Abstract
Purpose
Multigene testing for breast cancer recurrence risk became available in 2007, yet many eligible patients remain untested. This study evaluated variation in testing rates, and oncologist and organizational factors associated with variation, in a setting without financial influences on testing.
Methods
We conducted a retrospective cohort study using electronic data and oncologist surveys within Kaiser Permanente Northern California, a large integrated health care system. Analyses included all 2,974 test-eligible patients from 2013–15, 113 oncologists, and 15 practice groups. Receipt of multigene testing was evaluated with generalized linear mixed models.
Results
Overall, 39% of eligible patients had multigene testing, but rates varied widely among practice groups, ranging from 24 to 48% after case-mix adjustment. This 24% difference among practices was greater than the variation associated with most patient characteristics, including comorbidities and race/ethnicity, and similar to that associated with tumor size.
Practice group and oncologist factors were statistically significant contributors to the variation in testing after adjusting for patient factors. Patients were more likely to be tested if they had a female oncologist (aOR 1.60, 95% CI 1.21 – 2.12) or were in a practice whose chief had a high testing rate (aOR 1.20, 95% CI 1.12 – 1.29 per 10% increase in the percent tested).
Conclusions
Oncologist and leadership practices play a key role in the variation in genomic test use for cancer recurrence risk even in a healthcare system without financial barriers to testing, and could be a leverage point for implementing desired practice changes for new genomic advances.
Keywords: genetic testing, practice variation, practice change, genomics, recurrence risk
INTRODUCTION
Multigene testing for breast cancer recurrence risk became commercially available in 2007 for the diagnostic evaluation of patients with early stage, node negative, estrogen-receptor (ER) positive, human epidermal growth factor-2 (HER2) negative cancers. The primary goal of testing is to guide chemotherapy decisions by identifying patients at high risk of recurrence who would have the greatest treatment benefits, while reassuring those with low risk of recurrence that they could safely omit chemotherapy. However, despite longstanding inclusion of multigene testing in treatment guidelines [1,2], most eligible patients in the United States still do not receive this testing [3–6].
In prior research, the likelihood of being tested has been associated with non-modifiable patient characteristics, including age, race/ethnicity, socioeconomic status, and clinical characteristics such as tumor stage and comorbidities [3–8]. Other influences on testing use, such as organizational and physician factors, have not been evaluated. The characteristics and opinions of physicians and the settings within which they practice are typically key drivers of the adoption of new medical interventions [9].
This retrospective cohort study used linked data from electronic medical records, electronic organizational data, and physician surveys. We evaluated variation in breast cancer multigene (Oncotype DX) testing in a large integrated healthcare system without financial barriers to testing, and analyzed oncologist and practice group factors associated with variation. The results are intended to inform future efforts to enhance clinically appropriate adoption of this and other diagnostic genetic profiling assays developed to guide cancer care.
METHODS
Study setting
The setting for this study was Kaiser Permanente of Northern California (KPNC). Kaiser Permanente is a nonprofit integrated healthcare system that provides care to more than 10 million members nationwide, including four million from Northern California. The members are representative of the region’s general population in racial/ethnic diversity, and tend to have higher educational levels and employment rates [10,11]. All primary and specialty care is provided by salaried physicians working within The Permanente Medical Group (TPMG). This integrated regional system has more than 100 oncologists organized into 15 practice groups, each of which includes one or two of 21 medical centers. Each group has a chief of oncology who supervises the practice and coordinates with other chiefs on regional clinical plans. Multigene expression profile testing for breast cancer has been financially covered by KPNC since it became available for clinical use. This study was approved by the KPNC Institutional Research Review Board.
Population
The KPNC tumor registry, which reports to the California Cancer Registry and the National Cancer Institute Surveillance, Epidemiology, and End-Result (SEER) program[12], was used to identify female patients newly diagnosed with invasive, non-metastatic, incident primary breast cancer between January 1, 2013 through June 30, 2015. The population for this retrospective cohort study included all patients eligible for the multigene test and their oncologists. This study evaluates the use of Oncotype DX; no other multigene tests were used by this medical group during the study period. Test eligibility at KPNC followed professional guidelines [1,2] and included patients with stage 1 or 2 cancers with no lymph node involvement or only ≤ 2 mm axillary node micro-metastases, tumor size ≥ 0.5 cm, estrogen receptor positive (ER+, defined as ≥ 1% of cells positive on immunohistochemistry) and human epidermal growth factor 2 negative (HER2−).
Patient Data Collection
Patient clinical and demographic data were drawn from electronic medical records (EMRs) and other computerized databases. Data from the year before the cancer diagnosis were used to create a modified Deyo version of the Charlson comorbidity index [13]. Patients were assigned to a census block group based on their home address at the time of cancer diagnosis, and block group income and education variables were drawn from the 2006 to 2010 American Community Survey [14,15]. Each patient’s data was linked to data from her primary oncologist, defined as the oncologist with whom the patient had the most visits in the year post-diagnosis.
