Abstract
Mammalian studies have shaped our understanding of the endocrine control of appetite and body weight in vertebrates and provided the basic vertebrate model that involves central (brain) and peripheral signaling pathways as well as environmental cues. The hypothalamus has a crucial function in the control of food intake, but other parts of the brain are also involved. The description of a range of key neuropeptides and hormones as well as more details of their specific roles in appetite control continues to be in progress. Endocrine signals are based on hormones that can be divided into two groups: those that induce (orexigenic), and those that inhibit (anorexigenic) appetite and food consumption. Peripheral signals originate in the gastrointestinal tract, liver, adipose tissue, and other tissues and reach the hypothalamus through both endocrine and neuroendocrine actions. While many mammalian-like endocrine appetite-controlling networks and mechanisms have been described for some key model teleosts, mainly zebrafish and goldfish, very little knowledge exists on these systems in fishes as a group. Fishes represent over 30,000 species, and there is a large variability in their ecological niches and habitats as well as life history adaptations, transitions between life stages and feeding behaviors. In the context of food intake and appetite control, common adaptations to extended periods of starvation or periods of abundant food availability are of particular interest. This review summarizes the recent findings on endocrine appetite-controlling systems in fish, highlights their impact on growth and survival, and discusses the perspectives in this research field to shed light on the intriguing adaptations that exist in fish and their underlying mechanisms.
Keywords: appetite control, feed intake, hormones, neuropeptides, teleosts, adaptations, fasting, voracious feeding
Introduction
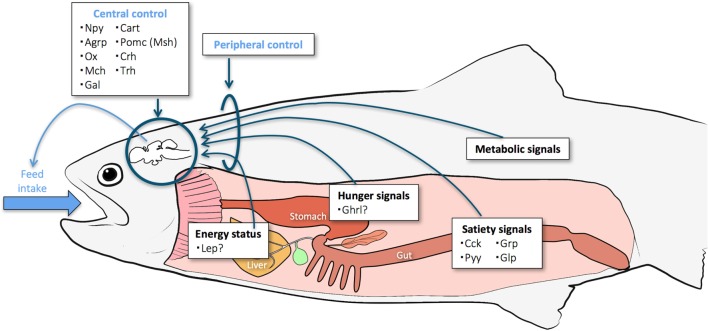
Control of food intake and energy metabolism is vital for the development and survival of an organism. These processes ensure optimal allocation of energy resources to cover the basic maintenance of metabolism and immune system, the cost of foraging and other daily activities, somatic growth, reproductive investment, and sufficient energy stores to survive periods of low food availability (1). Food intake is affected by external factors, such as temperature and photoperiod, stress, predators, and food availability, as well as by internal factors, such as genetics, life stage, gut filling, and stored energy. The hypothalamus is the hub that controls appetite and energy balance and integrates peripheral signals related to food intake and digestion, metabolism, and energy storage (Figure 1). These include not only endocrine signals (gut peptides, the focus of this review) but also other signals such as nutrient levels through central nutrient sensing systems and the presence/absence of food in the gastrointestinal (GI) tract through vagal afferents projecting to the brain.
Figure 1.
Key organs and signaling pathways believed to be involved in control of appetite in fish. Some of the central and peripheral endocrine factors explored so far are listed.
Fishes represent over 30,000 species with an enormous variation in their ecological niches and habitats as well as life history adaptations, transitions between life stages and feeding behaviors. In the context of food intake and appetite control, common adaptations to extended periods of starvation or periods of abundant food availability are of particular interest. Also, the large variations in appetite between species and within a species (individual variation) are intriguing. A large fraction of fish species has indeterminate growth, i.e., these species continue to grow during their whole life span. This contrasts with growth in mammals and other model animals including zebrafish (Danio rerio), which reach a maximum length size as adults. Thus, while control of appetite and food intake is often viewed as a behavioral component of maintaining an energy balance (2), the general concept of energy homeostasis needs to be used with caution.
This review summarizes the recent findings on appetite-controlling systems in fish with a focus on peptide hormones. A major goal is to discuss perspectives in this research field that can reveal how fish adapt to their specific ecological requirements.
Central Control
The physiological mechanisms that control appetite are relatively well conserved among vertebrates, and many of the neuropeptides and hormones involved in the central appetite regulation in mammals are also found in fish (3–7). However, differences in appetite-controlling systems can be found as a response to the large diversity in feeding habits of teleost species (8), yet the mechanisms for many of these adaptations remain unexplored.
Central signals arising in the hypothalamus are crucial for the control of food intake, and this brain area produces both orexigenic (appetite-stimulating) and anorexigenic (appetite-inhibiting) factors. The main hormones and neuropeptides so far described in teleosts and their possible involvement in the central control of appetite are presented in Figure 1 and described below.
