Abstract
Staphylococcus aureus (SA) and Pseudomonas aeruginosa (PA) are known to cause biofilm-related infections. MRSA and PA have been frequently isolated from chronically infected wounds, cystic fibrosis, chronic suppurative otitis media (CSOM), and from indwelling medical devices, and these bacteria co-exist; however, their interaction with each-other or with the host is not well known. In this study, we investigated MRSA and PA multi-species biofilm communities in vitro and their interaction with the host during in vivo colonization using an OM rat-model. In-vitro biofilm formation and in-vivo colonization were studied using CV-microtiter plate assay and OM rat-model respectively. The biofilms were viewed under scanning electron microscope and bacteria were enumerated using cfu counts. The differential gene expressions of rat mucosa colonized with single or multi-species of MRSA or PA were studied using RNA-sequencing of total transcriptome. In multi-species in-vitro biofilms PA partially inhibited SA growth. However, no significant inhibition of MRSA was detected during in-vivo colonization of multi-species in rat bullae. A total of 1,797 genes were significantly (p < 0.05) differentially expressed in MRSA or PA or MRSA + PA colonized rat middle ear mucosa with respect to the control. The poly-microbial colonization of MRSA and PA induced the differential expression of a significant number of genes that are involved in immune response, inflammation, signaling, development, and defense; these were not expressed with single species colonization by either MRSA or PA. Genes involved in defense, immune response, inflammatory response, and developmental process were exclusively up-regulated, and genes that are involved in nervous system signaling, development and transmission, regulation of cell growth and development, anatomical and system development, and cell differentiation were down-regulated after multi-species inoculation. These results indicate that poly-microbial colonization induces a host response that is different from that induced by single species infection.
Keywords: biofilms, planktonic, otitis media, methicillin resistant Staphylococcus aureus, Pseudomonas aeruginosa, poly-microbial, colonization
Introduction
Globally, Staphylococcus aureus and Pseudomonas aeruginosa (PA) are two major opportunistic pathogens that cause community-acquired and nosocomial infections. S. aureus and PA are the most prevalent pathogens that colonize structurally abnormal airways such as those in cystic fibrosis (CF) and other chronic obstructive lung diseases (Lyczak et al., 2002; Hubert et al., 2013). In addition, they are frequently found together in chronic wound infections (Gjødsbøl et al., 2006; Fazli et al., 2009). S. aureus and PA cause biofilm-related infections, and methicillin-resistant S. aureus (MRSA) has emerged as a clinically relevant pathogen because of its resistance to antibiotics and its ability to form biofilms (Chopra et al., 2015). Bacteria within biofilms are difficult to eradicate because being encased in a polymer matrix decreases their susceptibility to antimicrobials and immune defenses; this inherent antimicrobial resistance provides added resistance to antimicrobial therapy and host defense. In addition, during infection, the bacteria that originate in biofilms disperse as planktonic cells, which results in spread to secondary sites and progression of the infection (Hall-Stoodley and Stoodley, 2009; Lister and Horswill, 2014). MRSA and PA have been detected in biofilm-related infections such as chronic suppurative otitis media (CSOM) and chronic middle ear infections (Jung et al., 2009; Kim et al., 2015). S. aureus and PA have been isolated from upper respiratory tract infections including several chronic diseases such as chronic otitis media, cholesteatoma, chronic adenoiditis, chronic sinusitis, post-operative trampansomay, and nasal polyposis (Post et al., 2004; Bendouah et al., 2006; Boase et al., 2013). In chronic rhinosinusitis (CRS) patients, multi-species biofilms have been associated with enhanced mucosal inflammation, more severe osteitis, higher incidence of recurrent infection (Li et al., 2011; Dong et al., 2014), and postoperative outcomes (Singhal et al., 2011), and post-surgery progression (Bendouah et al., 2006). Furthermore, S. aureus and PA have been isolated from multi-species biofilms that are frequently found on indwelling medical devices such as prostheses, stents, implants, catheters, and endotracheal tube (Percival et al., 2015). Although the effect of poly-microbial infections of S. aureus and PA has not been well studied, some studies suggested that such poly-microbial infections are more virulent than single species infections (Hendricks et al., 2001; Pastar et al., 2013). Voggu et al. (2006) reported that in vitro interactions between S. aureus and PA are competitive and result in PA eradicating S. aureus (Voggu et al., 2006). However, it has been reported that during polymicrobial colonization of PA and S. aureus, PA does not completely inhibit the colonization of S. aureus; rather, S. aureus employs numerous defense strategies for its survival in the same ecological niche and grows as a small-colony variant (SCV) (Biswas et al., 2009). As a result of the competitive interactions between S. aureus and PA, an altered colony morphology strains called small colony variants (SCVs) emerges. Those SCVs are more persistent and more antibiotic-resistant strains than normal S. aureus (Nair et al., 2014).
Multi-species biofilm infections have important implications for management because this association will modify the clinical course of the disease, affecting the selection of antimicrobial therapy and the anticipated response to treatment. However, despite the gravity of these multi-species infections, their pathogenesis remains largely unknown. Therefore, in this study, we investigated S. aureus and PA multi-species biofilm communities in vitro, and assessed their interaction with the host during in vivo colonization using an otitis media rat model.
Materials and methods
Ethics statement
All animal experiments were performed in accordance with the guidelines of the Animal Research Committee, Korea University College of Medicine, Seoul, South Korea. The animal experiment protocol was approved by the Institute Review Board of Korea University, Guro Hospital, Seoul, South Korea (Permit Number, KOREA 2016-0019).
Bacterial strain and culture medium
Methicillin resistant S. aureus (MRSA) was purchased from the Culture Collection of Antimicrobial Resistant Microbes (CCARM 3903), Seoul, Korea. P. aeruginosa (ATCC 27853) was obtained from the American Type Culture Collection (Manassas, VA). The selective medium for MRSA was oxacillin resistance screening agar base (ORSAB) and Pseudomonas-specific medium was Pseudomonas agar base (PAB), and was purchased from Oxoid limited (Hampshire, UK). 2-n-heptyl-4-hydroxyquinoline-N-oxide (HQNO) was purchased from Santa Cruz Biotechnology (Dallas, Texas, USA).
In vitro single species or multispecies biofilm growth of MRSA or PA
Single- or multi-species in vitro biofilm growth of MRSA and PA was established in 24-well (flat-bottom) polystyrene tissue culture plates (BD Falcon, Sparks, MD, USA) using a static model and a previously described procedure (Christensen et al., 1985; Yadav et al., 2015b). The biofilm biomass was quantified using a crystal violet (CV) microtiter plate assay, and the bacterial loads within biofilms were enumerated by colony forming unit (CFU) counts. MRSA or PA cell suspensions (1 × 107), individually or in combination in TSB media, were inoculated (1 mL) in 24-well polystyrene plates. The plates were incubated at 37°C for 24 h. After incubation, medium was discarded, and plates were gently washed with 1 mL sterile water. Thereafter, plates were air-dried and stained with 200 μL CV (0.1%) for 15 min. Excess stain was decanted, and plates were washed three times with sterile distilled water. The biofilm was dissolved in 1 mL (95%) ethanol and the optical density (OD) at 570 nm was measured in an automatic spectrophotometer. All experiments were performed in triplicate and the average was calculated. The experiments were repeated three times.
Alternatively, MRSA, PA, or combinations of both species were grown in TSB medium under the same conditions. CFUs were counted to quantify the number of viable cells growing in the biofilms. Biofilms were dissolved with sonication at 50 W for 10 s, serially diluted, and plated on selective medium, specifically ORSAB or PAB with CN supplement, to determine CFU-values.
To characterize the biofilm matrix, 24-h pre-established biofilms of MRSA or PA were treated with 10 mM sodium metaperiodate (Sigma, St. Louis, MO, USA), 100 μg/mL DNase I (Roche, Mannheim, Germany), 100 μg/mL alginate lyase (sigma), and 100 μg/mL proteinase K (sigma) by procedure previously described (Gutiérrez et al., 2014). The control biofilms were treated with respective buffer. The biofilms biomass was quantified as described above.
Biofilm growth of SCVs of MRSA
S. aureus forms SCVs in the presence of HQNO produced by PA (Mitchell et al., 2010). To evaluate biofilm formation capability of SCVs of MRSA (3903), biofilms were grown with (20 μg/mL) HQNO, whereas the control biofilm was grown with DMSO supplementation. Biofilm biomass was quantified using a CV-microtiter plate assay as described above. The morphological variations in SCV biofilms grown with HQNO were examined using a scanning electron microscope (SEM).
In vivo single species or multispecies colonization of MRSA or PA in the rat middle ear
In vivo single species or multispecies colonization by MRSA or PA was assessed using a previously described otitis media (OM) model (Yadav et al., 2012, 2014, 2015a). Forty pathogen-free Sprague-Dawley rats weighing 150–200 g were obtained from Koatech (Pyeongtaek, South Korea). Before the experiment, all animals were examined for abnormalities in the middle ear and were housed in an infection-free zone for 2 weeks. The rats were divided into four experimental groups. Group 1 included rats inoculated with MRSA only (n = 11); Group 2 was rats inoculated with PA only (n = 11); Group 3 included rats inoculated with a mixed culture of MRSA + PA (n = 11); and Group 4 was rats inoculated with media only (vehicle control; n = 7). The bacterial cell suspensions were prepared in TSB medium. Cell suspension (50 μL) containing 1 × 107 CFU of either single species MRSA, PA, or mixed culture of the two species was injected into the middle ear cavity through the tympanic membrane of the right ear using a tuberculin syringe and a 27-gauge needle. The animals were euthanized using a 1:1 combination of nitrous oxide and oxygen. One week after inoculation, the rats were sacrificed using carbon dioxide inhalation, and the bulla was aseptically acquired. The tympanic membrane was removed, and the ears were irrigated to remove planktonic bacteria. The bullae from each group were dissected and cleaned, and the middle ear was visualized and photographed. The bullae were homogenized, serially diluted, and plated on selective plates to obtain CFU counts. For SEM analysis, the bullae were preserved in SEM solution.
Visualization of in-vitro biofilms and in-vivo colonization of MRSA or PA single species or multi-species using SEM
MRSA or PA single species or multispecies in vitro biofilms grown in 24-well tissue culture plates for 24 h were analyzed by SEM. After a 24-h incubation, the medium with planktonic cells were discarded, and the plates were gently washed twice with sterile PBS. Samples were pre-fixed by immersion in 2% glutaraldehyde and 2.5% paraformaldehyde solution, and post-fixed for 2 h in 1% osmic acid dissolved in PBS. Samples were treated with a graded series of ethanol and t-butyl alcohol. The samples were freeze-dried in a freeze dryer (ES-2030 Hitachi, Tokyo, Japan), followed by platinum coating using an IB-5 ion coater (Eiko, Kanagawa, Japan), and specimens were visualized using a S-4700 field emission scanning electron microscope (Hitachi).
Similarly, for SEM analysis of in vivo colonization by MRSA or PA, the rat bullae from representative groups were dissected and cleaned of unwanted tissue, and the middle ear was carefully opened. The bullae were preserved in glutaraldehyde and paraformaldehyde solution. The rest of the protocol (pre-fix, post-fix, dehydration, etc.) was the same as that described previously for in vitro biofilms.
Total RNA extraction and transcriptome sequencing using rat mucosa colonized with single or multi-species of MRSA or PA
Rats from representative groups were sacrificed as described above, and the bullae were acquired. Bullae were immediately dissected, and the middle ear was exposed and preserved in RNAlater (Qiagen, Hilden, Germany). The mucosal membrane of the bullae preserved in RNAlater solution was carefully scraped and preserved in fresh RNAlater solution until RNA extraction. Total RNA was isolated using a Qiagen RNeasy kit (Qiagen, Hilden, Germany) in accordance with the manufacturer's instructions. RNA was quantified using a nano-drop, and the RNA quality was assessed by analysis of rRNA band integrity using an Agilent RNA 6000 Nano kit (Agilent Technologies, Palo Alto, CA, USA). Ahead of cDNA library construction, 2 μg of total RNA and magnetic beads with Oligo (dT) were used to enrich poly(A)-containing mRNA. Subsequently, the purified mRNA was disrupted into short fragments, and double-stranded cDNA was immediately synthesized. The cDNA was subjected to end-repair and poly(A) addition, and was connected with sequencing adapters using the TruSeq RNA sample prep kit (Illumina, CA, USA). Suitable fragments that were automatically purified using a BluePippin 2% agarose gel cassette (Sage Science, MA, USA) were selected as templates for PCR amplification. The final library sizes and qualities were evaluated electrophoretically using an Agilent high sensitivity DNA kit (Agilent Technologies, CA, USA) and the fragment was determined to be between 350 and 450 bp. Subsequently, the library was sequenced using an Illumina HiSeq2500 sequencer (Illumina, CA, USA).
Low quality reads were filtered according to the following criteria; reads containing more than 10% of skipped bases (marked as “N”s), reads containing more than 40% of bases with quality scores <20, and reads with average quality scores <20. The entire filtering process was performed using the in-house scripts. Filtered reads were mapped to the human reference genome (Ensembl release 72) (Flicek et al., 2013) using aligner STAR v.2.3.0e (Dobin et al., 2013).