Oncologist Data Collection
Oncologist demographic and practice-level variables were available from computerized data for all 113 oncologists who were identified as the primary oncologist for any patient in the cohort. These data included physician gender, years since graduation from medical school, the number of breast cancer patients eligible for testing in the study period, and the ages of these patients, and the proportions who had multigene testing and chemotherapy.
For each practice group, we calculated the total number of patients eligible for testing, the number of oncologists, number of eligible patients per oncologist, median community-level income of eligible patients, and proportion of the oncology practice group chief’s eligible patients who were tested.
A 12-item survey (available on request from the authors) was developed to elicit other oncologist factors associated with multigene testing. The content was guided by results of six key informant interviews (3 external to KPNC and 3 within KPNC) and cognitive laboratory pretesting. The final survey used closed-ended questions to ask oncologists about their use of multigene testing, associated guidelines, and other factors that influenced testing decisions. One question asked, “For what % of patients do you use Adjuvant Online?”, referring to an internet-based risk prediction tool available for use at the time of the survey (https://www.adjuvantonline.com/). Another item asked, “In what percent of your patients did multigene testing neither assist the decision about chemotherapy nor reassure the patient?” Responses to this question were on a five-point scale corresponding to <10%, 11 to 25%, 25 to 50%, 50 to 75%, and 75% or more; the order was inverted during analysis for ease of interpretation.
The survey was sent in May 2015 via both online and via interoffice mail to all TPMG medical oncologists who had test-eligible study patients and were actively practicing at KPNC on the survey date. Of the 113 oncologists who had treated patients in the study cohort, 16 were no longer actively practicing at KPNC at the time of the survey and 97 were eligible (Figure 1). Among eligible oncologists, 84 completed the survey (response rate 87%). Most surveys (56%) were completed on paper; the rest were completed online.
Figure 1.
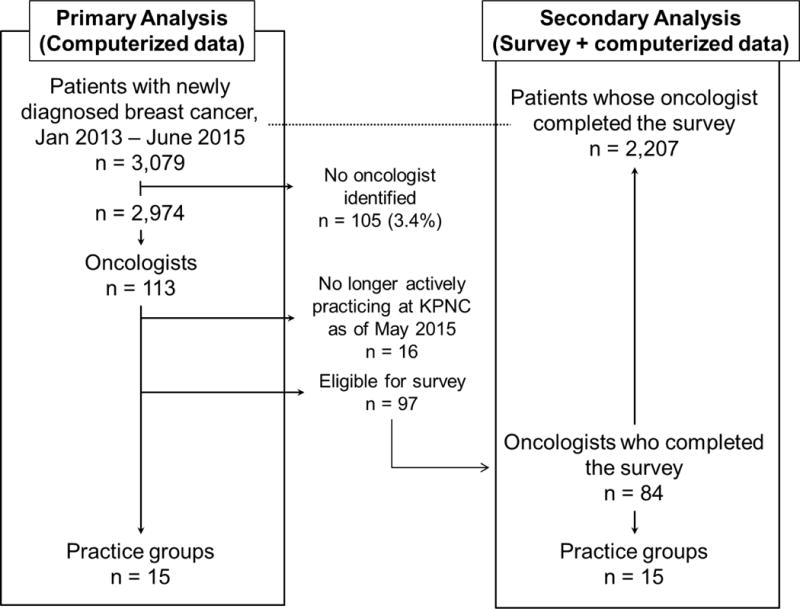
Practice groups, oncologists, and patients in primary and secondary analyses of predictors of breast cancer multigene testing
Final Sample and Statistical Analyses
The hierarchical structure of the data is depicted in Figure 1. The main analyses used EMR and organizational data and included the full study population of practice groups (n=15), oncologists (n=113), and eligible patients (n=2,974). A secondary analysis added the oncologist survey data to the main analysis, and was thus limited to those oncologists who had responded to the survey (n=84) and their practice groups (n=15) and patients (n=2,207).
All analyses modeled the dichotomous patient-level outcome indicating use of multigene testing (yes/no). Multilevel modeling techniques were used to predict patient-level test use, accounting for the hierarchical structure of the patient, oncologist, and practice group data. A generalized linear mixed model was fit with a logit link and random intercepts for oncologist and practice group. The statistical significance of the random effects for oncologist and practice group were assessed using likelihood ratio tests. Covariates for oncologist, practice group, and patient-level factors found significant at p<.10 in bivariate tests were entered into the model as fixed effects and retained if significant at p<.05. Patient-level factors were entered into the model first to adjust for case-mix. The significance of oncologist and practice group factors were then evaluated after adjustment for case mix.
