Abstract
Fufang Xueshuantong (FXT) is a well-known Chinese herbal formula which has been used to treat cardiovascular and ophthalmic diseases, especially diabetic retinopathy. Panax notoginseng (Burkill) F.H. Chen (PN) is the main herb of FXT, whose major bioactive constituents are ginsenosides. However, the scientific basis of the compatibility of FXT is still ambiguous. The present study investigated the scientific basis of the compatibility of FXT by comparing the pharmacokinetics of marker compounds after oral administrations of PN and FXT. A high performance liquid chromatography-electrospray ionization tandem mass spectrometry (HPLC-ESI-MS/MS) method was developed for simultaneous detection of notoginsenoside R1 (NR1), ginsenoside Rg1 (GRg1), and ginsenoside Rb1 (GRb1) in rat plasma. The pharmacokinetic studies of FXT and PN were performed using the established method with the pharmacokinetic parameters being determined by non-compartmental analysis. The results showed that the pharmacokinetic parameters (maximum concentration, area under the curve (AUC0–t), clearance, and mean residence time) of NR1, GRg1, and GRb1 were significantly different after oral administration of FXT (P<0.05) compared with PN. The AUC0–t values of GRg1 and GRb1 were 1.7-and 3.4-fold greater, respectively, in FXT than in PN. The compatible herbs of FXT could prolong the retention time and increase the systemic exposure of NR1, GRg1, and GRb1 compared with PN in vivo, providing some scientific basis for the compatibility and clinical use of FXT.
Keywords: Fufang Xueshuantong, Panax notoginseng, Pharmacokinetics, Compatibility
1. Introduction
Fufang Xueshuantong (FXT), a Chinese herbal formula, is composed of Panax notoginseng (Burkill) F.H. Chen (Araliaceae), Salvia miltiorrhiza Bunge (Lamiaceae), Astragalus membranaceus (Fisch.) Bunge (Leguminosae), and Scrophularia ningpoensis Hemsl (Scrophulariaceae). It was approved by the State Food and Drug Administration of China for the clinical treatment of cardiovascular disease in 2003 (State medical license No. Z20030017) (Sheng et al., 2014). It has also been widely used to treat ophthalmic diseases, especially diabetic retinopathy. Modern research has indicated that FXT has beneficial effects on retinal vein occlusion (Yuan et al., 2011), diabetic retinopathy (Duan et al., 2013), and diabetic nephropathy (Zhang et al., 2013).
Pharmacokinetic studies can help greatly in understanding the in vivo process of traditional Chinese medicine (TCM) and in investigating potential mechanisms of the compatibility of herbal formulas, as well as in providing guidance for drug development and clinical application (Yang et al., 2012; Wu et al., 2014). TCM formulas often contain a variety of different components which may interact during pharmacokinetic and pharmacodynamic processes (Lai et al., 2011). However, there has been limited investigation into the pharmacokinetics of FXT, or the scientific basis of its compatibility. Because of the complexity of the constituents in TCMs and the combined prescriptions, most research has selected one or several representative compounds to investigate the pharmacokinetic properties of a single herb or a whole prescription (Wang et al., 2014; Huang et al., 2015). Panax notoginseng (Burkill) F.H. Chen (PN), which contains notoginsenoside R1 (NR1), ginsenoside Rg1 (GRg1), and ginsenoside Rb1 (GRb1) as its major active components, is the main herb in FXT. It has been widely used to treat vascular disorders (Ng, 2006). Our previous research has demonstrated that NR1, GRg1, and GRb1 are contained in the core effective constituent of FXT (Jian et al., 2015). Therefore, NR1, GRg1, and GRb1 were selected as the marker compounds in the present study.
The aim of the present study was to investigate the scientific basis of the compatibility of FXT by comparing the pharmacokinetics of marker compounds after oral administrations of PN and FXT. To our knowledge, this is the first time that the scientific basis of the compatibility of FXT has been studied. The results will help to verify the compatibility and to improve the clinical application of FXT.
2. Materials and methods
2.1. Chemicals and reagents
FXT and PN (extract and residue) were provided by Guangdong Zhongsheng Pharmaceutical Co., Ltd. (No. 130703, Guangzhou, China). NR1 (No. 110766-200619, ≥98%), GRg1 (No. 110703-201027, ≥98%), and GRb1 (No. 110704-201122, ≥98%) were purchased from the National Institute for Food and Drug Control (Beijing, China). Palmatine chloride (MUST-12022707, internal standard (IS) ≥98%) was purchased from Chengdu MUST Bio-technology Co., Ltd. (Chengdu, China). The structures of R1, Rg1, Rb1, and IS are shown in Fig. 1. Methanol and acetonitrile were of high performance liquid chromatography (HPLC) grade (Fisher, Fair Lawn, NJ, USA). Formic acid was of analytical grade and was purchased from Beijing Chemical Works (Beijing, China). Heparin sodium and carboxymethyl cellulose sodium salt (both analytical grade) were purchased from Beijing BioDee Biotechnology Co., Ltd. (Beijing, China).
Fig. 1.