Gene expression levels were measured with Cufflinks v2.1.1 (Trapnell et al., 2010) using the gene annotation database of Ensembl release 72. The non-coding gene region was removed with the mask option. To improve the accuracy of measurement, multi-read-correction and fragbias-correct options were applied, whereas all other options were set to default values. For differential expression analysis, gene level count data were generated using the HT Seq-count v0.5.4p3 (Anders et al., 2015) tool with the option “-m intersection-nonempty” and -r option, considering paired-end sequences.
Based on the calculated read count data, differentially expressed genes (DEG) were identified using the R package TCC (Sun et al., 2013). The TCC package applies robust normalization strategies to compare tag count data. Normalization factors were calculated using the iterative DEGES/edgeR method. Q-values were calculated based on the p-value using the p.adjust function of the R package with default parameter settings. DEGs were identified based on a q-value threshold < 0.05. Gene ontology (GO) and KEGG pathway analysis of the DEGs were performed using STRING version 10.
Quantification of gene expression using real-time RT-PCR
RNA-sequencing gene expression results were confirmed by real-time RT-PCR. Eighteen DEGs, and one house-keeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), were analyzed by real-time RT-PCR. The gene sequences were downloaded from the NCBI gene bank, and primers were designed using primer express software (Table 1). cDNA synthesis was performed using a PrimeScript first strand cDNA synthesis kit according to the manufacturer's instructions (TaKaRa Bio Inc., Kusatsu, Japan). The amplification reaction was performed in an ABI Prism 7300 real-time PCR system (Applied Biosystems, Foster, CA, USA) in a total volume of 20 μL. The reaction mixture consisted of 10 μL of 2 × SYBR Green PCR Master Mix (Roche Applied Science, Indianapolis, IN, USA), 2.5 pmol of each forward and reverse primer, and 4 μL cDNA. The PCR reaction mixture was incubated as follows: initial denaturation at 95°C for 10 min, followed by 45 cycles of DNA denaturation at 95°C for 15 s, primer annealing and extension at 56°C for 1 min, and a final extension step at 72°C for 5 min. A control reaction without reverse transcriptase was included in each real-time RT-PCR experiment to check for cDNA contamination with genomic DNA. The relative quantification of gene expression in rat mucosa inoculated with S. aureus, PA, or mixed culture, with respect to the type of media, was performed using the 2−ΔΔCT method (Livak and Schmittgen, 2001), and the gene expression values were normalized to those of the housekeeping gene, GAPDH.
Table 1.
List of primers used in this study.
| Serial number | Gene name | Sequence | Base pairs | Amplicon size |
|---|---|---|---|---|
| 1 | KERA | F: CCG TCG AGG GGT TTT GAT GT | 20 | 246 |
| R: CAT CGG GAT TGG TGG CTT GA | 20 | |||
| 2 | GPD1 | F: AAC AGG TGC TGA CAT CCT GG | 20 | 254 |
| R: AGC CAA TGG TCG TCT CAC AG | 20 | |||
| 3 | ACTA1 | F: CTC TTG TGT GTG ACA ACG GC | 20 | 124 |
| R: CCC ATA CCG ACC ATG ACA CC | 20 | |||
| 4 | EEF1A2 | F: TCG GAT CCT CGT TAC GCC G | 19 | 195 |
| R: GCC GGC GGT TTT ATC TCT CT | 20 | |||
| 5 | HRC | F: CTT CCA GGA GCC ATG GTT GT | 20 | 138 |
| R: CCA GGG ATA CCT GCG TTG TT | 20 | |||
| 6 | FOXD1 | F: GGT ACT CTG CAC CAA GGG AC | 20 | 130 |
| R: CCC ATC CGT AGA AAG GAG CC | 20 | |||
| 7 | MYH1 | F: AGT TGC ATC CCT AAA GGC AGA | 21 | 148 |
| R: GGC TTG TTC TGA GCC TCG AT | 20 | |||
| 8 | SYNPO2L | F: GGG TAC CAG CAC CTC AAC TT | 20 | 233 |
| R: TAA GAG CTG GTC CCT CTC CC | 20 | |||
| 9 | TNMD | F: GTC CCA CAA GTG AAG GTG GA | 20 | 148 |
| R: TGC CTC GAC GGC AGT AAA TA | 20 | |||
| 10 | MYOM2 | F: GGT ACT CCT CAT CTT TCT GGG AA | 23 | 134 |
| R: TCG ATG CAT ATC GGT CCA GG | 20 | |||
| 11 | RPS9 | F: CGT TTC TCT TTG TCA CGG GC | 20 | 192 |
| R: CCT CCA CAC CTC ACG TTT GT | 20 | |||
| 12 | MMP12 | F: CTC CCA TGA ACG AGA GCG AA | 20 | 170 |
| R: GGT GTC CAG TTG CCC AGT TA | 20 | |||
| 13 | ITGB2 | F: TTG TCA ACA CCC ATC CCG AG | 20 | 215 |
| R: AAT TTC CTC CGG ACA GGC AG | 20 | |||
| 14 | CST7 | F: GCA TAC ACC TCA GAT TTT TGT TCC A | 25 | 235 |
| R:TAG TTC GGC CGA TTT CCA CC | 20 | |||
| 15 | SCTR | F: GTC ATT CGA GGG CCT GTG AT | 20 | 197 |
| R: GGG GAG AAG GCG AAG ACA AT | 20 | |||
| 16 | APOC1 | F: CAT AGT GGT GGG AGG TGG TG | 20 | 103 |
| R: AGG AAG TGC GAT GAA GAG CC | 20 | |||
| 17 | CD69 | F: GCGATATGCTGGTGGACTGA | 20 | 111 |
| R: GACCCTGTCACGTTGAACCA | 20 | |||
| 18 | CCNO | F: CCAGTCGTTGCAGCCCATTA | 20 | 261 |
| R: ACCTCTCGGCAAGTCAAAGG | 20 |
Statistical analysis
Values were calculated as the mean of individual experiments that were performed in triplicate, and compared to those of the control groups. Differences between mean values were assessed using a Student's t-test. Statistical significance was set at p < 0.05.
Results
In vitro single species or multispecies biofilm growth of MRSA or PA
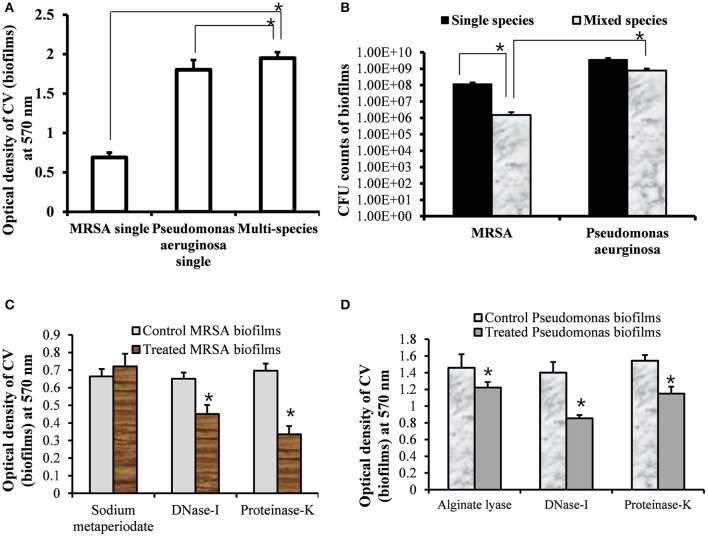
To assess the growth of MRSA and PA in single and multi-species biofilms, in vitro single and multi-species biofilms were grown on polystyrene plates that allowed for bacterial attachment and biofilm formation. The biofilms were quantified using a CV-microtiter plate assay after removing planktonic cells. The CV-microtiter plate assay detected significantly (p < 0.05) increased biofilm biomass in multi-species biofilms of MRSA and PA, compared to single species biofilms of either MRSA or PA (Figure 1A). The OD570-values of single species biofilms of MRSA and PA were 0.7 and 1.8, respectively; however, that of the multi-species biofilm was 2.0. These results indicated that total biomass of multi-species biofilms were higher than that of single species biofilms, which might be because of the accumulation of extracellular polysaccharide (EPS) and cells from both species. Thus, both species contributed to increased biofilm biomass.
Figure 1.
Quantification of single- and multi-species in vitro biofilms of methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa (PA). (A) Quantification of single-species and multi-species biofilms of MRSA or PA using crystal violet microtiter plate assays. (B) Colony forming unit (CFU) counts of bacteria within single- and multi-species biofilms of MRSA or PA. (C) Eradication of pre-established MRSA biofilms with sodium metaperiodate (SM), DNase I and proteinase K with respect to control (untreated). (D) Eradication of PA pre-established biofilms with alginate lyase, DNase I, and proteinase K. The error bars represent the standard deviation from the mean value. The results were compared using a Student's t-test (*p < 0.05).
To further evaluate the viable bacteria in single or multi-species biofilms, CFUs were enumerated. The CFU counts showed significantly higher numbers of bacteria in single species biofilms than in multi-species biofilms of either MRSA or PA (Figure 1B). In single species biofilms, the CFU counts for MRSA and PA were 1 × 108 and 4 × 109, respectively. In multi-species biofilms, PA inhibited the growth of MRSA. The CFU counts of MRSA and PA in multi-species biofilms were 1 × 106 and 8 × 108, respectively. The CFU counts for PA were significantly (p < 0.05, approximately 2.6 log values) higher than those of MRSA. These results indicated that PA and MRSA could exist together in multi-species in vitro biofilms. However, the decreased CFU counts for MRSA indicated that PA partially inhibited the growth of MRSA in multi-species biofilms.
The treatment of pre-established biofilm of MRSA with DNase I and Proteinase K significantly (p < 0.05) decreased biofilm biomass in compare to control biofilms (Figure 1C). However, no effect of sodium metaperiodate was detected. These results indicate that MRSA biofilms contains e-DNA and proteins, and the MRSA produce ica- independent biofilms. Previous studies reported no effect of sodium metaperiodate on MRSA biofilms (Fitzpatrick et al., 2005). Treatment of PA biofilms with DNase I and proteinase K significantly (p < 0.05) reduced biofilms biomass. However, very low biofilm eradication activity of alginate lyase was detected on pre-established biofilms of PA (Figure 1D). PA is known to produces at least three types of exopolysaccharides. The mucoid PA strains predominantly produce alginate exopolysaccharide (Hentzer et al., 2001), and non-mucoid strains produce the Pel and Psl polysaccharides for biofilm formation (Wozniak et al., 2003). ATCC 27853 is a standard non-mucoid strain (Li et al., 2001). These results indicate that PA (ATCC 27853) biofilms contains DNA and proteins along with polysaccharides primarily the Pel and Psl.
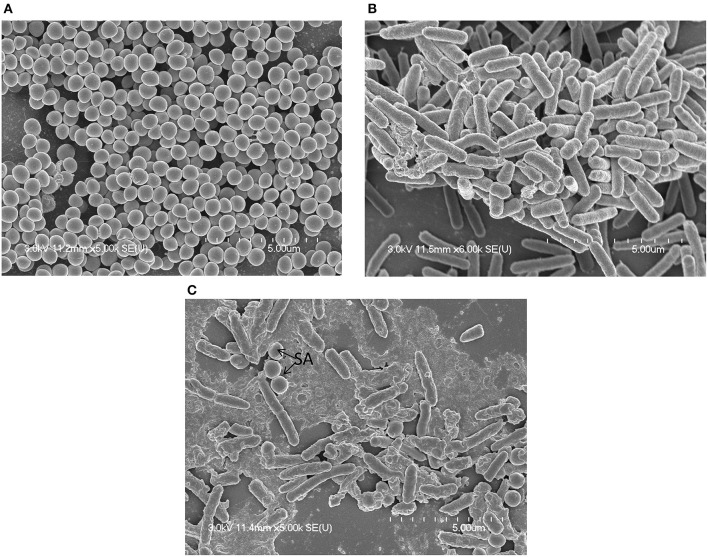
MRSA or PA single-species or multi-species in vitro biofilms were analyzed using SEM. SEM analysis revealed that single-species biofilms were compact, and cells were connected to the bottom of the plate and to each other (Figures 2A,B). The multi-species biofilms were also compact, and the bacteria were interconnected to each other and to the surface. In multi-species biofilms, the biofilm debris or may be EPS was visible that were not visible in single species biofilms of either MRSA or PA (Figure 2C). As expected in multi-species biofilms, fewer S. aureus bacteria were visible than PA, indicating that PA partially inhibited MRSA growth in the biofilm state.
Figure 2.
Scanning electron microscope (SEM) images of in vitro biofilms of single or multi-species methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa (PA). (A) Representative SEM image of MRSA single species biofilms. (B) Representative SEM images of PA single species biofilms. (C) Representative SEM images of multi-species biofilms of MRSA and PA.
