Xenotransplantation has retained its topicality since the resurgence of interest in the mid 1990s. Work continues in many scientific fields to overcome the obstacles. However, as old problems are solved, new ones present themselves for solution. In this brief review we discuss the need for xenotransplantation, issues relating to physiological compatibilities, current ideas on how to overcome the initial aggressive hyperacute rejection (HAR) and the later immunological difficulties as well as the risks and ethical issues.
THE CRISIS IN SUPPLY OF ORGANS FOR TRANSPLANTATION
For many patients with end-stage cardiac, respiratory or hepatic failure the only chance of survival is a transplant. In renal failure, although long-term dialysis is an option, transplantation offers better survival1 and quality of life2 as well as economic advantages3.
The major difficulty facing the transplant community currently is the shortage of organs for transplantation, and strategies to increase the supply include the deployment of specialist donor liaison nurses, the use of so-called marginal donors (whose organs would never previously have been considered for transplantation) and the encouragement of live donation. Consideration has even been given to altering the basis of cadaveric donation, which at present proceeds only when the permission of relatives, actively sought, is granted. The ‘presumed consent’ approach (whereby objectors must actively register their wish to opt out) is now lawful in several countries and has increased the cadaveric donation rate.
However, even with the successful implementation of all these initiatives the number of donations would still be insufficient. In 1991 there were 4815 patients on the transplant waiting list, but in 2000 the number waiting for solid organ transplants was 6823 (renal 6154, cardiothoracic 494, liver 175). Over the same period the number of donors actually fell, from 934 to 8454 (Figure 1).
Figure 1.
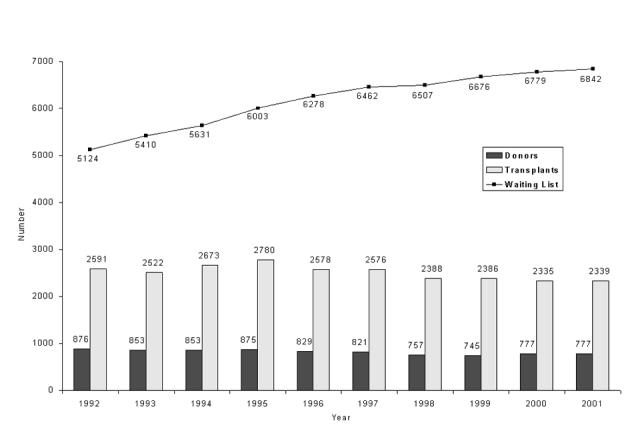
Numbers of cadaveric donors and transplants in the UK and Republic of Ireland, 1991-2000 and patients on the waiting list at 31 December. Statistics prepared by UK Transplant from the National Transplant Database maintained on behalf of transplant services in the UK and Republic of Ireland [www.uktransplant.nhs.uk] (Reproduced by permission)
Can we solve the problem by reducing the demand for transplantation? If we look only at the kidney, five groups of diseases accounted for about 70% of new starts on renal replacement therapy in 1992, according to the large registry of the European Dialysis and Transplantation Association—European Renal Association1. Diabetes mellitus and renovascular disease are actually becoming more prevalent, and, of the other three (glomerulonephritis, polycystic kidney disease, pyelonephritis), only the prevalence of the last is decreasing. Improvements in managing these patients are unlikely to reduce the need for renal transplantation, although screening programmes for diabetes may improve matters in the longer term. There is even less potential to decrease the demand for the transplantation of other organs, when one considers the minority of patients that are offered this option in cardiac and respiratory disease.
If we are unable, therefore, to solve the problem by increasing the level of donation or reducing demand we will have to look elsewhere. Increasing attention is being paid to other sources of organs for human transplantation. For heart failure implantable mechanical devices are being tried as are biomechanical support devices for other failed organs5. There is also much interest in cloning and stem cell differentiation research for tissue replacement6. However, these strategies are still much removed from the clinic. The greatest chance of providing an early solution is offered by xenotransplantation.
