Abstract
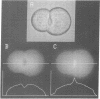
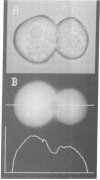
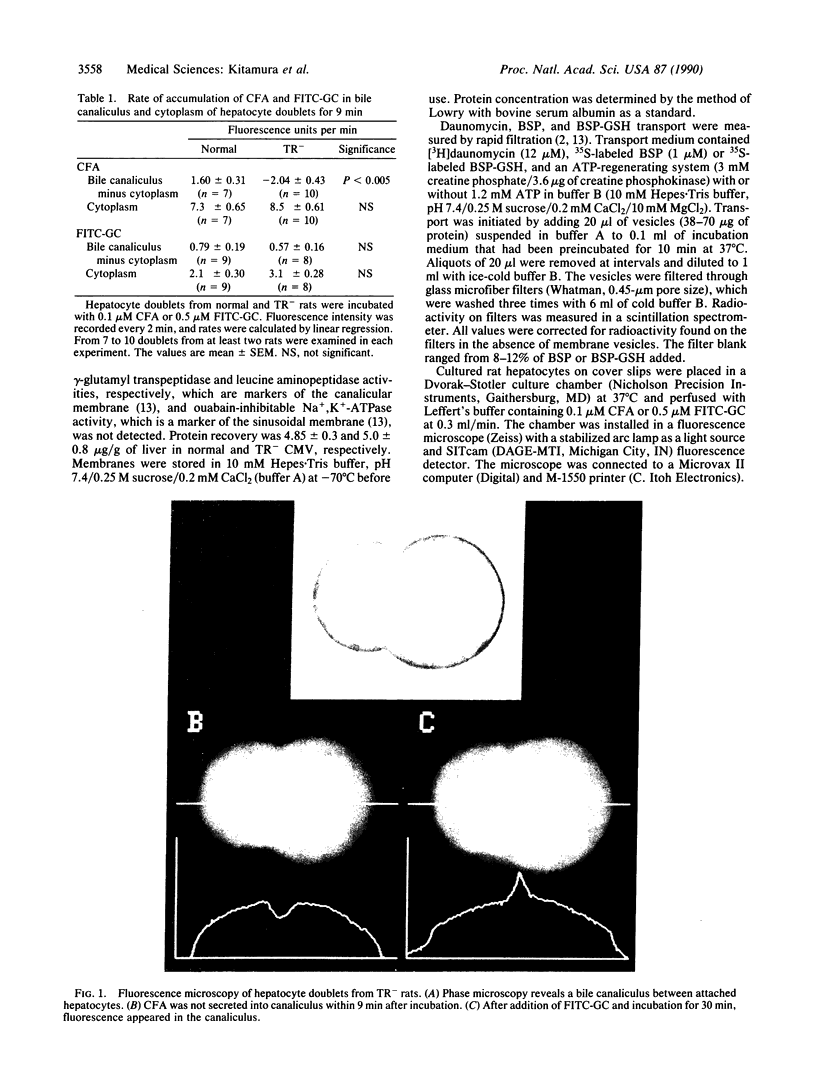
TR- mutant Wistar rats secrete markedly fewer organic anions other than bile acids from the liver into the bile than do control rats. Fluorescence-image analysis of isolated normal and TR- hepatocyte "doublets", which retain a bile canaliculus between them, revealed that normal hepatocytes readily transport a fluorescent bile acid (fluorescein isothiocyanate glycocholate) and a nonbile acid organic anion (carboxydichlorofluorescein diacetate) into the canaliculus. Hepatocyte doublets from TR- rats also transported fluorescein isothiocyanate glycocholate normally, but transport of carboxydichlorofluorescein diacetate into the canaliculus was negligible. Vesicles derived from the canicular domain of the plasma membrane of hepatocytes (CMV) from control and TR- rats were used to characterize the transport process for 35S-labeled bromosulphthalein and 35S-labeled bromosulphthalein glutathione, which represent nonbile acid organic anions. CMV from normal rat hepatocytes had an ATP- and temperature-dependent, saturable transport process for these 35S-labeled compounds that was absent in CMV from TR- rats. CMV from TR- rats retained normal ATP-dependent transport of daunomycin, and immunologic blots with a monoclonal antibody against the multidrug resistance gene product, P-glycoprotein, revealed no difference between normal and TR-CMV. These studies reveal that the bile canaliculus in normal rats contains an ATP-dependent organic anion transport system that is functionally absent in TR- mutant rats. The defect in TR- mutant rats is phenotypically similar to that seen in mutant Corriedale sheep and in the Dubin-Johnson syndrome in man.