SCVs of MRSA enhance biofilm formation
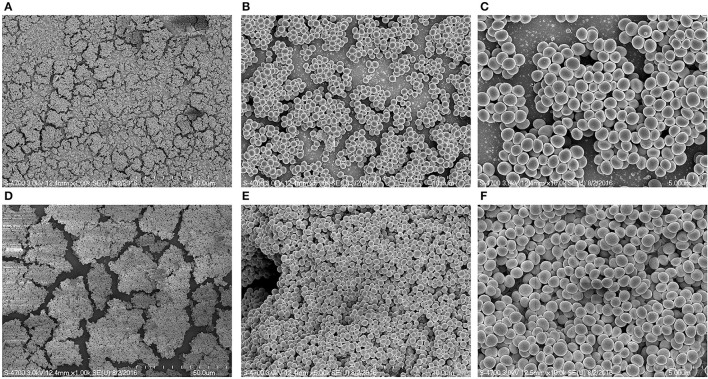
The in vitro biofilm formation ability of MRSA 3903 SCVs was evaluated by culturing biofilms in the presence or absence of HQNO. CV-microtiter plate assay results showed no significant decrease in MRSA biofilm biomass in the presence of 20 μg HQNO (Figure 3). SEM images revealed no significant difference between SCV biofilms and phenotypically normal MRSA biofilms (Figure 4). These results indicate that MRSA 3903 could form SCVs, and that MRSA could exist in multi-species biofilms with PA.
Figure 3.
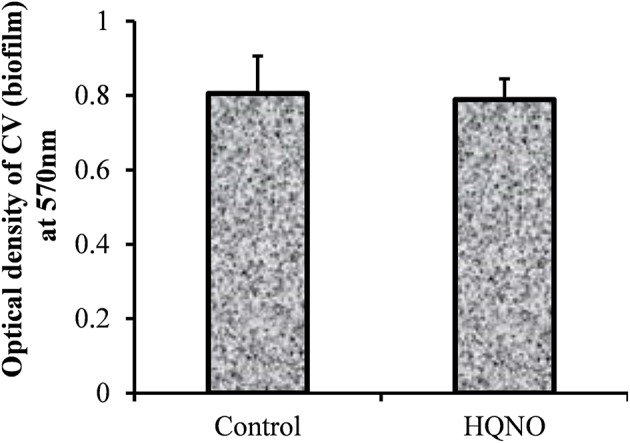
In vitro biofilm growth of methicillin-resistant Staphylococcus aureus (MRSA) small colony variant. In vitro biofilm growth of MRSA supplemented with HQNO (20 μg/mL) or without HQNO. The biofilm biomass was detected by a crystal violet microtiter plate assay. Error bars represent standard deviation from the mean value.
Figure 4.
Scanning electron microscopic (SEM) image of methicillin-resistant Staphylococcus aureus (MRSA) biofilms grown with and without HQNO. Images (A–C) are representative SEM images of control biofilms (DMSO). (D–F) Representative SEM images of biofilm growth with 20 μg/ml HQNO. Scale bar = 5, 10, 50 μm.
In vivo colonization of MRSA and PA alone or together in the rat middle ear
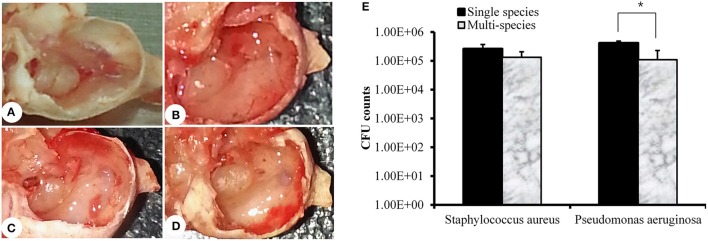
Rats were sacrificed 1 week after inoculation, and bullae were acquired. The representative rat bullae were dissected and photographed (Figures 5A–D). The rat bullae inoculated with media only (vehicle control) were clear with no sign of mucosal inflammation or bacterial colonization (Figure 5A). However, the rat bullae inoculated with MRSA or PA alone or together were filled with a sticky fluid and showed signs of bacterial colonization. The mucosa was thick, swollen, and showed signs of severe inflammation (Figures 5B–D). The mean CFU counts for the rat bullae inoculated with MRSA and PA alone were 2.63 × 105 (SD = 1.07 × 105) and 4.50 × 105 (SD = 7.07 × 104), respectively. A higher number of PA CFUs, compared to MRSA CFUs, was recovered from rat bullae inoculated with either MRSA or PA alone; however, this difference was not significant. The CFU counts for MRSA and PA in the rat bullae inoculated with both species were 1.34 × 105 (SD = 1.1 × 105) and 1.10 × 105 (SD = 7.3 × 104), respectively (Figure 5E). No significant difference in CFU counts between MRSA and PA were detected in multi-species-inoculated rat bullae. However, the CFU counts for PA alone were significantly high than those of PA in multi-species inoculations.
Figure 5.
Quantification of methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa (PA) in the rat middle ear. (A) Digital image of rat bulla inoculated with media only. (B) Digital image of rat bulla inoculated with MRSA only. (C) Digital images of rat bulla inoculated with PA only. (D) Digital image of rat bulla inoculated with multi-species (MRSA + PA). (E) Colony forming unit (CFU) counts for rat bullae inoculated with single or multi-species MRSA or PA in the rat middle ear.
Visualization of in vivo colonization of MRSA and PA alone or in combination
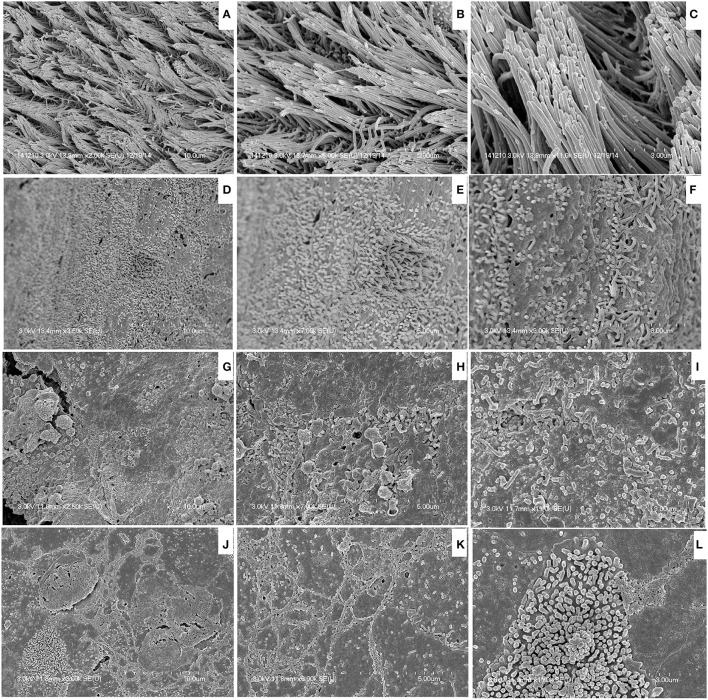
SEM images of rat middle ears inoculated with MRSA, PA, MRSA + PA, or control inoculum are shown in Figure 6. In the control middle ear, the ciliated epithelium in the hypotympanum area and Eustachian tube orifice were intact (Figures 6A–C). In the MRSA, PA, or MRSA + PA treated groups, the entire middle ear was covered with cell debris or biofilm EPS. The tips of the cilia were invisible due to the deposition of cell debris (Figures 6D–L).
Figure 6.
Scanning electron microscope (SEM) images of bullae inoculated with methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa (PA) as a single or multi-species inoculum. (A–C) Representative SEM images of no procedure control. (D–F) Representative SEM images of rat bullae inoculated with SA only. (G–I) Representative SEM images of rat bullae inoculated with PA only. (J–L) Representative SEM images of rat bullae inoculated with a mixed culture of MRSA and PA.
Transcriptome sequencing of rat mucosa inoculated with MRSA, PA, or a combination of the two
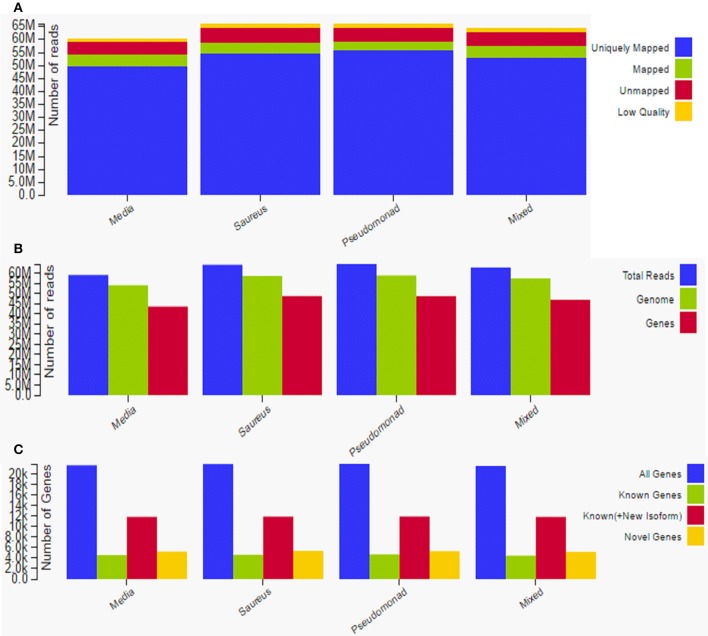
Total raw data reads in all samples ranged from 50 to 65 million, with an average of approximately 55 million raw reads per sample. The average mRNA insert size detected by sequencing and mapping was 159 bp. The uniquely mapped reads ranged between ~50 and 55 million; there were ~5 million mapped reads and ~6 million unmapped reads (Figure 7A). There were ~58 million total reads, 55 million genome reads, and 43 million gene reads (Figure 7B). The gene reads were ~20 K, known genes were ~5 K, known plus new isoforms were ~12 K, and novel genes were ~5 K (Figure 7C). A total of 1797 genes were significantly (p < 0.05) differentially expressed in all three treatments with respect to the control (media). Among them, 973 were upregulated > 1-fold and 824 were downregulated > 1-fold.
Figure 7.
Overview and summary of raw data analysis after transcriptome sequencing to assess gene expression in rat middle ear mucosae inoculated with methicillin-resistant Staphylococcus aureus (MRSA) or Pseudomonas aeruginosa (PA) as a single species or multi-species inoculum. (A) Graphical representation of uniquely mapped, mapped, and unmapped reads. (B) Graphical representation of total reads, gene reads, and genome reads. (C) Graphical representation of novel genes, known genes, and all genes.
Differential gene expression
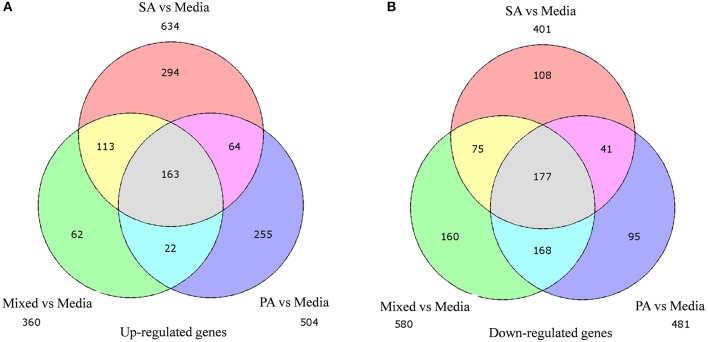
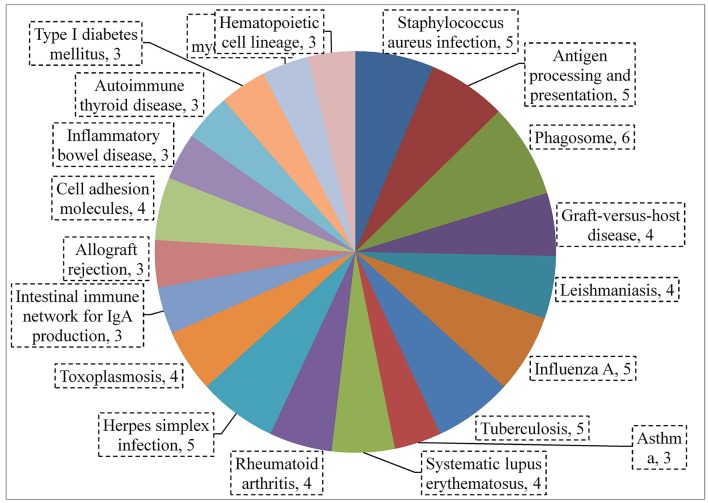
A total of 360, 634, and 504 genes were significantly (p < 0.05) upregulated in multi-species, MRSA and PA, colonized rat middle ear mucosae (Figure 8A). Among 360 upregulated genes in multi-species-colonized rat mucosae, 113 (31%) were similar to those of MRSA, and 22 (6.1%) were similar to those of PA. The three treatments shared 163 genes whose expression was upregulated. Moreover, 62 genes were exclusively upregulated during multi-species colonization. Among them, the 42 genes upregulated by ≥1.5-fold and encoding proteins of known functions are shown in Table 2. A total of 580, 401, and 481 genes were significantly (p < 0.05) downregulated in multi-species-, MRSA-, and PA-colonized rat middle ear mucosae (Figure 8B). Among the 580 downregulated genes after multi-species colonization, 75 genes (12.9%) were similar to those associated with MRSA, and 168 (28.9%) genes were similar to those associated with PA. Among 160 genes exclusively downregulated after multi-species colonization, 88 genes that were downregulated by ≥1.5-fold and encoding known proteins are shown in Table 3. The gene ontology (GO) of upregulated and downregulated genes in multi-species inoculated rat mucosae is shown in Figures 9A,B. The GO analysis of 42 protein-encoding genes that were upregulated (≥1.5-fold) after multi-species colonization revealed that genes involved in defense, immune response, inflammatory response, and developmental process were exclusively upregulated after multi-species inoculation (Figure 9A). The GO analysis of 88 protein-encoding genes that were downregulated (≥1.5-fold) revealed that genes that are involved in nervous system signaling, development and transmission, regulation of cell growth and development, anatomical and system development, and cell differentiation were significantly downregulated after multi-species inoculation (Figure 9B). Based on KEGG pathway analysis of upregulated genes after multi-species inoculation, genes involved in phagosome, antigen processing and presentation, influenza A infection, Staphylococcus infection, herpes virus infection, toxoplasmosis, and tuberculosis (and other) pathway genes were evaluated (Figure 10). These results revealed that colonization of the rat middle ear with MRSA or PA alone or in combination regulates a significant number of genes. In addition, multi-species colonization with MRSA and PA induced a different set of genes that were not induced by either MRSA or PA alone. This shows that the host response is different after multi-species colonization, which could enhance the pathogenicity of the bacteria.