THE EARLY DAYS OF XENOTRANSPLANTATION
The xeno in xenotransplantation is derived from the Greek for foreign or strange. Donor-recipient combinations can be classified, as was initially done by Calne, into ‘discordant’ (where transplant between species results in a rapid, hyperacute rejection) or ‘concordant’ (where rejection occurs at a pace similar to that of allotransplantation). We know that this difference is essentially due to the presence or absence of preformed antibodies.
Blood transfusions from animals to man were performed in England and France from the early seventeenth century. These were the first clinical attempts at xenotransplantation. Solid organ transplants, attempted in the 20th century, had one or two successes, again mainly in concordant transplants. Reemtsma and colleagues reported patients surviving up to nine months after kidney transplants from a chimpanzee7. They also showed that acute cellular rejection could be reversed by high doses of steroids. By contrast, organ transplants from non-primates have had little success, graft survival being measured in hours or minutes. With cells the picture is somewhat more promising: pig hepatocytes have been incorporated into extracorporeal liver assist devices, and non-primate tissue has also been used with varying success in treatment of diabetes8 and parkinsonism9.
IS THE PIG THE ANSWER?
While from numerous perspectives, non-human primates may be the preferred source of organs, they have several disadvantages: there is little experience in breeding these animals in captivity, and the cost of doing so would be large; they reproduce slowly; many of the relevant species are endangered (posing a conservation issue); and little work has been done on the genetic modification of such species. Most importantly, we should not discount the worries concerning inter-species transmission of infectious diseases: the more closely related the donor and recipient species, the more likely this is to arise. Finally, their very similarity to man poses ethical and moral dilemmas.
The present trend in research is towards use of the pig as donor. Transgenic pigs have been available for some years, and recent ‘knock out’ pigs10 have been generated by nuclear transfer techniques. This means that we are now capable of removing or adding proteins to and from potential donor animals—a luxury clearly unavailable in allotransplantation. Added to that, the pig is bred for slaughter and its use should not generate the objections that arise with non-human primates. We already have extensive knowledge of husbandry conditions, and studies have shown the possibility of producing pigs with little or no infectious diseases11. Because of their phylogenetic distance from man, the likelihood of cross-species transmission of infections is less. We shall return to this last point later.
CAN XENOTRANSPLANTATION WORK?
Physiology
Two issues with pig organs are size and longevity. The heart or kidney of a young pig, when of suitable size for donation, may still have potential for rapid growth; whether this will happen we do not know. Also, the natural lifespan of a pig is only some fifteen years, and nothing is known about ageing in xenotransplanted organs. What of hormonal factors? That porcine insulin can achieve glucose homoeostasis in man has long been known, but not all porcine hormones are effective across the species barrier. For example, porcine renin does not cleave human angiotensin12 and porcine erythropoietin does not stimulate human erythropoiesis. The liver produces over two thousand proteins, and it is clear that many of these will have incompatible or absent function across the species barrier. This makes hepatic xenotransplantation less promising than that of other organs.
Recent work with organs from transgenic pigs has allowed long enough survival to study the in vivo physiological compatibility of porcine organs13. Porcine renal xenografts in a primate model were able to sustain plasma electrolyte homoeostasis for as long as the grafts survived14, though not all the human kidney's functions were reproduced.
Another issue is temperature. The body temperature of pigs is roughly 39°C, whereas human body temperature is about 37°C14. The functional implications of this for the activity of porcine enzymes at this lower temperature remain unclear.
Hyperacute rejection
Until recently research focused on the phenomenon of hyperacute rejection. This, characterized histologically by the rapid onset of oedema, haemorrhage and vascular thrombosis, is caused by the presence of preformed antibodies, and occurs within minutes of transplantation.
The gal epitope
Xenoreactive natural antibodies (XNA) are similar to those produced naturally against blood group antigens. The epitope, which is the principal target of these antibodies, is the non-reducing trisaccharide group, galactosyl α-(1,3)-galactosyl β-1,4-N-acetyl glucosaminyl, commonly referred to as the gal epitope15. Man does not possess this epitope, because of the absence of the enzyme that generates it. Higher primates therefore recognize the gal epitope as ‘non-self’ and generate an immune response to it. Human beings are exposed to the antigen through the gut (the gal epitope is present on various microbes16) and generate anti-gal antibodies. XNA produce their effects primarily through activating complement, via natural killer (NK) cells17 and by altering the phenotype of the endothelium. Research has so far focused on reducing the impact of XNA.
