Abstract
Porcine circovirus type 2 (PCV2) has recently been reported to elicit the unfolded protein response (UPR) via activation of the PERK/eIF2α (RNA-activated protein kinase-like endoplasmic reticulum (ER) kinase/eukaryotic initiation factor 2α) pathway. This study attempted to examine which viral protein might be involved in inducing UPR and whether this cellular event would lead to apoptosis of the cells expressing the viral protein. By transient expression, we found that both replicase (Rep) and capsid (Cap) proteins of PCV2 could induce ER stress as shown by increased phosphorylation of PERK with subsequent activation of the eIF2α-ATF4 (activating transcription factor 4)-CHOP (CCAAT/enhancer-binding protein homologous protein) axis. Cap expression, but not Rep, significantly reduced anti-apoptotic B-cell lymphoma-2 (Bcl-2) and increased caspase-3 cleavage, possibly due to increased expression of CHOP. Since knockdown of PERK by RNA interference clearly reduced Cap-induced CHOP expression, caspase-3 cleavage, and apoptotic cell death possibly by partially rescuing Bcl-2 expression, we propose that there is connection between Cap-induced UPR and apoptosis via the PERK/eIF2α/ATF4/CHOP/Bcl-2 pathway. This study, together with our earlier studies, provides insight into the mechanisms underlying PCV2 pathogenesis.
Keywords: Porcine circovirus 2, Capsid protein, Unfolded protein response, Apoptosis
1. Introduction
Porcine circovirus type 2 (PCV2) is the causative agent of porcine circovirus-associated disease (PCVAD) (Meng, 2013). The disease is characterized by weight loss, respiratory or digestive disorders, reproductive failure, and enlarged lymph nodes with lymphocyte depletion and subsequent immunosuppression in pigs (Finsterbusch and Mankertz, 2009).
The PCV2 genome contains three main open reading frames (ORFs). ORF1 encodes replicase (Rep) proteins which are required for virus replication (Mankertz et al., 1998). ORF2 encodes the structural protein capsid (Cap) that forms the viral coat (Nawagitgul et al., 2000). Cap contains a nuclear localization signal of 41 amino acids at the N-terminus that determines its subcellular location during the viral life cycle (Liu et al., 2001). PCV2 Rep proteins are localized in the nuclei of infected PK-15 cells (Timmusk et al., 2006), whereas Cap can be detected in the nuclei and cytoplasm (Gilpin et al., 2003), indicating that the Cap protein shuttles between distinct cellular compartments during the infection cycle. The ORF3 protein remains controversial and appears to contribute to apoptosis both in vitro (Liu et al., 2005) and in vivo (Liu et al., 2006). Other putative ORFs are reportedly present in the PCV2 genome, such as ORF4 (He et al., 2013) and ORF5 (Lv et al., 2015).
Mounting evidence indicates that a wide variety of viruses could disturb endoplasmic reticulum (ER) homeostasis and lead to ER stress (Li et al., 2015). To cope with the stress, cells activate a series of adaptive mechanisms known as the unfolded protein response (UPR) (Hetz, 2012). When cells are overwhelmed due to prolonged stress, UPR leads to intrinsic mitochondrial apoptosis (Tabas and Ron, 2011). There is a complex relationship between host cells and virus infection with regard to UPR and apoptosis. In addition to our early reports showing that PCV2 infection could induce autophagy (Zhu et al., 2012a; 2012b; Gu et al., 2016), we have recently revealed that PCV2 could induce UPR by selective activation of the RNA-activated protein kinase-like ER kinase (PERK) pathway (Zhou et al., 2016). This study aimed to investigate which protein(s) of PCV2 could be responsible for activation of UPR and if apoptosis would be the eventual destiny of the cells undergoing UPR.
2. Materials and methods
2.1. Cell lines
The cell line PK-15 free of PCV1 contamination was used. The cells were cultured at 37 °C and 5% CO2 in Dulbecco’s minimal essential medium (HyClone, South Logan, UT, USA) supplemented with 10% (0.1 g/ml) heat-inactivated newborn calf serum (Gibco, Grand Island, NY, USA), 1% (0.01 g/ml) L-glutamine, 1% (0.01 g/ml) non-essential amino acids, 100 U/ml penicillin G, and 100 μg/ml streptomycin.
