Abstract
Background
Fast-acting insulin aspart (faster aspart) is insulin aspart set in a new formulation with faster initial absorption after subcutaneous administration. This study investigated the pharmacokinetic properties, including the absolute bioavailability, of faster aspart when administered subcutaneously in the abdomen, upper arm or thigh.
Methods
In a randomised, open-label, crossover trial, 21 healthy male subjects received a single injection of faster aspart at five dosing visits: 0.2 U/kg subcutaneously in the abdomen, upper arm and thigh, intramuscularly in the thigh and 0.02 U/kg intravenously. Blood sampling for pharmacokinetics was performed pre-dose and frequently thereafter until 12 h post-dose (8 h after intravenous administration).
Results
Onset of appearance (~3 min), time to 50% of maximum concentration (t Early 50% Cmax; ~20 min) and time to maximum concentration (t max; ~55 min) were all similar between injection regions. Early exposure within the first 2 h after injection (AUCIAsp,0–1h and AUCIAsp,0–2h) as well as maximum concentration (C max) were comparable for the abdomen and upper arm, but were ~25% lower for the thigh as seen previously for other mealtime insulin products. Total exposure (AUCIAsp,0–t) was similar for the abdomen, upper arm and thigh, and absolute bioavailability was ~80% after subcutaneous administration of faster aspart in all three injection regions.
Conclusion
The current study supports the ultra-fast pharmacokinetic characteristics of faster aspart across different injection regions, with administration in the abdomen and upper arm resulting in greater early exposure than in the thigh.
ClinicalTrials.gov identifier: NCT02089451.
Key Points
| Fast-acting insulin aspart has an ultra-fast onset of exposure independent of injection region (abdomen, upper arm or thigh). |
| As previously observed for other mealtime insulin products, early exposure (<2 h after administration) and maximum concentration of fast-acting insulin aspart are lower for the thigh than for the abdomen and upper arm, while total exposure is comparable between all three injection regions (absolute bioavailability of ~80%). |
| Fast-acting insulin aspart may be administered subcutaneously in the abdomen, upper arm or thigh; however, administration in the abdomen and upper arm leads to the fastest pharmacokinetic profile. |
Introduction
For practical, physiological and anatomical reasons, the preferred body region for subcutaneous injection of insulin varies among patients with diabetes [1]. Recommended injection regions include the abdomen, thigh, upper arm and buttock [2]. It is well recognised that the rate and/or extent of insulin absorption may differ between injection regions [3–7], e.g. slower absorption for the thigh versus the abdomen is a consistent finding [4, 5, 7]. Therefore, it is important for any new insulin that its pharmacokinetic properties are characterised when administered in different anatomical regions of the body.
Fast-acting insulin aspart (faster aspart) is insulin aspart (IAsp) designed in a new formulation including two additional excipients (l-arginine and niacinamide), serving to achieve a stable formulation that conveys earlier onset of appearance and faster initial absorption rate after subcutaneous injection. Faster aspart has twice-as-fast onset of appearance and two-fold higher early insulin exposure leading to faster onset of action and more than 50% greater early glucose-lowering effect compared with IAsp [8–10]. Consequently, use of faster aspart may lead to better postprandial glucose control relative to current rapid-acting insulins.
The current study aimed to investigate the pharmacokinetics, including the absolute bioavailability, of faster aspart administered subcutaneously in the abdomen, upper arm or thigh in healthy subjects.
Methods
Trial Design and Participants
This randomised, open-label, five-period, crossover trial was approved by Bundesinstitut für Arzneimittel und Medizinprodukte (the local health authority) and by Ärztekammer Nordrhein (an independent ethics committee), conducted according to the Declaration of Helsinki, Good Clinical Practice and guidelines on bioavailability trials [11, 12], and registered at ClinicalTrials.gov (NCT02089451). Informed consent was obtained from all individual participants included in the study.