Prevention of the anti-gal response in recipients
One approach has been to deplete xenograft natural antibodies by means of affinity columns, extracorporeal perfusion of excised organs or plasmapheresis. Unfortunately, anti-gal returns to normal levels within a few days18. Attempts have been made to prevent the anti-gal response through the use of a α-gal toxin19 to eliminate the plasma cells capable of producing this antibody. Results have been encouraging in the mouse model but its efficacy in higher models is still to be evaluated.
Complement
The main pathway through which the xenograft natural antibodies cause hyperacute rejection is the activation of complement and the consequent activation of the endothelial cells. Endothelial cells subsequently secrete various cytokines and platelet activating factor, and change from generating an anticoagulant milieu to a procoagulant one, causing thrombosis, haemorrhage and, quite rapidly, infarction.
One approach suggested to circumvent this is complement depletion with cobra venom factor and this has been shown to increase graft survival in rat-to-primate and pig-to-primate models20. An alternative strategy involves C1-inhibitor (C1-INH), the only physiological inhibitor of the first step in complement activation. Overexpression of this inhibitor has been shown to prevent hyperacute xenograft rejection in vitro21 and in vivo22. Unfortunately, both techniques would deprive the body of the benefits of a functional complement system. Hence, the generation of donor organs that express complement regulators only locally has been pursued.
In view of the putative species incompatibility of porcine complement regulating proteins, pigs have been generated which express human complement regulators. In vitro work showed that expression of these molecules protects cells from complement-induced lysis23. Three human membrane-bound inhibitors of complement function have been expressed in pigs—CD55 (decay accelerating factor), CD46 (monocyte chemoattractant protein) and CD49. These have been shown to increase graft survival in pig-to-primate renal and cardiac transplants24. This work, using both transgenic organs and immunosuppression, led to graft survival of up to 78 days: 4 of the 9 animals survived for more than nine days with intact kidney grafts25.
The Hanover group combined the two approaches outlined above, with encouraging results. Using a pig-to-primate kidney model with hDAF transgenic donor organs and postoperative immunosuppression, they found that episodes of acute vascular rejection were treated either with boluses of cyclophosphamide and steroids or with the same regimen supplemented by a three-day course of C1-INH. In all animals, one or more episodes of acute vascular rejection were observed. When, in 4 animals, C1-INH was added to the standard antirejection treatment regimen, acute vascular rejection was successfully reversed in six out of seven episodes26.
Other approaches
The ultimate cause of xenograft destruction in hyperacute rejection is thrombosis. Thrombin inhibition has been shown to prolong graft survival. Research is currently directed towards the expression of anticoagulant molecules on the endothelial cell to produce a local anti-thrombotic effect. Two groups have shown in vitro that the expression of these molecules on the cell surface can change the phenotype towards an anticoagulant one27,28. Our group is trying to develop an in vivo model for this approach. The difficulty is possibly compounded by species incompatibility: porcine tissue factor pathway inhibitor and porcine thrombomodulin may be ineffective in preventing the human coagulation cascade24.
Glycosyltransferase transgenes
Miyagawa et al. have produced both mice and pigs transgenic for the human β-D-mannoside β-1,4-N-acetyl-glucosaminyltransferase. Overexpression of this gene reduced expression of the gal epitope with a consequent reduction of complement-mediated and NK-cell lysis by up to 40% in the transgenic group. Immunohistochemistry with normal human serum as a source of XNA confirmed a reduction in the level of antigenicity. This has also been demonstrated in vivo in a pig-to-cynomolgus-monkey cardiac model29. A similar approach has been used to produce pigs transgenic for α1,2 fucosyltransferase30. This approach decreases α-gal expression by about 70%. But will this be enough?
Knock-out pigs
The complete prevention of expression of the epitope will only be achieved by the production of knock-out animals. A homozygous mouse strain with disrupted α1,3 galactosyl transferase genes has been generated. The mice lack the ability to synthesize α-gal epitopes and are capable of producing low amounts of the natural anti-gal antibody, although repeated immunization with the gal epitope yields anti-gal titres and specificity comparable with those observed in man31. Knock-out pigs have recently been created10,32, and data from these animals are keenly awaited.