2.2. Plasmid construction and siRNA
To construct p-ORF3-enhanced green fluorescent protein (EGFP), the genes orf3 and egfp were polymerase chain reaction (PCR)-amplified from the genomic DNA of the PCV2 isolate SY4 (PCV2b, GenBank accession No. GU325754) and the mammalian expression vector pcDNA3.1-EGFP (Invitrogen, Eugene, Oregon, USA) with gene-specific primers (Table 1), respectively. A flexible peptide linker GGSGG was introduced between ORF3 and EGFP. The orf3-egfp fusion fragment was obtained by overlap PCR and subcloned into the multiple cloning site of pcDNA3.1 (Invitrogen). For the construction of p-Rep-Flag and p-Cap-Flag, the orf1 and orf2 genes were amplified from the genomic DNA of the PCV2 and subcloned into pcDNA3.1-Flag (Invitrogen). All of the constructs were confirmed by DNA sequencing. Small interfering RNAs (siRNAs) against PERK and control scrambled siRNA were purchased from GenePharma (Shanghai, China). Four PERK-specific siRNAs (siPERKs) were used as a pool as previously described (Zhou et al., 2016).
Table 1.
Primers used for cloning
| Primer | Sequence (5'→3') |
| Cap-F | AAGGATCCATGACGTATCCAAGGAGGCGTT |
| Cap-R | CCCTCGAGTTAAGGGTTAAGTGGGGGGTCTT |
| Rep-F | AAGGATCCATGCCCCAGCAAGAAGAATGGA |
| Rep-R | CCTCGAGGTAATTTATTTCATATGGAAATTC |
| ORF3-F | CAAGCTTGCCACCATGGTAACCATCCCACCACTTGTT |
| ORF3-R | CAGCTCCTCGCCCTTGCTCACTCCTCCGCTTCCTCCCTGATCGAGTGTGGAGCTCCTA |
| EGFP-F | GGAGGAAGCGGAGGAGTGAGCAAGGGCGAGGAGCTGTT |
| EGFP-R | CGGATCCTTACTTGTACAGCTCGTCCATGCCG |
The underlined sequences indicate the enzyme cutting sites
2.3. Transfection
Each of the recombinant plasmids was delivered into PK-15 cells by transfection with lipofectamine 2000 (Invitrogen) according to the manufacturer’s instruction. For RNA interference, siPERK was delivered into PK-15 cells by transfection 24 h prior to transfection of the cells with p-Rep-Flag or p-Cap-Flag. All the samples were harvested at indicated time points and subjected to Western blotting of target protein molecules or labeled for flow cytometric analysis of apoptosis as described below.
2.4. Western blotting
Western blotting was performed as described previously (Zhu et al., 2012b). The membranes were blocked for 1 h in Tris-buffered saline containing 0.05% Tween 20 and 5% nonfat milk and then probed for 1 h with the following primary antibodies: mouse monoclonal anti-Cap IgG (produced in our laboratory),
mouse monoclonal anti-Bcl-2 (Abcam, Cambridge, UK), rabbit monoclonal antibodies to GRP78, eukaryotic initiation factor 2α (eIF2α) phospho and total eIF2α (Abcam), rabbit polyclonal antibodies to PERK phospho, total PERK, CCAAT/enhancer-binding protein homologous protein (CHOP) (Abcam), goat anti-activating transcription factor 4 (ATF4) polyclonal antibody (Abcam), and mouse monoclonal antibodies to β-actin, Flag and GFP (MultiSciences, Hangzhou, China). All antibodies were found to react with target molecules from porcine cell lines used in this study. Blots were washed and then incubated for another hour with goat anti-rabbit, anti-mouse or donkey anti-goat horseradish peroxidase-labeled antibodies (KPL, Gaithersburg, MD, USA). The blots were revealed using the ECL Plus detection system under conditions recommended by the manufacturer (Thermo, Marina, CA, USA). Images were captured by the Gel 3100 Chemiluminescent and Fluorescent Imaging System (Sagecreation, Beijing, China).
2.5. Flow cytometric analysis for apoptosis
PK-15 cells were trypsinized and collected, washed in phosphate buffered saline (PBS) twice, resuspended in 1× binding buffer, and stained with Annexin V-FITC and propidium iodide (PI) (MultiSciences, Hangzhou, China). After a 10-min incubation in the dark at room temperature, the labeled cells were quantified using FACS flow cytometry (BD Biosciences, USA) with CellQuest software within 1 h. Three independent experiments were performed.
2.6. Statistical analysis
All results in figures were presented, where appropriate, as mean±standard deviation (SD) from three independent experiments and analyzed using Student’s t-test.