NPY
Neuropeptide Y (NPY) is one of the strongest orexigenic signals in mammals, and the NPY/agouti-related peptide (AgRP) neurons in the arcuate nucleus (ARC) are the principal inducer of feeding (9, 10). The relative importance of NPY in feeding regulation seems to vary among teleosts. In goldfish (Carassius auratus) (11, 12), carp (Cyprinus carpio) (13), channel catfish (Ictalurus punctatus) (14), zebrafish (15), rainbow trout (Oncorhynchus mykiss) (16), and Nile (17) and red (18) tilapias (Oreochromis sp.), NPY injections increase feeding. Food deprivation increases brain npy expression in several species, including goldfish (19), chinook and Coho salmon (Oncorhynchus tshawytscha; Oncorhynchus kisutch) (20), zebrafish (15, 21), winter skate (Leucoraja ocellata) (22), tiger puffer (Takifugu rubripes) (23), and winter (24) and Brazilian (25) flounder (Pseudopleuronectes americanus; Paralichthys brasiliensis), suggesting an orexigenic role. In some species, such as Atlantic cod (Gadus morhua) (26), tiger puffer (23), snakeskin gourami (Trichogaster pectoralis) (27), Brazilian flounder (25), channel catfish (28), and cobia (Rachycentron canadum) (29), npy brain expression levels are high around feeding time and decrease post-feeding, further suggesting a role of Npy as a short-term appetite stimulator in fish. Npy treatments have also been shown to stimulate fish growth/growth hormone (GH) secretion both in vitro [goldfish (30)] and in vivo [tilapia (Oreochromis mossambicus) (17, 31); orange-spotted grouper (Epinephelus coioides) (32)].
However, in Atlantic cod, fasting does not affect npy brain expression (26), in cunner (Tautogolabrus adspersus), short-term fasting decreases npy brain expression (33), and in both Atlantic salmon (Salmo salar) (34) and larval Atlantic halibut (Hippoglossus hippoglossus) (35), npy expression increases after feeding, suggesting that Npy might have a minor role as a feeding stimulator in these species. GH transgenesis, which results in increased feeding rates, does not affect brain npy levels in Coho salmon (36) and carp (37) but decreases npy levels in zebrafish (38).
In goldfish (39), Senegalese sole (Solea senegalensis) larvae (40), rainbow trout (41), and both Atlantic cod larvae (42) and adults (43), npy brain expression is modulated by diet, which is consistent with the role of NPY containing neurons in sensing the metabolic status (e.g., glucose levels) as reported for mammals (10) and fish [e.g., tilapia (44)]. However, in cobia (29), npy expression does not appear to correlate with diet-induced changes in food intake.
CART
The peptide cocaine-amphetamine-related transcript (CART) was originally isolated from rat brain as a transcript regulated by acute administration of cocaine or amphetamine (45, 46). In goldfish, cart brain expression also increases following treatment with amphetamine (47). CART is a potent anorexigenic peptide in mammals (48–50) and birds (51), and CART injections inhibit food intake in goldfish (52).
Several cart genes have been identified in some fish species [e.g., two in goldfish (53), four in zebrafish (54), six in medaka (Oryzias latipes) (55), and seven in Senegalese sole (56)] whereas only one cart has been reported for others [e.g., Atlantic salmon (57), Atlantic cod (26), Atlantic halibut (35), and channel catfish (28)]. Post-feeding increases in cart brain expression have been reported for several fish species such as catfish (28), Atlantic salmon (34) and goldfish (53) suggesting that Cart acts as a short-term satiety factor in fish. Fasting has been shown to decrease cart brain expression in several fish species, and these changes are sometimes gene-specific. In goldfish, although the expression of both cart genes decreases after fasting, cart1 appears more affected than cart2 (53). In both zebrafish (58) and medaka (55), only one cart is affected by fasting, and in Senegalese sole, three out of seven cart genes are affected (56). However, fasting does not affect cart expression in other species such as winter flounder (24) or Atlantic halibut larvae (35), perhaps since only one gene has been identified in these species to date. Cart is also involved in sensing metabolic status, as hypothalamic cart mRNA levels change in response to changes in the levels of glucose in rainbow trout (41) or fatty acids in rainbow trout (59) and Senegalese sole (60).
Orexin
Orexins (OXs) A and B (or hypocretin 1 and 2) are neuropeptide products of a single gene precursor, prepro-orexin (pOX), through proteolytic cleavage. Two orexin receptors have been identified, OXR1 and OXR2. OX-A binds to both receptors with similar potencies whereas OX-B binds preferentially to OXR2 receptors (61). In mammals, orexins have been implicated in the regulation of many physiological functions, including feeding, sleep–wake cycles, reproduction, and cardiovascular function (62–65). Orexins and/or orexin receptors have been identified in several fish species, including goldfish (66), cavefish (Astyanax fasciatus mexicanus) (67), zebrafish (68), barfin flounder (Verasper moseri) (69), orange-spotted grouper (70), Atlantic cod (71), winter flounder (72), and dourado (Salminus brasiliensis) (73). Orexins have been shown to increase feeding and locomotor behavior in both mammals (74) and fish (75–81). Consistent with its role as an orexigenic peptide, ox brain mRNA expression increases following fasting [e.g., cavefish (67), goldfish (77, 82), zebrafish (68), winter flounder (72), Atlantic cod (71), and dourado (73)] and around feeding time [e.g., cavefish (67), orange-spotted grouper (70), and goldfish (83)].