We further assessed the association between oncologist-level and practice-group-level factors and multigene test use by examining the estimates of the variance components and the intra-class correlation coefficients (ICCs) [16]. For a dichotomous outcome, the ICCs represent the percentage of variance attributable to each random-effect level (oncologist and provider group) as defined in the context of an underlying latent variable distribution, conditional on the covariates in the model. ICCs were calculated first in a model adjusted for patient factors and calculated again after adding oncologist-level and practice group-level covariates.
Finally, to illustrate the relative influence of patient, oncologist, and practice group predictors on the probability of testing, we used the fitted final model to estimate the predicted probability of testing for a representative hypothetical patient. Each predictor was varied from its lowest to the highest-probability value, while holding all other predictors constant. In addition, we estimate the predicted probability of testing in each practice group for a representative case-mix of patients (those from the practice group with the lowest probability of testing), and used the fitted model with oncologist and practice group random effects. Analyses were conducted in SAS version 9.3 (Cary, NC).
RESULTS
Multigene-test eligible breast cancer patients had an average age of 63, and included diverse racial/ethnic groups. Most (77%) had Stage I disease, 70% had no comorbidities, and the average community-level socioeconomic status was high. Among the oncologists, 63 (48%) were female, and six of the 15 practice group chiefs were female. On average, female oncologists had fewer years since medical school graduation (mean 18.8 vs. 24.0, p<.01) and more patients in the study cohort than male oncologists (mean 29.2 vs. 16.7, p<.01).
Variation in Multigene Test Rates
Among the 15 practice groups, the unadjusted percent of eligible patients who were tested varied from 18% to 56% (Figure 2a). Although some of this variability was due to differences in case-mix, the adjusted rates still varied widely, from 24% to 48% (Figure 2b).
Figure 2a.
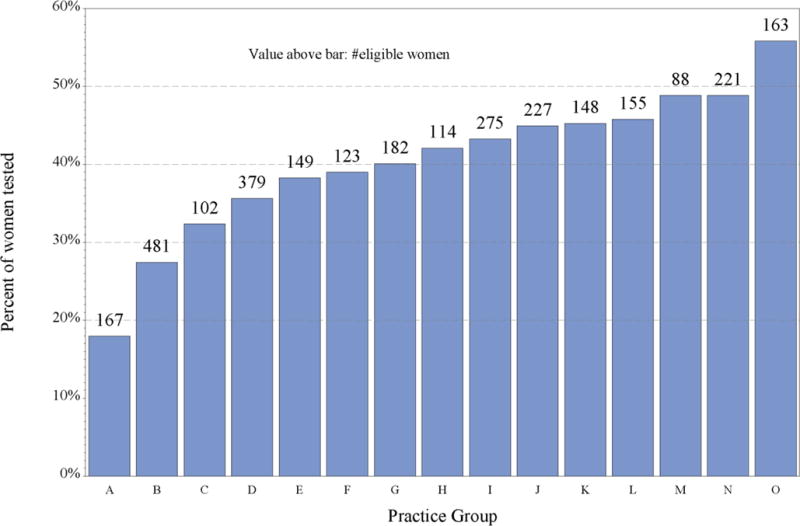
Unadjusted percent of eligible patients tested, by practice group, Kaiser Permanente Northern California, 2013–2015
b. Predicted testing rates adjusted for case-mix, by practice group, Kaiser Permanente Northern California, 2013–15. The adjusted rate represents the percent of eligible patients predicted to have the multigene test, if all eligible patients from practice group A were seen at the practice group indicated.
Figure 2b.
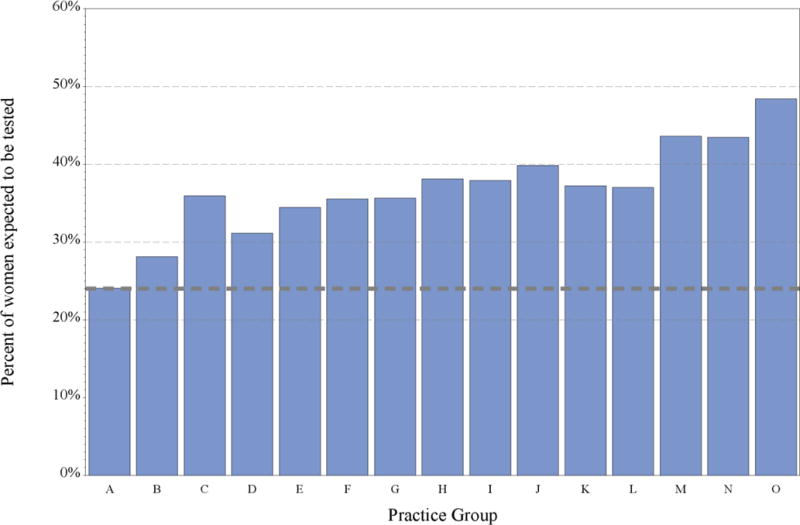
Predicted testing rates adjusted for case-mix, by practice group, Kaiser Permanente Northern California, 2013–15. The adjusted rate represents the percent of eligible patients predicted to have the multigene test, if all eligible patients from practice group A were seen at the practice group indicated.
