Abstract
The protein tyrosine phosphatase 1B (PTP1B) is an important regulator of metabolism. The relationship between PTP1B and tumors is quite complex. The purpose of this study is to explore the expression pattern and role of PTP1B in breast cancer. The expression of PTP1B was detected in 67 samples of breast cancer tissue by Western blot. Cell growth assay, Transwell migration assay, and Scratch motility assay were used to examine the proliferation and migration of MCF-7 with and without PTP1B. The total levels and phosphorylated levels of signal transduction and activator of transcription 3 (STAT3) and the expression of C-C motif chemokine ligand 5 (CCL5) were also examined by Western blot. PTP1B was overexpressed in over 70% of breast cancer tissues, correlating with patients with estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and human epidermal growth factor receptor 2 (HER2)-positive tumors. The data also showed that both tumor size and lymph node metastasis were significantly higher in patients with a higher level of PTP1B. The proliferation and migration of MCF-7 cells were found to be inhibited after knocking down the gene of PTP1B. Our data also showed that PTP1B could up-regulate the dephosphorylated level of STAT3, which could increase the expression of CCL5. These phenomena indicated that PTP1B may play a crucial role in the development of breast cancer.
Keywords: Protein tyrosine phosphatase 1B (PTP1B), Signal transduction and activator of transcription 3 (STAT3), Breast cancer, Tumorigenesis
1. Introduction
Tyrosyl phosphorylation is a reversible process. It plays an important role in a fundamental mechanism of eukaryotic cells. It is closely related to human health and disease (Mustelin et al., 2002). Many cellular functions, such as proliferation and metastasis, are under its control. Protein tyrosine phosphorylation and dephosphorylation can be regulated by both the protein tyrosine kinases and the protein tyrosine phosphatases (PTPs). PTPs comprise a large superfamily. Among them, protein tyrosine phosphatase 1B (PTP1B) is the first PTP purified from human placenta by Fischer et al. (1991). PTP1B, localized on the surface of endoplasmic reticulum, is an abundant cytoplasmic enzyme (Dubé and Tremblay, 2004). Down-regulating the signaling pathways of insulin and leptin is mainly contributed by PTP1B. The gene of PTPN1, encoding PTP1B, is located in the region from q13.1 to q13.2 in the human chromosome of No. 20. This region is found frequently amplified in cervical, gastric, and breast cancers, and is also closely related to poor prognosis (Bjorge et al., 2000; Scotto et al., 2008; Buffart et al., 2009). Recently, it was found that PTP1B may be involved with many kinds of tumor, but the specific function is controversial. Some research proposed that PTP1B may have the potential to inhibit the proliferation and development of tumor cells by counteracting the activity of protein tyrosine kinases. Research showed that tyrosine phosphorylation of some oncogenes, such as ErbB2 and Bcr-Abl, was suppressed by the overexpression of PTP1B in fibroblasts (Woodford-Thomas et al., 1992; LaMontagne et al., 1998; Liu et al., 1998), and Warabi et al. (2000) reported that PTP1B expression in esophageal cancer cells significantly decreased in comparison with normal esophageal tissue. All these suggested that PTP1B may act as a brake to metabolic and proliferative signals. However, recent data implied a positive role of PTP1B in growth signaling in some cancer cells, notably in a mice model of breast cancer which was induced by ErbB2 (Bentires-Alj and Neel, 2007). Some research also reported that the expression of PTP1B in gastric and colon tumor cells is higher than that in surrounding normal tissues (Zhu et al., 2007; Wang et al., 2012). Therefore, the relationship between PTP1B and tumors remains to be determined.
Breast cancer is the most common tumor and the second leading cause of cancer death among women (Baselga and Norton, 2002). In America, the incidence of breast cancer is just a little lower than that of non-melanomatous skin cancers and its mortality rate is second to that of lung cancer (ACS, 2010). The incidence of breast cancer has increased rapidly over the last 20 years, especially in Asia. However, the molecular mechanisms of development and metastasis in breast cancer are not yet clear. In order to clarify the role of PTP1B in breast cancer, we examined the expression of PTP1B in human breast cancer tissue, and used genetic approaches to investigate the function of PTP1B. Our data indicate that PTP1B represents a major mechanism contributing to tumorigenesis in breast cancer.