Galanin
Galanin (GAL) is a 29–30 amino acid peptide first identified in mammals (84) and shown to have actions in brain and peripheral tissues to increase appetite and regulate metabolism (85, 86). Gal has been isolated in several fish species [reviewed in Ref. (87)] and appears to act as an orexigenic peptide. Injections of GAL stimulate food intake in goldfish (88) and tench (Tinca tinca) (89). Although long-term food deprivation does not affect brain gal mRNA expression in goldfish, the expression levels of gal decrease after the scheduled feeding time in fed fish, suggesting that Gal is a short-term regulator of appetite (90). Related to its role in metabolism, high gal mRNA expression has been linked to increased locomotion in zebrafish (91).
MCH
Melanin-concentrating hormone (Mch) was first isolated from the salmon pituitary as a skin-paling factor (92, 93) and later isolated and identified as an orexigenic factor in mammals (94). In fish, the role of Mch in food intake regulation is still unclear. In goldfish, central injections of MCH inhibit appetite, and fasting induce a decrease in brain Mch-immunoreactive (ir) cells (95–97), suggesting an anorexigenic role. However, in other teleost species, such as winter flounder (98), barfin flounder (99), zebrafish (100), and Atlantic cod (101), fasting-induced increases mch mRNA levels and -ir cells, pointing to an orexigenic role.
CRH
The corticotropin-releasing hormone (CRH) family includes CRH [or corticotropin-releasing factor (CRF)], urocortin (Ucn), urocortin 2, and urocortin 3. Members of the CRF family of neuropeptides have been shown to decrease feed intake in mammals (102). In goldfish, Crf and urotensin I (UI, the homolog of UCN in mammals) stimulate the hypothalamic–pituitary–interrenal axis (the fish homolog to the hypothalamic–pituitary–adrenal axis) to induce secretion of glucocorticoids (e.g., cortisol) and act as anorexigenic factors (6, 103). Central injections of CRF (104–106) or UI inhibit food intake in goldfish. Similar effects have been shown in rainbow trout (106). In Ya fish (Schizothorax prenanti), fasting decreases crf brain expression levels (107), consistent with the anorexigenic role of Crf-related peptides in fish.
Melanocortin System
The vertebrate melanocortin system is phylogenetically well conserved, and it has been identified in fish, amphibians, and mammals (108–110). It consists of (1) melanocortin peptides, which includes melanocyte-stimulating hormones (α-, β-, and γ-MSH) and adrenocorticotropic hormone, all derived from the gene pro-opiomelancortin (Pomc), (2) five G protein-coupled melanocortin receptors (MCRs), and (3) endogenous melanocortin antagonists, agouti and AgRP (111). In vertebrates, components of the melanocortin system are involved in a diverse range of physiological functions, including regulation of food intake, appetite, and anticipatory behavior (112).
The melanocortins are posttranslational products of the POMC prohormone, which also gives rise to the opiate peptide β-endorphin. Posttranslational processing of the POMC prohormone is tissue-specific, which results in the production of different POMC peptides by different cell types and, therefore, multiple physiological functions. Pomc is a single copy gene in mammals and birds, but in most teleosts, there are two to three different pomc transcripts [e.g., zebrafish (113), carp (114), barfin flounder (115), gilthead sea bream (Sparus aurata) (116), and sockeye salmon (Oncorhynchus nerka) (117)], proposed to result from the whole or partial genome duplication (118). In salmonids, Atlantic salmon and rainbow trout, three copies of pomc gene and one splice variant have been described, i.e., pomc (-a1, -a2, -a2s, and -b) (119, 120). However, the functions of the fish pomc subtypes remain largely unexplored. In rainbow trout, fasting induces increased expression levels of both hypothalamic pomca1 and pomcb (121), whereas in olive flounder (Paralichthys olivaceus), pomc2 but not pomc1 and pomc3 mRNA levels increase with fasting (122), suggesting a form-specific response of pomc in some species.
The repertoire of MCRs (MC1R to MC5R) found at the target cells has undergone significant diversification and specialization. Therefore, MCRs differ in their affinity for the different melanocortins, agouti, and AgRP. Of importance to energy homeostasis are MC3R and MC4R that are expressed throughout the central nervous system (CNS). Fish Mcr and ligands are expressed in a highly conserved pattern relative to mammals (123, 124). This conservation is also seen in the melanocortin neural circuits involved in hypothalamic control of energy homeostasis, underlining that the melanocortin functions originated early in evolution (125). The presence of Mc4r in teleosts has been reported in several species [e.g., goldfish (126), zebrafish (127), spotted scat (Scatophagus argus) (128), snakeskin gourami (129), fugu (109), common carp (130), and Ya fish (131)]. In Atlantic salmon, several paralogs of Mcr have been described, mc1r (-p1 and -p2), mc2r, mc4r (-a-p1, -a-p2, -b-p1, and b-p2), mc5r, mrap2 (-p1 and -p2) (Lars Ebbesson, Uni Environment, Bergen, Norway, personal communication). Mc3r seems to have been lost early in teleost evolution and is not present in salmonids, as observed for pufferfishes, tiger puffer and tetraodon (Tetraodon nigroviridis) (132). The only known mc3r in teleosts is the zebrafish mc3r; however, mc3r has also been identified in the spiny dogfish (Squalus acanthias) (133). In snakeskin gourami, the mc4r mRNA expression varies during daily feeding and fasting period, and its correlation with npy expression indicates a role in feed intake control (27, 129). However, in barfin flounder and sea bass (Dicentrarchus labrax), progressive fasting did not modify the hypothalamic mc4r mRNA expression (134, 135). Intracerebroventricular injections of MCR agonist decrease food intake in juvenile rainbow trout (136) and in goldfish (126, 137) in a dose-dependent manner, whereas the injection of MCR antagonists increases food intake in rainbow trout and in goldfish (137). The importance of Mc4r in the regulation of fish growth is also emphasized by naturally occurring mutations of the Mc4r in swordtails (Xiphophorus nigrensis and Xiphophorus multilineatus), which dramatically affects growth (138, 139).