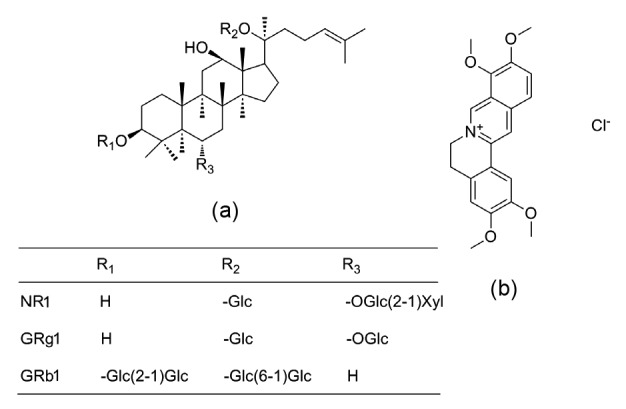
Chemical structures of NR1, GRg1, GRb1 (a), and IS (b)
2.2. Animals
Male Sprague-Dawley rats, weighing (300±20) g, were purchased from the Sibeifu Experimental Animal Science and Technology Co., Ltd. (Beijing, China). Rats were acclimatized for 7 d before the start of experiments. They were kept in a controlled environment (12-h light/12-h dark cycle, consistent temperature and humidity), with abundant food and water. Animal experiments were performed in accordance with the guidelines for the Care and Use of Laboratory Animals, and all experimental protocols were reviewed and approved by the Institutional Animal Experimentation Committee of Beijing University of Chinese Medicine (Beijing, China).
2.3. Instrumentation and analytical conditions
The liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) system consisted of a Surveyor AS autosampler, Surveyor LC pump, Surveyor MS pump, Accela solvent transport pump, and a TSQ Quantum Access tandem mass spectrometer (TSQ Quantum Access MAX, Thermo Fisher Scientific Inc., San Jose, CA, USA) with electrospray ionization (ESI) source.
The chromatographic separations were carried out using a Thermo Hypersil GOLD C18 column (2.1 mm×100 mm, 5 μm) by gradient elution with a mobile phase of acetonitrile and water (containing 1% formic acid) at a flow rate of 0.2 ml/min. The gradient conditions of the mobile phase were as follows: 0–9 min, 25%–52% acetonitrile; 9–10 min, 52% acetonitrile. The column temperature was maintained at 30 °C and the injection volume was 10 μl.
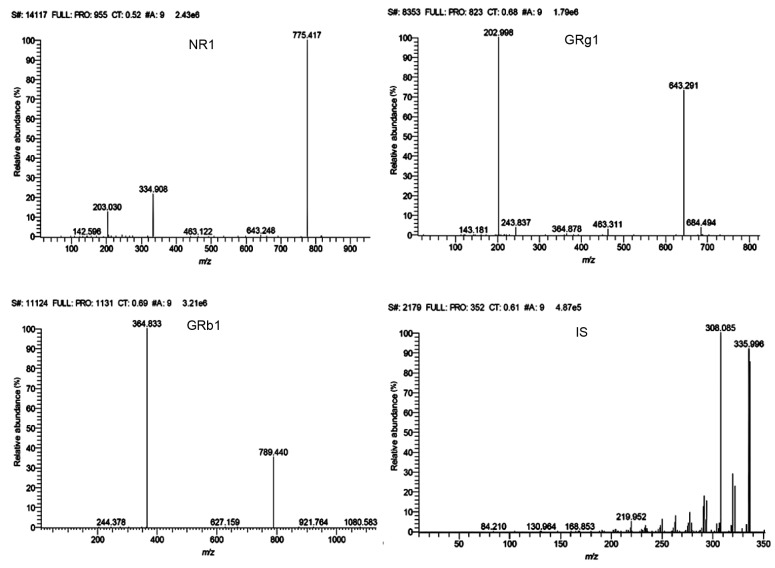
The mass spectrometer was operated in positive ionization mode using multiple reaction monitoring (MRM) to assess NR1, GRg1, GRb1, and IS: m/z 955.3–775.4 for NR1, m/z 823.3–643.3 for GRg1, m/z 1131.4–364.8 for GRb1, m/z 352.2–336.1 for IS. The optimized collision energies were 41, 30, 54, and 26 eV, respectively. A spray voltage of 3000 V was used. The capillary temperature was 380 °C and the vaporizer temperature was 200 °C. The pressures of sheath gas and auxiliary gas were 28 and 10 aribitrary unit, respectively. Data acquisition and processing were accomplished on a Thermo TSQ Xcalibur 2.0 workstation. The representative MS/MS spectra of NR1, GRg1, GRb1, and IS are shown in Fig. 2.
Fig. 2.
Representative MS/MS spectra of NR1, GRg1, GRb1, and palmatine chloride (IS)
2.4. Method validation
2.4.1 Preparation of calibration standards, IS, and quality control samples
Stock solutions for NR1, GRg1, and GRb1 were separately prepared by dissolving the accurately weighed reference standards in methanol to yield a concentration of 10.0, 9.5, and 13.3 μg/ml, respectively. A series of standard or quality control (QC) working solutions was prepared by mixing the stock solutions of NR1, GRg1, and GRb1 together and then serially diluting them with methanol. The IS working solution of palmatine chloride was prepared in the same way at a concentration of 104 ng/ml. All of the solutions were stored at 4 °C and brought to room temperature before use.