The work summarized above suggests that hyperacute rejection can be eliminated or controlled through various techniques. But what about immunological processes that occur days and weeks after the transplantation?
Acute humoral xenograft rejection
The next barrier to be surmounted is acute humoral xenograft rejection (AHXR), also known as delayed xenograft rejection. The main histopathological features of AHXR are endothelial swelling or disruption, vascular thrombosis with blood extravasation and interstitial oedema33. This normally arises within 24 hours of transplantation and progresses to destroy the graft over the next few days. The initial response is mediated by IgM, principally but not exclusively specific for the gal epitope, with a subsequent increase in IgG levels34. The presence of these xenograft natural antibodies alone leads to the production of a procoagulant state and eventually to disseminated intravascular coagulation35. These complications generally develop despite the best available measures for depletion of xenograft natural antibodies, inhibition of complement activation and suppression of T-cell and B-cell mediated immune responses. The mechanisms underlying the disseminated intravascular coagulation and thrombotic microangiopathy associated with delayed xenotransplant rejection remain unclear. AHXR is the least well understood of the early phases of xenograft rejection.
Preventing acute humoral xenograft rejection
Approaches to prevention of AHXR have included depletion of anti-gal antibodies through the use of an immunoaffinity column for extracorporeal immunoadsorption of plasma36. Robson et al. have shown that use of synthetic low-molecular-weight thrombin inhibitor can prolong survival, enhance function of the explanted organ, and improve histological features at the time of rejection37. Cooper's group in Boston have used soluble synthetic gal sugars or bovine serum albumin conjugated to multiple gal molecules to deplete the primate bloodstream of the antigal antibodies38. However, as yet no definitive therapeutic intervention for AHXR has emerged.
Cellular rejection
Thus far we have discussed the consequences for the xenograft of its interactions with preformed antibodies. Although products of the adaptive immune response, xenograft natural antibodies result from stimulation by cross-reactive antigens that happen to be present on the flora of the recipient. In addition, we have considered how the innate immune system, with its limited set of predefined specificities and responses, which do not change (adapt) in response to antigen exposure, has the potential to damage xenogeneic tissue. However, xenografts also interact with the adaptive immune system and stimulate their own specific immune response. In alloresponses, the immune system is able to recognize allogeneic MHC molecules directly by engaging T cell receptors with the MHC molecules (Figure 2). The direct xenoresponse is probably of comparable magnitude to the direct alloresponse. Thus it would require at least comparable levels of suppression39.
Figure 2.
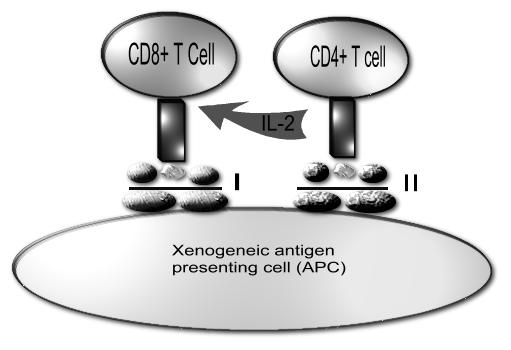
Direct xenorecognition. Xenogeneic APC presents peptide to the recipient CD4+ T cell via the xenogeneic MHC class II molecules. This results in the production of interleukin 2 (IL-2) by the CD4+ T cell. IL-2 acts on the CD8+ T cell which itself recognizes xenogeneic peptide presented via the MHC class I on the xenogeneic APC
However, it is known in allotransplantation that allogeneic MHC molecules, like any proteins, can be phagocytosed, broken down into peptides, presented on recipient type MHC molecules and generate an immune response. This is referred to as ‘indirect’ allorecognition (Figure 3). This also occurs in xenotransplantation. However, there are many more peptide differences between different species than between different members of the same species. Hence, the potential for indirect xenogeneic responses is much greater than for indirect allogeneic responses. This might need more immunosuppression than required in an alloresponse—perhaps more than is clinically acceptable. Given that such indirect responses appear to be increasingly important with time in allotransplantation, this may prove to be a major stumbling block in xenotransplantation.