Figure 8.
Venn diagram illustrating the number of genes commonly or uniquely differentially expressed in rat middle ear mucosa colonized with methicillin-resistant Staphylococcus aureus (MRSA) or Pseudomonas aeruginosa (PA) as a single species or multi-species inoculum. (A) Venn diagram showing significantly (p < 0.05) upregulated genes after colonization with MRSA, PA, or a combination of the two, with respect to the media (control). (B) Venn diagram showing significantly (p < 0.05) downregulated genes after MRSA, PA, or multi-species colonization, with respect to media control.
Table 2.
List of genes upregulated ≥1.5-fold (p < 0.05) in rat middle ear mucosae colonized with MRSA and PA and upregulated or downregulated with MRSA or PA single species colonization.
| Serial number | Gene accession | Gene ID | Gene name | Protein | Fold change in mixed inoculation | p-value | Fold change with single species SA | p-value | Fold change with single species PA | p-value |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | XLOC_007451 | TBIG007451 | CHI3L1 | Chitinase-3-like protein 1 | 1.5 | 0.0127 | 1.35 | 0.0061 | 1.21 | 0.0636 |
| 2 | XLOC_008245 | TBIG008245 | PPP2R3D | Serine/threonine-protein phosphatase 2A regulatory subunit B” subunit delta | 1.52 | 0.0412 | 1.67 | 0.00865 | 1.38 | 0.08015 |
| 3 | XLOC_010172 | TBIG010172 | JAK3 | Tyrosine-protein kinase JAK3 | 1.52 | 0.01095 | 1.31 | 0.01205 | 1.64 | 0.0179 |
| 4 | XLOC_016832 | TBIG016832 | FCN2 | Ficolin-2 | 1.53 | 0.04235 | 0.547 | 0.38185 | 0.917 | 0.2557 |
| 5 | XLOC_015362 | TBIG015362 | RT1-BA | Rano class II histocompatibility antigen, B alpha chain | 1.54 | 0.0146 | 1.42 | 0.00855 | 0.573 | 0.35145 |
| 6 | XLOC_026947 | TBIG026947 | TLR7 | Toll-like receptor 7 | 1.55 | 0.0207 | 1.68 | 0.0028 | 1.4 | 0.0428 |
| 7 | XLOC_023774 | TBIG023774 | SLA | Src-like-adapter | 1.55 | 0.01355 | 1.65 | 0.0038 | 1.47 | 0.04885 |
| 8 | XLOC_002817 | TBIG002817 | CORO1A | Coronin-1A | 1.56 | 0.0055 | 1.5 | 0.00155 | 1.43 | 0.019 |
| 9 | XLOC_014051 | TBIG014051 | CFI | Complement factor I | 1.57 | 0.04835 | 0.89 | 0.1797 | 1.96 | 0.0404 |
| 10 | XLOC_017708 | TBIG017708 | CTSZ | Cathepsin Z | 1.57 | 0.0143 | 1.36 | 0.0164 | 0.876 | 0.19125 |
| 11 | XLOC_013234 | TBIG013234 | CDH6 | Cadherin-6 | 1.61 | 0.0029 | 1.22 | 0.00625 | 2.64 | 5.00E−05 |
| 12 | XLOC_009200 | TBIG009200 | DNASE1L3 | Deoxyribonuclease gamma | 1.63 | 0.0323 | 1.61 | 0.01495 | 0.767 | 0.3716 |
| 13 | XLOC_004746 | TBIG004746 | PRSS22 | Brain-specific serine protease 4 | 1.66 | 0.0247 | 0.698 | 0.2435 | 2.12 | 0.0144 |
| 14 | XLOC_025304 | TBIG025304 | RPLP1 | 60S acidic ribosomal protein P1 | 1.67 | 0.00535 | 1.6 | 0.00365 | 0.472 | 0.41575 |
| 15 | XLOC_024595 | TBIG024595 | STRA6 | Stimulated by retinoic acid gene 6 protein homolog | 1.67 | 0.0355 | 0.526 | 0.4289 | 2.35 | 0.011 |
| 16 | XLOC_026676 | TBIG026676 | ST8SIA4 | CMP-N-acetylneuraminate-poly-alpha-2,8-sialyltransferase | 1.67 | 0.0333 | 1.43 | 0.02115 | 2.04 | 0.01315 |
| 17 | XLOC_006363 | TBIG006363 | TFRC | Transferrin receptor protein 1 | 1.69 | 0.0043 | 0.726 | 0.1144 | 0.124 | 0.82765 |
| 18 | XLOC_016540 | TBIG016540 | HCK | Tyrosine-protein kinase HCK | 1.7 | 0.00685 | 1.94 | 0.00045 | 1.36 | 0.04235 |
| 19 | XLOC_006164 | TBIG006164 | SAMSN1 | SAM domain-containing protein SAMSN-1 | 1.71 | 0.0231 | 1.8 | 0.00595 | 1.34 | 0.09725 |
| 20 | XLOC_019232 | TBIG019232 | ENV | Envelope glycoprotein gp70 | 1.71 | 0.0325 | 1.3 | 0.0364 | 1.87 | 0.0289 |
| 21 | XLOC_007736 | TBIG007736 | LAMB3 | Laminin subunit beta-3 | 1.71 | 0.0046 | 1.01 | 0.0415 | 2.27 | 0.0012 |
| 22 | XLOC_014992 | TBIG014992 | RT1-BB | Rano class II histocompatibility antigen, B-1 beta chain | 1.72 | 0.0213 | 1.54 | 0.0081 | 0.402 | 0.48745 |
| 23 | XLOC_014993 | TBIG014993 | RT1-DB1 | Rano class II histocompatibility antigen, D-1 beta chain | 1.74 | 0.00385 | 1.59 | 0.00255 | 1.3 | 0.0427 |
| 24 | XLOC_020229 | TBIG020229 | CNR2 | Cannabinoid receptor 2 | 1.74 | 0.04435 | 1.69 | 0.0167 | 1.57 | 0.12025 |
| 25 | XLOC_002804 | TBIG002804 | LAT | Linker for activation of T-cells family member 1 | 1.77 | 0.0202 | 1.37 | 0.0229 | 1.34 | 0.10215 |
| 26 | XLOC_016951 | TBIG016951 | RPS17 | 40S ribosomal protein S17 | 1.8 | 0.04525 | 1.42 | 0.06125 | −0.406 | 0.725 |
| 27 | XLOC_023263 | TBIG023263 | KRT7 | Keratin, type II cytoskeletal 7 | 1.81 | 0.003 | 0.839 | 0.10965 | 2.18 | 0.00285 |
| 28 | XLOC_025817 | TBIG025817 | C3 | Complement C3 | 1.85 | 0.0068 | 1.81 | 0.00065 | 0 | 1 |
| 29 | XLOC_015648 | TBIG015648 | ELL3 | RNA polymerase II elongation factor ELL3 | 1.9 | 0.04245 | 1.08 | 0.12345 | 0.566 | 0.57055 |
| 30 | XLOC_002984 | TBIG002984 | GAL | Galanin peptides | 1.91 | 0.03065 | 0.656 | 0.36215 | −0.583 | 0.604 |
| 31 | XLOC_016373 | TBIG016373 | NMES1 | Normal mucosa of esophagus-specific gene 1 protein | 2.04 | 0.01855 | 1.79 | 0.0102 | 1.43 | 0.1201 |
| 32 | XLOC_018558 | TBIG018558 | KLRD1 | Natural killer cells antigen CD94 | 2.07 | 0.0351 | 1.83 | 0.05635 | 0.31 | 0.8115 |
| 33 | XLOC_001187 | TBIG001187 | SRCRM_HUMAN | Putative scavenger receptor cysteine-rich domain-containing protein LOC619207 | 2.07 | 0.0019 | 2.57 | 5.00E−05 | 0.401 | 0.5927 |
| 34 | XLOC_018355 | TBIG018355 | CD8A | T-cell surface glycoprotein CD8 alpha chain | 2.11 | 0.0217 | 2.51 | 0.00255 | 1.2 | 0.23315 |
| 35 | XLOC_020260 | TBIG020260 | PLA2G2D | Group IID secretory phospholipase A2 | 2.25 | 0.01785 | 1.44 | 0.0665 | 0.51 | 0.6369 |
| 36 | XLOC_023454 | TBIG023454 | GZMM | Granzyme M | 2.41 | 0.0116 | 2.33 | 0.0055 | −0.876 | 0.44375 |
| 37 | XLOC_005212 | TBIG005212 | CCL5 | C-C motif chemokine 5 | 2.42 | 0.01385 | 2.05 | 0.00985 | −0.559 | 0.6301 |
| 38 | XLOC_022420 | TBIG022420 | HVM17_MOUSE | Ig heavy chain V region MOPC 47A | 2.51 | 0.02705 | 1.16 | 0.22905 | 0.871 | 0.51305 |
| 39 | XLOC_015317 | TBIG015317 | UBD | Ubiquitin D | 2.7 | 0.00275 | 1.93 | 0.00665 | 1.14 | 0.2022 |
| 40 | XLOC_011426 | TBIG011426 | TCC2_MOUSE | T-cell receptor gamma chain C region C7.5 | 2.72 | 0.0168 | 3.31 | 0.00035 | 0.352 | 0.78555 |
| 41 | XLOC_006104 | TBIG006104 | LV2B_MOUSE | Ig lambda-2 chain V region MOPC 315 | 2.8 | 0.002 | 0.785 | 0.2881 | 0.937 | 0.33275 |
| 42 | XLOC_018648 | TBIG018648 | KLRB1F | Killer cell lectin-like receptor subfamily B member 1F | 2.82 | 0.02 | 2.45 | 0.02085 | 0.0885 | 0.9476 |
| 43 | XLOC_012586 | TBIG012586 | CLEC3A | C-type lectin domain family 3 member A | 3.07 | 0.04855 | 3.67 | 0.014 | 1.08 | 0.46495 |
| 44 | XLOC_022400 | TBIG022400 | HVM43_MOUSE | Ig heavy chain V region MOPC 141 | 3.15 | 0.04835 | 3.26 | 0.06595 | 0.34 | 0.8163 |
Table 3.