The calibration samples were prepared by spiking 200 μl blank plasma with 200 μl standard working solutions and 200 μl IS working solutions to obtain final concentrations. The final concentrations were 1.00–800.00, 0.95–760.00, and 1.33–1330.00 ng/ml for NR1, GRg1, and GRb1, respectively. Three QC plasma samples were prepared in the same way to yield concentrations of 25.00, 100.00, 300.00 ng/ml for NR1; 23.75, 95.00, 285.00 ng/ml for GRg1; and 33.25, 133.00, 399.00 ng/ml for GRb1.
2.4.2 Selectivity
To investigate the selectivity of the assay, blank plasma, blank plasma spiked with NR1, GRg1, GRb1, and IS, and plasma samples obtained from rats after oral administration of FXT were analyzed for the exclusion of any endogenous co-eluting interference at or close to the expected retention time of NR1, GRg1, GRb1, and IS.
2.4.3 Linearity and lower limit of quantification
The linearity was determined by analyzing a series of standard plasma samples with different concentrations. The calibration curves were fitted by least squares linear regression of the peak area ratios of each analyte to IS obtained against the corresponding concentrations (C) with a weighting factor of 1/C 2. The lower limit of quantification (LLOQ) was defined as the lowest concentration on the calibration curve with an acceptable relative standard deviation (RSD) below 20%.
2.4.4 Accuracy and precision
The intra-and inter-day precisions, defined as the RSD, were evaluated by analyzing QC samples at three different concentrations in five replicates on the same day and five consecutive validation days. The assay accuracy was expressed as relative error (RE), which was calculated by (calculated concentration−nominal concentration)/nominal concentration×100%.
2.4.5 Extraction recovery and matrix effect
The extraction recoveries of the three analytes from rat plasma at three QC levels were measured individually by comparing the mean peak areas obtained from the blank plasma samples spiked with the analytes prior to extraction to those added after extraction (n=5). The ratio gives the percentage recovery.
The matrix effect (ME) was evaluated by comparing the peak areas in post-extracted blank plasma spiked with NR1, GRg1, GRb1, and IS to those of equivalent concentrations of the pure standard solutions at three QC levels. The IS normalized ME (IS-ME) was calculated by dividing the ME of the analyte by the ME of the IS, which means IS-ME=matrix effect of each analyte/matrix effect of internal standard×100%.
2.4.6 Stability
Three concentration levels of QC plasma samples (low, medium, and high) in five duplicates were subjected to analysis using the conditions given below. The short-term stability was assessed by analyzing QC plasma samples kept at room temperature for 18 h. The freeze-thaw stability of plasma was investigated after one freeze (−20 °C)-thaw (room temperature) cycle.
2.5. Preparation of PN and FXT
FXT was produced by Zhongsheng Pharmacy according to the Chinese Pharmacopoeia (National Pharmacopoeia Committee, 2015), which contains PN extract (24.0%), PN residue (30.0%), extracts from three other herbs (19.6%), and excipients (26.4%). The FXT solution was prepared using the powder of the capsule. The powder (1.05 g) was precisely weighed and suspended in 0.5% (5 g/L) carboxymethylcellulose sodium (CMC-Na) solution giving a final volume of 10 ml.
The PN extract and residue were obtained from the same batch of FXT. The PN solution was prepared by combining 0.252 g extract and 0.315 g residue in the same proportion as used in FXT, and then 0.5% CMC-Na solution was added giving a final volume of 10 ml.
2.6. Pharmacokinetic study
The rats were randomly divided into two groups (n=6) and were orally administered PN (0.567 g/kg) or FXT (1.050 g/kg), respectively. The rats were fasted overnight and received water ad libitum before and during the pharmacokinetic study. Blood samples (400 μl) from the orbital vein were collected into heparinized tubes at 0.083, 0.167, 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, 8, 12, 24, 36, 48, and 72 h after oral administration, and were immediately separated by centrifugation at 3500g for 10 min. The collected supernatant was then stored at −20 °C until analysis.
2.7. Sample preparation
The IS solution (104 ng/ml, 200 μl) was added into 2.0 ml centrifuge tubes and evaporated to dryness. Then, 200 μl aliquots of plasma samples were added and the solution was vortexed for 1 min. Afterwards, 600 μl of methanol was added and vortexed for 1 min. The mixed samples were separated by centrifugation at 6000g for 10 min. The clean supernatant was collected and evaporated to dryness. Then the residue was re-dissolved by 150 μl of the mobile phase in the proportions used at the start (25:75 (v/v), acetonitrile: 1% formic acid aqueous solution), vortexed for 1 min, and centrifuged at 9000g for 20 min. The clean supernatant was collected and stored at −20 °C until further analysis.
2.8. Data analysis
The plasma concentration-time profile was processed by non-compartmental analysis using the Winnonlin 4.1 package. Statistical significance was assessed by an unpaired Student’s t-test and the significance level adopted for all statistical comparisons was P<0.05. All results were expressed as mean±standard deviation (SD).