Eligible subjects were healthy men and women (however, all enrolled subjects were men) aged 18–64 years, with a body mass index of 20.0–28.0 kg/m2 and fasting plasma glucose ≤5.6 mmol/L. Individuals with abnormal clinical laboratory results, those using prescription or non-prescription drugs (except topical medication, oral contraceptives, routine vitamins and occasional use of ibuprofen and paracetamol), smokers and pregnant or breastfeeding women were excluded.
Procedures
The visit structure is shown in Fig. 1. Subjects received faster aspart single-dosing at five visits: 0.2 U/kg subcutaneously in the abdomen (lifted skin fold of the lower abdominal wall above the inguinal area), upper arm (lifted skin fold of the outer aspect of the upper arm) and thigh (lifted skin fold of the anterior surface of the thigh), 0.2 U/kg intramuscularly in the thigh and 0.02 U/kg intravenously (1-min injection through a catheter inserted into a hand or forearm vein). Faster aspart (100 U/mL; Novo Nordisk, Bagsværd, Denmark) was provided in a PDS290 pen-injector prefilled pen (Novo Nordisk) for subcutaneous administration and in a 3-mL Penfill® cartridge for intramuscular and intravenous administration.
Fig. 1.
Study design and subject disposition. Each subject participated in a total of five dosing visits in randomised sequence. All dosing visits were separated by a washout period of 3–12 days. The three randomised subjects who did not complete the trial were all due to withdrawal of consent (one before first dosing, one after subcutaneous administration in the thigh and one after intravenous administration and subcutaneous administration in the abdomen and upper arm). I.m. intramuscularly, i.v. intravenously, N number of subjects, s.c. subcutaneously
At the dosing visits, subjects came to the clinic in the morning after an overnight fast and received faster aspart. For safety reasons, to maintain blood glucose within the normal range, a euglycaemic clamp (ClampArt®; Profil, Neuss, Germany) was performed [9]. The clamp target was 4.5 mmol/L and clamp duration was up to 12 h post-dosing (8 h after intravenous administration).
For subcutaneous and intramuscular administration, blood was sampled for pharmacokinetics within 2 min pre-dose, then every 2 min from dosing until 20 min post-dose, every 5 min until 80 min, every 10 min until 2 h, every 15 min until 3 h, and then at 3.5, 4, 5, 6, 7, 8, 10 and 12 h post-dose. For intravenous administration, the same schedule applied until 2.5 h, followed by sampling at 3, 4, 6 and 8 h post-dose. Free (unbound) serum IAsp concentration was assessed by polyethylene glycol precipitation using an IAsp-specific enzyme-linked immunosorbent assay [lower limit of quantification (LLOQ) of 10 pmol/L] validated according to relevant guidelines [13, 14]. Safety assessments included adverse events, local tolerability at the injection site, hypoglycaemic episodes (defined as ‘confirmed’ when they were either ‘severe’ according to the American Diabetes Association [15] or verified by plasma glucose <3.1 mmol/L), laboratory safety parameters, physical examination, vital signs and electrocardiogram.
Statistical Analyses
Statistical analysis was performed using SAS version 9.3 (SAS Institute, Cary, NC, USA).
Assuming a residual standard deviation for the primary endpoint (total exposure; AUCIAsp,0–t) of 0.25 [8], 18 completers would expectedly yield a 95% confidence interval (CI) of (0.85–1.18) for the geometric mean ratio of AUCIAsp,0–t between any two subcutaneous injection regions, if the observed ratio was 1.00. This was considered sufficiently narrow to support the primary objective of comparing total exposure between administration in the abdomen, upper arm and thigh.
Endpoints related to onset of exposure included onset of appearance (time from faster aspart administration until the first time of insulin concentration ≥LLOQ), time to 50% of maximum concentration (t Early 50% Cmax) and time to maximum concentration (t max). Endpoints to evaluate early exposure were areas under the curve (AUC) for IAsp from 0 to 1 h (AUCIAsp,0–1h) and 0 to 2 h (AUCIAsp,0–2h). Overall exposure was assessed by AUCIAsp,0–t, maximum concentration (C max) and the IAsp AUC from 0 to infinity (AUCIAsp,0–∞).