An interesting fact is the existence of two endogenous antagonists in the melanocortin system, agouti and AgRP. These proteins are paracrine-signaling molecules and act as subtype-selective endogenous antagonists. AgRP exerts its major physiological function in the hypothalamus, where it acts as a potent orexigenic factor (140) due to its ability to antagonize the MC3R and MC4R (141). agrp genes have been identified in several fish species (57, 124, 126, 130, 131, 142–144). Hypothalamic agrp expression in goldfish (137), sea bass (agrp1, not agrp2) (144), and zebrafish (124) dramatically increased during fasting. In addition, GH-transgenic common carp has higher feed intake and higher hypothalamic agrp1 mRNA expression levels than non-transgenic fish (37). agrp mRNA abundance in the hypothalamus of rainbow trout (59) and Senegalese sole (60) also responds to changes in the levels of specific fatty acids. Altogether, it is suggested that the role of AgRP in energy homeostasis and its relation to the melanocortin system is conserved across vertebrates (51, 145).
Anatomical Locations of Central Appetite Control Systems
Control of appetite is an evolutionarily conserved process resulting from a close interplay between multiple neuronal and peripheral signals, which are integrated in the hypothalamus and processed in a specific spatial and temporal order to regulate hunger and satiety (4, 146). The mammalian hypothalamus consists of numerous interconnecting nuclei organized into complex neuronal networks where ARC nucleus, ventromedial nucleus (VMN), dorsomedial nucleus (DMN), paraventricular nucleus (PVN), and lateral hypothalamus (LH) play crucial roles in food intake control and energy expenditure [reviewed in Ref. (146)]. The ARC contains two distinct neuronal populations referred to as “first order” neurons, releasing appetite stimulators NPY/AgRP and appetite suppressors POMC/CART (1, 147). Neuronal projections from the first order neurons connect to other hypothalamic nuclei (PVN, DMN, VMN, and LH) (148). These “second order” nuclei express potent orexigenic factors such as orexins and MCH in the LH, and anorexigenic neuropeptides such as CRH and thyrotropin-releasing hormone (TRH) in the PVN. Lesioning studies in these nuclei have long recognized their functional significance in generating satiety and hunger responses [reviewed in Ref. (149)].
The existence of a functional (and to lesser extent anatomical) equivalence of appetite-controlling brain regions in fish has been demonstrated, based on electrical stimulation and brain lesion studies [reviewed in Ref. (4)]. The teleostean hypothalamic neurons are organized in a similar fashion as their mammalian counterparts and are distributed in conserved clusters within the ventral diencephalon (150–153). Yet, very little is known about the fish anatomical homologs to mammalian hypothalamic VMN, DMN, PVN, and LH nuclei, owing to the lack of specific neuronal molecular markers for distinct neuronal classes. In addition, expression domains of fish appetite control genes do not appear anatomically confined to their putative hypothalamic homologous areas.
The lateral tuberal nucleus (NLT; also known as ventral periventricular hypothalamus Hv) might be a feeding center and the teleostean homolog of the mammalian ARC [reviewed in Ref. (153)]. pomc, agrp, and leptin receptor transcripts are found in neurons within the NLT of goldfish (126, 137) and zebrafish (154), and ir and/or gene expression studies have identified Npy in the NLT of several teleosts (155, 156), as well as sturgeon [Acipenser transmontanus (157) and elasmobranch fish (158)]. npy and cart transcripts are also present in the NLT of juvenile Atlantic cod (159). In addition, Msh-α and Agrp-ir-cells are found in discrete populations in the NLT of zebrafish (125).
A recent study shows high homology between the zebrafish neurosecretory preoptic area (POA) and the mammalian PVN (153, 160). This homology is consistent with the presence of fish trh and crh ortholog genes in the POA, although their expression is not exclusive to the POA (161–164). The mammalian PVN is an important site of NPY synthesis and release (146, 165), and recent evidence indicates that Npy-ir cells and npy mRNAs are also present in the POA of fish (159, 166), further supporting functional homology between PVN and POA structures.
