabricated with a multi-head deposition system for bone tissue engineering. J Biomat Sci Polym Ed. 2010;21(6-7):951–962. doi: 10.1163/156856209X458380. (Available from: http://dx.doi.org/10.1163/156856209X458380) [DOI] [PubMed] [Google Scholar]
- 33.Kim TH, Yun YP, Park YE, et al. In vitro and in vivo evaluation of bone formation using solid freeform fabrication-based bone morphogenic protein-2 releasing PCL/PLGA scaffolds. Biomed Mater. 2014;9(2):025008. doi: 10.1088/1748-6041/9/2/025008. (Available from: http://dx.doi.org/10.1088/1748-6041/9/2/025008) [DOI] [PubMed] [Google Scholar]
- 34.Korpela J, Kokkari A, Korhonen H, et al. Biodegradable and bioactive porous scaffold structures prepared using fused deposition modeling. J Biomed Mater Res B Appl Biomater. 2013;101B(4):610–619. doi: 10.1002/jbm.b.32863. (Available from: http://dx.doi.org/10.1002/jbm.b.32863) [DOI] [PubMed] [Google Scholar]
- 35.Landers R, Mülhaupt R. Desktop manufacturing of complex objects, prototypes and biomedical scaffolds by means of computer-assisted design combined with computer-guided 3D plotting of polymers and reactive oligomers. Macromol Mater Eng. 2000;282(1):17–21. (Available from: http://dx.doi.org/10.1002/1439-2054(20001001)282:1<17::AID-MAME17>3.0.CO;2-8) [Google Scholar]
- 36.Lee JW, Kim JY, Cho DW. Solid free-form fabrication technology and its application to bone tissue engineering. Int J Stem Cells. 2010;3(2):85–95. doi: 10.15283/ijsc.2010.3.2.85. (Available from: http://dx.doi.org/10.15283/ijsc.2010.3.2.85) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee JW, Kang KS, Lee SH, et al. Bone regeneration using a microstereolithography-produced customized poly(propylene fumarate)/diethyl fumarate photopolymer 3D scaffold incorporating BMP-2 loaded PLGA microspheres. Biomaterials. 2011;32(3):744–752. doi: 10.1016/j.biomaterials.2010.09.035. (Available from: http://dx.doi.org/10.1016/j.biomaterials.2010.09.035) [DOI] [PubMed] [Google Scholar]
- 38.Lee JY, Choi B, Wu B, et al. Customized biomimetic scaffolds created by indirect three-dimensional printing for tissue engineering. Biofabrication. 2013;5(4):045003. doi: 10.1088/1758-5082/5/4/045003. (Available from: http://dx.doi.org/10.1088/1758-5082/5/4/045003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li JP, Habibovic P, van den Doel M, et al. Bone ingrowth in porous titanium implants produced by 3D fiber deposition. Biomaterials. 2007;28(18):2810–2820. doi: 10.1016/j.biomaterials.2007.02.020. (Available from: http://dx.doi.org/10.1016/j.biomaterials.2007.02.020) [DOI] [PubMed] [Google Scholar]
- 40.Li JP, Habibovic P, Yuan H, et al. Biological performance in goats of a porous titanium alloy-biphasic calcium phosphate composite. Biomaterials. 2007;28(29):4209–4218. doi: 10.1016/j.biomaterials.2007.05.042. (Available from: http://dx.doi.org/10.1016/j.biomaterials.2007.05.042) [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Wu Z, Li X, et al. A polycaprolactone-tricalcium phosphate composite scaffold as an autograft-free spinal fusion cage in a sheep model. Biomaterials. 2014;35(22):5647–5659. doi: 10.1016/j.biomaterials.2014.03.075. (Available from: http://dx.doi.org/10.1016/j.biomaterials.2014.03.075) [DOI] [PubMed] [Google Scholar]