List of genes downregulated by ≥1.5-fold (p < 0.05) in rat middle ear mucosae colonized by MRSA and PA and either downregulated or upregulated with MRSA or PA single species colonization.
| Serial number | Gene accession | Gene ID | Gene name | Protein | Fold change in mixed inoculation | p-value | Fold change with single species SA | p-value | Fold change with single species PA | p-value |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | XLOC_000033 | TBIG000033 | TXLNB | Beta-taxilin | −7.41 | 0.04245 | −1.39 | 0.01125 | −7.68 | 0.07995 |
| 2 | XLOC_005477 | TBIG005477 | SCN4A | Sodium channel protein type 4 subunit alpha | −5.49 | 0.02615 | −1.34 | 0.074 | −4.26 | 0.11755 |
| 3 | XLOC_021210 | TBIG021210 | GAG | Gag polyprotein | −5.22 | 0.044 | 0.78 | 0.2248 | −0.722 | 0.4373 |
| 4 | XLOC_027720 | TBIG027720 | IGSF1 | Immunoglobulin superfamily member 1 | −4.53 | 0.0182 | −1.13 | 0.19385 | −5.06 | 0.14935 |
| 5 | XLOC_013130 | TBIG013130 | LDLRAD1 | Low-density lipoprotein receptor class A domain-containing protein 1 | −4.36 | 0.0083 | −1.15 | 0.2058 | −1.93 | 0.15705 |
| 6 | XLOC_027382 | TBIG027382 | POL | Retrovirus-related Pol polyprotein LINE-1 | −4.29 | 0.0183 | −1.76 | 0.07165 | −4.89 | 0.095 |
| 7 | XLOC_012347 | TBIG012347 | GAG-POL | Gag-Pol polyprotein | −4.25 | 0.00015 | 0.841 | 0.0705 | −0.867 | 0.15245 |
| 8 | XLOC_015004 | TBIG015004 | TNXB | Tenascin-X | −4.14 | 0.0376 | −2.21 | 0.25835 | −3 | 0.0938 |
| 9 | XLOC_024947 | TBIG024947 | GRIA4 | Glutamate receptor 4 | −4.06 | 0.04955 | −1.7 | 0.0813 | −2.26 | 0.1075 |
| 10 | XLOC_014321 | TBIG014321 | TTC24 | Tetratricopeptide repeat protein 24 | −4.04 | 0.0285 | −0.657 | 0.4197 | −1.2 | 0.28155 |
| 11 | XLOC_026757 | TBIG026757 | POL | Pol polyprotein | −4.03 | 0.02135 | 0.779 | 0.21675 | 1.18 | 0.15825 |
| 12 | XLOC_020111 | TBIG020111 | NT5C1A | Cytosolic 5'-nucleotidase 1A | −3.93 | 0.00125 | −1.39 | 0.0508 | −2.43 | 0.0548 |
| 13 | XLOC_002039 | TBIG002039 | MARK4 | MAP/microtubule affinity-regulating kinase 4 | −3.65 | 0.0475 | −1.43 | 0.4849 | −3.48 | 0.0536 |
| 14 | XLOC_007497 | TBIG007497 | BRINP3 | BMP/retinoic acid-inducible neural-specific protein 3 | −3.63 | 0.0429 | −1.46 | 0.1324 | −3.66 | 0.13615 |
| 15 | XLOC_024201 | TBIG024201 | POL | Pol polyprotein | −3.57 | 0.0373 | −0.937 | 0.2889 | −1.6 | 0.2579 |
| 16 | XLOC_006129 | TBIG006129 | POL | Pol polyprotein | −3.38 | 0.04745 | −1.18 | 0.207 | −1.52 | 0.34225 |
| 17 | XLOC_005475 | TBIG005475 | GH1 | Somatotropin | −3.34 | 0.04165 | 0.851 | 0.2985 | −0.925 | 0.49845 |
| 18 | XLOC_024602 | TBIG024602 | HCN4 | Potassium/sodium hyperpolarization-activated cyclic nucleotide-gated channel 4 | −3.27 | 0.03825 | −0.742 | 0.37845 | −1.53 | 0.27585 |
| 19 | XLOC_021698 | TBIG021698 | RGS6 | Regulator of G-protein signaling 6 | −3.25 | 0.02615 | −1.38 | 0.14185 | −1.96 | 0.20475 |
| 20 | XLOC_006065 | TBIG006065 | OSTN | Osteocrin | −3.23 | 0.04325 | −1.79 | 0.04165 | −2.8 | 0.081 |
| 21 | XLOC_021434 | TBIG021434 | ALK | ALK tyrosine kinase receptor | −3.18 | 0.0397 | −1.86 | 0.0675 | −3.07 | 0.08475 |
| 22 | XLOC_026950 | TBIG026950 | EGFL6 | Epidermal growth factor-like protein 6 | −3.13 | 0.0404 | −0.986 | 0.2498 | −1.45 | 0.2599 |
| 23 | XLOC_021001 | TBIG021001 | NCMAP | Noncompact myelin-associated protein | −3.13 | 0.0497 | −1.11 | 0.50945 | −1.23 | 0.5089 |
| 24 | XLOC_004000 | TBIG004000 | GRIA1 | Glutamate receptor 1 | −3.05 | 0.0488 | −2.95 | 0.0636 | −2.59 | 0.0773 |
| 25 | XLOC_021987 | TBIG021987 | KCNK3 | Potassium channel subfamily K member 3 | −2.98 | 0.03695 | −2.13 | 0.0539 | −2.07 | 0.1183 |
| 26 | XLOC_008308 | TBIG008308 | NAA11 | N-alpha-acetyltransferase 11 | −2.97 | 0.02785 | −1.27 | 0.1347 | −1.53 | 0.1725 |
| 27 | XLOC_003922 | TBIG003922 | TRIM7 | Tripartite motif-containing protein 7 | −2.93 | 0.0133 | −1.02 | 0.11075 | −1.47 | 0.10675 |
| 28 | XLOC_010040 | TBIG010040 | POL | Pol polyprotein | −2.86 | 0.01455 | −1.46 | 0.06215 | −2.43 | 0.0726 |
| 29 | XLOC_017690 | TBIG017690 | BCAS1 | Breast carcinoma-amplified sequence 1 homolog | −2.65 | 0.04765 | −1.54 | 0.07675 | −0.974 | 0.3948 |
| 30 | XLOC_002797 | TBIG002797 | SNRPN | Small nuclear ribonucleoprotein-associated protein N | −2.65 | 0.0201 | −1.4 | 0.06855 | −2.02 | 0.06625 |
| 31 | XLOC_023030 | TBIG023030 | COL14A1 | Collagen alpha-1(XIV) chain | −2.62 | 0.0069 | −0.904 | 0.16355 | −1.16 | 0.15975 |
| 32 | XLOC_023753 | TBIG023753 | KLHL38 | Kelch-like protein 38 | −2.62 | 0.0302 | −0.989 | 0.17215 | −2.69 | 0.0851 |
| 33 | XLOC_002608 | TBIG002608 | HBB2_RAT | Hemoglobin subunit beta-2 | −2.55 | 0.0001 | −2.36 | 5.00E−05 | −3.34 | 5.00E−05 |
| 34 | XLOC_024012 | TBIG024012 | COL2A1 | Collagen alpha-1(II) chain | −2.51 | 0.00715 | −0.616 | 0.24645 | 1.09 | 0.10775 |
| 35 | XLOC_007817 | TBIG007817 | NFASC | Neurofascin | −2.46 | 0.02025 | −1.47 | 0.09665 | −2.29 | 0.08025 |
| 37 | XLOC_018133 | TBIG018133 | MEST | Mesoderm-specific transcript homolog protein | −2.43 | 0.03235 | −1.11 | 0.1112 | −1.91 | 0.07395 |
| 38 | XLOC_022758 | TBIG022758 | LINGO3 | Leucine-rich repeat and immunoglobulin-like domain-containing nogo receptor-interacting protein 3 | −2.35 | 0.0387 | −1.42 | 0.0885 | −1.4 | 0.18525 |
| 39 | XLOC_005055 | TBIG005055 | CHRNB1 | Acetylcholine receptor subunit beta | −2.25 | 0.0174 | −1.12 | 0.10445 | −1.7 | 0.08505 |
| 40 | XLOC_009184 | TBIG009184 | CDHR3 | Cadherin-related family member 3 | −2.25 | 0.04445 | −0.978 | 0.13985 | −1.47 | 0.15415 |
| 41 | XLOC_014711 | TBIG014711 | CAPSL | Calcyphosin-like protein | −2.24 | 0.04895 | −0.886 | 0.25825 | −0.976 | 0.37135 |
| 42 | XLOC_003432 | TBIG003432 | POL | Pol polyprotein | −2.11 | 0.0243 | −1.35 | 0.0438 | −1.65 | 0.07025 |
| 43 | XLOC_022093 | TBIG022093 | EGLN3 | Egl nine homolog 3 | −2.09 | 0.02785 | −0.962 | 0.11 | −0.67 | 0.37785 |
| 44 | XLOC_017633 | TBIG017633 | MATN4 | Matrilin-4 [Source:SWISS;Acc:O89029] | −2.02 | 0.0068 | −0.87 | 0.0838 | −0.925 | 0.15585 |
| 45 | XLOC_017934 | TBIG017934 | GAG-POL | Gag-Pol polyprotein | −1.92 | 0.0217 | −0.381 | 0.4882 | −0.885 | 0.2449 |
| 46 | XLOC_000295 | TBIG000295 | SSC5D | Soluble scavenger receptor cysteine-rich domain-containing protein SSC5D | −1.91 | 0.00235 | −0.86 | 0.062 | −1.13 | 0.05595 |
| 47 | XLOC_020236 | TBIG020236 | TCEA3 | Transcription elongation factor A protein 3 | −1.8 | 0.04715 | −0.75 | 0.2413 | −1.45 | 0.1176 |
| 48 | XLOC_019566 | TBIG019566 | POL | Pol polyprotein | −1.75 | 0.00645 | −0.592 | 0.29525 | −1.36 | 0.0589 |
| 49 | XLOC_024924 | TBIG024924 | FAM198A | Protein FAM198A | −1.68 | 0.04485 | −0.975 | 0.104 | −1.57 | 0.0906 |
| 50 | XLOC_016802 | TBIG016802 | - | - | −1.68 | 0.01715 | −0.262 | 0.6802 | −1.08 | 0.13725 |
| 51 | XLOC_010859 | TBIG010859 | AGTPBP1 | Cytosolic carboxypeptidase 1 | −1.67 | 0.01575 | −0.817 | 0.1006 | −1.66 | 0.0367 |
| 52 | XLOC_002382 | TBIG002382 | ST8SIA2 | Alpha-2,8-sialyltransferase 8B | −1.67 | 0.04965 | −0.846 | 0.171 | −1.09 | 0.23515 |
| 53 | XLOC_026579 | TBIG026579 | CCDC108 | Coiled-coil domain-containing protein 108 | −1.66 | 0.01635 | −0.31 | 0.5204 | −0.735 | 0.2972 |
| 54 | XLOC_027640 | TBIG027640 | CHRDL1 | Chordin-like protein 1 | −1.62 | 0.00485 | −0.842 | 0.0639 | −1.81 | 0.0037 |
| 55 | XLOC_002898 | TBIG002898 | ADAM8 | Disintegrin and metalloproteinase domain-containing protein 8 | −1.59 | 0.00935 | −0.908 | 0.0545 | −0.97 | 0.12135 |
| 56 | XLOC_021028 | TBIG021028 | UBXN10 | UBX domain-containing protein 10 | −1.58 | 0.01805 | −0.353 | 0.5061 | −1.22 | 0.08675 |
| 57 | XLOC_013884 | TBIG013884 | C1ORF173 | Uncharacterized protein C1orf173 | −1.56 | 0.019 | −0.068 | 0.88615 | −0.855 | 0.18325 |
| 58 | XLOC_024898 | TBIG024898 | DLEC1 | Deleted in lung and esophageal cancer protein 1 | −1.56 | 0.03495 | −0.467 | 0.3517 | −1.07 | 0.14125 |
| 59 | XLOC_001829 | TBIG001829 | SLC22A3 | Solute carrier family 22 member 3 | inf | 5.00E−05 | −1.36 | 0.2606 | −3.68 | 0.19145 |
| 60 | XLOC_003476 | TBIG003476 | POL | Pol polyprotein | inf | 5.00E05 | −0.582 | 0.23855 | −10.4 | 0.2431 |
| 61 | XLOC_004811 | TBIG004811 | RPS2 | 40S ribosomal protein S2 | inf | 5.00E−05 | 0.17 | 0.71865 | inf | 5.00E−05 |
| 62 | XLOC_006114 | TBIG006114 | RPL9 | 60S ribosomal protein L9 | inf | 5.00E−05 | −0.538 | 0.5414 | inf | 5.00E−05 |
| 63 | XLOC_006289 | TBIG006289 | MYH15 | Myosin-15 | inf | 5.00E−05 | −1.09 | 0.28855 | −6.22 | 0.2431 |
| 64 | XLOC_006508 | TBIG006508 | GAG | Gag polyprotein | inf | 5.00E−05 | −0.187 | 0.8494 | −3.29 | 0.21805 |
| 65 | XLOC_009108 | TBIG009108 | GAG-POL | Gag-Pol polyprotein | inf | 5.00E−05 | −0.5 | 0.5663 | −6.64 | 0.24325 |
| 66 | XLOC_013224 | TBIG013224 | RPS2 | 40S ribosomal protein S2 | inf | 5.00E−05 | 0.11 | 0.8856 | −7.12 | 0.24315 |
| 67 | XLOC_017320 | TBIG017320 | ACTC1 | Actin, alpha cardiac muscle 1 | inf | 5.00E−05 | −0.929 | 0.0515 | −11.1 | 0.2431 |
| 68 | XLOC_022977 | TBIG022977 | STAC3 | SH3 and cysteine-rich domain-containing protein 3 | inf | 5.00E−05 | −1.31 | 0.0224 | −8.87 | 0.1708 |
| 69 | XLOC_023915 | TBIG023915 | CYP2D1 | Cytochrome P450 2D1 | inf | 5.00E−05 | 1.29 | 0.09275 | −4.64 | 0.16405 |
| 70 | XLOC_026493 | TBIG026493 | MSTN | Growth/differentiation factor 8 | inf | 5.00E−05 | −2.2 | 0.1506 | −5.46 | 0.2456 |
| 71 | XLOC_027253 | TBIG027253 | CX064_MOUSE | Uncharacterized protein CXorf64 homolog | inf | 5.00E−05 | −1.48 | 0.1895 | −5.75 | 0.2454 |
| 72 | XLOC_022618 | TBIG022618 | ENV | Envelope glycoprotein | inf | 0.0004 | −0.993 | 0.41135 | inf | 0.0008 |
| 73 | XLOC_005431 | TBIG005431 | NEDD4 | E3 ubiquitin-protein ligase NEDD4 | inf | 0.00345 | −1.81 | 0.2551 | −4.4 | 0.25485 |
| 74 | XLOC_016973 | TBIG016973 | NEB | Nebulin | inf | 0.00495 | −0.73 | 0.57485 | −2.83 | 0.2479 |
| 75 | XLOC_019744 | TBIG019744 | CALB1 | Calbindin | inf | 0.0053 | −1.73 | 0.2773 | −3.48 | 0.2558 |
| 76 | XLOC_020636 | TBIG020636 | 15.5 KDA FABP | Major urinary protein | inf | 0.0053 | −3.54 | 0.1979 | −2.95 | 0.2434 |
| 77 | XLOC_022462 | TBIG022462 | HV208_HUMAN | Ig heavy chain V-II region SESS | inf | 0.0053 | −0.717 | 0.578 | −4.71 | 0.2508 |
| 78 | XLOC_027795 | TBIG027795 | POL | Pol polyprotein | inf | 0.0053 | −3 | 0.1555 | −3.64 | 0.25175 |
| 79 | XLOC_007006 | TBIG007006 | KIF19 | Kinesin-like protein KIF19 | inf | 0.0058 | −1.9 | 0.2472 | −4.39 | 0.2531 |
| 80 | XLOC_017904 | TBIG017904 | POL | Retrovirus-related Pol polyprotein LINE-1 | inf | 0.00575 | −3.28 | 0.2009 | −1.76 | 0.3774 |
| 81 | XLOC_019217 | TBIG019217 | OXTR | Oxytocin receptor | inf | 0.0058 | −2.29 | 0.19495 | −4.46 | 0.2524 |
| 82 | XLOC_007150 | TBIG007150 | DNAH10 | Dynein heavy chain 10, axonemal | inf | 0.01035 | −3.34 | 0.26695 | −2.62 | 0.2963 |
| 83 | XLOC_016972 | TBIG016972 | NEB | Nebulin | inf | 0.0097 | −2.55 | 0.23375 | −3.61 | 0.2679 |
| 84 | XLOC_009140 | TBIG009140 | DUSP13 | Dual specificity protein phosphatase 13 isoform A | inf | 0.0117 | −0.865 | 0.6321 | −3.34 | 0.27455 |
| 85 | XLOC_006921 | TBIG006921 | CD209L2 | CD209 antigen-like protein 2 | inf | 0.01335 | −0.951 | 0.6269 | −3.07 | 0.2863 |
| 86 | XLOC_009994 | TBIG009994 | GAG | Gag polyprotein | inf | 0.01335 | 1.01 | 0.46895 | 0.352 | 0.8164 |
| 87 | XLOC_009143 | TBIG009143 | DUSP13 | Dual specificity protein phosphatase 13 isoform A | inf | 0.0252 | −0.929 | 0.6643 | −2.67 | 0.42285 |
| 88 | XLOC_007324 | TBIG007324 | POL | Pol polyprotein | inf | 0.0387 | 0.404 | 0.83325 | 0.731 | 0.77005 |
Figure 9.