Functional and to some extent anatomical homologies could also exist between the mammalian and fish LH. In mammals, LH is an important site of orexins and MCH expression and believed to act as a “feeding center” (146). The LH is the site of transit for neuronal fibers interconnecting hypothalamic nuclei and forebrain to midbrain structures. A similar neuronal pattern has been observed in the LH of zebrafish, where pOx-expressing neurons send projections to the midbrain and the spinal cord (167, 168). In addition to the LH, the POA and the rostral NLT are also important sites of pOx expression in fish, as recently observed by double-fluorescence in situ hybridization in Atlantic cod larvae, in which the caudal domain of pOx-expressing neurons in the POA overlaps with the rostral-most cart cell population in the NLT (159). pOx mRNA expression in the POA has also been reported in zebrafish (169).
Furthermore, the strong expression of cart mRNAs and the absence of orexigenic modulators such as npy or pOx in the diffuse nucleus of the inferior hypothalamic lobe of Atlantic cod has recently led to the hypothesis that this nucleus may be the VMN homolog and that may serve as “satiety center” in fish (159) as in mammals (149, 167).
mRNAs of several appetite signals have been detected in the brain of different fish in extra-hypothalamic areas analogous to those characterized in mammals, suggesting a functional relationship between them (26, 41, 58, 159, 170, 171). It is, however, important to underline that canonical appetite genes (e.g., Npy and Cart) in mammals are modulated by many factors and their wide brain distribution may reflect various physiological roles and responses to changing environmental conditions (45, 172). All these mechanisms are still largely unknown in fish.
Peripheral Signals
The GI-Tract
The GI-tract is the largest endocrine organ in vertebrates and produces around 30 different neuropeptides and hormones. These peptides act on several tissues, including the GI-tract itself, exocrine glands, and the CNS (173, 174). Most of the GI peptides are sensitive to the gut nutrient content, and some of them are important in the control of appetite and meal size (174, 175). GI peptides may act on the CNS via an endocrine action by traveling in the blood, which requires that they pass the blood–brain barrier, and/or by stimulating afferent vagal nerve fibers (174, 176, 177). Studies on rainbow trout show that appetite returns when 80–90% of the stomach content has been emptied (178), indicating that gut filling, feed digestion, and transit rates may affect appetite control with both hunger and satiety signals. Indeed, most of the gut-derived appetite-regulating factors are also involved in digestion, thus coordinating these two processes (179).
GHRL
Ghrelin (GHRL) is mainly produced in the stomach of fish and mammals, or in the intestine of some stomachless species (180). Ghrl has been shown to have an orexigenic function in several fish species, including goldfish (177, 181), tilapia (182), brown trout (Salmo trutta) (183), and grass carp (Ctenopharyngodon idellus) (184), which is consistent with its role in mammals (185, 186). However, in rainbow trout, opposite effects of Ghrl on feed intake have been reported from two independent studies: one showed that central injection of Ghrl increased feed intake after 24 h (187) whereas the other study showed that short-term (1 h) central and long-term (weeks) peripheral administration of Ghrl suppressed appetite (174). The different time scales may, at least partly, explain the contradictory results. Recently, an anorexigenic response was also reported in channel catfish after Ghrl administration (188). In goldfish, appetite-regulating neuropeptides in the CNS, such as Npy and Ox, seem to mediate Ghrl-induced feeding (181, 189), but interactions between Ghrl and central appetite regulators are inconsistent in other examined fish species. For example, Ghrl increased (in tilapia and rainbow trout) (182, 187), decreased (in rainbow trout) (190), or did not affect (in brown trout and channel catfish) (183, 188) hypothalamic npy expression. Moreover, Ghrl decreased (in rainbow trout) (187) or had no effect (in channel catfish) (188) on pomc expression. A CRH receptor antagonist (α-helical CRF 9–41) abolished Ghrl-induced feeding (191) whereas Ghrl administration did not affect central crh expression in rainbow trout (187). In goldfish, it appears that peripheral Ghrl may stimulate feeding by acting on gastric vagal afferents that transmit information to brain appetite centers (177). Indirect effects on food intake, through stimulatory actions on digestion, could subsequently affect onset of feeding. For instance, rat GHRL evoked intestinal contraction in zebrafish (192, 193), but homologous Ghrl did not affect GI-tract contractility in goldfish and rainbow trout (194). The presence of GH secretagogue receptor in the fish pituitary and brain (particularly hypothalamus and telencephalon) also suggests a direct action of octanoylated Ghrl in these tissues (195, 196).
CCK
Cholecystokinin (CCK) is secreted by the proximal intestine and mainly acts as a short-term satiety factor at the same time as it promotes digestion through its many actions on the digestive system of vertebrates (174, 197). CCK is characterized by an evolutionary conserved biologically active C-terminal octapeptide (CCK-8) among vertebrates (198, 199), and Cck-ir cells have been observed in the intestine of most fish groups (174). Central or peripheral administration of sulfated CCK-8 suppresses food intake in goldfish (200) and channel catfish (14). Oral CCK administration inhibits feed intake in sea bass (201), while oral treatment with CCK antagonists increases food intake in rainbow trout (202). A single cck gene has been cloned in several teleost species, including yellowtail (Seriola quinqueradiata) (203), Atlantic herring (Clupea harengus) (204), and pirapitinga (Piaractus brachypomus) (205). However, two different cck sequences were identified in Japanese flounder (Paralichthys olivaceus), tetraodon (206), Atlantic salmon (207), and white sea bream (Diplodus sargus) (208), and three distinct cck genes exists in rainbow trout (209). All the identified cck genes in teleosts are predominantly expressed in the GI-tract and brain, including hypothalamus, telencephalon, and optic tectum.