- 42.Lin H, Zhang D, Alexander PG, et al. Application of visible light-based projection stereolithography for live cell-scaffold fabrication with designed architecture. Biomaterials. 2013;34(2):331–339. doi: 10.1016/j.biomaterials.2012.09.048. (Available from: http://dx.doi.org/10.1016/j.biomaterials.2012.09.048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu H, Webster TJ. Enhanced biological and mechanical properties of well-dispersed nanophase ceramics in polymer composites: from 2D to 3D printed structures. Mater Sci Eng C. 2011;31(2):77–89. (Available from: http://dx.doi.org/10.1016/j.msec.2010.07.013) [Google Scholar]
- 44.Lu L, Zhang Q, Wootton D, et al. Biocompatibility and biodegradation studies of PCL/β-TCP bone tissue scaffold fabricated by structural porogen method. J Mater Sci Mater Med. 2012;23(9):2217–2226. doi: 10.1007/s10856-012-4695-2. (Available from: http://dx.doi.org/10.1007/s10856-012-4695-2) [DOI] [PubMed] [Google Scholar]
- 45.Ma PX, Zhang R, Xiao G, et al. Engineering new bone tissue in vitro on highly porous poly(α-hydroxyl acids)/hydroxyapatite composite scaffolds. J Biomed Mater Res. 2001;54(2):284–293. doi: 10.1002/1097-4636(200102)54:2<284::aid-jbm16>3.0.co;2-w. (Available from: http://dx.doi.org/10.1002/1097-4636(200102)54:2<284::AID-JBM16>3.0.CO;2-W) [DOI] [PubMed] [Google Scholar]
- 46.Melchels FPW, Feijen J, Grijpma DW. A review on stereolithography and its applications in biomedical engineering. Biomaterials. 2010;31(24):6121–6130. doi: 10.1016/j.biomaterials.2010.04.050. (Available from: http://dx.doi.org/10.1016/j.biomaterials.2010.04.050) [DOI] [PubMed] [Google Scholar]
- 47.Melchels FPW, Domingos MAN, Klein TJ, et al. Additive manufacturing of tissues and organs. Prog Polym Sci. 2012;37(8):1079–1104. (Available from: http://dx.doi.org/10.1016/j.progpolymsci.2011.11.007) [Google Scholar]
- 48.Mithal A, Dhingra V, Lau E, et al. The Asian Audit Epidemiology, Costs and Burden of Osteoporosis in Asia. International Osteoporosis Foundation, Switzerland.2009. [Google Scholar]
- 49.Montjovent MO, Mathieu L, Hinz B, et al. Biocompatibility of bioresorbable poly(L-lactic acid) composite scaffolds obtained by supercritical gas foaming with human fetal bone cells. Tissue Eng. 2005;11(11-12):1640–1649. doi: 10.1089/ten.2005.11.1640. (Available from: http://dx.doi.org/10.1089/ten.2005.11.1640) [DOI] [PubMed] [Google Scholar]
- 50.Montjovent MO, Mathieu L, Schmoekel H, et al. Repair of critical size defects in the rat cranium using ceramic-reinforced PLA scaffolds obtained by supercritical gas foaming. J Biomed Mater Res A. 2007;83A(1):41–51. doi: 10.1002/jbm.a.31208. (Available from: http://dx.doi.org/10.1002/jbm.a.31208) [DOI] [PubMed] [Google Scholar]
- 51.Narayan R. Rapid prototyping of biomaterials: principles and applications. In: Chua CK, Leong KF, An J, editors. Introduction to Rapid Prototyping of Biomaterials. London: Woodhead Publishing; 2014. pp. 1–5. [Google Scholar]
- 52.Orthoworld, Inc. Orthopaedic Industry Annual Report. 2011. [Google Scholar]
- 53.Orthoworld, Inc. Orthopaedic Industry Annual Report. 2014. [Google Scholar]
- 54.Oryan A, Alidadi S, Moshiri A, et al. Bone regenerative medicine: classic options, novel strategies, and future directions. J Orthop Surg Res. 2014;9(1):18. doi: 10.1186/1749-799X-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Owen R, Sherborne C, Paterson T, et al. Emulsion templated scaffolds with tunable mechanical properties for bone tissue engineering. J Mech Behav Biomed Mater. 2016;54:159–172. doi: 10.1016/j.jmbbm.2015.09.019. (Available from: http://dx.doi.org/10.1016/j.jmbbm.2015.09.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Polo-Corrales L, Latorre-Esteves M, Ramirez-Vick JE. Scaffold design for bone regeneration. J Nanosci Nanotechnol. 2014;14(1):15–56. doi: 10.1166/jnn.2014.9127. (Available from: http://dx.doi.org/10.1166/jnn.2014.9127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qian C, Zhang F, Sun J. Fabrication of Ti/HA composite and functionally graded implant by three-dimensional printing. Bio-Med Mater Eng. 2015;25(2):127–136. doi: 10.3233/BME-151263. (Available from: http://dx.doi.org/10.3233/BME-151263) [DOI] [PubMed] [Google Scholar]