For determination of onset of appearance and AUC endpoints after subcutaneous administration, fitted curves based on compartmental modelling were used from the time of administration until the first quantifiable concentration, as previously described [9]. After intravenous administration, the initial part of the AUC was derived using back-extrapolation to 1 min (end of intravenous injection) and setting the concentration to zero at t = 0 (while excluding the 2-min sample since appropriate distribution of trial product had not been achieved at this time point). Between the first and last quantifiable concentration, AUCs were calculated using the linear trapezoidal technique. After the last quantifiable concentration, extrapolation based on the terminal slope was applied until the last pharmacokinetic sampling time point (for AUCIAsp,0–t) or until infinity (for AUCIAsp,0–∞).
Onset and early exposure endpoints were compared between subcutaneous injection regions using descriptive statistics. AUCIAsp,0–t and C max were log-transformed and compared between subcutaneous injection regions in an analysis of variance with treatment and period as fixed effects and subject as random effect. A covariance model allowing for different variances, but identical correlation between treatments for each subject, was used to account for heteroscedasticity. In order to assess the absolute bioavailability of faster aspart after subcutaneous administration, AUCIAsp,0–∞ was log-transformed and analysed using the same model as for AUCIAsp,0–t and C max. The estimated treatment ratios and 95% CIs for AUCIAsp,0–∞ were calculated between each of the subcutaneous injection regions and intravenous administration (after dose-adjustment to 0.2 U/kg for intravenous administration).
Results
Subjects
Of 33 subjects screened, 22 were randomised, 21 were exposed to faster aspart and 19 subjects completed the trial (Fig. 1). Subject characteristics are shown in Table 1.
Table 1.
Subject characteristics (N = 21)
| Characteristic | Value |
|---|---|
| Age, years | 32.8 (7.7) |
| Sex | |
| Female, N (%) | 0 (0.0) |
| Male, N (%) | 21 (100.0) |
| Race | |
| White, N (%) | 20 (95.2) |
| Othera, N (%) | 1 (4.8) |
| Height, m | 1.82 (0.07) |
| Body weight, kg | 84.0 (7.7) |
| BMI, kg/m2 | 25.4 (1.8) |
| Fasting plasma glucose, mmol/L | 5.0 (0.3) |
Data are mean (standard deviation) unless otherwise stated
BMI body mass index, N number of subjects
aMixed Asian and White
Pharmacokinetics
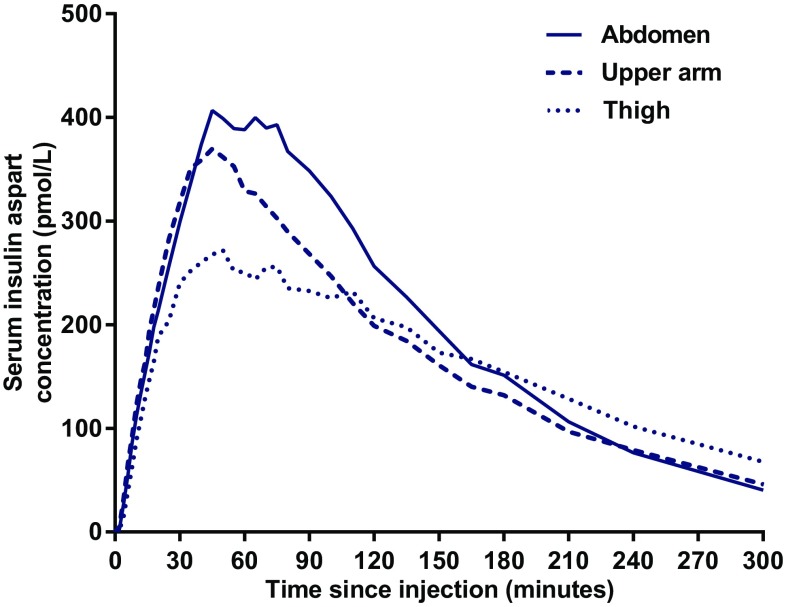
Observed pharmacokinetic time-profiles for subcutaneously administered faster aspart are shown in Fig. 2. Onset of appearance, t Early 50% Cmax and t max were all similar between injection regions (Table 2). AUCIAsp,0–1h and AUCIAsp,0–2h were comparable for the abdomen and upper arm, but lower for the thigh.