Both circulating levels of Cck and cck gene expression are influenced by macronutrients, although these effects appear to be species-specific. For example, rainbow trout fed a high fat diet had higher plasma Cck levels compared with fish fed a high protein diet (210) and oral administration of single bolus of fat (oleic acid) or protein (casein), but not carbohydrate (starch), increased cck expression in yellowtail gut (211). In addition, cck expression levels increased following a meal in yellowtail pyloric caeca (212) and circulating Cck levels increase postprandially in rainbow trout (213). Fasting decreases gene expression or protein levels of Cck in the gut of yellowtail and white sea bream (203, 208). These results support the anorexigenic function of Cck and the conservation of this function in the teleost lineage. Some studies, however, show opposite effects; in Coho salmon, cck gene expression in the gut increased during winter fasting (214). In Atlantic salmon, on the other hand, intestinal cck mRNA expression was unchanged after 6 days of fasting (207). Furthermore, there are variations in the distribution pattern of Cck-producing cells within the intestinal segments among species (204, 215, 216) as well as in the fasting response among cck isoforms (207–209) suggesting diverging roles among species and cck isoforms. The action of CCK is initiated by its binding to two subtypes of cognate receptors (CCK-1R and CCK-2R), which results in satiety (197). Cck receptor genes have been isolated in yellowtail (cck-1r) (217), Atlantic salmon (cck-1r, cck-2r1, and cck-2r2) (218), and goldfish (cck-1r and cck-2r) (219). The primary structure of fish Cck receptors as well as their tissue distribution patterns is highly conserved; cck-1r is widely distributed within the GI-tract, while cck-2r is mainly expressed in the brain. Furthermore, cck-1r expression levels increased after feeding in yellowtail pyloric caeca (217), suggesting that Cck-1r mediates the effects of Cck on appetite, as in mammals (220). Further studies on Cck receptors are required to elucidate the detailed mechanisms underlying the anorexigenic function of Cck in fish.
PYY
Peptide YY (PYY) is a member of the NPY family. But, while NPY is well known to have a strong orexigenic function in the CNS (1), peripheral PYY mainly produced in the distal intestine (221) inhibits food intake in mammals (222). PYY consists of two forms: 36 (PYY1–36) or 34 (PYY3–36) amino acids (223). Two isoforms of the gene pyy, pyya, and pyyb (previously named py) (224) have been identified in teleost species, including sea bass (155), Atlantic salmon (207), and piranha (Pygocentrus nattereri) (225). To date, the pyy gene expression patterns are similar among the studied fish species, being predominantly expressed in the brain and GI-tract (203, 226). On the other hand, controversial results have been reported when analyzing intestinal segments from fed versus fasted fish. Fasting decreased (in piranha) (225), increased (in yellowtail) (203), or did not affect (in Atlantic salmon) (207) pyy expression. After feeding, GI-tract pyy mRNA expression increased in grass carp (227), while it decreased in yellowtail (212). These observations suggest that pyy response to fasting/feeding might be species-specific (225). Central and peripheral Pyy1–36 injection reduced food intake in goldfish (228), while administration of the truncated form Pyy3–36 had no effect on food intake in channel catfish (188) or goldfish (228). These results suggest that Pyy3–36 is not a major endogenous form of Pyy in fish (228, 229). The current mammalian model indicates that PYY suppresses appetite through the inhibition of NPY and subsequent activation of POMC neurons (230); however, the effects of GI-tract-derived Pyy on CNS are still uncertain in fish. PYY inhibits GI motility and pancreatic exocrine activity in mammals (175), and a similar digestive function has also been suggested for Pyy in teleosts (207, 211).
GRP
Gastrin-releasing peptide (GRP) is a homolog of the amphibian bombesin (Bbs) and is released from the GI-tract. In mammals, GRP decreases feed intake (231) and stimulates gastric acid secretion and motility (232). Bbs/Grp also appears to stimulate gastric secretion and motility in teleosts (233–235). In teleost species, Bbs/Grp-like peptides have been detected in the GI-tract of rainbow trout (236) and chub (Squalius cephalus) (237), and bbs/grp cDNA sequences have been published for goldfish (238), zebrafish (239), and Atlantic cod (240). Restricted feeding decreased grp expression in the gut of Atlantic cod (240) and zebrafish, but the grp decreasing pattern was reversed in the latter after refeeding (239). Central or peripheral injections of Bbs suppress feed intake in goldfish (200), which might be attributed to Bbs-induced reduction in ghrl gut expression (241). In addition, peripheral injections of Bbs/Grp decrease feeding in channel catfish (188) and Coho salmon (242). On the other hand, feeding status or diet composition does not seem to influence plasma Grp levels in rainbow trout (210). These observations indicate that teleost peripheral (gut) Grp may have an anorexigenic function and its signaling pathway is not endocrine but via neuronal circuits or local paracrine action, as proposed for the mammalian model (231).