Full text
PDF
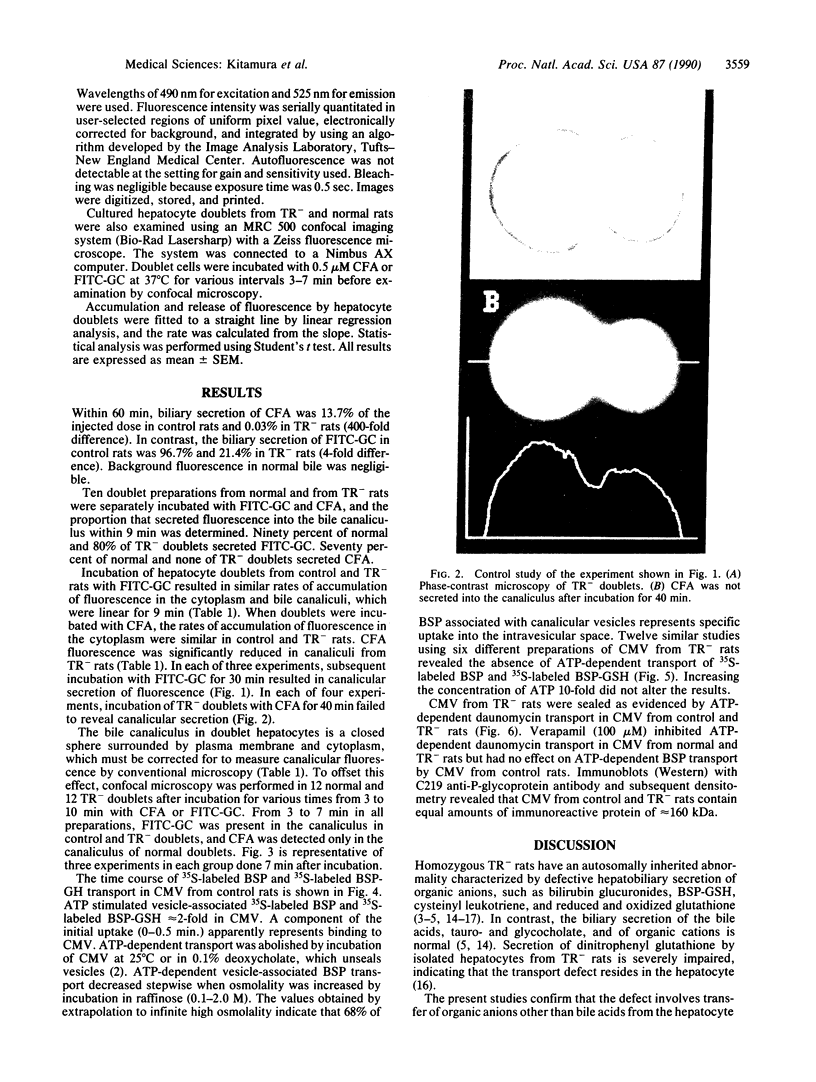
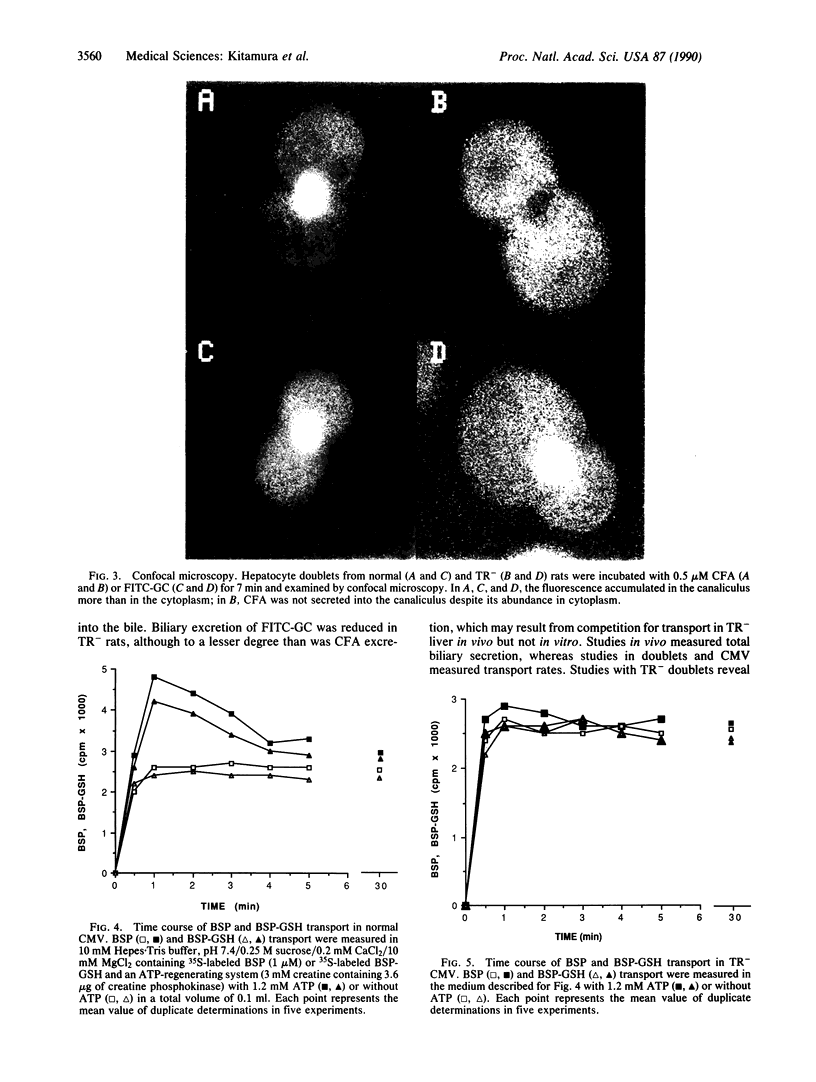
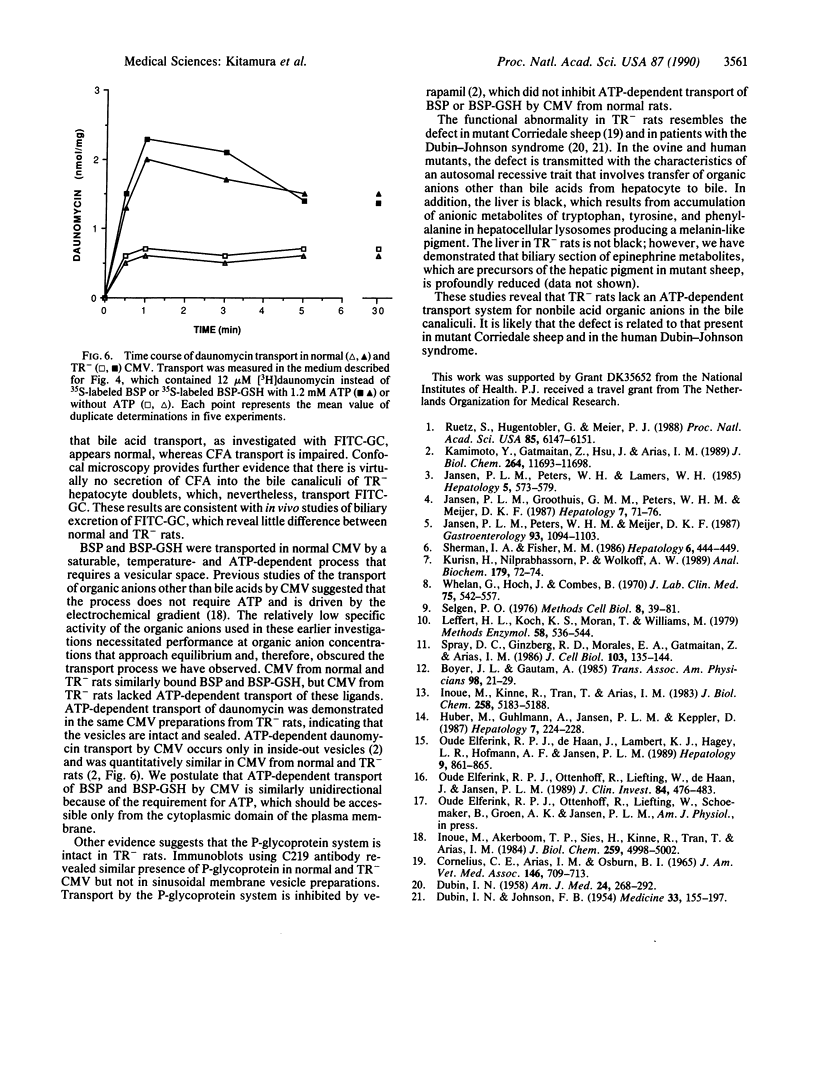
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyer J. L., Ng O. C., Gautam A. Formation of canalicular spaces in isolated rat hepatocyte couplets. Trans Assoc Am Physicians. 1985;98:21–29. [PubMed] [Google Scholar]
- CORNELIUS C. E., ARIAS I. M., OSBURN B. I. HEPATIC PIGMENTATION WITH PHOTOSENSITIVITY: A SYNDROME IN CORRIEDALE SHEEP RESEMBLING DUBIN-JOHNSON SYNDROME IN MAN. J Am Vet Med Assoc. 1965 Apr 1;146:709–713. [PubMed] [Google Scholar]
- DUBIN I. N. Chronic idiopathic jaundice; a review of fifty cases. Am J Med. 1958 Feb;24(2):268–292. doi: 10.1016/0002-9343(58)90315-2. [DOI] [PubMed] [Google Scholar]
- DUBIN I. N., JOHNSON F. B. Chronic idiopathic jaundice with unidentified pigment in liver cells; a new clinicopathologic entity with a report of 12 cases. Medicine (Baltimore) 1954 Sep;33(3):155–197. doi: 10.1097/00005792-195409000-00001. [DOI] [PubMed] [Google Scholar]
- Elferink R. P., Ottenhoff R., Liefting W., de Haan J., Jansen P. L. Hepatobiliary transport of glutathione and glutathione conjugate in rats with hereditary hyperbilirubinemia. J Clin Invest. 1989 Aug;84(2):476–483. doi: 10.1172/JCI114189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber M., Guhlmann A., Jansen P. L., Keppler D. Hereditary defect of hepatobiliary cysteinyl leukotriene elimination in mutant rats with defective hepatic anion excretion. Hepatology. 1987 Mar-Apr;7(2):224–228. doi: 10.1002/hep.1840070204. [DOI] [PubMed] [Google Scholar]