Fig. 2.
Mean observed serum insulin aspart concentration-time profiles for 0.2 U/kg faster aspart administered subcutaneously in the abdomen, upper arm or thigh
Table 2.
Onset of exposure and early exposure for 0.2 U/kg faster aspart administered subcutaneously in the abdomen, upper arm or thigh (N = 21)
| Abdomen | Upper arm | Thigh | |
|---|---|---|---|
| Onset of exposure | |||
| Onset of appearance (min) | 2.8 (1.3–5.0) | 2.3 (1.1–5.3) | 3.4 (1.8–5.9) |
| t Early 50% Cmax (min) | 25.0 (12.0–35.0) | 18.0 (8.0–30.0) | 20.0 (12.0–40.0)a |
| t max (min) | 55.0 (30.0–100.0) | 50.0 (30.0–100.0) | 57.5 (20.0–210.0) |
| Early exposure | |||
| AUCIAsp,0–1h (pmol·h/L) | 265.1 ± 121.5 | 261.6 ± 136.2 | 192.4 ± 114.2 |
| AUCIAsp,0–2h (pmol·h/L) | 607.9 ± 259.0 | 529.4 ± 213.9 | 426.3 ± 174.1 |
Data for onset of exposure endpoints are median (minimum–maximum) and data for early exposure endpoints are mean ± standard deviation
AUC area under the curve, IAsp insulin aspart, N number of subjects, t Early 50% Cmax time to 50% of maximum insulin aspart concentration in the early part of the pharmacokinetic profile, t max time to maximum insulin aspart concentration
aComparison with abdomen and upper arm should be interpreted with caution due to the maximum concentration being lower for thigh compared to abdomen and upper arm (see Table 3)
Total exposure (AUCIAsp,0–t) was similar for all three injection regions, while C max was comparable between the abdomen and upper arm but lower for the thigh (Table 3).
Table 3.
Total exposure and maximum concentration for 0.2 U/kg faster aspart administered subcutaneously in the abdomen, upper arm or thigh (n = 21)
| LS mean | Treatment ratio (95% CI) | P value | |
|---|---|---|---|
| Total exposure (AUCIAsp,0–t) (pmol·h/L) | |||
| Abdomen | 1000.9 | ||
| Upper arm | 921.9 | ||
| Thigh | 926.5 | ||
| Upper arm/abdomen | 0.92 (0.84–1.01) | 0.070 | |
| Thigh/abdomen | 0.93 (0.85–1.01) | 0.092 | |
| Thigh/upper arm | 1.00 (0.92–1.09) | 0.907 | |
| Maximum concentration (C max) (pmol/L) | |||
| Abdomen | 394.6 | ||
| Upper arm | 363.8 | ||
| Thigh | 275.7 | ||
| Upper arm/abdomen | 0.92 (0.74–1.14) | 0.447 | |
| Thigh/abdomen | 0.70 (0.56–0.87) | 0.002 | |
| Thigh/upper arm | 0.76 (0.61–0.95) | 0.016 | |
AUC area under the curve, CI confidence interval, C max maximum concentration, IAsp insulin aspart, LS Mean least square mean, N number of subjects
Treatment ratios (95% CI) for AUCIAsp,0–∞ for the abdomen, upper arm and thigh versus intravenous administration were 0.83 (0.74–0.93), 0.77 (0.68–0.87) and 0.77 (0.68–0.87), respectively. Thus, absolute bioavailability of faster aspart was close to 80% after subcutaneous administration in all three injection regions (abdomen 83%; upper arm 77%; thigh 77%).
Intramuscular administration of faster aspart resulted in a median onset of appearance of 2.6 min (minimum–maximum: 1.1–5.3), t Early 50% Cmax of 14.0 min (10.0–35.0) and t max of 45.0 min (25.0–90.0), mean ± standard deviation AUCIAsp,0–1h and AUCIAsp,0–2h of 198.9±80.0 and 414.1±141.9 pmol·h/L, and least square mean total exposure of 696.7 pmol·h/L and C max of 270.1 pmol/L.