The Evolution of Leptin Teleost Genes
The leptin gene (Ob) was first identified in double mutant (Ob/Ob) mice (243) and presented an obese phenotype associated with impaired metabolic functions. Since obesity is linked to several comorbidities in humans, including type II diabetes and cardiovascular disease (244, 245), leptin has been extensively investigated in both humans and murine models. The first fish leptin was identified in 2005 (246). Leptin orthologs and several duplicated paralogs, originating from the whole-genome duplication (WGD) events, have recently been identified in teleost species (247, 248). These include 3R-leptin duplicated paralogs (A and B) in zebrafish (249), medaka (250), orange-spotted grouper (251), tilapia (252), chub mackerel [Scomber japonicas (253)], and European and Japanese eel [Anguilla anguilla and Anguilla japonica (254)], as well as two conserved leptin paralogs [lepAI/lepAII and lepA1/lepA2 (255, 256)]; in common carp and goldfish, as a result of the ancestral lepA doubling at the basal root of cyprinids (256, 257) about 8 million years ago (258). In salmonids, additional “recent” 4R-leptin duplicates have been identified consistently with the (pseudo) tetraploid state of their genome (259–261).
Leptin functions are mediated via class-I helical cytokine receptors (long-form LEPR) through intracellular JAK/STAT signal transduction pathways (262, 263), in an evolutionarily conserved manner as suggested by transfection assay studies for carp (264), rainbow trout (265), and tilapia (252) receptors. In humans, alternative splicing of the LEPR gene leads to expression of long (LEPRb) and short (LEPRa, -Rc, -Rd) isoforms (266).
Single leptin receptors have been identified in most fishes (154, 250, 251, 267, 268), but two 3R-duplicated lepR genes are present in the ancestral teleost eel. This suggests that a loss of the second lepR (lepRB) may have occurred after the clupeocephals/elopomorphs split during teleost radiation (254). At the root of extant salmonids, the lepRA was then further duplicated by the 4R-WGD as deduced by the recently cloned lepRA2 in Atlantic salmon (269). Like mammals, LepR isoforms that arise from alternative splicing of the C-terminal exon have been identified in fish (260, 264, 270, 271). LepR splice variants encode for circulating soluble binding proteins (LepBPs) that may function in leptin modulation, transport, and clearance (265, 271, 272). The characterization of the leptin-lepR system in the context of WGD(s) in teleost genomes and overall evaluation of their functional significance are instrumental to understand to which extent leptin duplicates have contributed to species-specific feeding adaptations.
Leptin Signaling—The Liver and Adipose Tissue
In mammals, leptin is an anorexigenic hormone released into the blood stream mainly by adipocytes. It acts as a lipostatic factor in a negative feedback loop between fat tissue and hypothalamic brain regions so that the organism can maintain energy balance and adequate fat mass reservoirs (273–276). Leptin signaling in the CNS is exerted on different hypothalamic neurons to inhibit the expression of the orexigenic NPY and AgRP and stimulate anorexigenic POMC and CART (120, 277–280). In fish, liver is the main secretory source of LepA (249, 250, 260, 270, 281–283), although some studies reported moderate mRNA expression and secretion from the adipose tissue (260, 270, 281, 284, 285). Central and peripheral administration of recombinant leptin, using homologous or heterologous leptin, produces anorectic effects in several fish species, suggesting that the regulatory role of leptin on appetite is well conserved in vertebrates (120, 279, 282, 286–289).
Leptin variations in response to feeding status (postprandial, short- and long-term fasting/food restriction) have been reported at the level of gene expression and protein among fish orthologs as well as among paralogs. For instance, postprandial increases in hepatic lepA and lepB expression are observed within 9 h in common carp (255), and hepatic lepA in orange-spotted grouper (251) and mandarin fish [Siniperca chuatsi (289)], suggesting that leptins may act as a satiety signal. In longer-term fasting (after 7 days and after 3 weeks), a significant increase in hepatic lepA expression was observed in orange-spotted grouper, but not in carp (289). Prolonged feed restriction induced hepatic upregulation of lepA expression in salmonids (290–292) and chub mackerel (253). In contrast, liver lepA expression decreases during catabolic states in striped bass (Morone saxatilis) (282), and hepatic mRNA expression of lep1, lep2, lepRa, and lepRb does not correlate to feeding status in eels (254).
lepB expression is low or absent in the liver of several teleosts and is mostly found in the CNS (253, 261, 289). The brain expression profiling of lepA-B paralogs in relation to feeding status shows species-specific variations among orthologs, paralogs, and time exposure to catabolic states. For instance, short-term fasting induces a downregulation of both lepA and lepB in the brain of mandarin fish (289), whereas it has no effect on leptin(s)/lepR in orange-spotted grouper (251). Long-term fasting has no effect on either lepA or lepB in Nile tilapia, Oreochromis niloticus (252), and eel (254), while in salmon, it induces upregulation of lepA1 and leprA1 expression and downregulation of lepB1–2 genes in the brain (269). The increases in lepA1 and leprA1 mRNA upon fasting are in line with most studies on plasma leptin in salmonids (291–293). Also, in Mozambique tilapia (Oreochromis mossambicus), hepatic lepA mRNA as well as circulating LepA is higher in fasted than fed fish (294), as is seen with salmonids. Rising leptin plasma levels could be adaptive during catabolic states inducing anorexigenic effects at the level of the CNS, and a consequent reduction of energy-demanding foraging behavior during periods of limited food availability (291, 295). Interestingly, in burbot (Lota lota), plasma leptin levels decrease following fasting at 2°C but not at 10°C, implying that metabolic rate may influence leptin in catabolic conditions (296).