Gene ontology (GO) of differentially expressed genes in rat middle ear mucosa colonized with methicillin-resistant Staphylococcus aureus (MRSA) or Pseudomonas aeruginosa (PA) as a single or multi-species inoculum. (A) GO category of significantly (p < 0.05) upregulated genes (by ≥1.5-fold) with multi-species colonization. (B) GO category of significantly (p < 0.05) downregulated genes (≥1.5-fold) after multi-species colonization.
Figure 10.
KEGG pathway analysis using string showing significantly (p < 0.05) upregulated genes (by ≥1.5-fold) after multi-species colonization of the rat middle ear mucosa.
Quantification of gene expression using real-time RT-PCR
The results of real time-PCR were in complete agreement with RNA sequencing gene expression results. The expression of RPS9, MMP12, ITGB2, CST7, SCTR, APOC1, CD69, and CCNO genes was significantly (p < 0.05) upregulated (by >1.5-fold) in rat mucosae colonized with MRSA or PA alone or in combination based on both real time RT-PCR and RNA sequencing (Table 4). Similarly, the expression of KERA, GPD1, ACTA1, EEF1A2, HRC, FOXD1, MYH1, SYNPO2L, TNMD, and MYOM2, based on real time RT-PCR and RNA sequencing, was significantly (p < 0.05) decreased by more than 1.5-fold (Table 4).
Table 4.
Confirmation of RNA sequencing results by real-time RT-PCR.
| Gene name | Rat colonized with multi-species culture | Rat colonized with MRSA single species | Rat colonized with PA single species | ||||
|---|---|---|---|---|---|---|---|
| Fold change in RNA seq | Fold change in real-time PCR | Fold change in RNA seq | Fold change in real-time PCR | Fold change in RNA seq | Fold change in real-time PCR | ||
| 1 | RPS9 | 4.27 | 3.23 | 3.72 | 1.87 | 3.97 | 2.71 |
| 2 | MMP12 | 2.67 | 1.34 | 4.2 | 1.11 | 2.51 | 2.0 |
| 3 | ITGB2 | 2.02 | 25.28 | 2.42 | 12.55 | 2.15 | 7.36 |
| 4 | CST7 | 3.09 | 7.2 | 3.12 | 13.45 | 2.09 | 1.7 |
| 5 | SCTR | 4.26 | 15.78 | 5.18 | 20.11 | 4.13 | 18.25 |
| 6 | APOC1 | 3.4 | 11.55 | 3.95 | 4.99 | 2.88 | 2.9 |
| 7 | CD69 | 3.36 | 12.04 | 3.44 | 14.32 | 2.71 | 1.7 |
| 8 | CCNO | 2.8 | 5.6 | 3.68 | 11.31 | 3.74 | 3.78 |
| 9 | KERA | −9.37 | −5.55 | −2.19 | −7.69 | −5.66 | −6.6 |
| 10 | GPD1 | −6.4 | −6.66 | −1.62 | −8.33 | −6.1 | −5.82 |
| 11 | EEF1A2 | −7.31 | −3.3 | −1.36 | −4.76 | −6.93 | −1.5 |
| 12 | HRC | −6.77 | −4 | −1.62 | −6.2 | −6.73 | −5.8 |
| 13 | FOXD1 | −4.94 | −4.34 | −2.16 | −8.33 | −4.37 | −6.25 |
| 14 | MYH1 | −10.9 | −3.33 | −2.36 | −4.76 | −10.5 | −4 |
| 15 | SYNPO21 | −5.98 | −5.26 | −1.42 | −7.1 | −5.59 | −3.84 |
| 16 | TNMD | −7.12 | −9 | −1.88 | −8.3 | −7.3 | −7.69 |
| 17 | MYOM2 | −7.1 | −7.6 | −2.61 | −5.2 | −6.65 | −4 |
| 18 | ACTA1 | −11.5 | −5.55 | −1.36 | −16.66 | −10.1 | −2.70 |
Discussion
S. aureus and PA are known to cause biofilm-related infections and are frequently found together in chronically infected wounds, CF, CSOM, and on indwelling medical devices such as prostheses, stents, implants, catheters, and endotracheal tubes (Chole and Faddis, 2002; Hubert et al., 2013; Percival et al., 2015). Previous studies using a wound infection model suggested that poly-microbial infections of MRSA and PA were more virulent than single species infections and that synergism exists between these species, which increases virulence (Hendricks et al., 2001; Pastar et al., 2013). In various types of chronic ear infections, MRSA and PA have been frequently isolated, and these bacteria co-exist; however, their interaction with each-other or with the host was previously unknown (Klein and Chan, 2010; Kim et al., 2015). In this study, we investigated MRSA and PA multi-species biofilm communities in vitro and their interaction with the host during in vivo colonization using an OM rat model.
CV microtiter plate assay results showed significantly increased biofilm biomass in multi-species biofilms of MRSA and PA in comparison with that of single species biofilms of either MRSA or PA. It was speculated that the matrix produced by the two bacterial species and cellular components of the bacteria contributes to increased biomass. It is suggested that Staphylococcus and Pseudomonas biofilm matrix consist of extracellular DNA (e-DNA) along with exopolysaccharides (Whitchurch et al., 2002; Eckhart et al., 2007). Staphylococcal biofilm accumulation is mediated by polysaccharide intercellular adhesion (PIA), modulated by gene products encoded by ica operon (Cramton et al., 1999), and can also form ica-independent biofilm, mediated by proteins binding of the extracellular matrix (Vergara-Irigaray et al., 2009). MRSA isolates are known to produce ica-independent biofilms (Fitzpatrick et al., 2005). While the PA biofilms matrix consists of alginate (a negatively charged polymer of guluronic and mannuronic acid), Psl (a neutral polysaccharide consisting of a pentasaccharide repeat containing glucose, mannose, and rhamnose), and Pel (positively charged exopolysaccharide composed of partially acetylated 1 → 4 glycosidic linkages of N-acetylgalactosamine and N-acetylglucosamine) (Franklin et al., 2011). However, MRSA CFU counts were significantly lower (by >2-fold) than those of PA. These results are in agreement with previous studies that demonstrated that S. aureus and PA are frequently found together in human infections, and that PA kills S. aureus when the two species are grown together in nutrient rich medium in vitro (Palmer et al., 2005, 2007; Voggu et al., 2006). The killing of S. aureus has been attributed to various exoproducts of PA such as LasA protease (Mansito et al., 1987), HQNO (Hoffman et al., 2006), pel and psl products (Qin et al., 2009), and pyocyanin (Dietrich et al., 2006). SEM images also confirmed the presence of few MRSA bacteria in multi-species biofilms; however, increased EPS matrix was visible in multi-species biofilms, compared to that in single species biofilms. Many studies have suggested that S. aureus can overcome this suppression and escape PA-mediated cell death by growing as SCVs, which are characterized as a respiration-defective subpopulation with an altered phenotype (Biswas et al., 2009; Nair et al., 2014). Moreover, S. aureus forms SCVs in the presence of HQNO produced by PA (Hoffman et al., 2006). In addition, these SCVs that were shown to form under stress of HQNO formed robust in vitro biofilms through the activation of SigB and the repression of agr (Mitchell et al., 2010). Our results also showed robust biofilm formation by MRSA 3903 in the presence of HQNO, when compared to that in the control. SEM results provided further evidence of significant biofilm formation in the presence of HQNO. This process has been suggested to be due to the activation of SigB and the repression of agr, which upregulates various cell surface proteins such as FnBA, which are involved in biofilm formation (Mitchell et al., 2008, 2009, 2010).
S. aureus and PA frequently colonize the upper respiratory tract and cause several chronic diseases including chronic OM, cholesteatoma, chronic adenoiditis, chronic sinusitis, post-operative trampansomay, and nasal polyposis (Bendouah et al., 2006; Boase et al., 2013). Furthermore, MRSA and PA have been frequently isolated from chronic ear infections such as CSOM (Jung et al., 2009; Kim et al., 2015). In this study, we used the OM rat model to study the interaction between MRSA and PA in vivo. We previously used this model to study the colonization of S. aureus and Streptococcus pneumoniae separately in the rat middle ear (Yadav et al., 2012, 2014, 2015a). Herein, we first evaluated the utility of the OM rat model for multi-species colonization. The results showed consistent recovery of MRSA or PA from the rat middle ear, which was associated with significant inflammation. We choose the OM rat model over other animal models because the rat middle ear is a natural habitat for S. aureus and PA, and because inflammation in the middle ear mucosa (due to bacterial challenge or infection) results in severe swelling that can be easily visualized by SEM; this tissue can also be easily recovered for gene expression studies.