3. Results
3.1. Rep and Cap, but not ORF3, induced unfolded protein response via PERK activation
We have previously shown that persistent PCV2 infection could induce UPR via selective activation of the PERK pathway (Zhou et al., 2016). In order to identify the specific PCV2 protein(s) involved in UPR induction, we investigated the involvement of viral proteins in activating UPR. By using a transient transfection system with PK-15 cells, we found that expression of Rep or Cap caused significant elevation of the phosphorylated form of PERK and eIF2α over a time course of 12, 24, 36, and 48 h, while the levels of total PERK and eIF2α remained unchanged. GRP78, a well-established marker of ER stress, was induced progressively and remained elevated at 48 h post-transfection (hpt) in cells expressing Cap protein, as compared to mock-transfected cells (Fig. 1a). While ORF3 protein failed to induce UPR since the expression of GRP78, p-PERK and p-eIF2α were relatively stable (Fig. 1b). These results suggest that Rep and Cap are primarily responsible for UPR induction during PCV2 infection.
Fig. 1.
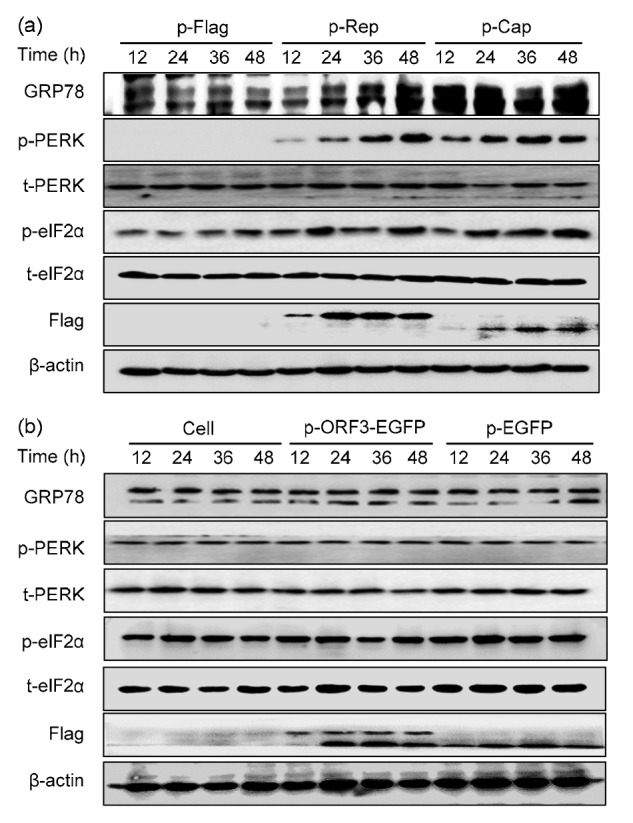
Rep and Cap, but not ORF3, induced unfolded protein response via PERK activation
PK-15 cells were transfected with the indicated plasmids (p-Flag stands for pcDNA3.1-Flag, p-EGFP for pcDNA3.1-EGFP, p-ORF3-EGFP for pcDNA3.1-ORF3-EGFP, p-Rep for p-Rep-Flag, p-Cap and p-Cap-Flag, the same for the following figures) for 12, 24, 36, and 48 h. Western blotting was performed to visualize phosphorylated forms of PERK (p-PERK) and eIF2α (p-eIF2α), total PERK (t-PERK) and total eIF2α (t-eIF2α), and ER stress marker GRP78 in lysates of cells expressing Rep or Cap (a) and of those expressing ORF3 (b). Expressions of the fusion proteins Cap, Rep, and ORF3 were revealed by antibodies to Flag or EGFP
3.2. Rep and Cap induced ATF4 and CHOP expressions
Phosphorylation of eIF2α inhibits global protein translation but selectively induces translation of ATF4 mRNA during ER stress (Moreno et al., 2012). ATF4 accumulates in the nuclei and upregulates the genes that attempt to rescue the cells (Dey et al., 2012) as well as the chop gene encoding the pro-apoptotic transcription factor CHOP (Palam et al., 2011). Therefore, we attempted to analyze ATF4 and CHOP in Rep-or Cap-expressing cells. Fig. 2 shows that both Rep and Cap proteins led to increased expressions of ATF4 and CHOP starting from 24 to 48 hpt. ATF4 and CHOP were not detectable in mock-transfected cells.
Fig. 2.