3. Results and discussion
3.1. Method validation
3.1.1 Selectivity
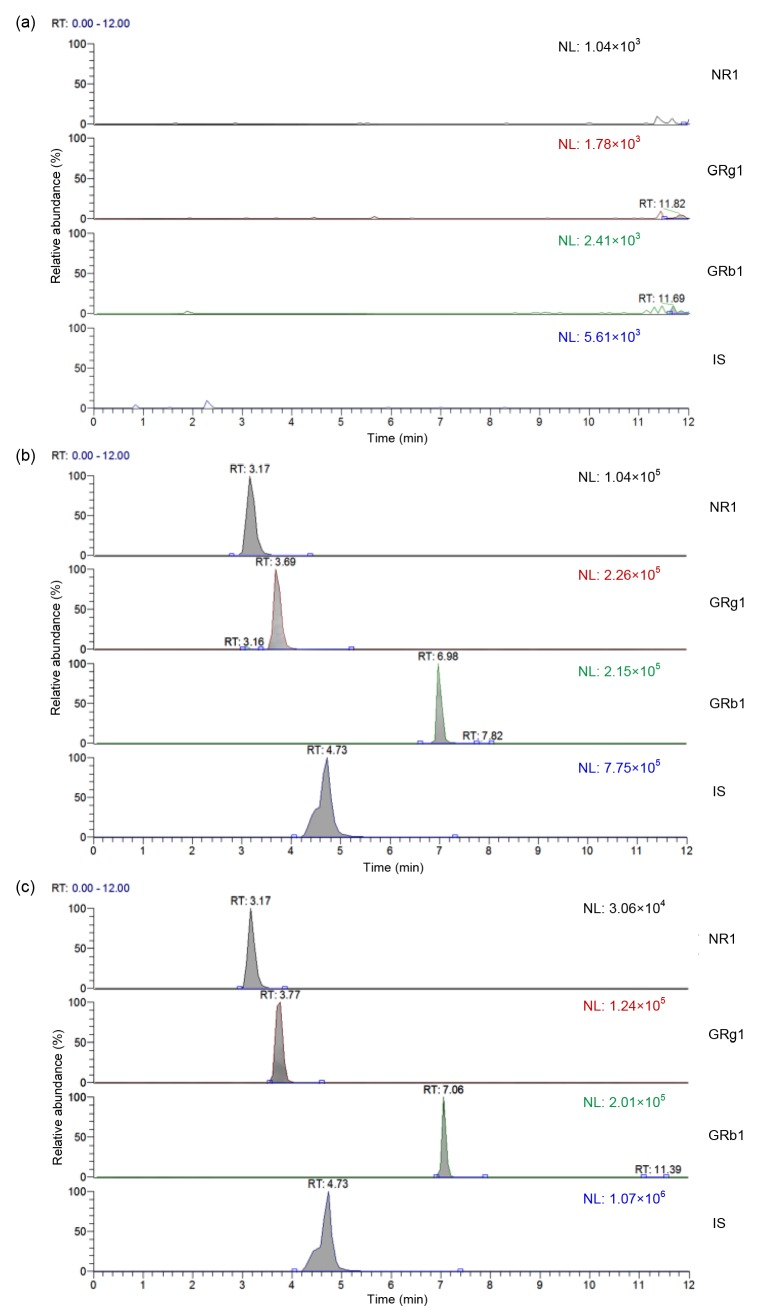
Under the LC-MS/MS conditions described, a good separation of the analytes was achieved and no significant endogenous peak to interfere with NR1, GRg1, and GRb1 was observed, as shown in Fig. 3.
Fig. 3.
Representative MRM chromatograms of NR1, GRg1, GRb1, and IS in rat plasma samples
(a) Blank plasma; (b) Blank plasma spiked with analytes at a high level of QC, and IS (104 ng/ml); (c) Plasma sample collected from a rat at 1 h after oral administration of FXT
3.1.2 Linearity and LLOQ
The linear ranges, regression equations, correlation coefficients, and LLOQ are listed in Table 1. Excellent linearity was observed over these concentration ranges with a correlation coefficient (r) higher than 0.99. The LLOQ values of NR1, GRg1, and GRb1 were acceptable, with RSD of less than 20%.
Table 1.
Linearity and LLOQ of the assay for NR1, GRg1, and GRb1
| Analyte | Linear equation | r | Linear range (ng/ml) | LLOQ (ng/ml) | RSD (%) |
| NR1 | y=2.37×10−5 x+2.50×10−5 | 0.9910 | 1.00–800.00 | 1.00 | 17.28 |
| GRg1 | y=6.80×10−5 x+1.54×10−4 | 0.9980 | 0.95–760.00 | 0.95 | 8.67 |
| GRb1 | y=1.65×10−5 x+1.13×10−5 | 0.9938 | 1.33–1330.00 | 1.33 | 16.94 |
LLOQ: lower limit of quantification; RSD: relative standard deviation
3.1.3 Accuracy and precision
The results of intra-day (n=5) and inter-day (n=5) accuracies and precisions of three analytes estimated by evaluating three concentrations of QC samples (25, 100, 300 ng/ml) are shown in Table 2. All results for the samples tested are within the acceptable criteria of ±15%.