The middle ear is a natural habitat for many bacteria including normal body flora or opportunistic pathogens. Both S. aureus and PA have been isolated from middle ear infections (Brook, 2003). Our in vivo results showed marked recovery of MRSA or PA after multi-species and single species inoculation with significant inflammation in the rat middle ear. In addition, no significant differences in CFU counts between MRSA and PA were observed after multi-species inoculation. These results indicate that MRSA and PA successfully colonized the rat middle ear with no significant inhibition of either species. Using wound infection and rabbit models, DeLeon et al. (2014) reported no inhibition of S. aureus in the presence of PA during multi-species in vivo colonization (DeLeon et al., 2014). Other studies also showed a lack of inhibition in the absence of nutrient rich medium, which could be due to the presence of SCVs of S. aureus (McNamara and Proctor, 2000; Filkins et al., 2015). Inflammation in the middle ear is a sign of infection; our results also detected significant inflammation in this tissue after inoculation with either MRSA or PA alone or in combination. The significant number of bacteria recovered (based on CFU counts) indicates that multi-species bacterial colonization was established in the middle ear. SEM analysis also confirmed the presence of biofilm-like structures that were deposited on the cilia of the middle ear after single or multi-species inoculation. However, those of MRSA or PA alone were indistinguishable. Similarly, Kaya et al. (2013) also detected poly-microbial biofilms of S. aureus and PA in the middle ear (Saunders et al., 2011; Kaya et al., 2013). In CRS patients, multi-species biofilms have been associated with increased mucosal inflammation, more severe osteitis, higher incidence of recurrent infection (Li et al., 2011; Dong et al., 2014) and postoperative outcome (Singhal et al., 2011), and post-surgery progressions (Bendouah et al., 2006).
To analyze differential gene expression in rat middle ear mucosae inoculated with MRSA or PA alone or in combination, RNA sequencing of the total transcriptome was performed. RNA-seq results detected significantly increased expression of defense-, immune-, response-, and inflammatory-related genes after multi-species colonization and decreased expression of signaling, development, and communication pathway genes. Interestingly, a total of 122 genes (62 upregulated and 160 downregulated) were exclusively differentially regulated by multi-species colonization, and these genes were not expressed with either MRSA or PA inoculation. Gene pathway analysis revealed that those genes are involved in the phagosome, antigen processing and presentation, influenza A infection, Staphylococcus infection, herpes virus infection, toxoplasmosis, and tuberculosis among others.
FCN2, CFI, KLRD1, KLRB1F, UBD, CCL5, and GAL were significantly upregulated after multi-species inoculation and were either downregulated or insignificantly upregulated with MRSA or PA single inoculation. The ficolin-2-encoding gene FCN2, was upregulated by 1.52-fold (P = 0.04) with multi-species infection and was downregulated after inoculation with MRSA or PA alone. Ficolin activates the lectin pathway of complement by specifically binding to lipoteichoic acid, a cell wall constituent of gram-positive bacteria, and initiates an innate anti-microbial immune response (Lynch et al., 2004). The CFI gene was significantly upregulated in multi-species infections and was downregulated with MRSA or PA alone. CFI encodes complement factor I, which is involved in complement activation during cochlear responses to acoustic trauma (Patel et al., 2013). The KLRD1 and KLRB1F genes encode the killer cell lectin-like receptor subfamily K, member 1 protein and killer cell lectin like receptor B1, which are involved in the activation of immune responses and cytotoxicity, were upregulated by 2.1- and 2.0-fold, respectively, after mixed culture inoculation, and were downregulated with MRSA or PA alone (Taniguchi et al., 2015). The UBD gene, encoding the protein ubiquitin D, which is involved in cytokine-induced apoptosis in rat, was upregulated by mixed cultures and MRSA and was downregulated with PA alone (Brozzi et al., 2016). Similarly, the CCL5 gene encodes chemokine (C-C Motif) ligand 5 protein. These chemokine proteins are involved in immunoregulatory and inflammatory processes. CCL5 is an essential factor for the induction and maintenance of protective pneumococcal immunity (Palaniappan et al., 2006). The GAL gene encodes galanin peptides, and was upregulated by 1.9-fold or repressed with SA or PA, respectively. The galanin protein is involved in post-traumatic stress disorder and mild blast-induced traumatic brain injury (Kawa et al., 2016). The upregulation of important immune response- and cytotoxicity-related genes after multispecies inoculation indicates an enhanced host response, when compared to that after inoculation with MRSA and PA alone.
DLECI, ST8SIA2, and SSC5d are important genes, which were downregulated with multi-species inoculation and either upregulated or insignificantly downregulated with MRSA or PA alone. The DLEC1 gene encodes the deleted in lung and esophageal cancer 1 protein (DLECI) that has tumor suppressive activities. The downregulation of this gene has been observed in several human cancers including lung, esophageal, renal, and head and neck squamous cell carcinoma (Zhang et al., 2015). Similarly, the ST8SIA2 gene encodes alpha-2,8-sialyltransferase 2, which is involved in neuronal plasticity (Shaw et al., 2014). The SSC5D gene encodes scavenger receptor cysteine rich family member with 5 domains protein, which induces bacterial and fungal aggregation and subsequent inhibition of PAMP-induced cytokine release, and was also downregulated after multi-species inoculation. These results indicate that multi-species colonization triggers host responses that are different from those induced by infection with MRSA or PA alone. It has been previously reported that poly-microbial biofilms of S. aureus and PA, in a chronic wound infection model, significantly impair wound healing relative to that observed with their single-species biofilm counterparts. Multi-species biofilms also trigger enhanced host inflammatory responses through the expression of IL-1β and TNF-α (Seth et al., 2012). Similarly, Pastar et al. (2013) also detected significantly decreased re-epithelization with mixed species biofilms, via the suppression of keratinocyte growth factor-1. Moreover, in poly-microbial wound infections, the presence of PA induced the expression of S. aureus virulence factors including Panton-Valentine leucocidin and α-hemolysin (Pastar et al., 2013).
Conclusion
This study demonstrated that MRSA and PA could co-exist in poly-microbial biofilms in vitro, and colonize the rat middle ear in vivo. The poly-microbial colonization of MRSA and PA induced the differential expression of a significant number of genes that are involved in immune response, inflammation, signaling, development, and defense; these were not expressed with single species colonization by either MSA or PA. These results indicate that poly-microbial colonization induces a host response that is different from that induced by single species infection. It is probable that poly-microbial infections were more virulent than single species infections. Thus, poly-microbial infections could modify the clinical course of disease, affecting the selection of antimicrobial therapy and the anticipated response to treatment.
Author contributions
MY, designed research, did experiment, results analysis wrote manuscript. SC, evaluated results, chemical supply, facility arrangement, check manuscript. YG, protocol approval, animal work design, arrange reagent, and chemical facility. GI, protocol design, manuscript reading, manuscript review. JS, Protocol design, results analysis, manuscript review.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was supported by Korean Health Technology Research and Development Project, Ministry of Health and Welfare (HI15C2835), South Korea.
References
- Anders S., Pyl P. T., Huber W. (2015). HTSeq–a python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169. 10.1093/bioinformatics/btu638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendouah Z., Barbeau J., Hamad W. A., Desrosiers M. (2006). Biofilm formation by Staphylococcus aureus and Pseudomonas aeruginosa is associated with an unfavorable evolution after surgery for chronic sinusitis and nasal polyposis. Otolaryngol. Head Neck Surg. 134, 991–996. 10.1016/j.otohns.2006.03.001 [DOI] [PubMed] [Google Scholar]
- Biswas L., Biswas R., Schlag M., Bertram R., Götz F. (2009). Small-colony variant selection as a survival strategy for Staphylococcus aureus in the presence of Pseudomonas aeruginosa. Appl. Environ. Microbiol. 75, 6910–6912. 10.1128/AEM.01211-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boase S., Foreman A., Cleland E., Tan L., Melton-Kreft R., Pant H., et al. (2013). The microbiome of chronic rhinosinusitis: culture, molecular diagnostics and biofilm detection. BMC Infect. Dis. 13:210. 10.1186/1471-2334-13-210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook I. (2003). Microbiology and management of chronic suppurative otitis media in children. J. Trop. Pediatr. 49, 196–200. 10.1093/tropej/49.4.196 [DOI] [PubMed] [Google Scholar]
- Brozzi F., Gerlo S., Grieco F. A., Juusola M., Balhuizen A., Lievens S., et al. (2016). Ubiquitin D regulates IRE1α/c-Jun N-terminal Kinase (JNK) protein-dependent apoptosis in pancreatic beta cells. J. Biol. Chem. 291, 12040–12056. 10.1074/jbc.M115.704619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chole R., Faddis B. (2002). Evidence for microbial biofilms in cholesteatomas. Arch. Otolaryngol. Head Neck Surg. 128, 1129–1133. 10.1001/archotol.128.10.1129 [DOI] [PubMed] [Google Scholar]
- Chopra S., Harjai K., Chhibber S. (2015). Antibiotic susceptibility of ica-positive and ica-negative MRSA in different phases of biofilm growth. J. Antibiot. 68, 15–22. 10.1038/ja.2014.96 [DOI] [PubMed] [Google Scholar]
- Christensen G. D., Simpson W. A., Younger J. J., Baddour L. M., Barrett F. F., Melton D. M., et al. (1985). Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J. Clin. Microbiol. 22, 996–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramton S. E., Gerke C., Schnell N. F., Nichols W. W., Götz F. (1999). The intercellular adhesion (ICA) locus Is present in Staphylococcus aureus and is required for biofilm formation. Infect. Immun. 67, 5427–5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeon S., Clinton A., Fowler H., Everett J., Horswill A. R., Rumbaugh K. P. (2014). Synergistic interactions of Pseudomonas aeruginosa and Staphylococcus aureus in an in vitro wound model. Infect. Immun. 82, 4718–4728. 10.1128/IAI.02198-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich L. E. P., Price-Whelan A., Petersen A., Whiteley M., Newman D. K. (2006). The phenazine pyocyanin is a terminal signalling factor in the quorum sensing network of Pseudomonas aeruginosa. Mol. Microbiol. 61, 1308–1321. 10.1111/j.1365-2958.2006.05306.x [DOI] [PubMed] [Google Scholar]
- Dobin A., Davis C. A., Schlesinger F., Drenkow J., Zaleski C., Jha S., et al. (2013). STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21. 10.1093/bioinformatics/bts635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong D., Yulin Z., Xiao W., Hongyan Z., Jia L., Yan X., et al. (2014). Correlation between bacterial biofilms and osteitis in patients with chronic rhinosinusitis. Laryngoscope 124, 1071–1077. 10.1002/lary.24424 [DOI] [PubMed] [Google Scholar]
- Eckhart L., Fischer H., Barken K. B., Tolker-Nielsen T., Tschachler E. (2007). DNase1L2 suppresses biofilm formation by Pseudomonas aeruginosa and Staphylococcus aureus. Br. J. Dermatol. 156, 1342–1345. 10.1111/j.1365-2133.2007.07886.x [DOI] [PubMed] [Google Scholar]
- Fazli M., Bjarnsholt T., Kirketerp-Møller K., Jørgensen B., Andersen A. S., Krogfelt K. A., et al. (2009). Nonrandom distribution of Pseudomonas aeruginosa and Staphylococcus aureus in chronic wounds. J. Clin. Microbiol. 47, 4084–4089. 10.1128/JCM.01395-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filkins L. M., Graber J. A., Olson D. G., Dolben E. L., Lynd L. R., Bhuju S., et al. (2015). Coculture of Staphylococcus aureus with Pseudomonas aeruginosa drives S. aureus towards fermentative metabolism and reduced viability in a cystic fibrosis model. J. Bacteriol. 197, 2252–2264. 10.1128/JB.00059-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick F., Humphreys H., O'Gara J. P. (2005). Evidence for ica ADBC_independent biofilm development mechanism in methicillin-resistant Staphylococcus aureus clinical isolates. J. Clin. Microbiol. 43, 1973–1976. 10.1128/JCM.43.4.1973-1976.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flicek P., Ahmed I., Amode M. R., Barrell D., Beal K., Brent S., et al. (2013). Ensembl 2013. Nucleic Acids Res. 41, D48–D55. 10.1093/nar/gks1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin M. J., Nivens D. E., Weadge J. T., Howell P. L. (2011). Biosynthesis of the Pseudomonas aeruginosa extracellular polysaccharides, alginate, Pel, and Psl. Front. Microbiol. 2:167. 10.3389/fmicb.2011.00167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjødsbøl K., Christensen J. J., Karlsmark T., Jørgensen B., Klein B. M., Krogfelt K. A. (2006). Multiple bacterial species reside in chronic wounds: a longitudinal study. Int. Wound J. 3, 225–231. 10.1111/j.1742-481X.2006.00159.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez D., Ruas-Madiedo P., Martínez B., Rodríguez A., García P. (2014). Effective removal of staphylococcal biofilms by the endolysin LysH5. Rohde H. PLoS ONE 9:e107307. 10.1371/journal.pone.0107307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall-Stoodley L., Stoodley P. (2009). Evolving concepts in biofilm infections. Cell. Microbiol. 11, 1034–1043. 10.1111/j.1462-5822.2009.01323.x [DOI] [PubMed] [Google Scholar]