- Inoue M., Akerboom T. P., Sies H., Kinne R., Thao T., Arias I. M. Biliary transport of glutathione S-conjugate by rat liver canalicular membrane vesicles. J Biol Chem. 1984 Apr 25;259(8):4998–5002. [PubMed] [Google Scholar]
- Inoue M., Kinne R., Tran T., Biempica L., Arias I. M. Rat liver canalicular membrane vesicles. Isolation and topological characterization. J Biol Chem. 1983 Apr 25;258(8):5183–5188. [PubMed] [Google Scholar]
- Jansen P. L., Groothuis G. M., Peters W. H., Meijer D. F. Selective hepatobiliary transport defect for organic anions and neutral steroids in mutant rats with hereditary-conjugated hyperbilirubinemia. Hepatology. 1987 Jan-Feb;7(1):71–76. doi: 10.1002/hep.1840070116. [DOI] [PubMed] [Google Scholar]
- Jansen P. L., Peters W. H., Lamers W. H. Hereditary chronic conjugated hyperbilirubinemia in mutant rats caused by defective hepatic anion transport. Hepatology. 1985 Jul-Aug;5(4):573–579. doi: 10.1002/hep.1840050408. [DOI] [PubMed] [Google Scholar]
- Jansen P. L., Peters W. H., Meijer D. K. Hepatobiliary excretion of organic anions in double-mutant rats with a combination of defective canalicular transport and uridine 5'-diphosphate-glucuronyltransferase deficiency. Gastroenterology. 1987 Nov;93(5):1094–1103. doi: 10.1016/0016-5085(87)90574-9. [DOI] [PubMed] [Google Scholar]
- Kamimoto Y., Gatmaitan Z., Hsu J., Arias I. M. The function of Gp170, the multidrug resistance gene product, in rat liver canalicular membrane vesicles. J Biol Chem. 1989 Jul 15;264(20):11693–11698. [PubMed] [Google Scholar]