Practice Group and Oncologist Predictors of Multigene Testing
Oncologist and practice group characteristics were associated with test use in bivariate analyses (Table 1). In the final multivariate model that adjusted for patient characteristics, patients were more likely be tested if their oncologist was female (vs. male) (aOR 1.6, 95% CI 1.2 – 2.1) or the practice group oncology chief had higher test use (aOR 1.2, 95% CI 1.1 – 1.3 for each increase of 10% in the proportion of patients tested (Table 2). The oncologist’s years since medical graduation was associated with both gender and test use. However, when years since medical school graduation was added to the model, the association of oncologist gender with testing was only slightly attenuated [17] (OR for gender decreased from 1.60 to 1.50), suggesting that this variable does not explain the variation by gender. Other differences between female and male oncologists were not associated with the odds of test use after adjusting for other factors.
Table 1.
Oncologist And Practice Group Characteristics By Breast Cancer Multigene Test Use, Kaiser Permanente Northern California, January 2013 – June 2015
| Level/Predictor | Tested patients (n=1,158) | Non-tested patients (n=1,816) | pa |
|---|---|---|---|
| Oncologist-level characteristics | |||
| Gender, n (%) | |||
| Female | 785 (43) | 1,053 (57) | <0.01 |
| Male | 373 (33) | 763 (67) | |
| Years since medical school graduation, n (%) | |||
| 1 – <10 | 136 (42) | 188 (58) | 0.07 |
| 10 – <20 | 490 (40) | 724 (60) | |
| 20 – <30 | 289 (39) | 450 (61) | |
| 30 or more | 243 (35) | 454 (65) | |
| Age of oncologist’s test-eligible patients, mean (sd) | 63 (3) | 63 (3) | <0.01 |
| Number of oncologist’s patients eligible for testing, mean (sd) | 35 (18) | 34 (17) | <0.01 |
| For this patient’s oncologist, the % of their test-eligible patients who had chemotherapy, based on computerized data, n (%) | |||
| <5% | 93 (39) | 146 (61) | 0.03 |
| 5–<15% | 443 (37) | 753 (63) | |
| 15–<25% | 401 (39) | 633 (61) | |
| 25–<35% | 167 (40) | 250 (60) | |
| 35–<50% | 44 (59) | 30 (41) | |
| 50% or more | 10 (71) | 4 (29) | |
| For this patient’s oncologist, the % of their test-eligible patients who were tested, based on computerized data, n (%)b | |||
| <5% | 2 (3) | 77 (97) | <0.01 |
| 5–<15% | 18 (13) | 124 (87) | |
| 15–<25% | 59 (19) | 256 (81) | |
| 25–<35% | 181 (29) | 435 (71) | |
| 35–<50% | 431 (42) | 584 (58) | |
| 50–<75% | 419 (56) | 327 (44) | |
| 75% or more | 48 (79) | 13 (21) | |
| Practice Group Predictors | |||
| Number of patients eligible for testing, mean (sd) | 238 (118) | 263 (133) | <0.01 |
| Number of oncologists, mean (sd) | 8 (3) | 9 (3) | <0.01 |
| Number of patients per oncologist, mean (sd) | 29 (8) | 30 (9) | <0.01 |
| Community-level median family income of patients, mean (sd) | 92812 (13314) | 91198 (13440) | <0.01 |
| Chief of oncology, % of patients tested | 43 (21) | 36 (21) | <0.01 |
|
| |||
| Oncologist survey predictorsc | Tested patients (n=848) | Non-tested patients (n=1,359) | |
| For this patient’s oncologist, in what % of patients does the oncologist use Adjuvant!Online, based on survey self-reportd | |||
| <25% | 123 (47) | 139 (53) | 0.02 |
| 25 – <50% | 93 (38) | 150 (62) | |
| 50 – <75% | 115 (40) | 171 (60) | |
| 75% or more | 495 (37) | 851 (63) | |
| Does not use Adjuvant Online | 22 (31) | 48 (69) | |
| For this patient’s oncologist, in what % of patients they have tested did the test not assist the decision and not reassure the patient? | |||
| <10% | 437 (42) | 606 (58) | <0.01 |
| 10 – <25% | 266 (38) | 431 (62) | |
| 25 – <50% | 105 (40) | 157 (60) | |
| 50 – <75% | 26 (19) | 108 (81) | |
| 75% or more | 8 (17) | 38 (83) | |
| Not answered | 6 (24) | 19 (76) | |
p values are from bivariate analyses comparing tested and non-tested patients. P values for categorical variables are based on chi-square tests and for continuous variables are based on t-tests. The Kruskal-Wallis test was used for the oncologist-level variables proportion of patients with chemotherapy and proportion of patients with multigene testing.