Figure 3.
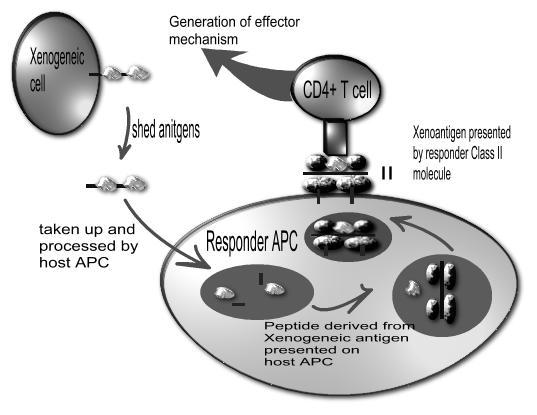
Indirect xenorecognition. Shed antigens from the xenogeneic cell are taken up by the responder antigen presenting cell (APC) to be presented to the CD4+ T cell via MHC class II molecule, which results in generation of the effector mechanism
One possible answer to this is to attempt to induce donor-specific non-responsiveness, or ‘immunological tolerance’. Much effort has been expended in trying to generate tolerance by haematopoietic chimerism. The term mixed chimerism refers to the coexistence of donor and recipient haematopoietic cells. The development of a protocol to generate a stable state of mixed chimerism without subjecting the recipient to a toxic myeloablative regimen has been the focus of much research. Initial protocols involved the non-specific elimination with antibodies of pre-existing mature donor-reactive T cells and NK cells. More recently, models have been developed in which it appears possible to inactivate and eliminate only donor specific T cells while leaving the remaining T cell repertoire essentially intact, by use of co-stimulatory blocking reagents to induce peripheral clonal deletion after bone marrow transplantation. After the peripheral immune system has been eliminated, donor stem cells are infused intravenously, and engraft in the bone marrow compartment of the recipient where they coexist with recipient stem cells and give rise to cells of all haematopoietic lineages. Within the thymus, T cells deemed to be potentially self-reactive are deleted. This process is at least in part mediated by cells seeded from haematopoietic progenitor cells originating from the bone marrow. In mixed chimeras, haematopoietic cells from both the recipient and the donor locate to the thymus and hence mediate the elimination of both host-reactive and donor-reactive T cells.
The induction of mixed haematopoietic chimerism has been shown to lead to stable tolerance in allogeneic and closely related xenogeneic combinations40. Early data suggest that this may also be possible in a highly disparate pig-to-mouse model41.
Cosimi et al. have induced tolerance to allotransplanted kidneys in monkeys by use of mixed haematopoietic chimerism42. The tolerance persists even after cessation of immunosuppressive therapy. This work is not only applicable to xenotransplantation but could also be of great benefit in allotransplantation where conventional immunosuppression leads to complications such as infection and malignancy. The small numbers of patients who have been given marrow and a kidney from the same donor have shown robust tolerance14.
Another approach to inducing T cell tolerance is by transplanting pig thymic tissue into the recipient primate. This approach has been successful in the pig-to-mouse model43.
The main concern regarding this approach is the risk of graft-versus-host disease—an attack by donor immune cells on the recipient's tissues. However, it remains one of the most exciting areas of research activity.
MICROBIOLOGICAL RISK
The risk of transmission of infectious agents across the species barrier is a major anxiety about this whole approach of xenotransplantation. Many such agents can be eliminated from the pig herd through scrupulous husbandry methods. Such methods include the sterilization of both feed and drinking water and the elimination of all mammalian protein from the feed to prevent prion infection. Unfortunately, this does not eliminate the risk of transmitting viruses whose DNA is integrated into the nucleus of transplanted cells, such as porcine endogenous retroviruses (PERVs).
There is a long history of using porcine valves and insulin in the treatment of disease without generating any infectious complications. However, this represents experience in a cell-free situation. In vitro studies suggest that PERV can, in fact, infect human cells44. However, in vivo studies of 160 patients who have been exposed to living porcine cells or tissue have shown no evidence of PERV transmission; thus such transfer must be at least a rare event45. In vivo organ transplants in mice can generate transmission of PERVs. But whole-organ transplants, in patients who are likely to be immunosuppressed, have yet to be assessed.