Table 2.
Accuracy and precision of the analytes at three different concentrations in rat plasma (n=5)
| Analyte | Concentration (ng/ml) | Accuracy (RE) (%) | Precision (RSD) (%) |
|
| Intra-day | Inter-day | |||
| NR1 | 25.00 | 3.06 | 13.91 | 10.47 |
| 100.00 | 17.60 | 5.73 | 8.00 | |
| 300.00 | 6.07 | 5.93 | 2.03 | |
| GRg1 | 23.75 | 7.44 | 12.82 | 9.27 |
| 95.00 | 7.69 | 4.78 | 2.48 | |
| 285.00 | −1.77 | 1.54 | 3.59 | |
| GRb1 | 33.25 | −14.30 | 9.77 | 6.53 |
| 133.00 | 16.30 | 5.04 | 9.88 | |
| 399.00 | 8.71 | 5.67 | 2.89 | |
RSD: relative standard deviation; RE: relative error
3.1.4 Extraction recovery and matrix effect
The mean extraction recoveries of NR1, GRg1 and GRb1 ranged from 53.72% to 74.11% (Table 3). Although these were a little low, they were consistent with the extraction recovery of IS (62.11%) and fluctuated within ±15%.
Table 3.
Extraction recovery and IS-ME of the analytes at three different concentrations in rat plasma (n=5)
| Analyte | Spiked C (ng/ml) | Extraction recovery (%) | IS-ME (%) |
| NR1 | 25.00 | 73.89±5.59 | 112.15±5.09 |
| 100.00 | 72.36±1.96 | 115.72±10.24 | |
| 300.00 | 67.25±3.62 | 113.27±4.48 | |
| GRg1 | 23.75 | 74.11±8.64 | 115.71±10.15 |
| 95.00 | 68.83±2.64 | 115.17±6.12 | |
| 285.00 | 71.24±3.46 | 112.50±8.77 | |
| GRb1 | 33.25 | 56.16±2.35 | 115.57±2.37 |
| 133.00 | 53.72±1.80 | 117.63±3.01 | |
| 399.00 | 62.41±1.93 | 107.27±2.83 |
C: concentration; IS-ME: IS normalized matrix effect. Data are presented as mean±SD
The detailed matrix effect results are shown in Table 3. Despite the heavy absolute ME of the analytes within the range of 120%–150%, the IS-ME values were 107%–118%, which indicated that IS eliminated the absolute matrix effect of the three analytes to some extent.
3.1.5 Stability
The stability of notoginsenosides in rat plasma under different conditions was investigated and summarized in Table 4. The concentrations of NR1, GRg1, and GRb1 in plasma under different conditions deviated less than 15% compared with those in the freshly spiked plasma, which showed good stabilities of NR1, GRg1, and GRb1 in the overall steps of the determination.
Table 4.
Stabilities of the analytes in rat plasma (n=5)
| Analyte | Spiked C (ng/ml) | Short-term stability |
Freeze-thaw stability |
||
| Measured C (ng/ml)* | RE (%) | Measured C (ng/ml)* | RE (%) | ||
| NR1 | 25.00 | 24.92±2.36 | −0.30 | 23.17±3.54 | −7.32 |
| 100.00 | 103.37±13.97 | 3.37 | 100.74±9.63 | 0.74 | |
| 300.00 | 308.37±28.89 | 2.79 | 314.76±7.07 | 4.92 | |
| GRg1 | 23.75 | 23.26±3.45 | −2.05 | 27.06±2.11 | 13.94 |
| 95.00 | 92.70±8.40 | −2.42 | 99.81±8.27 | 5.06 | |
| 285.00 | 251.04±9.27 | −11.92 | 241.90±25.10 | −15.12 | |
| GRb1 | 33.25 | 31.14±4.14 | −6.32 | 31.72±1.33 | −4.60 |
| 133.00 | 123.97±21.51 | −6.79 | 110.40±6.21 | −16.99 | |
| 399.00 | 369.77±55.09 | −7.33 | 424.14±18.01 | 6.30 | |
C: concentration; RE: relative error.
Data are presented as mean±SD
3.2. Pharmacokinetic study
The validated method was successfully applied to pharmacokinetic studies of NR1, GRg1, and GRb1 in rat plasma after oral administrations of PN and FXT. The mean plasma concentration-time profiles are shown in Fig. 4, and the pharmacokinetic parameters are presented in Table 5.
Fig. 4.