2. Materials and methods
2.1. Antibodies and reagents
Anti-PTP1B, anti-C-C motif chemokine ligand 5 (anti-CCL5), unphosphorylated signal transduction and activator of transcription 3 (U-STAT3) antibodies, anti-tubulin and phospho-STAT3 antibodies were purchased from Cell Signaling Technology (CST Co., Ltd., Danvers, MA, USA). Rabbit and mouse special secondary antibodies were purchased from Santa Cruz Biotechnology (SCB Co., Ltd., Santa Cruz, CA, USA).
2.2. Tissue sample collection
From January 2009 to August 2010, 67 samples of breast cancer tissues and surrounding normal tissues were collected from the Department of Breast and Thyroid Surgery in the Renmin Hospital and Zhongnan Hospital of Wuhan University, China. All patients with breast cancer were diagnosed by pathological examination, and the pathologic tumor staging was developed by the American Joint Committee on Cancer’s Tumor-Node-Metastasis Classification in 2003. All patients involved in this study have signed informed consent, and this study has been approved by the Ethics Committee at Wuhan University, China.
2.3. Cell culture
The human breast cancer cell line MCF-7 was maintained in RPMI 1640 medium (HyClone, Thermo Scientific, Logan, UT, USA) supplemented with 10% (v/v) of fetal calf serum and 1% (v/v) of penicillin and streptomycin at 37 °C with an atmosphere of 95% air/5% CO2 in a humidified incubator.
2.4. Lentivirus infections
The lentiviral particle productions, PTP1B-RNAi-LV and NC-RNAi-LV respectively containing PTP1B short hairpin RNA (shRNA) and non-target control shRNA, were purchased from GeneChem Co., Ltd. (Shanghai, China). Lentiviral knockdown of PTP1B proteins was achieved by infecting 30%–50% confluent MCF-7 cells with 1.2×104 TU/μl (TU: transducing unit) lentivirus in the presence of 10 μg/ml polybrene. The infection medium was replaced after 18 h. Then culture with fresh medium was supplemented with 5 μg/ml puromycin for 4 weeks to allow for efficient PTP1B protein knockdown. Stably transfected clones were collected for use in this study.
2.5. Western blot
Proteins were extracted as described by Boute et al. (2003). Protein samples were resolved by 10% (0.1 g/ml) sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to polyvinylidene fluoride (PVDF) membranes. The PVDF membranes were blocked by 1% (0.01 g/ml) bovine serum albumin (BSA) and hybridized with corresponding primary antibody at 4 °C overnight, and incubated with specific secondary antibody for 2 h at room temperature. They were detected by a chemiluminescence system. The density of autoradiograms was quantified by Quantity One software (Bio-Rad, USA). Relative protein levels were calculated by reference to the amount of tubulin protein.
2.6. Cell growth assay
MCF-7 cells were respectively planted at about 2×103 per well in 96-well plates in quintuplicate. The cell number was counted every 12 h using Cell-Counting Kit-8 (R & D Systems, Minneapolis, MN, USA). The optical density (OD) values of absorbance were measured by a microplate reader (Multiskan FC, Thermo Scientific, USA), and reported as mean±standard deviation (SD).
2.7. Transwell migration assay
Serum-free media with 5×104 suspension cells were added into the upper chamber of 24-well Transwell plates, and 500 μl RPMI 1640 medium with 10% fetal calf serum was added to the bottom chamber as chemoattractant. After incubation at 37 °C with 5% CO2 for 24 h, cells on the upper chamber were removed by sterile swabs, and cells in the lower chamber were fixed by methanol for 20 min and stained with crystal violet staining solution for 30 min, and three preset fields per insert were photographed. Then they were eluted by 33% (v/v) acetic acid, and microplate reader was used to measure the OD values of absorbance in staining-positive cells.
2.8. Scratch motility assay
Cells of 5×105 per well were seeded into six-well plates and cultured in RPMI 1640 medium supplemented with 10% fetal calf serum at 37 °C with 5% CO2 overnight. Next day, the monolayer was scratched with a pipette tip, washed with phosphate-buffered saline (PBS) three times to remove floating cells, and further cultured in RPMI 1640 medium supplemented with 10% fetal calf serum. The scratched area was photographed at 0 and 24 h with 10× objective lens. The average scratch width was calculated at five random points and reported as mean±SD.