The risk must be assessed both on an individual basis (the risk of infection versus the benefit of a viable organ) and for the public in general—spread of a new pathogen throughout the population. We have already witnessed the disaster over the transmission of bovine spongiform encephalopathy in humans, and HIV is thought to have originated in monkeys.
It is impossible to prove a negative—that a novel pathogen could never be transferred from pigs to man as a result of xenotransplantation. And any clinical development of xenotransplantation must be accompanied by rigorous and lifelong microbiological monitoring of recipients.
CONCLUSIONS
Although headway has been made in overcoming the initial hurdle of hyperacute rejection through modulation of the local immune response, we now have to deal with the other aspects of the immune system. Current work is mainly directed towards the production of transgenic and knockout pigs. Alongside this is the exciting possibility of inducing tolerance through mixed haematopoietic chimerism.
Anxiety over the risk of infection may be diminished by data confirming the lack of transmission in well-controlled experiments, or by the identification of pig strains incapable of transmitting PERVs. However, there will always be concerns that experiments have failed to exclude transmission of pathogens with a very long lag time and the transmission of pathogens as yet unknown. Xenotransplantation does offer a way to meet the shortfall in organs available for transplantation, though the results may be inferior to those of allotransplantation: the greater immunological incompatibility, with need for stronger immunotherapy, could mean lower life expectancy and shorter graft survival. Against all these issues, xenotransplantation offers the potential to make available functional solid organs, on tap, to patients who at present have little or no chance of receiving a transplant.
References
- 1.Valderrabano F, Jones EH, Mallick NP. Report on management of renal failure in Europe, XXIV, 1993. Nephrology Dial Transplant 1995; 10(suppl 5): 1-25 [DOI] [PubMed] [Google Scholar]
- 2.Gokal R. Quality of life in patients undergoing renal replacement therapy. Kidney Int 1993;40: S23-7 [PubMed] [Google Scholar]
- 3.Aranzabal J, Perdigo L, Mijares J, Villar F. Renal transplantation costs: an economic analysis and comparison with dialysis costs. Transplant Proc 1991;23: 2574. [PubMed] [Google Scholar]
- 4.UK Transplant. United Kingdom Preliminary Data. Bristol: UK Transplant, 2002
- 5.Duncan BW. Mechanical circulatory support for infants and children with cardiac disease. Ann Thorac Surg 2002;73: 1670-7 [DOI] [PubMed] [Google Scholar]
- 6.Devine SM. Mesenchymal stem cells: will they have a role in the clinic? J Cell Biochem 2002;(suppl 38): 73-9 [DOI] [PubMed]
- 7.Reemtsma K. Renal heterotransplantation. Adv Surg 1966;2: 285-93 [PubMed] [Google Scholar]
- 8.Collins BH. Porcine islets as an alternative to human islets for transplantation. J Invest Med 2001;49: 576-9 [DOI] [PubMed] [Google Scholar]
- 9.Larsson LC, Widner H. Neural tissue xenografting. Scand J Immunol 2000;52: 249-56 [DOI] [PubMed] [Google Scholar]
- 10.Frankish H. Pig organ transplantation brought one step closer. Lancet 2002;359: 137. [DOI] [PubMed] [Google Scholar]
- 11.Weiss RA, Magre S, Takeuchi Y. Infection hazards of xenotransplantation. J Infect 2000;40: 21-5 [DOI] [PubMed] [Google Scholar]