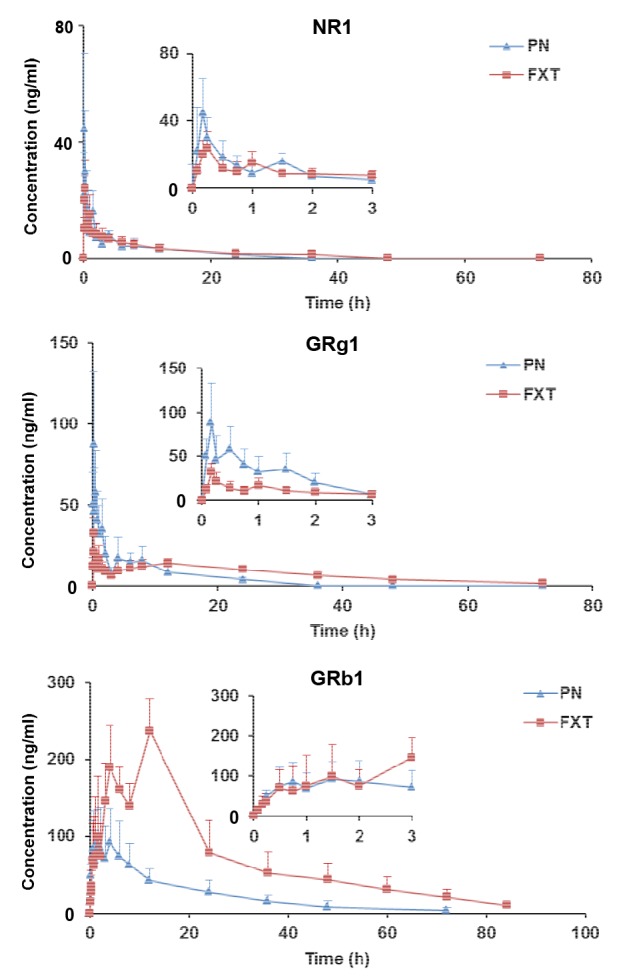
Mean concentration-time profiles of NR1, GRg1, and GRb1 in rat plasma after oral administrations of PN and FXT
Partial enlarged drawings of 0–3 h are shown in each image. Date are expressed as mean±SD (n=6)
Table 5.
Pharmacokinetic parameters for NR1, GRg1, and GRb1 after oral administrations of PN and FXT (n=6)
| Analyte | Administration | K e (h−1) | T 1/2 (h) | T max (h) | C max (ng/ml) | AUC0–t (h·ng/ml) |
| NR1 | PN | 0.089±0.037 | 9.17±4.21 | 0.17±0.00 | 44.67±25.79 | 105.53±20.38 |
| FXT | 0.065±0.035 | 12.64±4.68 | 0.22±0.04* | 24.80±8.80 | 109.60±26.00 | |
| GRg1 | PN | 0.084±0.019 | 8.73±2.40 | 0.28±0.17 | 90.82±42.00 | 274.63±101.27 |
| FXT | 0.060±0.014* | 11.98±2.66 | 0.17±0.00 | 32.48±9.46* | 453.04±76.42** | |
| GRb1 | PN | 0.053±0.017 | 14.07±3.99 | 2.71±1.47 | 102.17±42.66 | 1730±918 |
| FXT | 0.039±0.017 | 20.87±8.48 | 12.00±0.00** | 237.08±42.42** | 5797±1908** | |
|
| ||||||
|
| ||||||
| Analyte | Administration | AUC0–∞ (h·ng/ml) | V d (ml/kg) | CL (ml/(h·kg)) | T MR0–t (h) | T MR0–∞ (h) |
|
| ||||||
| NR1 | PN | 119.41±22.09 | 390 609±183 186 | 29 440±5974 | 8.40±2.32 | 12.41±4.55 |
| FXT | 133.90±26.20 | 423 902±188 784 | 23 227±5360 | 9.21±2.85 | 16.51±5.68 | |
| GRg1 | PN | 320.87±105.96 | 1 152 179±463 127 | 90 327±26 839 | 7.72±1.11 | 12.19±3.23 |
| FXT | 478.22±96.99* | 835 441±149 883 | 49 507±9290** | 21.53±3.05** | 24.19±4.92** | |
| GRb1 | PN | 1836±988 | 233 381±105 370 | 12 490±6590 | 17.13±2.48 | 20.65±3.21 |
| FXT | 6117±1843** | 84 423±46 266** | 2647±606* | 24.85±1.80** | 30.33±4.41** | |
K e: elimination rate; T 1/2: half-life time; T max: peak time; C max: peak concentration; AUC: area under the curve; V d: apparent volume of distribution; CL: clearance; T MR: mean residence time.
P<0.05;
P<0.01 compared with the PN group. Data are presented as mean±SD
Double peaks are present in the plasma concentration time curves. This phenomenon is very common in pharmacokinetic studies of TCM (Long et al., 2014). Possible reasons include enterohepatic recirculation (Pedersen and Miller, 1980), delayed gastric emptying (Oberle and Amidon, 1987), variable absorption within different regions of the gut (Lennernas and Regardh, 1993), reabsorption after distribution to tissues, and so on. Owing to their different structures, NR1 and GRg1 are 20(s)-protopanaxatrial saponins (Ppts) whereas GRb1 is a 20(s)-protopanaxadiol saponin (Ppd). NR1 and GRg1 showed quick absorption with peak time (T max) about 0.2 h, while the absorption rate of GRb1 was relatively slow with T max about 2 h. A similar phenomenon can also be seen in previous studies (Xu et al., 2003; Deng et al., 2009).