- 58.Ratner BD, Hoffman AS, Schoen FJ, et al. Biomaterials Science: An Introduction to Materials in Medicine. San Diego: Elsevier Academic Press; 2004. [Google Scholar]
- 59.Ronca A, Ambrosio L, Grijpma DW. Design of porous three-dimensional PDLLA/nano-HAP composite scaffolds using stereolithography. J Appl Biomater Funct Mater. 2012;10(3):249–258. doi: 10.5301/JABFM.2012.10211. (Available from: http://dx.doi.org/10.5301/JABFM.2012.10211) [DOI] [PubMed] [Google Scholar]
- 60.Roskies M, Jordan JO, Fang D, et al. Improving PEEK bioactivity for craniofacial reconstruction using a 3D printed scaffold embedded with mesenchymal stem cells. J Biomater Appl. 2016;31(1):132–139. doi: 10.1177/0885328216638636. (Available from: http://dx.doi.org/10.1177/0885328216638636) [DOI] [PubMed] [Google Scholar]
- 61.Sachlos E, Czernuszka JT. Making tissue engineering scaffolds work. Review: the application of solid freeform fabrication technology to the production of tissue engineering scaffolds. Eur Cell Mater. 2003;5:29–40. doi: 10.22203/ecm.v005a03. (Available from: http://dx.doi.org/10.22203/eCM.v005a03) [DOI] [PubMed] [Google Scholar]
- 62.Saiz E, Zimmermann EA, Lee JS, et al. Perspectives on the role of nanotechnology in bone tissue engineering. Dental Mater. 2013;29(1):103–115. doi: 10.1016/j.dental.2012.08.001. (Available from: http://dx.doi.org/10.1016/j.dental.2012.08.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schuurman W, Khristov V, Pot MW, et al. Bioprinting of hybrid tissue constructs with tailorable mechanical properties. Biofabrication. 2011;3(2):021001. doi: 10.1088/1758-5082/3/2/021001. (Available from: http://dx.doi.org/10.1088/1758-5082/3/2/021001) [DOI] [PubMed] [Google Scholar]
- 64.Seitz H, Rieder W, Irsen S, et al. Three-dimensional printing of porous ceramic scaffolds for bone tissue engineering. J Biomed Mater Res B Appl Biomater. 2005;74B(2):782–788. doi: 10.1002/jbm.b.30291. (Available from: http://dx.doi.org/10.1002/jbm.b.30291) [DOI] [PubMed] [Google Scholar]
- 65.Shor L, Guceri S, Wen X, et al. Fabrication of three-dimensional polycaprolactone/hydroxyapatite tissue scaffolds and osteoblast-scaffold interactions in vitro. Biomaterials. 2007;28(35):5291–5297. doi: 10.1016/j.biomaterials.2007.08.018. (Available from: http://dx.doi.org/10.1016/j.biomaterials.2007.08.018) [DOI] [PubMed] [Google Scholar]
- 66.Shor L, Guceri S, Chang R, et al. Precision extruding deposition (PED) fabrication of polycaprolactone (PCL) scaffolds for bone tissue engineering. Biofabrication. 2009;1(1):015003. doi: 10.1088/1758-5082/1/1/015003. (Available from: http://dx.doi.org/10.1088/1758-5082/1/1/015003) [DOI] [PubMed] [Google Scholar]
- 67.Simpson RL, Wiria FE, Amis AA, et al. Development of a 95/5 poly(L -lactide-co-glycolide)/hydroxylapatite and β-tricalcium phosphate scaffold as bone replacement material via selective laser sintering. J Biomed Mater Res B Appl Biomater. 2008;84B(1):17–25. doi: 10.1002/jbm.b.30839. (Available from: http://dx.doi.org/10.1002/jbm.b.30839) [DOI] [PubMed] [Google Scholar]