- 12.Evans DB, Cornette JC, Sawyer TK, Staples DJ, de Vaux AE, Sharma SK. Substrate specificity and inhibitor structure-activity relationships of recombinant human renin: implications in the in vivo evaluation of renin inhibitors. Biotechnol Appl Biochem 1990;12: 161-75 [PubMed] [Google Scholar]
- 13.Soin B, Smith KG, Zaidi A, Cozzi E, Bradley JR, Ostlie DJ et al. Physiological aspects of pig-to-primate renal xenotransplantation. Kidney Int 2001;60: 1592-7 [DOI] [PubMed] [Google Scholar]
- 14.Cooper DK, Gollackner B, Sachs DH. Will the pig solve the transplantation backlog? Annu Rev Med 2002;53: 133-47 [DOI] [PubMed] [Google Scholar]
- 15.Sandrin MS, Vaughan HA, Dabkowski PL, McKenzie IF. Anti-pig IgM antibodies in human serum react predominantly with Gal(alpha 1-3)Gal epitopes. Proc Natl Acad Sci USA 1993;90: 11391-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galili U, Mandrell RE, Hamadeh RM, Shohet SB, Griffiss JM. Interaction between human natural anti-alpha-galactosyl immunoglobulin G and bacteria of the human flora. Infect Immun 1988;56: 1730-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Artrip JH, Kwiatkowski P, Michler RE, et al. Target cell susceptibility to lysis by human natural killer cells is augmented by alpha(1,3)-galactosyltransferase and reduced by alpha(1,2)-fucosyltransferase. [erratum appears in J Biol Chem 1999;274:15292.] J Biol Chem 1999;274: 10717-22 [DOI] [PubMed] [Google Scholar]
- 18.Azimzadeh A, Meyer C, Watier H, et al. Removal of primate xenoreactive natural antibodies by extracorporeal perfusion of pig kidneys and livers. Transpl Immunol 1998;6: 13-22 [DOI] [PubMed] [Google Scholar]
- 19.Tanemura M, Yin D, DiSesa VJ, Galili U. Preventing anti-Gal response in xenograft recipients by an alpha-Gal toxin. Transplant Proc 2001;33: 699-700 [DOI] [PubMed] [Google Scholar]
- 20.Leventhal JR, Dalmasso AP, Cromwell JW, et al. Prolongation of cardiac xenograft survival by depletion of complement. Transplantation 1993;55: 857-65 [DOI] [PubMed] [Google Scholar]
- 21.Dalmasso AP, Platt JL. Prevention of complement-mediated activation of xenogeneic endothelial cells in an in vitro model of xenograft hyperacute rejection by C1 inhibitor. Transplantation 1993;56: 1171-6 [DOI] [PubMed] [Google Scholar]
- 22.Hecker JM, Lorenz R, Appiah R, et al. C1-inhibitor for prophylaxis of xenograft rejection after pig to cynomolgus monkey kidney transplantation. Transplantation 2002;73: 688-94 [DOI] [PubMed] [Google Scholar]
- 23.Huang J, Gou D, Zhen C, et al. Protection of xenogeneic cells from human complement-mediated lysis by the expression of human DAF, CD59 and MCP. FEMS Immunol Med Microbiol 2001;31: 203-9 [DOI] [PubMed] [Google Scholar]
- 24.Bach FH, Robson SC, Winkler H, et al. Barriers to xenotransplantation. Nature Med 1995;1: 869-73 [DOI] [PubMed] [Google Scholar]
- 25.Cozzi E, Bhatti F, Schmoeckel M, et al. Long-term survival of nonhuman primates receiving life-supporting transgenic porcine kidney xenografts. Transplantation 2000;70: 15-21 [PubMed] [Google Scholar]
- 26.Vangerow B, Hecker JM, Lorenz R, et al. C1-inhibitor for treatment of acute vascular xenograft rejection in cynomolgus recipients of h-DAF transgenic porcine kidneys. Xenotransplantation 2001;8: 266-72 [DOI] [PubMed] [Google Scholar]
- 27.Bach FH, Robson SC, Wrighton CJ, Stuhlmeier K, Ferran C, Winkler H. Genetic engineering of endothelial cells to ameliorate xenograft rejection. Clin Transplant 1996;10(1 pt 2): 124-7 [PubMed] [Google Scholar]
- 28.Riesbeck K, Chen D, Kemball-Cook G, et al. Expression of hirudin fusion proteins in mammalian cells: a strategy for prevention of intravascular thrombosis. Circulation 1998;98: 2744-52 [DOI] [PubMed] [Google Scholar]