Significant pharmacokinetic differences were found between PN and FXT. In comparison with oral administration of PN, the pharmacokinetic parameters (maximum concentration (C max), area under the curve (AUC0–t, AUC0–∞), clearance (CL), mean residence time (T MR0–t, T MR0–∞)), particularly for GRg1 and GRb1, were significantly different after oral administration of FXT (P<0.05). FXT administration markedly improved the systemic levels of GRg1 and GRb1 in vivo, with AUC0–t increasing from 274.63 to 453.04 h·ng/ml and from 1730 to 5797 h·ng/ml, respectively. Moreover, the CL of GRg1 and GRb1 decreased from 90 327 to 49 507 ml/(h·kg) and from 12 490 to 2647 ml/(h·kg), respectively. The mean retention time (T MR0–t) of GRg1 and GRb1 increased from 7.72 to 21.53 h and 17.13 to 24.85 h, respectively. In addition, the absorption rate of NR1 in PN was significantly higher than that in FXT (T max increased from 0.17 to 0.22 h).
The compatibility of PN with other herbs in FXT and possible herb-herb interactions may account for these pharmacokinetic differences. To our knowledge, pharmacokinetic herb-herb interactions can be partly explained by regulation of liver microsomal cytochrome P450-mediated metabolism. It was reported that the main metabolism enzymes of P. notoginseng Saponins are CYP1A2 and/or CYP3A4 (Hao et al., 2010; Liu et al., 2012), which could be inhibited by some components of the herb extract. Some studies have shown that the extract of S. miltiorrhiza exhibited an inhibitory effect on liver microsomal CYP1A2 and CYP3A in vitro (Wang and Yeung, 2010; 2012). Another study showed that the extract of A. membranaceus exerted an inhibitory effect on liver microsomal CYP3A4 (Lau et al., 2013). In addition, it has been reported that the hydrophilic properties and large molecular weights of NR1, GRg1, and GRb1 lead to their poor intestinal permeabilities and low bioavailabilities (Han et al., 2007). However, the water-soluble components of FXT might enhance the solubility and promote the absorption of ginsenosides in the intestine. It has been reported that the water-soluble components of S. miltiorrhiza can increase the solubility of other water-soluble components (Song et al., 2007). The results of our pharmacokinetic comparison studies infer that the compatible herbs in FXT could increase the absorption and reduce the rate of metabolism of the major ginsenosides of PN (monarch herb) in vivo; however, further studies on the compatibility mechanisms of FXT are needed.
4. Conclusions
We have developed and validated an LC-MS/MS method for simultaneous determination of NR1, GRg1, and GRb1 in rat plasma. We used it to perform pharmacokinetic studies of NR1, GRg1, and GRb1 in rat plasma after oral administration of PN or FXT. Our results show that the pharmacokinetic properties of NR1, GRg1, and GRb1 differ significantly between PN and FXT. The compatible herbs in FXT prolonged the retention time and elevated the systemic levels of NR1, GRg1, and GRb1 in vivo. This study provided valuable information for the compatibility and clinical application of FXT.
Footnotes
Project supported by the Ministry of Science and Technology of China (No. 2011ZX09201-201-22)
Compliance with ethics guidelines: Huan-huan PANG, Meng-yi LI, Yuan WANG, Min-ke TANG, Chang-hua MA, and Jian-mei HUANG declare that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Deng G, Wang D, Meng M, et al. Simultaneous determination of notoginsenoside R1, ginsenoside Rg1, Re, Rb1 and icariin in rat plasma by ultra-performance liquid chromatography-tandem mass spectrometry. J Chromatogr B. 2009;877(22):2113–2122. doi: 10.1016/j.jchromb.2009.06.003. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 2.Duan H, Huang J, Li W, et al. Protective effects of Fufang Xueshuantong on diabetic retinopathy in rats. Evid-Based Compl Alt. 2013;2013:408268. doi: 10.1155/2013/408268. (Available from: ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han M, Fu S, Fang XL. Comparison between the characteristics of absorption and pharmacokinetic behavior of ginsenoside Rg1 and ginsenoside Rb1 of Panax notoginseng saponins. Acta Pharmaceut Sin. 2007;42(8):849–853. (in Chinese) [PubMed] [Google Scholar]
- 4.Hao H, Lai L, Zheng C, et al. Microsomal cytochrome P450-mediated metabolism of protopanaxatriol ginsenosides: metabolite profile, reaction phenotyping, and structure-metabolism relationship. Drug Metab Dispos. 2010;38(10):1731–1739. doi: 10.1124/dmd.110.033845. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 5.Huang H, Yang Y, Lv C, et al. Pharmacokinetics and tissue distribution of five bufadienolides from the Shexiang Baoxin pill following oral administration to mice. J Ethnopharmacol. 2015;161:175–185. doi: 10.1016/j.jep.2014.07.056. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 6.Jian W, Yu S, Tang M, et al. A combination of the main constituents of Fufang Xueshuantong Capsules shows protective effects against streptozotocin-induced retinal lesions in rats. J Ethnopharmacol. 