2.9. Statistical analysis
SPSS 18.0 software was used for statistical analysis. Results are expressed as mean±SD. The chi-squared test was used to test the correlation between PTP1B expression in tumors and clinicopathologic variables, and the t-test was used to test the difference for the independent samples.
3. Results
3.1. Expression of PTP1B in breast cancer tissue
To explore the role of PTP1B in breast cancer development, we detected the expression of PTP1B in 67 samples of breast cancer tissue and surrounding normal tissue by Western blot. Forty-nine of 67 patients had a high expression of PTP1B in breast cancer tissue, and the level of PTP1B in breast cancer tissue was lower than that in surrounding normal tissue in the other 18 patients. When the densities of autoradiograms were quantified by Quantity One software (Fig. 1), we found that the level of PTP1B was much higher in breast cancer tissue than in the surrounding normal tissue and the difference has significance (P<0.05, n=67).
Fig. 1.
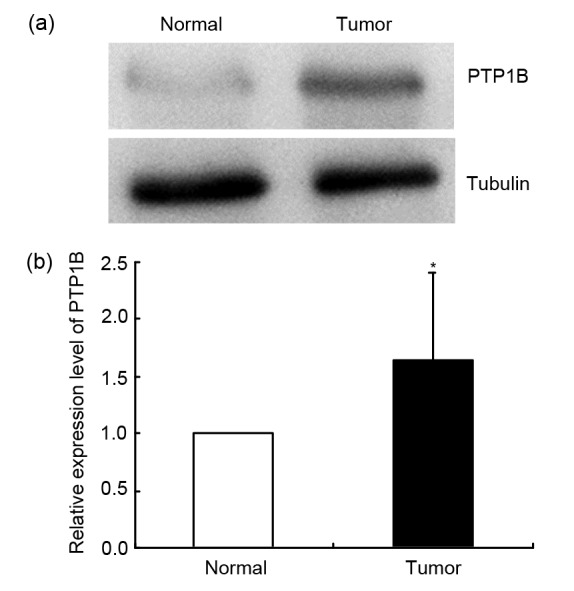
Expression of PTP1B in breast cancer tissue
(a) The expression of PTP1B in breast cancer tissue and the surrounding normal tissue was examined by Western blot; (b) Quantifications of PTP1B autoradiograms are represented as mean±SD (n=67). * P<0.05, vs. normal tissue
In order to assess the relationship between PTP1B expression level and clinicopathologic factors in breast cancer, 67 samples of tumor tissue and surrounding normal tissue were analyzed. Patients’ clinicopathologic conditions are presented in Tables 1 and 2. Our research showed that the positive rate of PTP1B was increased with the degree of malignancy. From Table 1, we can see that with the increase of tumor stage from T1 to T4, the positive rate of PTP1B significantly increased from 50% to 100% (P=0.032). With the increase of lymph node stage from N0 to N3, the positive rate of PTP1B also significantly increased from 52% to 100% (P=0.014). Moreover, PTP1B was highly expressed in all breast cancer patients with distant metastasis. However, no significant correlation was found between the level of PTP1B and the patients with metastasis (P=0.388). From Table 2, the PTP1B level in T2+T3+T4 was higher than that in T1 (P=0.029, relative risk (RR)=3.818, 95% confidence interval (CI) 1.105–13.198). The patients with lymph node metastasis (N1+N2+N3) have a higher PTP1B level than those without lymph node metastasis (P=0.003, RR=5.538, 95% CI 1.724–17.797). We also found that advanced breast cancer (II+III+IV) tissue had a higher PTP1B level than the early stage breast cancer tissue. The data from Table 1 also showed that the higher PTP1B level was correlated with estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and human epidermal growth factor receptor 2 (HER2)-positive tumors.
Table 1.