- 68.Sultana N, Wang M. Fabrication of HA/PHBV composite scaffolds through the emulsion freezing/freeze-drying process and characterisation of the scaffolds. J Mater Sci Mater Med. 2008;19(7):2555–2561. doi: 10.1007/s10856-007-3214-3. (Available from: http://dx.doi.org/10.1007/s10856-007-3214-3) [DOI] [PubMed] [Google Scholar]
- 69.Sultana N, Wang M. PHBV/PLLA-based composite scaffolds fabricated using an emulsion freezing/freeze-drying technique for bone tissue engineering: surface modification and in vitro biological evaluation. Biofabrication. 2012;4(1):015003. doi: 10.1088/1758-5082/4/1/015003. (Available from: http://dx.doi.org/10.1088/1758-5082/4/1/015003) [DOI] [PubMed] [Google Scholar]
- 70.Tabata Y. Biomaterial technology for tissue engineering applications. J R Soc Interface. 2009;6(Suppl. 3):S311–S324. doi: 10.1098/rsif.2008.0448.focus. (Available from: http://dx.doi.org/10.1098/rsif.2008.0448.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tan KH, Chua CK, Leong KF, et al. Selective laser sintering of biocompatible polymers for applications in tissue engineering. Bio-Med Mater Eng. 2005;15(1-2):113–124. [PubMed] [Google Scholar]
- 72.Tarafder S, Bose S. Polycaprolactone-coated 3D printed tricalcium phosphate scaffolds for bone tissue engineering: in vitro alendronate release behavior and local delivery effect on in vivo osteogenesis. ACS Appl Mater Interfaces. 2014;6(13):9955–9965. doi: 10.1021/am501048n. (Available from: http://dx.doi.org/10.1021/am501048n) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tarafder S, Davies NM, Bandyopadhyay A, et al. 3D printed tricalcium phosphate scaffolds: effect of SrO and MgO doping on osteogenesis in a rat distal femoral defect model. Biomater Sci. 2013;1(12):1250–1259. doi: 10.1039/C3BM60132C. (Available from: http://dx.doi.org/10.1039/c3bm60132c) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thadavirul N, Pavasant P, Supaphol P. Improvement of dual-leached polycaprolactone porous scaffolds by incorporating with hydroxyapatite for bone tissue regeneration. J Biomater Sci Polym Ed. 2014;25(17):1986–2008. doi: 10.1080/09205063.2014.966800. (Available from: http://dx.doi.org/10.1080/09205063.2014.966800) [DOI] [PubMed] [Google Scholar]
- 75.Wang C, Meng G, Zhang L, et al. Physical properties and biocompatibility of a core-sheath structure composite scaffold for bone tissue engineering in vitro. J Biomed Biotechnol. 2012;2012:579141. doi: 10.1155/2012/579141. (Available from: http://dx.doi.org/10.1155/2012/579141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang F, Shor L, Darling A, et al. Precision extruding deposition and characterization of cellular poly-epsiloncaprolactone tissue scaffolds. Rapid Prototyping J. 2004;10(1):42–49. (Available from: http://dx.doi.org/10.1108/13552540410512525) [Google Scholar]
- 77.Williams JM, Adewunmi A, Schek RM, et al. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials. 2005;26(23):4817–4827. doi: 10.1016/j.biomaterials.2004.11.057. (Available from: http://dx.doi.org/10.1016/j.biomaterials.2004.11.057) [DOI] [PubMed] [Google Scholar]
- 78.Wiria FE, Leong KF, Chua CK, et al. Poly-epsilon-caprolactone/hydroxyapatite for tissue engineering scaffold fabrication via selective laser sintering. Acta Biomater. 2007;3(1):1–12. doi: 10.1016/j.actbio.2006.07.008. (Available from: http://dx.doi.org/10.1016/j.actbio.2006.07.008) [DOI] [PubMed] [Google Scholar]