2015;182:50–56. doi: 10.1016/j.jep.2015.11.021. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 7.Lai X, Zhang L, Li J, et al. Comparative pharmacokinetic and bioavailability studies of three salvianolic acids after the administration of Salviae miltiorrhizae alone or with synthetical borneol in rats. Fitoterapia. 2011;82(6):883–888. doi: 10.1016/j.fitote.2011.04.015. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 8.Lau C, Mooiman KD, Maas-Bakker RF, et al. Effect of Chinese herbs on CYP3A4 activity and expression in vitro. J Ethnopharmacol. 2013;149(2):543–549. doi: 10.1016/j.jep.2013.07.014. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 9.Lennernas H, Regardh C. Evidence for an interaction between the β-blocker pafenolol and bile salts in the intestinal lumen of the rat leading to dose-dependent oral absorption and double peaks in the plasma concentration-time profile. Pharmaceut Res. 1993;10:819–883. doi: 10.1023/a:1018965328626. [DOI] [PubMed] [Google Scholar]
- 10.Liu R, Qin M, Hang P, et al. Effects of Panax notoginseng Saponins on the activities of CYP1A2, CYP2C9, CYP2D6 and CYP3A4 in rats in vivo. Phytother Res. 2012;26(8):1113–1118. doi: 10.1002/ptr.3688. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 11.Long W, Zhang S, Wen L, et al. In vivo distribution and pharmacokinetics of multiple active components from Danshen and Sanqi and their combination via inner ear administration. J Ethnopharmacol. 2014;156:199–208. doi: 10.1016/j.jep.2014.08.041. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 12.National Pharmacopoeia Committee. Chinese Pharmacopoeia (2015 Chinese version) Part 1. China Medical Science Press, Beijing. 2015:1223–1224. (in Chinese) [Google Scholar]
- 13.Ng TB. Pharmacological activity of sanchi ginseng (Panax notoginseng) J Pharm Pharmacol. 2006;58(8):1007–1019. doi: 10.1211/jpp.58.8.0001. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 14.Oberle RL, Amidon GL. The influence of variable gastric emptying and intestinal transit rates on the plasma level curve of cimetidine; an explanation for the double peak phenomenon. J Pharmacokinet Biop. 1987;15(5):529–544. doi: 10.1007/BF01061761. [DOI] [PubMed] [Google Scholar]
- 15.Pedersen PV, Miller R. Pharmacokinetics and bioavailability of cimetidine in humans. J Pharm Sci. 1980;69(4):394–398. doi: 10.1002/jps.2600690408. [DOI] [PubMed] [Google Scholar]
- 16.Sheng S, Wang J, Wang L, et al. Network pharmacology analyses of the antithrombotic pharmacological mechanism of Fufang Xueshuantong Capsule with experimental support using disseminated intravascular coagulation rats. J Ethnopharmacol. 2014;154(3):735–744. doi: 10.1016/j.jep.2014.04.048. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 17.Song M, Hang TJ, Zhang ZX. Pharmacokinetic interactions between the main components in the extracts of Salvia miltiorrhiza Bge. in rat. Acta Pharm Sin. 2007;42(3):301–307. (in Chinese) [PubMed] [Google Scholar]
- 18.Wang Q, Jiang P, Ye F, et al. Identification and pharmacokinetics of multiple constituents in rat plasma after oral administration of Yinchenzhufu decoction. J Ethnopharmacol. 2014;153(3):714–724. doi: 10.1016/j.jep.2014.03.039. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Yeung JHK. Effects of the aqueous extract from Salvia miltiorrhiza Bunge on caffeine pharmacokinetics and liver microsomal CYP1A2 activity in humans and rats. J Pharm Pharmacol. 2010;62(8):1077–1083. doi: 10.1111/j.2042-7158.2010.01127.x. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Yeung JH. Investigation of cytochrome P450 1A2 and 3A inhibitory properties of Danshen tincture. Phytomedicine. 2012;19(3-4):348–354. doi: 10.1016/j.phymed.2011.09.075. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 21.Wu K, Wang Z, Liu D, et al. Pharmacokinetics, brain distribution, release and blood-brain barrier transport of Shunaoxin pills. J Ethnopharmacol. 2014;151(3):1133–1140. doi: 10.1016/j.jep.2013.12.027. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 22.Xu QF, Fang XL, Chen DF. Pharmacokinetics and bioavailability of ginsenoside Rb1 and Rg1 from Panax notoginseng in rats. J Ethnopharmacol. 2003;84(2-3):187–192. doi: 10.1016/S0378-8741(02)00317-3. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 23.Yang S, Zhang K, Lin X, et al. Pharmacokinetic comparisons of single herb extract of Fufang Danshen preparation with different combinations of its constituent herbs in rats. J Pharmaceut Biomed. 2012;67-68:77–85. doi: 10.1016/j.jpba.2012.03.058. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 24.Yuan Y, Yuan F, Xu Q, et al. Effect of Fufang Xueshuantong Capsule on a rat model of retinal vein occlusion. Chin J Integr Med. 2011;17(4):296–301. doi: 10.1007/s11655-011-0690-6. (Available from: ) [DOI] [PubMed] [Google Scholar]
- 25.Zhang Q, Xiao X, Li M, et al. Attenuating effect of Fufang Xueshuantong Capsule on kidney function in diabetic nephropathy model. J Nat Med. 2013;67(1):86–97. doi: 10.1007/s11418-012-0654-y. (Available from: ) [DOI] [PubMed] [Google Scholar]