Expression of PTP1B in breast cancer tissues and its correlation with clinicopathologic characteristics
| Variable | PTP1B*
|
Overall P | |
| Negative | Positive | ||
| Tumor stage (T) | 0.032 | ||
| T1 | 7 (50%) | 7 (50%) | |
| T2 | 9 (23%) | 30 (77%) | |
| T3 | 2 (18%) | 9 (82%) | |
| T4 | 0 (0%) | 3 (100%) | |
| Lymph node (N) | 0.014 | ||
| N0 | 12 (48%) | 13 (52%) | |
| N1 | 2 (10%) | 18 (90%) | |
| N2 | 4 (27%) | 11 (73%) | |
| N3 | 0 (0%) | 7 (100%) | |
| Metastasis (M) | 0.388 | ||
| M0 | 18 (28%) | 47 (72%) | |
| M1 | 0 (0%) | 2 (100%) | |
| TNM stage | 0.018 | ||
| I | 7 (54%) | 6 (46%) | |
| II | 7 (24%) | 22 (76%) | |
| III | 4 (17%) | 19 (83%) | |
| IV | 0 (0%) | 2 (100%) | |
| ER status | 0.001 | ||
| Positive | 15 (47%) | 17 (53%) | |
| Negative | 3 (9%) | 32 (91%) | |
| PR status | 0.002 | ||
| Positive | 11 (52%) | 10 (48%) | |
| Negative | 7 (16%) | 37 (84%) | |
| Unknown | 0 (0%) | 2 (100%) | |
| HER2 status | <0.001 | ||
| Positive | 7 (14%) | 42 (86%) | |
| Negative | 11 (61%) | 7 (39%) | |
Data are expressed as number (percent)
Table 2.
Relationship between PTP1B expression and TNM stage in breast cancer
| Variable | PTP1B*
|
Overall P | RR | 95% CI | |
| Negative | Positive | ||||
| Tumor stage (T) | 0.029 | 3.818 | 1.105–13.198 | ||
| T1 | 7 (50%) | 7 (50%) | |||
| T2+T3+T4 | 11 (21%) | 42 (79%) | |||
| Lymph node (N) | 0.003 | 5.538 | 1.724–17.797 | ||
| N0 | 12 (48%) | 13 (52%) | |||
| N1+N2+N3 | 6 (14%) | 36 (86%) | |||
| TNM stage | 0.015 | 4.561 | 1.273–16.334 | ||
| I | 7 (54%) | 6 (46%) | |||
| II+III+IV | 11 (20%) | 43 (80%) | |||
Data are expressed as number (percent)
3.2. Effect of PTP1B on the proliferation of breast cancer cells
To delineate the proliferation role of PTP1B in human breast cancer cells, we used lentiviral delivery of shRNA to knock down PTP1B. As shown in Figs. 2a and 2b, effective and selective knockdown of PTP1B was achieved. The expression of PTP1B in MCF-7/PTP1B shRNA was lower than that in MCF-7 cells. The proliferation of MCF-7 cells was much faster than that of MCF-7/PTP1B shRNA cells. The proliferation ability decreased with time, especially after 24 h (Fig. 2c).
Fig. 2.

Effect of PTP1B on the proliferation of MCF-7 cells
(a) Western blotting analysis showed that PTP1B protein expression in MCF-7 cells was much higher than that in MCF-7/PTP1B shRNA cells; (b) Quantifications of PTP1B autoradiograms are represented as mean±SD (* P<0.05, vs. non-target shRNA); (c) Cell growth (mean±SD) was assessed with a cell-counting kit 8 (CCK8). Knocking down the PTP1B expression decreased the proliferation of cells (* P<0.05, vs. non-target shRNA). All results were representative of three independent experiments
3.3. Effect of PTP1B on migration of breast cancer cells
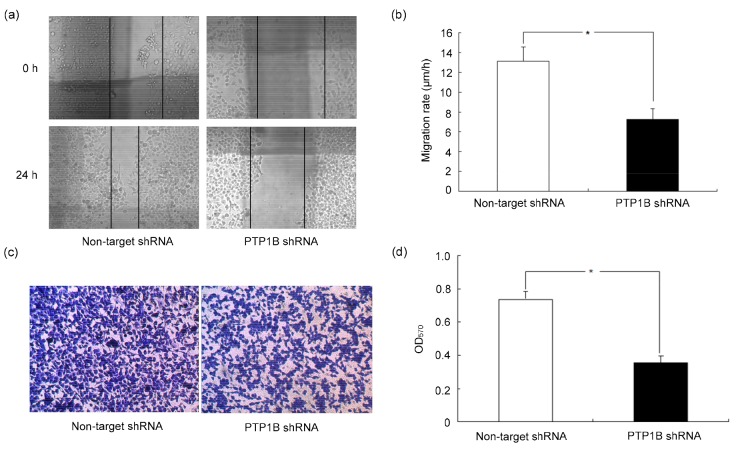
To investigate the effect of PTP1B on migration of breast cancer cells, we used two different methods, scratch motility assay and Transwell migration assay. For the scratch motility assay, the wound closure was observed after the monolayer cell was scratched for 24 h. A significantly enhanced wound closure was observed in MCF-7 cells (Fig. 3a). The motility of MCF-7 was almost twice of that in MCF-7/PTP1B shRNA cells (MCF-7 vs. MCF-7/PTP1B shRNA cells: 13.13 μm/h vs. 7.24 μm/h). In addition, Transwell assay also indicated that the invasiveness and migration had association with PTP1B (Fig. 3c). The migration and invasiveness of MCF-7/PTP1B shRNA cells reduced by 51.78% compared to MCF-7 cells.