Safety
Faster aspart was well tolerated with no safety issues identified during the trial. A total of 11 adverse events (AEs) were reported (10 mild and 1 moderate). All were non-serious and no obvious pattern occurred across injection region or route of administration. Headache was the most frequently reported AE (5 events). There were no clinically significant findings in safety laboratory parameters, vital signs, physical examination or electrocardiogram. No confirmed hypoglycaemic episodes or injection site reactions were reported.
Discussion
Among the key pharmacological properties of faster aspart are its faster onset of exposure and glucose-lowering effect versus IAsp [8–10]. It was therefore reassuring in the present study that onset of exposure for faster aspart was unaffected by subcutaneous injection region. Total exposure for faster aspart was comparable between injection regions, as previously seen for insulin lispro, insulin glulisine and IAsp (Table 4). Pharmacodynamics were not assessed in the current study. However, a robust correlation between pharmacokinetics and pharmacodynamics was recently shown for faster aspart [9], suggesting the present pharmacokinetic results may likely translate to similar pharmacodynamics for different injection regions.
Table 4.
Differences between subcutaneous injection regions for total exposure and maximum concentration of insulin lispro, insulin glulisine, insulin aspart and faster aspart
| Meana | Ratio (%) | ||||
|---|---|---|---|---|---|
| Abdomen | Upper arm | Thigh | Upper arm/abdomen | Thigh/abdomen | |
| Total exposure (pmol·h/L) | |||||
| Insulin lispro [5] | 1388 | 1313 | 1277 | 95 | 92 |
| Insulin glulisine [7] | 2182 | 2119 | 2021 | 97 | 93 |
| Insulin aspart [6] | 1300 | 1361 | 1265 | 105 | 97 |
| Faster aspart | 1001b | 922b | 927b | 92 | 93 |
| Maximum concentration (pmol/L) | |||||
| Insulin lispro [5] | 589 | 395 | 458 | 67 | 78 |
| Insulin glulisine [7] | 1003 | 821 | 684 | 82 | 68 |
| Insulin aspart [6] | 501 | 506 | 422 | 101 | 84 |
| Faster aspart | 395b | 364b | 276b | 92 | 70 |
All data are for a dose of 0.2 U/kg. Insulin glulisine data have been dose-adjusted from 0.1 U/kg assuming dose-proportionality
aAbsolute levels of total exposure and maximum concentration should be compared with caution between the different insulin products, since results originate from different studies
bAbsolute levels of total exposure and maximum concentration for faster aspart are not comparable to the other insulin products, since free (i.e. unbound), rather than total (i.e. bound plus unbound), insulin concentration was measured in the current study
The lower early exposure and C max of faster aspart for the thigh compared with the abdomen was expected, since a similar difference was shown in several previous studies with regular human insulin [3–6] and with rapid-acting insulin analogues (Table 4). For mealtime insulins, early exposure and C max are important factors determining the ability to control postprandial glucose. Therefore, it is expected for faster aspart, and for other mealtime insulins, that abdominal administration is better able to reduce postprandial glucose compared with administration in the thigh.
Early exposure and C max for faster aspart were comparable for the abdomen versus the upper arm (Tables 2 and 3). The same was observed for C max of IAsp in a previous study [6] (Table 4). In contrast, for insulin lispro and insulin glulisine, C max appears lower for the upper arm versus the abdomen (Table 4). Moreover, for insulin glulisine, early exposure within 1 h after administration is ~20% lower for the upper arm than for the abdomen (and ~40% lower for the thigh than for the abdomen) [7]. These findings imply that faster aspart may be administered in the abdomen or upper arm with no difference in its ultra-fast pharmacokinetic properties. In contrast, for insulin lispro and insulin glulisine, some difference in early pharmacokinetic response, and thereby in postprandial glucose-lowering effect, may be expected for administration in the abdomen versus the upper arm. Differences in insulin absorption rate between injection regions are due to subcutaneous anthropometry and blood flow [16]. It is speculated that faster aspart absorption is less prone to variations in these factors for some yet unidentified reason.