The characteristics described are those of the oncologist of each patient in the indicated group (tested or non-tested). Thus, they may differ from the individual patient’s actual experience. For example, among patients who were tested, 48 had oncologists who tested 75% or more of their test-eligible patients.
From a survey completed by 84 oncologists (87% of the 97 oncologists eligible for the survey). Analyses compared the response of the oncologist linked to each patient, between tested and non-tested patients.
Use of Adjuvant! Online was based on the oncologist survey and represents the oncologist’s self-report of their general use, rather than an individual patient-level variable.
Table 2.
Adjusted Odds of Breast Cancer Multigene Testing (Computerized Data Onlya) Kaiser Permanente Northern California, January 2013 – June 2015
| Predictor | Odds Ratio (95% Confidence Interval) |
|---|---|
| Age at diagnosis | |
| <40 | 12.07 (6.95,20.99)* |
| 40–<50 | 14.58 (10.46,20.32)* |
| 50–<60 | 12.05 (9.03,16.09)* |
| 60–<70 | 5.99 (4.60,7.80)* |
| 70+ | REF |
| Race/Ethnicity | |
| Asian | 0.95 (0.74,1.21) |
| Black | 0.60 (0.41,0.88)* |
| Other or unknown | 2.04 (0.79,5.28) |
| White, Hispanic | 0.96 (0.71,1.32) |
| White, non-Hispanic | REF |
| Tumor size | |
| >0.5 cm to <=1.0 cm | 0.29 (0.22,0.37)* |
| >1.0 cm to <=2.0 | 1.13 (0.91,1.40) |
| >2.0 cm | REF |
| Charlson comorbidity score | 0.89 (0.81,0.98)* |
| Community-level median family incomeb | 1.05 (1.02,1.07)* |
| Oncologist gender | |
| Female | 1.60 (1.21,2.12)* |
| Male | REF |
| Practice group oncology chief: proportion of patients testedc | 1.20 (1.12,1.29)* |
| Percent of variance unexplained by fixed effects that is explained by this level effect | |
| Oncologist level | 7.93% |
| Practice group level | 0.25% |
Results from a generalized linear mixed model with data from 2,974 patients, 113 oncologists, and 15 practice groups.
Community-level is from census block group characteristics.
Odds ratios represent increased odds for each absolute 10% increase in the percent of patients the oncology chief tested.
Significant at p<=0.05
A multivariate model adjusted for patient factors indicated that random oncologist-level effects explained more of the remaining variance (ICC=9.20%) than random practice group-level effects (ICC = 4.69%), but both random effects were statistically significant. The oncologist-level random effect was still statistically significant in the final model that included oncologist gender (ICC=7.93%), indicating that oncologist gender explained only a small amount of the heterogeneity in testing by oncologist. In contrast, the practice group-level random effect was no longer statistically significant in the final model that included the practice group chief’s test use (ICC=0.25%), indicating that the practice group chief’s test use explained virtually all of the heterogeneity in testing by practice group.
Oncologist Survey Predictors of Test Use
Patients were more likely to have been tested if their oncologist rated the test as having a higher (vs. lower) likelihood of assisting chemotherapy decisions or reassuring patients (OR 1.37, 95% CI 1.16–1.62 for each increase across the five response categories), and if the practice group oncology chief had higher test use (OR 1.23, 95% CI 1.13–1.34 for each increase of 10% in the proportion of patients tested), after considering other covariates (model not shown).
Predicted Probabilities of Testing
Using a standard reference patient to evaluate differences in predicted testing rates, we found that patients of female oncologists had a 50% predicted probability of being tested, compared with a 38% predicted probability for those of male oncologists, a 12% adjusted difference (Table 3). The practice group oncology chief’s testing rate was associated with a larger adjusted difference (14%) than patient race/ethnicity (11%), comorbidity score (8%), or community-level family income (5%). The adjusted difference in the testing rate among practice groups observed in the earlier analysis (24%, in Figure 2b) was greater than that due to patient race/ethnicity and comorbidities and similar to that for tumor size.
Table 3.