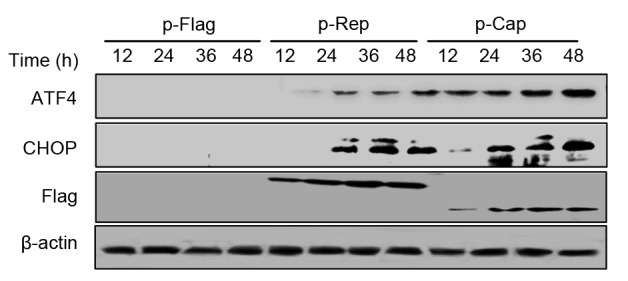
Rep and Cap increased expressions of ATF4 and CHOP
PK-15 cells were transfected with the indicated plasmids for 12, 24, 36, and 48 h. Whole cell lysates were then subjected to Western blotting for ATF4, CHOP, Flag, and (-actin
3.3. Knockdown of PERK reduced Rep-or Cap-induced expressions of ATF4 and CHOP
To examine if PERK would play a role in subsequent activation of transcriptional factors AFT4 and CHOP downstream of eIF2α in cells experiencing Rep-or Cap-activated UPR, the siRNA knockdown approach was adopted. Transfection of siPERK markedly reduced the expressions of the p-eIF2α, ATF4, and CHOP, compared to the cells transfected with control siRNA, suggesting that PERK is essential for activation of ATF4 and CHOP by Rep or Cap (Fig. 3).
Fig. 3.
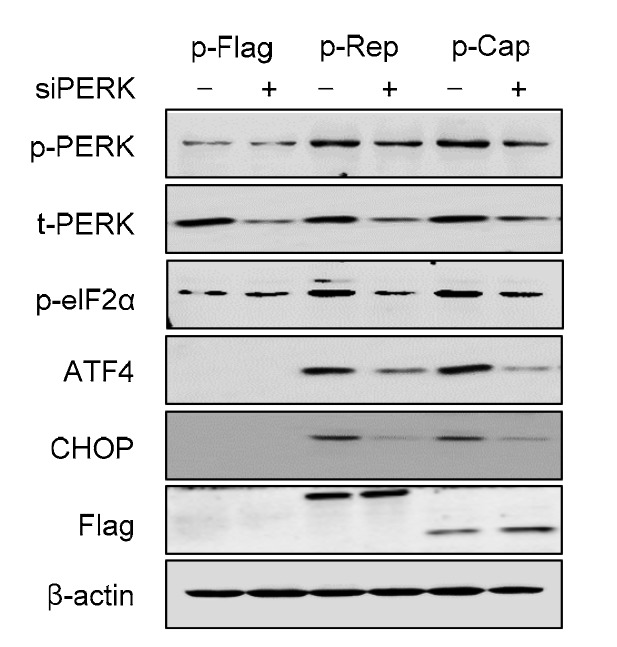
Knockdown of PERK reduced Rep-or Cap-induced expressions of ATF4 and CHOP
PK-15 cells were first transfected with PERK-specific siRNA (siPERK). Scrambled RNA was used as control (−). After 24 h of transfection, the cells were transfected with p-Rep-Flag, p-Cap-Flag, or control vector for 36 h. Cells were harvested and subjected to Western blotting for phosphorylated forms of PERK (p-PERK) and eIF2α (p-eIF2α), total PERK (t-PERK), ATF4, CHOP, Flag, and (-actin. Expression of Rep or Cap fusion protein was revealed by anti-Flag antibody
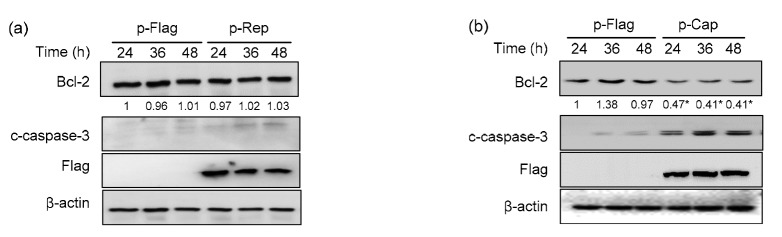
3.4. Cap downregulated Bcl-2 and induced caspase-3 cleavage
One of the acknowledged mechanisms of CHOP-induced apoptosis is through the suppression of the pro-survival protein Bcl-2, which exerts its function by antagonizing pro-apoptotic proteins such as Bax/Bak (Oyadomari and Mori, 2004). We were interested to see if Rep or Cap would modulate activation of the apoptosis executer caspase-3 or expression of Bcl-2. Fig. 4 reveals that Bcl-2 expression was significantly decreased in Cap-transfected cells (P<0.05), but relatively unchanged in Rep-transfected cells. Proteolytic cleavage of caspase-3 precursor into the active form (c-caspase-3) is used as a marker of apoptosis. Western blotting showed that Cap clearly induced caspase-3 cleavage from 24 to 48 hpt, while such cleavage was barely detectable in Rep-expressing cells. These results suggest that Cap, but not Rep, is the apoptosis inducer of PCV2.