- Hendricks K. J., Burd T. A., Anglen J. O., Simpson A. W., Christensen G. D., Gainor B. J. (2001). Synergy between Staphylococcus aureus and Pseudomonas aeruginosa in a rat model of complex orthopaedic wounds. J. Bone Joint Surg. 83, 855–861. 10.2106/00004623-200106000-00006 [DOI] [PubMed] [Google Scholar]
- Hentzer M., Teitzel G. M., Balzer G. J., Heydorn A., Molin S., Givskov M., et al. (2001). Alginate overproduction affects Pseudomonas aeruginosa biofilm structure and function. overproduction affects Pseudomonas aeruginosa biofilm structure and function. J. Bacteriol. 183, 5395–401. 10.1128/JB.183.18.5395-5401.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman L. R., Deziel E., D'Argenio D. A., Lepine F., Emerson J., McNamara S., et al. (2006). Selection for Staphylococcus aureus small-colony variants due to growth in the presence of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U.S.A. 103, 19890–19895. 10.1073/pnas.0606756104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert D., Réglier-Poupet H., Sermet-Gaudelus I., Ferroni A., Le Bourgeois M., Burgel P.-R., et al. (2013). Association between Staphylococcus aureus alone or combined with Pseudomonas aeruginosa and the clinical condition of patients with cystic fibrosis. J. Cyst. Fibros. 12, 497–503. 10.1016/j.jcf.2012.12.003 [DOI] [PubMed] [Google Scholar]
- Jung H., Lee S. K., Cha S.-H., Byun J. Y., Park M. S., Yeo S. G. (2009). Current bacteriology of chronic otitis media with effusion: high rate of nosocomial infection and decreased antibiotic sensitivity. J. Infect. 59, 308–316. 10.1016/j.jinf.2009.08.013 [DOI] [PubMed] [Google Scholar]
- Kawa L., Barde S., Arborelius U. P., Theodorsson E., Agoston D., Risling M., et al. (2016). Expression of galanin and its receptors are perturbed in a rodent model of mild, blast-induced traumatic brain injury. Exp. Neurol. 279, 159–167. 10.1016/j.expneurol.2016.02.019 [DOI] [PubMed] [Google Scholar]
- Kaya E., Dag I., Incesulu A., Gurbuz M. K., Acar M., Birdane L. (2013). Investigation of the presence of biofilms in chronic suppurative otitis media, nonsuppurative otitis media, and chronic otitis media with cholesteatoma by scanning electron microscopy. Sci. World J. 2013:638715. 10.1155/2013/638715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. H., Kim M. G., Kim S. S., Cha S. H., Yeo S. G. (2015). Change in detection rate of methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa and their antibiotic sensitivities in patients with chronic suppurative otitis media. J. Int. Adv. Otol. 11, 151–156. 10.5152/iao.2015.1106 [DOI] [PubMed] [Google Scholar]
- Klein J., Chan S. (2010). Methicillin-resistant Staphylococcus aureus in middle ear fluid of children. Clin. Pediatr. 49, 66–68. 10.1177/0009922809342463 [DOI] [PubMed] [Google Scholar]
- Li H., Wang D., Sun X., Hu L., Yu H., Wang J. (2011). Relationship between bacterial biofilm and clinical features of patients with chronic rhinosinusitis. Eur. Arch. Oto Rhino Laryngol. 269, 155–163. 10.1007/s00405-011-1683-y [DOI] [PubMed] [Google Scholar]
- Li J., Turnidge J., Milne R., Nation R. L., Coulthard K. (2001). In vitro pharmacodynamic properties of colistin and colistin methanesulfonate against Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Antimicrob. Agents Chem. 45, 781–785. 10.1128/AAC.45.3.781-785.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister J. L., Horswill A. R. (2014). Staphylococcus aureus biofilms: recent developments in biofilm dispersal. Front. Cell. Infect. Microbiol. 4:178. 10.3389/fcimb.2014.00178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 25, 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Lyczak J. B., Cannon C. L., Pier G. B. (2002). Lung infections associated with cystic fibrosis. Clin. Microbiol. Rev. 15, 194–222. 10.1128/CMR.15.2.194-222.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch N. J., Roscher S., Hartung T., Morath S., Matsushita M., Maennel D. N., et al. (2004). L-Ficolin specifically binds to lipoteichoic acid, a cell wall constituent of gram-positive bacteria, and activates the lectin pathway of complement. J. Immunol. 172, 1198–1202. 10.4049/jimmunol.172.2.1198 [DOI] [PubMed] [Google Scholar]
- Mansito T. B., Falcón M. A., Moreno J., Carnicero A., Gutierrez-Navarro A. M. (1987). Effects of staphylolytic enzymes from Pseudomonas aeruginosa on the growth and ultrastructure of Staphylococcus aureus. Microbios 49, 55–64. [PubMed] [Google Scholar]
- McNamara P. J., Proctor R. A. (2000). Staphylococcus aureus small colony variants, electron transport and persistent infections. Int. J. Antimicrob. Agents 14, 117–122. 10.1016/S0924-8579(99)00170-3 [DOI] [PubMed] [Google Scholar]
- Mitchell G., Brouillette E., Seguin D. L., Asselin A. E., Jacob C. L., Malouin F. (2009). A role for sigma factor B in the emergence of Staphylococcus aureus small-colony variants and elevated biofilm production resulting from an exposure to aminoglycosides. Microb. Pathog. 48, 18–27. 10.1016/j.micpath.2009.10.003 [DOI] [PubMed] [Google Scholar]
- Mitchell G., Lamontagne C. A., Brouillette E., Grondin G., Talbot B. G., Grandbois M., et al. (2008). Staphylococcus aureus SigB activity promotes a strong fibronectin-bacterium interaction which may sustain host tissue colonization by small-colony variants isolated from cystic fibrosis patients. Mol. Microbiol. 70, 1540–1555. 10.1111/j.1365-2958.2008.06511.x [DOI] [PubMed] [Google Scholar]
- Mitchell G., Séguin D. L., Asselin A.-E., Déziel E., Cantin A. M., Frost E. H., et al. (2010). Staphylococcus aureus sigma B-dependent emergence of small-colony variants and biofilm production following exposure to Pseudomonas aeruginosa 4-hydroxy-2-heptylquinoline-N- oxide. BMC Microbiol. 10:33. 10.1186/1471-2180-10-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair N., Biswas R., Götz F., Biswas L. (2014). Impact of Staphylococcus aureus on pathogenesis in polymicrobial infections. Infect. Immun. 82, 2162–2169. 10.1128/IAI.00059-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaniappan R., Singh S., Singh U. P., Singh R., Ades E. W., Briles D. E., et al. (2006). CCL5 Modulates pneumococcal immunity and carriage. J. Immunol. 176, 2346–2356. 10.4049/jimmunol.176.4.2346 [DOI] [PubMed] [Google Scholar]
- Palmer K. L., Aye L. M., Whiteley M. (2007). Nutritional cues control Pseudomonas aeruginosa multicellular behavior in cystic fibrosis sputum. J. Bacteriol. 189, 8079–8087. 10.1128/JB.01138-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer K. L., Mashburn L. M., Singh P. K., Whiteley M. (2005). Cystic fibrosis sputum supports growth and cues key aspects of Pseudomonas aeruginosa physiology. J. Bacteriol. 187, 5267–5277. 10.1128/JB.187.15.5267-5277.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastar I., Nusbaum A. G., Gil J., Patel S. B., Chen J., Valdes J., et al. (2013). Interactions of methicillin resistant Staphylococcus aureus USA300 and Pseudomonas aeruginosa in polymicrobial wound infection. PLoS ONE 8:e56846. 10.1371/journal.pone.0056846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M., Hu Z., Bard J., Jamison J., Cai Q., Hu B. H. (2013). Transcriptome characterization by RNA-Seq reveals the involvement of the complement components in noise-traumatized rat cochleae. Neuroscience 248, 1–16. 10.1016/j.neuroscience.2013.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percival S. L., Suleman L., Vuotto C., Donelli G. (2015). Healthcare-associated infections, medical devices and biofilms: risk, tolerance and control. J. Med. Microbiol. 64, 323–334. 10.1099/jmm.0.000032 [DOI] [PubMed] [Google Scholar]
- Post J. C., Stoodley P., Hall–Stoodley L., Ehrlich G. D. (2004). The role of biofilms in otolaryngologic infections. Curr. Opin. Otolaryngol. Head Neck Surg. 12, 185–190. 10.1097/01.moo.0000124936.46948.6a [DOI] [PubMed] [Google Scholar]
- Qin Z., Yang L., Qu D., Molin S., Tolker-Nielsen T. (2009). Pseudomonas aeruginosa extracellular products inhibit staphylococcal growth, and disrupt established biofilms produced by Staphylococcus epidermidis. Microbiology 155, 2148–2156. 10.1099/mic.0.028001-0 [DOI] [PubMed] [Google Scholar]
- Saunders J., Murray M., Alleman A. (2011). Biofilms in chronic suppurative otitis media and cholesteatoma: scanning electron microscopy findings. Am. J. Otolaryngol. 32, 32–37. 10.1016/j.amjoto.2009.09.010 [DOI] [PubMed] [Google Scholar]
- Seth A. K., Geringer M. R., Hong S. J., Leung K. P., Galiano R. D., Mustoe T. A. (2012). Comparative analysis of single-species and polybacterial wound biofilms using a quantitative, in vivo rabbit ear model. PLoS ONE 7:e42897. 10.1371/journal.pone.0042897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw A. D., Tiwari Y., Kaplan W., Heath A., Mitchell P. B., Schofield P. R., et al. (2014). Characterisation of genetic variation in ST8SIA2 and its interaction region in NCAM1 in patients with bipolar disorder. PLoS ONE 9:e92556. 10.1371/journal.pone.0092556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal D., Foreman A., Bardy J.-J., Wormald P.-J. (2011). Staphylococcus aureus biofilms. Laryngoscope 121, 1578–1583. 10.1002/lary.21805 [DOI] [PubMed] [Google Scholar]
- Sun J., Nishiyama T., Shimizu K., Kadota K. (2013). TCC: an R package for comparing tag count data with robust normalization strategies. BMC Bioinformatics 14:219. 10.1186/1471-2105-14-219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi R., Koyano S., Suzutani T., Goishi K., Ito Y., Morioka I., et al. (2015). A Thr72Ala polymorphism in the NKG2D gene is associated with early symptomatic congenital cytomegalovirus disease. Infection 43, 353–359. 10.1007/s15010-015-0774-x [DOI] [PubMed] [Google Scholar]
- Trapnell C., Williams B. A., Pertea G., Mortazavi A., Kwan G., van Baren M. J., et al. (2010). Transcript assembly and abundance estimation from RNA-Seq reveals thousands of new transcripts and switching among isoforms. Nat. Biotechnol. 28, 511–515. 10.1038/nbt.1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara-Irigaray M., Valle J., Merino N., Latasa C., García B., Ruiz-de L. M., et al. (2009). Relevant role of fibronectin-binding proteins in Staphylococcus aureus biofilm-associated foreign-body infections. Infect. Immun. 77, 3978–3991. 10.1128/IAI.00616-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voggu L., Schlag S., Biswas R., Rosenstein R., Rausch C., Götz F. (2006). Microevolution of cytochrome bd oxidase in staphylococci and its implication in resistance to respiratory toxins released by pseudomonas. J. Bacteriol. 188, 8079–8086. 10.1128/JB.00858-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitchurch C. B., Tolker-Nielsen T., Ragas P. C., Mattick J. S. (2002). Extracellular DNA required for bacterial biofilm formation. Science 295:1487. 10.1126/science.295.5559.1487 [DOI] [PubMed] [Google Scholar]
- Wozniak D. J., Wyckoff T. J., Starkey M., Keyser R., Azadi P., O'Toole G. A., et al. (2003). Alginate is not a significant component of the extracellular polysaccharide matrix of PA14 and PAO1. Pseudomonas aeruginosa biofilms. Proc. Natl. Acad. Sci. U.S.A. 100, 7907–7912. 10.1073/pnas.1231792100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav M. K., Chae S.-W., Im G. J., Chung J.-W., Song J.-J. (2015a). Eugenol: a phyto-compound effective against methicillin-resistant and methicillin-sensitive Staphylococcus aureus clinical strain biofilms. PLoS ONE 10:e0119564. 10.1371/journal.pone.0119564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav M. K., Chae S.-W., Song J.-J. (2012). In Vitro Streptococcus pneumoniae biofilm formation and in vivo middle ear mucosal biofilm in a rat model of acute otitis induced by S. pneumoniae. Clin. Exp. Otorhinolaryngol. 5, 139–144. 10.3342/ceo.2012.5.3.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav M. K., Go Y. Y., Chae S.-W., Song J.-J. (2015b). The small molecule dam inhibitor, pyrimidinedione, disrupts Streptococcus pneumoniae biofilm growth in vitro. PLoS ONE 10:e0139238. 10.1371/journal.pone.0139238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav M. K., Park S.-W., Chae S.-W., Song J.-J. (2014). Sinefungin, a natural nucleoside analogue of s-adenosylmethionine, inhibits Streptococcus pneumoniae biofilm growth. Biomed Res. Int. 2014:156987. 10.1155/2014/156987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Zhang Q., Li L., Wang Z., Ying J., Fan Y., et al. (2015). DLEC1, a 3p tumor suppressor, represses NF-κB signaling and is methylated in prostate cancer. J. Mol. Med. 93, 691–701. 10.1007/s00109-015-1255-5 [DOI] [PubMed] [Google Scholar]