Relationship of Patient, Oncologist, and Practice Group Variables to the Probability of Having the Multigene Test, Relative to a Standard Reference Patient with Other Variables Held Constant
| Variable | Variable Valuea | Probability of multigene testing, % | Difference in probability between the variable lowest- to highest-risk value |
|---|---|---|---|
| Age (years) | 70+ | 9% | 51% |
| 60–70 | 38% | ||
| 40–50 | 60% | ||
| Race/ethnicity | Black | 28% | 11% |
| Asian | 38% | ||
| White, non-Hispanic | 39% | ||
| Tumor size | >0.5 cm to <=1.0 cm | 15% | 26% |
| >2.0 cm | 38% | ||
| >1.0 cm to <=2.0 | 41% | ||
| Charlson comorbidity score | 3 | 33% | 8% |
| 1 | 38% | ||
| 0 | 41% | ||
| Community-level median family incomeb | 64,000 | 36% | 5% |
| 87,000 | 38% | ||
| 113,000 | 41% | ||
| Oncologist gender | Male | 38% | 12% |
| Female | 50% | ||
| Practice group oncology chief’s proportion of patients getting tested | 20% | 30% | 14% |
| 40% | 38% | ||
| 53% | 44% |
Values in bold represent the characteristics of the standard patient, who has an estimated probability of testing of 38% using the fitted multilevel regression model. Each probability estimate represents the effect of changing the value of a single variable while the other values in bold were held constant. Variable values were chosen to illustrate maximum range from lowest to highest probability of testing.
Community-level is from census block group characteristics.
DISCUSSION
To our knowledge, this is the first study to evaluate the role of oncologist and practice organizational factors in adoption of a validated multigene test for cancer treatment decision making. The results indicate that receiving care in an integrated care setting does not guarantee consistency in genomic test use. We observed wide variation in testing rates among practice groups. even without financial barriers to testing. Further, this variation persisted after adjusting for patient factors and was associated with oncologist and practice group characteristics. The odds of multigene testing were higher among breast cancer patients cared for by female oncologists. Finally, being cared for in a practice where the chief of oncology adopted higher rates of testing increased the chances that a patient would receive testing from the other oncologists in the practice.
The finding that patients of female oncologists were more likely to have multigene testing has not been reported previously. This observation is consistent with a recent finding that patients of female internists have lower mortality and readmissions than those of male internists [18], and with studies in the 1990s that found that patients of female primary care physicians were more likely to have mammography and Pap smears than those of male physicians [19–22]. The differences in practice patterns are postulated to be related in part to differences in female and male physicians’ attitudes toward care, communication styles, and differences in the types of patients who selected female physicians [23]. More generally, the business literature suggests that women tend to gather more information than men before making decisions [24]. It is likely that the observed gender differences in genomic testing were due to unmeasured factors such as decision-making or communication styles. It will be important to determine if our findings are present in other settings and populations to better understand how provider gender and other characteristics affect adoption of multigene testing.
Since the optimal rate of testing is not known, some variation in testing rates may be justifiable based on oncologist and patient preferences. The variation we observed in oncologists’ perceptions of the test’s usefulness is consistent with the ongoing controversy about selection of patients for this test and may also be related to differences in the perceived value of multigene test results in some patient subgroups based on other risk stratification elements [2]. In addition, this variation might stem, in part, from the uncertainty about whether to use chemotherapy for patients with intermediate-risk recurrence scores. The ongoing TAILORx randomized trial, which has enrolled more than 10,000 women, is expected to produce information that addresses this gap.(https://clinicaltrials.gov/show/NCT00310180). It is possible that those results will diminish variation in oncologist perceptions and rates of actual test use.
The finding that the testing rate of the practice group chief was associated with a patient’s likelihood of being tested is consistent with studies showing that physicians tend to adopt practices similar to those of opinion leaders [9]. These findings may generalize to other groups where oncologists have either formal structural relationships or informal cultural norms that support following the practices of opinion leaders. In the medical group studied, it may be possible to involve the practice group leaders or others such as the group’s regional leaders in breast cancer in efforts to optimize testing rates. However, existing research has not produced clear guidance on how to maximize the effectiveness of such opinion leaders in disseminating new technology [9].
This analysis has several important strengths including the large, integrated healthcare setting, linked patient, provider, and practice data and information about oncologists beliefs about multigene testing, and robust analyses. However, there are several caveats that should be considered in evaluating the results. First, patients generally do not face financial barriers to obtaining cancer services within this integrated care setting, although some have high-deductible health plans, and oncologists have no individual-level financial incentives for or against testing. Thus, results may vary in other practice settings and organizational structures. However, increasing numbers of physicians are practicing in accountable care organizations or other integrated systems, and the observed rate of test use in eligible patients (39%) was similar to or higher than that in most other settings [3,6,8,25–27], and has increased from earlier reported rates in this setting [5]. On balance, the variability we observed in testing rates among practice groups suggests that financial access alone will not ensure consistent use of cancer genomic tests. Next, while we had robust data about patients from electronic medical records, we did not have data on patient’s attitudes towards for or preferences about testing or individual data on socioeconomic factors. However, the findings for oncology and practice effects on testing use were robust even after consideration of patient age and clinical characteristics.