Fig. 4.
Cap, but not Rep, downregulated Bcl-2 and induced caspase-3 cleavage
PK-15 cells were transfected with the p-Rep-Flag, p-Cap-Flag, or control vector for 24, 36, and 48 h. Rep-expressing (a) and Cap-expressing (b) cells were subjected to Western blotting for Bcl-2, cleaved caspase-3 (c-caspase-3), Flag, and β-actin. The numbers below the blots “Bcl-2” represent average densitometric ratios of Bcl-2 to (-actin of three independent experiments. The ratio of p-Flag transfected cells at 24 h was considered as 1 and used for normalization with other time points of the p-Flag control cells or with p-Rep or p-Cap expressing cells (* P<0.05, as compared with mock-transfected cells at the same time points)
3.5. Knockdown of PERK reduced Cap-induced caspase-3 activation and apoptosis
Non-resolving ER stress-induced apoptosis is becoming increasingly recognized as an important molecular mechanism in a number of viral diseases such as Hepatitis C virus infection (Benali-Furet et al., 2005) and Influenza A virus infection (Roberson et al., 2012). Given that Cap expression resulted in significant downregulation of Bcl-2 and activation of caspase-3 cleavage, we focused our attention on this structural protein. We silenced PERK in Cap-expressing cells to elucidate its role in Cap-mediated apoptosis. Fig. 5a shows that Bcl-2 level was partially restored in siPERK-transfected cells. In contrast to significant caspase-3 cleavage detected in Cap-expressing cells, transfection with siPERK showed a reduced level of caspase-3 cleavage. To further confirm this observation, flow cytometric analysis of apoptosis was conducted. Fig. 5b shows that the percentage of annexinV+/PI− apoptotic cells was lower in PERK-knockdown Cap-expressing cells than in controls. Lower level of apoptotic response accorded with reduced expression of CHOP and increased expression of Bcl-2 as a result of PERK silencing. Taken together, these results indicate that PERK is involved in Cap-induced apoptosis via upregulation of CHOP and downregulation of Bcl-2.
Fig. 5.
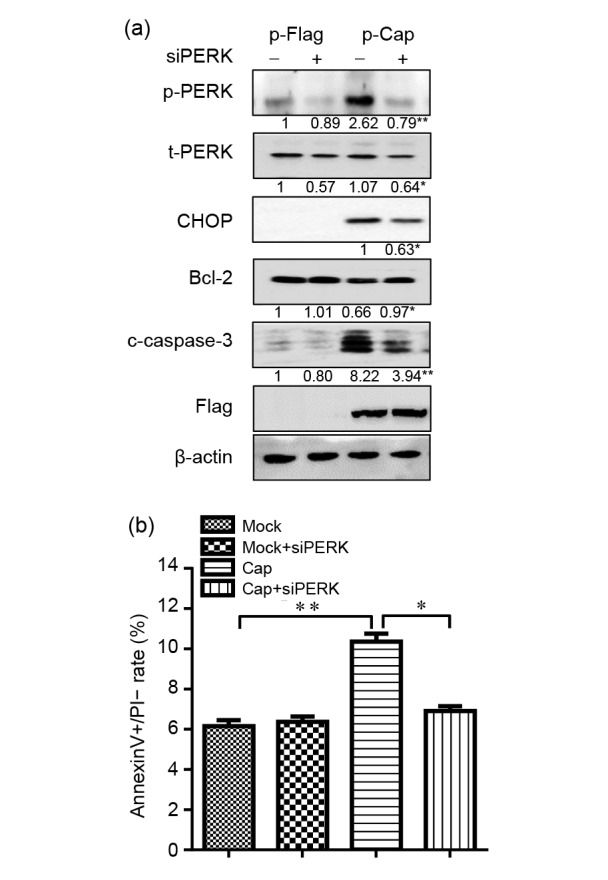
Silencing of PERK reduced Cap-induced caspase-3 activation and apoptosis
PK-15 cells were first transfected with PERK-specific siRNA (siPERK). Scrambled RNA was used as control (−). After 24 h of transfection, the cells were transfected with p-Cap-Flag or control vector for 36 h. Cells were harvested and subjected to Western blotting or labeled for flow cytometric analysis of apoptosis. (a) Western blotting of phosphorylated forms of PERK (p-PERK), total PERK (t-PERK), CHOP, Bcl-2, cleaved caspase-3 (c-caspase-3), Flag, and (-actin. The numbers below the blots represent average densitometric ratios of target molecules to (-actin of three independent experiments. The ratio of p-Flag transfected cells was considered as 1 and used for normalization with other treatments (** P<0.01 as compared with scrambled RNA-transfected Cap-expressing cells). (b) Percentage of apoptotic cells from flow cytometric analysis shown as mean±SD from three independent experiments (* P<0.05; ** P<0.01)
4. Discussion