Fig. 3.
Effect of PTP1B on the migration of breast cancer cells
Low level of PTP1B decreased the migration of breast cancer cells. (a) Scratch motility assay; (b) The velocity of cell migration into the scratched area was counted at five randomly selected field and represented as mean±SD; (c) Transwell migration assay; (d) Optical density values at 570 nm (OD570) in staining positive cell were represented as mean±SD. * P<0.05. All results were representative of three independent experiments
3.4. Effect of PTP1B on the production of CCL5
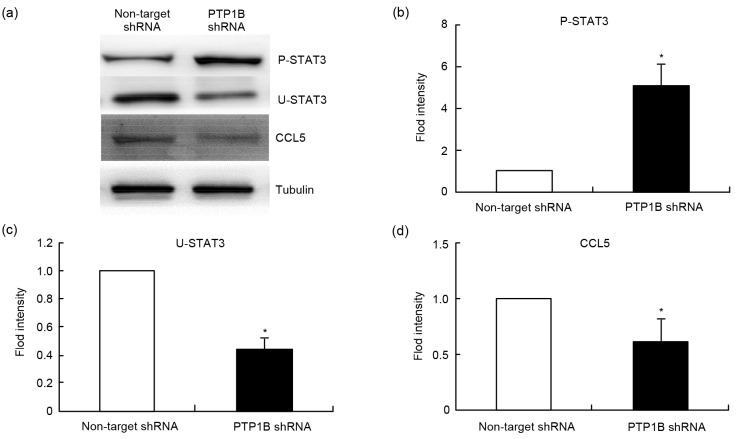
In the previous study, we found that the proliferation and migration of breast cancer are closely correlated with CCL5 (Zhang et al., 2009). Research has also shown that U-STAT3 can effectively compete with non-phosphorylated inhibitory κB (IκB) in binding with nuclear factor (NF)-κB to form a new transcription factor. The new transcription factor could induce the expression of CCL5 (Yang et al., 2005; 2007; Yang and Stark, 2008; Zhang et al., 2014). Therefore, we detect the expressions of phosphorylated STAT3, U-STAT3, and CCL5 in both MCF-7 cells and MCF-7/PTP1B shRNA cells by Western blot. The results are shown in Fig. 4. When the gene of PTP1B was knocked down, the phosphorylated level of STAT3 was significantly increased, but the levels of U-STAT3 and CCL5 were significantly decreased. Therefore, we postulated that the expression of CCL5 might be mediated through PTP1B.
Fig. 4.