- 79.Wiria FE, Chua CK, Leong KF, et al. Improved biocomposite development of poly(vinyl alcohol) and hydroxyapatite for tissue engineering scaffold fabrication using selective laser sintering. J Mater Sci Mater Med. 2008;19(3):989–996. doi: 10.1007/s10856-007-3176-5. (Available from: http://dx.doi.org/10.1007/s10856-007-3176-5) [DOI] [PubMed] [Google Scholar]
- 80.Wohlers Associates, Inc. Wohlers Reports 2015. 2015. [Google Scholar]
- 81.Woodfield TB, Malda J, de Wijn J, et al. Design of porous scaffolds for cartilage tissue engineering using a three-dimensional fiber-deposition technique. Biomaterials. 2004;25(18):4149–4161. doi: 10.1016/j.biomaterials.2003.10.056. (Available from: http://dx.doi.org/10.1016/j.biomaterials.2003.10.056) [DOI] [PubMed] [Google Scholar]
- 82.Xia Y, Zhou P, Cheng X, et al. Selective laser sintering fabrication of nano-hydroxyapatite/poly-epsilon-caprolactone scaffolds for bone tissue engineering applications. Int J Nanomed. 2013;8:4197–4213. doi: 10.2147/IJN.S50685. (Available from: http://dx.doi.org/10.2147/IJN.S50685) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xiong Z, Yan YN, Zhang RJ, et al. Fabrication of porous poly(L-lactic acid) scaffolds for bone tissue engineering via precise extrusion. Scripta Mater. 2001;45(7):773–779. (Available from: http://dx.doi.org/10.1016/S1359-6462(01)01094-6) [Google Scholar]
- 84.Xiong Z, Yan Y, Wang S, et al. Fabrication of porous scaffolds for bone tissue engineering via low-temperature deposition. Scripta Mater. 2002;46(11):771–776. (Available from: http://dx.doi.org/10.1016/S1359-6462(02)00071-4) [Google Scholar]
- 85.Xu M, Li Y, Suo H, et al. Fabricating a pearl/PLGA composite scaffold by the low-temperature deposition manufacturing technique for bone tissue engineering. Biofabrication. 2010;2(2):025002. doi: 10.1088/1758-5082/2/2/025002. (Available from: http://dx.doi.org/10.1088/1758-5082/2/2/025002) [DOI] [PubMed] [Google Scholar]
- 86.Xu N, Ye X, Wei D, et al. 3D artificial bones for bone repair prepared by computed tomography-guided fused deposition modeling for bone repair. ACS Appl Mater Interfaces. 2014;6(17):14952–14963. doi: 10.1021/am502716t. (Available from: http://dx.doi.org/10.1021/am502716t) [DOI] [PubMed] [Google Scholar]
- 87.Ye L, Zeng X, Li H, et al. Fabrication and biocompatibility of nano non-stoichiometric apatite and poly (epsilon-caprolactone) composite scaffold by using prototyping controlled process. J Mater Sci Mater Med. 2010;21(2):753–760. doi: 10.1007/s10856-009-3872-4. (Available from: http://dx.doi.org/10.1007/s10856-009-3872-4) [DOI] [PubMed] [Google Scholar]
- 88.Yen H, Tseng C, Hsu S, et al. Evaluation of chondrocyte growth in the highly porous scaffolds made by fused deposition manufacturing (FDM) filled with type II collagen. Biomed Microdev. 2009;11(3):615–624. doi: 10.1007/s10544-008-9271-7. (Available from: http://dx.doi.org/10.1007/s10544-008-9271-7) [DOI] [PubMed] [Google Scholar]
- 89.Zein I, Hutmacher DW, Tan KC, et al. Fused deposition modeling of novel scaffold architectures for tissue engineering applications. Biomaterials. 2002;23(4):1169–1185. doi: 10.1016/s0142-9612(01)00232-0. (Available from: http://dx.doi.org/10.1016/S0142-9612(01)00232-0) [DOI] [PubMed] [Google Scholar]
- 90.Zhou WY, Lee SH, Wang M, et al. Selective laser sintering of porous tissue engineering scaffolds from poly (L-lactide)/carbonated hydroxyapatite nanocomposite microspheres. J Mater Sci Mater Med. 2008;19(7):2535–2540. doi: 10.1007/s10856-007-3089-3. (Available from: http://dx.doi.org/10.1007/s10856-007-3089-3) [DOI] [PubMed] [Google Scholar]