Both RNA viruses such as hepatitis C virus (Benali-Furet et al., 2005), Japanese encephalitis virus (Su et al., 2002) and DNA viruses such as hepatitis B virus (Li et al., 2007), human cytomegalovirus (Isler et al., 2005) can induce ER stress. For virus-triggered ER stress, the three UPR transmembrane sensors are differentially activated (Li et al., 2015). UPR could be beneficial or detrimental to viral replication, depending on the pathways and cellular contexts involved (Ambrose and Mackenzie, 2011; 2013). To survive and propagate in host cells, viruses have evolved specific mechanisms to modulate or even subvert UPR (Li et al., 2015). In our recent report, we showed that PCV2 could deploy UPR to enhance its replication via PERK/eIF2α signaling pathway (Zhou et al., 2016). However, the mechanisms utilized by PCV2 to benefit its replication under stress conditions as well as the viral protein(s) involved in induction of UPR have yet to be elucidated. In this current report, we provide further evidence that Cap is the major contributor of PCV2 to UPR and subsequent apoptotic response, most likely via the PERK/eIF2α/ATF4/CHOP/Bcl-2 pathway.
First, we examined chaperon GRP78, which works as a sensor of unfolded proteins in the ER and regulates the activation of ER stress transducers. Upregulation of GRP78 in PK-15 cells with transient expression of Rep or Cap is consistent with that observed in PCV2 infection (Zhou et al., 2016). Next, we examined the PERK pathway as it is selectively activated during PCV2 infection. Both Rep and Cap activated PERK and eIF2α phosphorylation as well as their downstream molecules ATF4 and CHOP. By silencing PERK, we confirmed that PERK is essential for induction of CHOP. PERK knockdown resulted in marked reduction of eIF2α phosphorylation and increased expressions of ATF4 and CHOP, indicating the linkage of PERK/eIF2α/ATF4/CHOP axis as seen in PCV2 infection-induced UPR (Zhou et al., 2016). Increased phosphorylation of eIF2α upon ER stress inhibits general protein translation, with the exception of ATF4 that is actually activated to promote many adaptive responses to restore ER function and maintain cell survival (Hetz, 2012). However, sustained stress also activates the transcription of CHOP, one of the key UPR pro-apoptotic players, probably by downregulating the anti-apoptotic protein Bcl-2 (Oyadomari and Mori, 2004). Therefore, we suspected that there might be a connection between UPR and apoptosis in Cap-expressing cells.
We did observe significant decrease of the anti-apoptotic Bcl-2 expression concomitant with increased cleavage of caspase-3 in Cap-expressing cells, but not in cells expressing Rep. Reduced Bcl-2 could be due to increased expression of CHOP (Oyadomari and Mori, 2004). Various viruses regulate apoptosis through CHOP. For example, hepatitis C virus elicits UPR and triggers apoptosis through induction of CHOP and ER calcium depletion (Benali-Furet et al., 2005); hepatitis C virus envelope proteins E1 and E2 could activate the CHOP promoter (Chan and Egan, 2005); and infectious bronchitis virus activates the eIF2α-ATF4-CHOP pathway and upregulation of CHOP plays a critical role in apoptosis (Liao et al., 2013). By RNA silencing, we found that Cap-induced caspase-3 cleavage and apoptosis were reduced in cells transfected with siPERK and the Bcl-2 level was partially restored, indicating that Cap induces apoptotic cell death by sending the message through the PERK/eIF2α/ATF4/CHOP/Bcl-2 pathway down to the mitochondria that ultimately converge on executioner caspase-3 (Tabas and Ron, 2011). Thus, we propose that PCV2 infection induces UPR with subsequent apoptotic response via its Cap protein. This might explain the mechanism why transient Cap protein expressions of PCV2a and PCV2b induce cell death (Walia et al., 2014). This is also different from earlier reports showing that ORF3 seemed to induce apoptosis via caspase-3 activation (Liu et al., 2005; 2006), suggesting that PCV2 is able to deploy two distinct mechanisms in inducing apoptotic response.