Effect of PTP1B on the expressions of proteins P-STAT3, U-STAT3, and CCL5
(a) Western blotting analysis showed that knocking down the PTP1B increased the phosphorylation levels of STAT3 and decreased the levels of U-STAT3 and CCL5. (b, c, d) Quantifications of P-STAT3, U-STAT3, and CCL5 autoradiograms are represented as mean±SD. * P<0.05, vs. non-target shRNA. All results were representative of three independent experiments. P-STAT3: phosphorylated STAT3; U-STAT3: unphosphorylated STAT3; CCL5: C-C motif chemokine ligand 5
4. Discussion
Previous studies of PTP1B expression in different kinds of cancer and tumor cells have found variable expression levels and conflicting effects of this phosphatase in oncogenesis (Warabi et al., 2000; Dubé et al., 2005; Julien et al., 2007; Zhu et al., 2007). Up to now, the function of PTP1B is still unclear. Some studies have found that PTP1B is required for HER2/Neu-induced breast cancer, but the correlation between the expression of PTP1B and clinicopathologic factor was not clarified in breast cancer. To answer this question, we designed this experiment. We found PTP1B was overexpressed in over 70% breast cancer tissues. The result also provides evidence that the expression of PTP1B may adversely alter the presentation of breast cancer. The data from our study showed that both tumor size and lymph node metastasis have a significantly higher stage in patients with a higher level of PTP1B; the RR is 3.818 and 5.538, respectively. We also found tumors at an advanced stage (II+III+IV) expressed a high level of PTP1B. When we compared the expression of PTP1B and the clinicopathologic characteristics of 67 breast cancer patients, the results also showed that a higher level of PTP1B was correlated with patients with ER-negative, PR-negative and HER2-positive tumors. These phenomena indicated that PTP1B may play a crucial role in the development of breast cancer. To investigate the role of PTP1B in proliferation and migration of breast cancer, we knocked down the gene of PTP1B in MCF-7 cells. Our results showed that knocking down the gene of PTP1B will inhibit the proliferation and migration of MCF-7 cells. From the current study, we consider that PTP1B could up-regulate the dephosphorylated level of STAT3, which could increase the expression of CCL5.
Some studies also found that PTP1B could promote the development of breast cancer. Bentires-Alj and Neel (2007) have reported that transgenic overexpression of an activated form of HER2 (NeuNT) in the PTP1B+/+ mice mammary gland, under the control of mouse mammary tumor virus (MMTV) promoter (MMTV-NeuNT mice), causes glandular hyperplasia followed by mammary adenocarcinoma in fewer than 13 months on average, but PTP1B−/− mice developed mammary tumors over a 3-year period. Julien et al. (2007) found that inhibiting the expression of PTP1B might prevent the occurrence of breast cancer. His study also indicated that PTP1B has association with the lung metastasis of breast cancer. This phenomenon was also found in other tumors, such as gastric and colon cancers (Zhu et al., 2007; Wang et al., 2012).
Currently, there are several mechanisms by which PTP1B could promote tumor development. Some studies have found that high expression of PTP1B will abnormally activate some oncogenes (Nanney et al., 1997). Another mechanism is that PTP1B could catalyze Src dephosphorylation and Src Tyr530 phosphorylation could reduce Src activation. Therefore, PTP1B might promote the activation of Src. The activity of Src was overexpressed in several types of human cancers and played critical roles in a variety of cellular signal transduction pathways (Zhu et al., 2007; Arias-Romero et al., 2009). In the study of Arias-Romero et al. (2009), their results suggested that Src was a key target in mediating the positive role of PTP1B in ErbB2 oncogenesis. Furthermore, PTP1B is also proposed to dephosphorylate the scaffolding adapter p62DOK, which binds p120 Ras GTPase-activating protein (RasGAP) and promotes Ras/extracellular signal-regulated kinase (ERK) pathway inactivation (Dubé et al., 2004; Bentires-Alj and Neel, 2007). Bentires-Alj and Neel (2007) found that PTP1B mainly exists in HER2-positive breast cancer tissue, which is similar to our conclusion, and they considered that ErbB2 is another target of PTP1B in contributing to tumorigenesis in breast cancer. Moreover, PTP1B can also regulate insulin-like growth factor 2 (IGF-2)-induced MCF-7 cell migration. Herein, we described a novel mechanism: PTP1B could increase the expression of U-STAT3, which can effectively compete with non-phosphorylated IκB in binding with NF-κB to form a new transcription factor, and a new transcription factor could induce the expression of CCL5 Yang et al., 2005; 2007; Yang and Stark, 2008). It has been reported that the proliferation and migration of breast cancer have a close relationship with CCL5, and CCL5 is considered as an independent prognostic factor in breast cancer (Karnoub et al., 2007; Pinilla et al., 2009; Zhang et al., 2009).