- 29.Miyagawa S, Murakami H, Takahagi Y, et al. Remodeling of the major pig xenoantigen by N-acetylglucosaminyltransferase III in transgenic pig. J Biol Chem 2001;276: 39310-19 [DOI] [PubMed] [Google Scholar]
- 30.Sharma A, Okabe J, Birch P, et al. Reduction in the level of Gal(alpha1,3)Gal in transgenic mice and pigs by the expression of an alpha(1,2)fucosyltransferase. Proc Natl Acad Sci USA 1996;93: 7190-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LaTemple DC, Galili U. Adult and neonatal anti-Gal response in knock-out mice for alpha1,3galactosyltransferase. Xenotransplantation 1998;5: 191-6 [DOI] [PubMed] [Google Scholar]
- 32.Lai L, Kolber-Simonds D, Park KW, et al. Production of alpha-1, 3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science 2002;295: 1089-92 [DOI] [PubMed] [Google Scholar]
- 33.Shimizu A, Meehan SM, Kozlowski T, et al. Acute humoral xenograft rejection: destruction of the microvascular capillary endothelium in pig-to-nonhuman primate renal grafts. Lab Invest 2000;80: 815-30 [DOI] [PubMed] [Google Scholar]
- 34.Holmes BJ, Richards A, McLaughlin M, et al. Antibody responses in early graft rejection in pig-to-primate renal xenotransplantation. Transplant Proc 2001;33: 717-18 [DOI] [PubMed] [Google Scholar]
- 35.Robson SC, Cooper DK, d'Apice AJ. Disordered regulation of coagulation and platelet activation in xenotransplantation. Xenotransplantation 2000;7: 166-76 [DOI] [PubMed] [Google Scholar]
- 36.Taniguchi S, Neethling FA, Korchagina EY, et al. In vivo immunoadsorption of antipig antibodies in baboons using a specific Gal(alpha)1-3Gal column. Transplantation 1996;62: 1379-84 [DOI] [PubMed] [Google Scholar]
- 37.Robson SC, Young VK, Cook NS, et al. Thrombin inhibition in an ex vivo model of porcine heart xenograft hyperacute rejection. Transplantation 1996;61: 862-8 [DOI] [PubMed] [Google Scholar]
- 38.Teranishi K, Gollackner B, Buhler I, et al. Depletion of anti-gal antibodies in baboons by intravenous therapy with bovine serum albumin conjugated to gal oligosaccharides. Transplantation 2002;73: 129-39 [DOI] [PubMed] [Google Scholar]
- 39.Squinto SP. Genetically modified animal organs for human transplantation. World J Surg 1997;21: 939-42 [DOI] [PubMed] [Google Scholar]
- 40.Ildstad ST, Sachs DH. Reconstitution with syngeneic plus allogeneic or xenogeneic bone marrow leads to specific acceptance of allografts or xenografts. Nature 1984;307: 168-70 [DOI] [PubMed] [Google Scholar]
- 41.Abe M, Qi J, Sykes M, Yang YG. Mixed chimerism induces donor-specific T-cell tolerance across a highly disparate xenogeneic barrier. Blood 2002;99: 3823-9 [DOI] [PubMed] [Google Scholar]
- 42.Sablinski T, Gianello PR, Bailin M, et al. Pig to monkey bone marrow and kidney xenotransplantation. Surgery 1997;121: 381-91 [DOI] [PubMed] [Google Scholar]
- 43.Yamada K, Shimizu A, Utsugi R, et al. Thymic transplantation in miniature swine. II. Induction of tolerance by transplantation of composite thymokidneys to thymectomized recipients. J Immunol 2000;164: 3079-86 [DOI] [PubMed] [Google Scholar]
- 44.Specke V, Rubant S, Denner J. Productive infection of human primary cells and cell lines with porcine endogenous retroviruses. Virology 2001;285: 177-80 [DOI] [PubMed] [Google Scholar]
- 45.Paradis K, Langford G, Long Z, et al. Search for cross-species transmission of porcine endogenous retrovirus in patients treated with living pig tissue. The XEN 111 Study Group. Science 1999;285: 1236-41 [DOI] [PubMed] [Google Scholar]