One limitation of the current study was that pharmacodynamics were not assessed. In line with regulatory guidance, healthy subjects were included in the current trial [11, 12]. Consequently, pharmacodynamics would presumably have been affected by endogenous insulin secretion of the healthy subjects. It was therefore decided prospectively to include a glucose clamp only for safety precaution. Another limitation was that all subjects participating in the present study were males (although both males and females were eligible). A third limitation was related to the interpretation of t Early 50% Cmax after administration in the thigh relative to the abdomen and upper arm, which should be made with caution since C max was lower for the thigh versus the abdomen and upper arm [17].
Intramuscular administration in the thigh was only included for regulatory purposes. Compared with subcutaneous administration in the thigh, intramuscular administration resulted in similar onset of appearance, shorter t Early 50% Cmax, shorter t max, similar early exposure and C max, and lower total exposure. The clinical relevance is, however, assessed to be somewhat limited as faster aspart will not be indicated for intramuscular administration.
Conclusion
This study showed similar ultra-fast onset of exposure of faster aspart after subcutaneous administration in the abdomen, upper arm and thigh. As previously observed for other mealtime insulin products, early exposure and maximum concentration were lower for the thigh than for the abdomen and upper arm. The current study supports the option of administering faster aspart in the abdomen, upper arm or thigh, with the abdomen and upper arm providing greater early exposure and, hence, taking full advantage of the more rapid pharmacokinetic properties of this new formulation.
Acknowledgements
The authors are grateful to Alexandru L. Dinita, MD, Novo Nordisk, for reviewing and providing input to the manuscript and Carsten Roepstorff, PhD, CR Pharma Consult, Copenhagen, Denmark for contributing with medical writing support, which was funded by Novo Nordisk.
Compliance with Ethical Standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Funding
This study was funded by Novo Nordisk.
Conflict of interest
Tim Heise is a shareholder in Profil, which received research funds from Adocia, AstraZeneca, Becton Dickinson, Biocon, Boehringer Ingelheim, Dance Biopharm, Eli Lilly, Grünenthal, Gulf Pharmaceutical Industries, Johnson & Johnson, Marvel, MedImmune, Medtronic, Novartis, Novo Nordisk, Roche Diagnostics, Sanofi, Senseonics and Zealand Pharma. In addition, Tim Heise is a member of advisory panels for Novo Nordisk and received speaker honoraria and travel grants from Eli Lilly, Mylan and Novo Nordisk. Karen Margrete Due Thomsen and Hanne Haahr are employees and shareholders of Novo Nordisk. Ulrike Hövelmann, Leszek Nosek and Bettina Sassenfeld declare no conflicts of interest.
Footnotes
A comment to this article is available at http://dx.doi.org/10.1007/s40261-017-0538-8.
References
- 1.De Coninck C, Frid A, Gaspar R, et al. Results and analysis of the 2008–2009 Insulin Injection Technique Questionnaire survey. J Diabetes. 2010;2:168–179. doi: 10.1111/j.1753-0407.2010.00077.x. [DOI] [PubMed] [Google Scholar]
- 2.Frid AH, Kreugel G, Grassi G, et al. New insulin delivery recommendations. Mayo Clin Proc. 2016;91:1231–1255. doi: 10.1016/j.mayocp.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Berger M, Cüppers HJ, Hegner H, Jörgens V, Berchtold P. Absorption kinetics and biologic effects of subcutaneously injected insulin preparations. Diabetes Care. 1982;5:77–91. doi: 10.2337/diacare.5.2.77. [DOI] [PubMed] [Google Scholar]
- 4.Bantle JP, Neal L, Frankamp LM. Effects of the anatomical region used for insulin injections on glycemia in type I diabetes subjects. Diabetes Care. 1993;16:1592–1597. doi: 10.2337/diacare.16.12.1592. [DOI] [PubMed] [Google Scholar]
- 5.ter Braak EW, Woodworth JR, Bianchi R, et al. Injection site effects on the pharmacokinetics and glucodynamics of insulin lispro and regular insulin. Diabetes Care. 1996;19:1437–1440. doi: 10.2337/diacare.19.12.1437. [DOI] [PubMed] [Google Scholar]
- 6.Mudaliar SR, Lindberg FA, Joyce M, et al. Insulin aspart (B28 asp-insulin): a fast-acting analog of human insulin: absorption kinetics and action profile compared with regular human insulin in healthy nondiabetic subjects. Diabetes Care. 1999;22:1501–1506. doi: 10.2337/diacare.22.9.1501. [DOI] [PubMed] [Google Scholar]
- 7.Apidra (insulin glulisine). CHMP Assessment Report. Procedure No. EMEA/H/C/557/X/0023. 12 January 2010. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/000557/WC500090084.pdf. Accessed 19 Jan 2017.