- Kurisu H., Nilprabhassorn P., Wolkoff A. W. Preparation of [35S]sulfobromophthalein of high specific activity. Anal Biochem. 1989 May 15;179(1):72–74. doi: 10.1016/0003-2697(89)90202-9. [DOI] [PubMed] [Google Scholar]
- Leffert H. L., Koch K. S., Moran T., Williams M. Liver cells. Methods Enzymol. 1979;58:536–544. doi: 10.1016/s0076-6879(79)58168-3. [DOI] [PubMed] [Google Scholar]
- Oude Elferink R. P., de Haan J., Lambert K. J., Hagey L. R., Hofmann A. F., Jansen P. L. Selective hepatobiliary transport of nordeoxycholate side chain conjugates in mutant rats with a canalicular transport defect. Hepatology. 1989 Jun;9(6):861–865. doi: 10.1002/hep.1840090612. [DOI] [PubMed] [Google Scholar]
- Ruetz S., Hugentobler G., Meier P. J. Functional reconstitution of the canalicular bile salt transport system of rat liver. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6147–6151. doi: 10.1073/pnas.85.16.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman I. A., Fisher M. M. Hepatic transport of fluorescent molecules: in vivo studies using intravital TV microscopy. Hepatology. 1986 May-Jun;6(3):444–449. doi: 10.1002/hep.1840060321. [DOI] [PubMed] [Google Scholar]
- Spray D. C., Ginzberg R. D., Morales E. A., Gatmaitan Z., Arias I. M. Electrophysiological properties of gap junctions between dissociated pairs of rat hepatocytes. J Cell Biol. 1986 Jul;103(1):135–144. doi: 10.1083/jcb.103.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan G., Hoch J., Combes B. A direct assessment of the importance of conjugation for biliary transport of sulfobromophthalein sodium. J Lab Clin Med. 1970 Apr;75(4):542–557. [PubMed] [Google Scholar]