5. Conclusions
The findings in this study complemented our recent report revealing induction of UPR by PCV2 infection. Both PCV2 Rep and Cap proteins could activate UPR by activating PERK. However, only Cap-induced UPR led to apoptotic response via the PERK/eIF2α/ATF4/CHOP/Bcl-2 pathway. Further research would be to investigate whether these cellular events would occur in PCV2 permissive cells of the pigs, if there is cross-talk or switching mechanisms among UPR, autophagy, and apoptosis, and whether the viral proteins including the newly found proteins, such as ORF4 or ORF5, interact in deciding the fate of PCV2-infected cells.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 31272534) and the Department of Education of Zhejiang Province (No. Y201635576), China
Compliance with ethics guidelines: Ying-shan ZHOU, Yuan-xing GU, Bao-zhu QI, Yi-kai ZHANG, Xiao-liang LI, and Wei-huan FANG declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Ambrose RL, Mackenzie JM. West Nile virus differentially modulates the unfolded protein response to facilitate replication and immune evasion. J Virol. 2011;85(6):2723–2732. doi: 10.1128/JVI.02050-10. (Available from: ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambrose RL, Mackenzie JM. ATF6 signaling is required for efficient West Nile virus replication by promoting cell survival and inhibition of innate immune responses. J Virol. 2013;87(4):2206–2214. doi: 10.1128/JVI.02097-12. (Available from: ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benali-Furet NL, Chami M, Houel L, et al. Hepatitis C virus core triggers apoptosis in liver cells by inducing ER stress and ER calcium depletion. Oncogene. 2005;24(31):4921–4933. doi: 10.1038/sj.onc.1208673. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 4.Chan SW, Egan PA. Hepatitis C virus envelope proteins regulate CHOP via induction of the unfolded protein response. FASEB J. 2005;19(11):1510–1512. doi: 10.1096/fj.04-3455fje. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 5.Dey S, Savant S, Teske BF, et al. Transcriptional repression of ATF4 gene by CCAAT/enhancer-binding protein β (C/EBPβ) differentially regulates integrated stress response. J Biol Chem. 2012;287(26):21936–21949. doi: 10.1074/jbc.M112.351783. (Available from: ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finsterbusch T, Mankertz A. Porcine circoviruses–small but powerful. Virus Res. 2009;143(2):177–183. doi: 10.1016/j.virusres.2009.02.009. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 7.Gilpin DF, McCullough K, Meehan BM, et al. In vitro studies on the infection and replication of porcine circovirus type 2 in cells of the porcine immune system. Vet Immunol Immunopathol. 2003;94(3-4):149–161. doi: 10.1016/S0165-2427(03)00087-4. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 8.Gu Y, Qi B, Zhou Y, et al. Porcine circovirus type 2 activates CaMMKβ to initiate autophagy in PK-15 cells by increasing cytosolic calcium. Viruses. 2016;8(5):135. doi: 10.3390/v8050135. (Available from: ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He J, Cao J, Zhou N, et al. Identification and functional analysis of the novel ORF4 protein encoded by porcine circovirus type 2. J Virol. 2013;87(3):1420–1429. doi: 10.1128/JVI.01443-12. (Available from: ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13(2):89–102. doi: 10.1038/nrm3270. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 11.Isler JA, Skalet AH, Alwine JC. Human cytomegalovirus infection activates and regulates the unfolded protein response. J Virol. 2005;79(11):6890–6899. doi: 10.1128/JVI.79.11.6890-6899.2005. (Available from: ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li B, Gao B, Ye L, et al. Hepatitis B virus X protein (HBx) activates ATF6 and IRE1-XBP1 pathways of unfolded protein response. Virus Res. 2007;124(1-2):44–49. doi: 10.1016/j.virusres.2006.09.011. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 13.Li S, Kong L, Yu X. The expanding roles of endoplasmic reticulum stress in virus replication and pathogenesis. Crit Rev Microbiol. 2015;41(2):150–164. doi: 10.3109/1040841X.2013.813899. (Available from: ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao Y, Fung TS, Huang M, et al. Upregulation of CHOP/GADD153 during coronavirus infectious bronchitis virus infection modulates apoptosis by restricting activation of the extracellular signal-regulated kinase pathway. J Virol. 