Overall, this research suggests that the adoption of new cancer-related genetic tests may be influenced by oncologists’ gender and beliefs, and practice group culture based on key opinion leaders. These observations underscore that oncologist and practice group decision-making patterns are key leverage points for ensuring that genomic tests are optimally used in breast and other types of cancer.
Acknowledgments
We are grateful to the many TPMG oncology leaders who offered helpful guidance as we planned this study and interpreted its results, including David Baer, MD, Lou Fehrenbacher, MD, Ming Kuan, MD, Chunnan Liu, MD, Eva Thomas, MD, and all of the TPMG oncology practice group chiefs. We appreciate the excellent contributions of our research staff members Alice Ansfield, Pete Bogdanos, and Lillian Pacheco. We are grateful to Lawrence Kushi, ScD for laying key scientific groundwork for this study. We are deeply indebted to the many patients and oncologists who contributed information for this study.
Funding: This work was funded by grant #U01CA183081 from the National Cancer Institute at the National Institutes of Health. This work was also supported in part by the following National Cancer Institute grants: R01 CA105274; U19 CA079689; U24 CA171524; U01 CA152958; UC2 CA148471; R35CA197289; and U01CA199218
Footnotes
Disclaimer: All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the National Institutes of Health.
Conflict of interest: All authors declare that they have no conflict of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent for the survey of oncologists was considered to be confirmed via the cover letter to the survey and their return of the survey.
Contributor Information
Tracy A. Lieu, Division of Research, Kaiser Permanente Northern California, 2000 Broadway, Oakland, CA.
G. Thomas Ray, Division of Research, Kaiser Permanente Northern California, 2000 Broadway, Oakland, CA.
Stephanie R. Prausnitz, Division of Research, Kaiser Permanente Northern California, 2000 Broadway, Oakland, CA.
Laurel A. Habel, Division of Research, Kaiser Permanente Northern California, 2000 Broadway, Oakland, CA.
Stacey Alexeeff, Division of Research, Kaiser Permanente Northern California, 2000 Broadway, Oakland, CA.
Yan Li, Department of Oncology, Kaiser Permanente Oakland Medical Center, Oakland, CA Ramsey: Fred Hutchinson Cancer Research Center, Seattle, Washington.
Scott D. Ramsey, Fred Hutchinson Cancer Research Center, Seattle, Washington.
Charles E. Phelps, University of Rochester, Rochester, NY.
Neetu Chawla, Division of Research, Kaiser Permanente Northern California, 2000 Broadway, Oakland, CA.
Suzanne O’Neill, Georgetown University Medical Center, Lombardi Comprehensive Cancer Center, Department of Oncology, Washington, DC.
Jeanne S. Mandelblatt, Georgetown University Medical Center, Lombardi Comprehensive Cancer Center, Department of Oncology, Washington, DC.
References
- 1.Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25(33):5287–5312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 2.Carlson RW, Allred DC, Anderson BO, et al. Invasive breast cancer. J Natl Compr Canc Netw. 2011;9(2):136–222. doi: 10.6004/jnccn.2011.0016. [DOI] [PubMed] [Google Scholar]
- 3.Enewold L, Geiger AM, Zujewski J, Harlan LC. Oncotype Dx assay and breast cancer in the United States: usage and concordance with chemotherapy. Breast Cancer Res Treat. 2015;151(1):149–156. doi: 10.1007/s10549-015-3366-7. [DOI] [PubMed] [Google Scholar]
- 4.Dinan MA, Mi X, Reed SD, Lyman GH, Curtis LH. Association Between Use of the 21-Gene Recurrence Score Assay and Receipt of Chemotherapy Among Medicare Beneficiaries With Early-Stage Breast Cancer, 2005–2009. JAMA Oncol. 2015;1(8):1098–1109. doi: 10.1001/jamaoncol.2015.2722. [DOI] [PubMed] [Google Scholar]
- 5.Ray GT, Mandelblatt J, Habel LA, et al. Breast cancer multigene testing trends and impact on chemotherapy use. Am J Manag Care. 2016;22(5):e153–160. [PMC free article] [PubMed] [Google Scholar]