In conclusion, PTP1B was overexpressed in breast cancer tissues, and the level of PTP1B in breast cancer was significantly correlated with the tumor and node stage. Our results also showed that the deficiency of PTP1B will inhibit the proliferation and migration of MCF-7 cells. Our study indicated that the expression of PTP1B may adversely alter the presentation of breast cancer. In recent years, several pharmaceutical companies have programs to develop PTP1B inhibitors to treat obesity, diabetes, and other important medical disorders (Klaman et al., 2000; Bence et al., 2006). Our study suggests that a PTP1B inhibitor can be used for the treatment of breast cancer.
Footnotes
Project supported by the Research Foundation of Public Health Bureau of Hubei Province (No. JX3A14), China
Compliance with ethics guidelines: Shi-chong LIAO, Jin-xin LI, Li YU, and Sheng-rong SUN declare that they have no conflict of interest.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all patients for being included in the study.
References
- 1.ACS (American Cancer Society) Breast Cancer Facts & Figures 2009–2010. Atlanta: American Cancer Society; 2010. p. 28. [Google Scholar]
- 2.Arias-Romero LE, Saha S, Villamar-Cruz O, et al. Activation of Src by protein tyrosine phosphatase 1B is required for ErbB2 transformation of human breast epithelial cells. Cancer Res. 2009;69(11):4582–4588. doi: 10.1158/0008-5472.CAN-08-4001. (Available from: ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baselga J, Norton L. Focus on breast cancer. Cancer Cell. 2002;1(4):319–322. doi: 10.1016/S1535-6108(02)00066-1. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 4.Bence KK, Delibegovic M, Xue B, et al. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat Med. 2006;12(8):917–924. doi: 10.1038/nm1435. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 5.Bentires-Alj M, Neel BG. Protein-tyrosine phosphatase 1B is required for HER2/Neu-induced breast cancer. Cancer Res. 2007;67(6):2420–2424. doi: 10.1158/0008-5472.CAN-06-4610. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 6.Bjorge JD, Pang A, Fujita DJ. Identification of protein-tyrosine phosphatase 1B as the major tyrosine phosphatase activity capable of dephosphorylating and activating c-Src in several human breast cancer cell lines. J Biol Chem. 2000;275(52):41439–41446. doi: 10.1074/jbc.M004852200. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 7.Boute N, Boubekeur S, Lacasa D, et al. Dynamics of the interaction between the insulin receptor and protein tyrosine-phosphatase 1B in living cells. EMBO Rep. 2003;4(3):313–319. doi: 10.1038/sj.embor.embor767. (Available from: ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buffart TE, van Grieken NC, Tijssen M, et al. High resolution analysis of DNA copy-number aberrations of chromosomes 8, 13, and 20 in gastric cancers. Virchows Arch. 2009;455(3):213–223. doi: 10.1007/s00428-009-0814-y. (Available from: ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubé N, Tremblay ML. Beyond the metabolic function of PTP1B. Cell Cycle. 2004;3(5):548–551. doi: 10.4161/cc.3.5.851. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 10.Dubé N, Cheng A, Tremblay ML. The role of protein tyrosine phosphatase 1B in Ras signaling. Proc Natl Acad Sci USA. 2004;101(7):1834–1839. doi: 10.1073/pnas.0304242101. (Available from: ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubé N, Bourdeau A, Heinonen KM, et al. Genetic ablation of protein tyrosine phosphatase 1B accelerates lymphomagenesis of p53-null mice through the regulation of B-cell development. Cancer Res. 2005;65(21):10088–10095. doi: 10.1158/0008-5472.CAN-05-1353. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 12.Fischer EH, Charbonneau H, Tonks NK. Protein tyrosine phosphatases: a diverse family of intracellular and transmembrane enzymes. Science. 1991;253(5018):401–406. doi: 10.1126/science.1650499. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 13.Julien SG, Dubé N, Read M, et al. Protein tyrosine phosphatase 1B deficiency or inhibition delays ErbB2-induced mammary tumorigenesis and protects from lung metastasis. Nat Genet. 2007;39(3):338–346. doi: 10.1038/ng1963. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 14.Karnoub AE, Dash AB, Vo AP, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449(7162):557–563. doi: 10.1038/nature06188. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 15.Klaman LD, Boss O, Peroni OD, et al. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol Cell Biol. 2000;20(15):5479–5489. doi: 10.1128/MCB.20.15.5479-5489.2000. (Available from: ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LaMontagne KRJr, Hannon G, Tonks NK. Protein tyrosine phosphatase PTP1B suppresses p210 bcr-abl-induced transformation of Rat-1 fibroblasts and promotes differentiation of K562 cells. Proc Natl Acad Sci USA. 1998;95(24):14094–14099. doi: 10.1073/pnas.95.24.14094. (Available from: ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu F, Sells MA, Chernoff J. Transformation suppression by protein tyrosine phosphatase 1B requires a functional SH3 ligand. Mol Cell Biol. 1998;18(1):250–259. doi: 10.1128/MCB.18.1.250. (Available from: ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mustelin T, Feng GS, Bottini N, et al. Protein tyrosine phosphatases. Front Biosci. 2002;7:d85–d142. doi: 10.2741/A770. [DOI] [PubMed] [Google Scholar]
- 19.Nanney LB, Davidson MK, Gates RE, et al. Altered distribution and expression of protein tyrosine phosphatases in normal human skin as compared to squamous cell carcinomas. J Cutan Pathol. 1997;24(9):521–532. doi: 10.1111/j.1600-0560.1997.tb01456.x. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 20.Pinilla S, Alt E, Abdul Khalek FJ, et al. Tissue resident stem cells produce CCL5 under the influence of cancer cells and thereby promote breast cancer cell invasion. Cancer Lett. 2009;284(1):80–85. doi: 10.1016/j.canlet.2009.04.013. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 21.Scotto L, Narayan G, Nandula SV, et al. Identification of copy number gain and overexpressed genes on chromosome arm 20q by an integrative genomic approach in cervical cancer: potential role in progression. Genes Chromosomes Cancer. 2008;47(9):755–765. doi: 10.1002/gcc.20577. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Liu B, Chen X, et al. PTP1B expression contributes to gastric cancer progression. Med Oncol. 2012;29(2):948–956. doi: 10.1007/s12032-011-9911-2. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 23.Warabi M, Nemoto T, Ohashi K, et al. Expression of protein tyrosine phosphatases and its significance in esophageal cancer. Exp Mol Pathol. 2000;68(3):187–195. doi: 10.1006/exmp.2000.2303. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 24.Woodford-Thomas TA, Rhodes JD, Dixon JE. Expression of a protein tyrosine phosphatase in normal and v-src-transformed mouse 3T3 fibroblasts. J Cell Biol. 1992;117(2):401–414. doi: 10.1083/jcb.117.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang J, Stark GR. Roles of unphosphorylated STATs in signaling. Cell Res. 2008;18(4):443–451. doi: 10.1038/cr.2008.41. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 26.Yang J, Chatterjee-Kishore M, Staugaitis SM, et al. Novel roles of unphosphorylated STAT3 in oncogenesis and transcriptional regulation. Cancer Res. 2005;65(3):939–947. [PubMed] [Google Scholar]
- 27.Yang J, Liao X, Agarwal MK, et al. Unphosphorylated STAT3 accumulates in response to IL-6 and activates transcription by binding to NFκB. Genes Dev. 2007;21(11):1396–1408. doi: 10.1101/gad.1553707. (Available from: ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Yao F, Yao X, et al. Role of CCL5 in invasion, proliferation and proportion of CD44+/CD24− phenotype of MCF-7 cells and correlation of CCL5 and CCR5 expression with breast cancer progression. Oncol Rep. 2009;21(4):1113–1121. doi: 10.3892/or_00000331. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Liao S, Fan W, et al. Tunicamycin-induced ER stress regulates chemokine CCL5 expression and secretion via STAT3 followed by decreased transmigration of MCF-7 breast cancer cells. Oncol Rep. 2014;32(6):2769–2776. doi: 10.3892/or.2014.3479. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 30.Zhu S, Bjorge JD, Fujita DJ. PTP1B contributes to the oncogenic properties of colon cancer cells through Src activation. Cancer Res. 2007;67(21):10129–10137. doi: 10.1158/0008-5472.CAN-06-4338. (Available from: ) [DOI] [PubMed] [Google Scholar]