2013;87(14):8124–8134. doi: 10.1128/JVI.00626-13. (Available from: ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, Chen I, Kwang J. Characterization of a previously unidentified viral protein in porcine circovirus type 2-infected cells and its role in virus-induced apoptosis. J Virol. 2005;79(13):8262–8274. doi: 10.1128/JVI.79.13.8262-8274.2005. (Available from: ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J, Chen I, Du Q, et al. The ORF3 protein of porcine circovirus type 2 is involved in viral pathogenesis in vivo. J Virol. 2006;80(10):5065–5073. doi: 10.1128/JVI.80.10.5065-5073.2006. (Available from: ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Q, Tikoo SK, Babiuk LA. Nuclear localization of the ORF2 protein encoded by porcine circovirus type 2. Virology. 2001;285(1):91–99. doi: 10.1006/viro.2001.0922. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 18.Lv Q, Guo K, Xu H, et al. Identification of putative ORF5 protein of porcine circovirus type 2 and functional analysis of GFP-fused ORF5 protein. PLoS ONE. 2015;10(6):e0127859. doi: 10.1371/journal.pone.0127859. (Available from: ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mankertz A, Mankertz J, Wolf K, et al. Identification of a protein essential for replication of porcine circovirus. J Gen Virol. 1998;79(Pt 2):381–384. doi: 10.1099/0022-1317-79-2-381. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 20.Meng XJ. Porcine circovirus type 2 (PCV2): pathogenesis and interaction with the immune system. Annu Rev Anim Biosci. 2013;1(1):43–64. doi: 10.1146/annurev-animal-031412-103720. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 21.Moreno JA, Radford H, Peretti D, et al. Sustained translational repression by eIF2α-P mediates prion neurodegeneration. Nature. 2012;485(7399):507–511. doi: 10.1038/nature11058. (Available from: ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nawagitgul P, Morozov I, Bolin SR, et al. Open reading frame 2 of porcine circovirus type 2 encodes a major capsid protein. J Gen Virol. 2000;81(Pt 9):2281–2287. doi: 10.1099/0022-1317-81-9-2281. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 23.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11(4):381–389. doi: 10.1038/sj.cdd.4401373. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 24.Palam LR, Baird TD, Wek RC. Phosphorylation of eIF2 facilitates ribosomal bypass of an inhibitory upstream ORF to enhance CHOP translation. J Biol Chem. 2011;286(13):10939–10949. doi: 10.1074/jbc.M110.216093. (Available from: ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberson EC, Tully JE, Guala AS, et al. Influenza induces endoplasmic reticulum stress, caspase-12-dependent apoptosis, and c-Jun N-terminal kinase-mediated transforming growth factor-β release in lung epithelial cells. Am J Resp Cell Mol. 2012;46(5):573–581. doi: 10.1165/rcmb.2010-0460OC. (Available from: ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su HL, Liao CL, Lin YL. Japanese encephalitis virus infection initiates endoplasmic reticulum stress and an unfolded protein response. J Virol. 2002;76(9):4162–4171. doi: 10.1128/JVI.76.9.4162-4171.2002. (Available from: ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13(3):184–190. doi: 10.1038/ncb0311-184. (Available from: ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Timmusk S, Fossum C, Berg M. Porcine circovirus type 2 replicase binds the capsid protein and an intermediate filament-like protein. J Gen Virol. 2006;87(Pt 11):3215–3223. doi: 10.1099/vir.0.81785-0. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 29.Walia R, Dardari R, Chaiyakul M, et al. Porcine circovirus-2 capsid protein induces cell death in PK15 cells. Virology. 2014;468-470:126–132. doi: 10.1016/j.virol.2014.07.051. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 30.Zhou Y, Qi B, Gu Y, et al. Porcine circovirus 2 deploys PERK pathway and GRP78 for its enhanced replication in PK-15 cells. Viruses. 2016;8(2):56. doi: 10.3390/v8020056. (Available from: ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu B, Xu F, Li J, et al. Porcine circovirus type 2 explores the autophagic machinery for replication in PK-15 cells. Virus Res. 2012;163(2):476–485. doi: 10.1016/j.virusres.2011.11.012. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 32.Zhu B, Zhou Y, Xu F, et al. Porcine circovirus type 2 induces autophagy via the AMPK/ERK/TSC2/mTOR signaling pathway in PK-15 cells. J Virol. 2012;86(22):12003–12012. doi: 10.1128/JVI.01434-12. (Available from: ) [DOI] [PMC free article] [PubMed] [Google Scholar]