- 6.Jasem J, Amini A, Rabinovitch R, et al. 21-Gene Recurrence Score Assay As a Predictor of Adjuvant Chemotherapy Administration for Early-Stage Breast Cancer: An Analysis of Use, Therapeutic Implications, and Disparity Profile. J Clin Oncol. 2016;34(17):1995–2002. doi: 10.1200/JCO.2015.65.0887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Epstein AJ, Wong YN, Mitra N, et al. Adjuvant Chemotherapy Use and Health Care Costs After Introduction of Genomic Testing in Breast Cancer. J Clin Oncol. 2015;33(36):4259–4267. doi: 10.1200/JCO.2015.61.9023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hassett MJ, Silver SM, Hughes ME, et al. Adoption of gene expression profile testing and association with use of chemotherapy among women with breast cancer. J Clin Oncol. 2012;30(18):2218–2226. doi: 10.1200/JCO.2011.38.5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flodgren G, Parmelli E, Doumit G, et al. Local opinion leaders: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2011;8:CD000125. doi: 10.1002/14651858.CD000125.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon N, Lin T. The Kaiser Permanente Northern California Adult Member Health Survey. Perm J. 2016;20:15–225. doi: 10.7812/TPP/15-225. 08/19/2016 ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon NP. Similarity of the adult Kaiser Permanente membership in Northern California to the insured and general population in Northern California: Statistics from the 2011 California Health Interview Survey report. 2015 [Google Scholar]
- 12.Oehrli MD, Quesenberry CP. Northern California Cancer Registry: 2015 Annual Report on Trends, Incidence, and Outcomes. Oakland, CA: Kaiser Permanente; 2015. [Google Scholar]
- 13.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 14.Census Bureau US. A compass for understaning an using American community survey data: what general data users need to know. 2008 https://www.census.gov/content/dam/Census/library/publications/2008/acs/ACSGeneralHandbook.pdf.
- 15.U.S. Census Bureau. American Community Survey. 2012 https://www.census.gov/programs-surveys/acs/. Accessed September 4, 2016.
- 16.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. Second. John Wiley & Sons; 2012. Generalized linear mixed effects models; pp. 395–439. [Google Scholar]
- 17.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129(1):125–137. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 18.Tsugawa Y, Jena AB, Figueroa JF, Orav EJ, Blumenthal DM, Jha AK. Comparison of Hospital Mortality and Readmission Rates for Medicare Patients Treated by Male vs Female Physicians. JAMA Intern Med. 2016 doi: 10.1001/jamainternmed.2016.7875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lurie N, Slater J, McGovern P, Ekstrum J, Quam L, Margolis K. Preventive care for women. Does the sex of the physician matter? N Engl J Med. 1993;329(7):478–482. doi: 10.1056/NEJM199308123290707. [DOI] [PubMed] [Google Scholar]
- 20.Franks P, Clancy CM. Physician gender bias in clinical decisionmaking: screening for cancer in primary care. Med Care. 1993;31(3):213–218. doi: 10.1097/00005650-199303000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Kreuter MW, Strecher VJ, Harris R, Kobrin SC, Skinner CS. Are patients of women physicians screened more aggressively? A prospective study of physician gender and screening. J Gen Intern Med. 1995;10(3):119–125. doi: 10.1007/BF02599664. [DOI] [PubMed] [Google Scholar]
- 22.Bertakis KD, Helms LJ, Callahan EJ, Azari R, Robbins JA. The influence of gender on physician practice style. Med Care. 1995;33(4):407–416. doi: 10.1097/00005650-199504000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Lurie N, Margolis KL, McGovern PG, Mink PJ, Slater JS. Why do patients of female physicians have higher rates of breast and cervical cancer screening? J Gen Intern Med. 1997;12(1):34–43. doi: 10.1046/j.1525-1497.1997.12102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benko CP,B. How women decide. Harv Bus Rev. 2013 [Google Scholar]
- 25.Roberts MC, Dusetzina SB. Use and Costs for Tumor Gene Expression Profiling Panels in the Management of Breast Cancer From 2006 to 2012: Implications for Genomic Test Adoption Among Private Payers. J Oncol Pract. 2015;11(4):273–277. doi: 10.1200/JOP.2015.003624. [DOI] [PubMed] [Google Scholar]
- 26.Chen C, Dhanda R, Tseng WY, Forsyth M, Patt DA. Evaluating use characteristics for the oncotype dx 21-gene recurrence score and concordance with chemotherapy use in early-stage breast cancer. J Oncol Pract. 2013;9(4):182–187. doi: 10.1200/JOP.2012.000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Afghahi A, Mathur M, Thompson CA, et al. Use of Gene Expression Profiling and Chemotherapy in Early-Stage Breast Cancer: A Study of Linked Electronic Medical Records, Cancer Registry Data, and Genomic Data Across Two Health Care Systems. J Oncol Pract. 2016;12(6):e697–709. doi: 10.1200/JOP.2015.009803. [DOI] [PMC free article] [PubMed] [Google Scholar]