- 8.Heise T, Hövelmann U, Brøndsted L, Adrian CL, Nosek L, Haahr H. Faster-acting insulin aspart: earlier onset of appearance and greater early pharmacokinetic and pharmacodynamic effects than insulin aspart. Diabetes Obes Metab. 2015;17:682–688. doi: 10.1111/dom.12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heise T, Stender-Petersen K, Hövelmann U, et al. Pharmacokinetic and pharmacodynamic properties of faster-acting insulin aspart versus insulin aspart across a clinically relevant dose range in subjects with type 1 diabetes mellitus. Clinical Pharmacokinet. 2016. doi:10.1007/s40262-016-0473-5. [DOI] [PMC free article] [PubMed]
- 10.Heise T, Pieber TR, Danne T, Erichsen L, Haahr H. A pooled analysis of clinical pharmacology trials investigating the pharmacokinetic and pharmacodynamic characteristics of fast-acting insulin aspart in adults with type 1 diabetes. Clinical Pharmacokinet. 2017. doi:10.1007/s40262-017-0514-8. [DOI] [PMC free article] [PubMed]
- 11.European Medicines Agency. Committee for Medicinal Products for Human Use. Guideline on the Investigation of Bioequivalence. CPMP/EWP/QWP/1401/98 Rev. 1/Corr. 20 January 2010. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/01/WC500070039.pdf. Accessed 19 Jan 2017.
- 12.Food and Drug Administration. Code of Federal Regulations. 21 CFR Part 320. Bioavailability and Bioequivalence Requirements. Available from: http://www.ecfr.gov/cgi-bin/text-idx?SID=b77b9e6e4aea3cdbd753c6bbd1b98077&mc=true&node=pt21.5.320&rgn=div5. Accessed 19 Jan 2017.
- 13.European Medicines Agency. Committee for Medicinal Products for Human Use. Guideline on Bioanalytical Method Validation. EMEA/CHMP/EWP/192217/2009 Rev. 1 Corr. 2. 21 July 2011. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/08/WC500109686.pdf. Accessed 19 Jan 2017.
- 14.Food and Drug Administration. Center for Drug Evaluation and Research. Center for Veterinary Medicine. Guidance for Industry. Bioanalytical Method Validation. May 2001. Available from: http://www.fda.gov/downloads/Drugs/Guidance/ucm070107.pdf. Accessed 19 Jan 2017.
- 15.American Diabetes Association Defining and reporting hypoglycaemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycaemia. Diabetes Care. 2005;28:1245–1249. doi: 10.2337/diacare.28.5.1245. [DOI] [PubMed] [Google Scholar]
- 16.Vora JP, Burch A, Peters JR, Owens DR. Relationship between absorption of radiolabeled soluble insulin, subcutaneous blood flow, and anthropometry. Diabetes Care. 1992;15:1484–1493. doi: 10.2337/diacare.15.11.1484. [DOI] [PubMed] [Google Scholar]
- 17.Jain L, Parks MH, Sahajwalla C. Determination of time to onset and rate of action of insulin products: importance and new approaches. J Pharm Sci. 2013;102:271–279. doi: 10.1002/jps.23355. [DOI] [PubMed] [Google Scholar]