Given the lipostatic role of leptin in mammals, putative similar roles have been investigated in teleosts. The lepB gene has been proposed to be involved in lipid metabolism in chub mackerel (253) and mandarin fish (289). However, plasma levels do not correlate with body adiposity in salmonids (293, 297). Leptin patterns in adipose tissue vary widely among species and between duplicates; in salmon, only lepA1–2 are found with lepA1 type being higher expressed (260, 261). Low lepAI–II expression has been reported in visceral adipose tissue of common carp (298). The differential leptin expression in adipose tissue between fish species and mammals may be a result of the divergent fat allocation patterns observed for the various species but also related to differences between endotherm and ectotherms.
In vivo recombinant LepA treatments suggest anti-adipogenic effects and stimulatory actions on fat metabolism in several teleosts (287, 299, 300). Consistently, LepA treatment in vitro stimulates lipolysis in rainbow trout adipocytes (284). In addition, lepr-deficient medaka exhibit increased visceral fat depots compared to wild types, which is consistent with the body composition of the leptin receptor-deficient db/db mice and Zucker obese rats (243, 301).
While these findings suggest that leptin is involved in mobilization of lipid stores in fish, emerging literature suggests that rather than a canonic “lipostat” signaling for adipostasis (as in mammals), leptin might be important in other metabolic processes. Recent fish studies suggest roles of leptin in glucose homeostasis (302–304) and in the coordination of energy metabolism and somatic growth (305). Leptin receptor-deficient zebrafish do not exhibit increased appetite or adiposity but display β-cell hyperplasia and increased levels of insulin mRNA and alterations in glucose homeostasis, suggesting that leptin might act as a glucostat rather than a lipostat in fish. In both rainbow trout (303) and tilapia (304), either peripheral or central treatment of homologous LepA induces hyperglycemia and glycogenolysis. In tilapia, lipase gene expression was not altered, suggesting the hormone is important in mobilizing glucose. Thus, the contradictory leptin data attained so far on gene expression, in vivo and in vitro recombinant leptin administrations or leptin plasma levels in response to different feeding status, suggest an independent evolution of leptin functions among teleosts. Species-specific responses among orthologs may reflect defined metabolic adaptations to the widely diverse fish life histories. Similarly, leptin duplicates may be under different selective processes and respond to modulation of nutritional status in a spatiotemporal specific manner.
Other Tissues
In mammalian species, there is a range of other peripheral tissues that produce and release factors (peptides/cytokines) that affect appetite, such as the thyroid and pancreatic hormones.
Thyroid
The thyroid axis consists of hypothalamic TRH, pituitary thyrotropin (TSH), and thyroid hormones [thyroxin (T4) and tri-iodothyronine (T3)]. In mammals, the thyroid axis plays a significant role in energy expenditure, as it increase basal metabolic rate, control appetite, and food intake and regulate body weight (306, 307). The few studies that have targeted the role of the thyroid axis on fish feeding suggest a stimulatory effect. For instance, in goldfish, injections of either TRH or T4 increase feeding and locomotion (82, 308), and treatment with the antifouling agent tributyltin increases weight gain and food intake, as well as serum thyroid hormone levels (309). In Amur sturgeon (Acipenser schrenckii), low feeding rates result in low thyroid hormones serum levels (310). In both winter flounder (72) and goldfish (82), fasting induces increases in hypothalamic trh mRNA expression, further suggesting an orexigenic role.
Pancreas
The pancreas secretes mainly insulin and glucagon-related peptides, which have been shown to affect metabolism in fish (311). Plasma insulin and glucagon levels increase after feeding in fish; however, their specific role in the food intake regulation is largely unknown.
Complete isletectomy in the goby (Gillichthys mirabilis) results in hyperphagia (312), and in rainbow trout, intraperitoneal injections of insulin decrease food intake (313), suggesting an anorexigenic role for insulin in fish.
The vertebrate proglucagon (Pg) gene encodes three peptide hormones, namely, glucagon, glucagon-like peptide 1 (GLP-1), and glucagon-like peptide 2 (GLP-2) (314). In mammals, GLP-1 and GLP-2 are satiety signals, mainly produced by the GI-tract (315, 316). In fishes, the pancreas synthesizes glucagon and Glp-1, and the intestine releases glucagon, Glp-1, and Glp-2 (317). To date, the pg gene has also been isolated in several teleost species (314), and duplicate pg genes have been identified in all teleost species for which the genomic sequencing has been completed (318). Although, to our knowledge, there is no information on glucagon and Glp2, Glp-1 appears to act as an anorexigenic factor in fish. In channel catfish, central administration of GLP-1 has a potent inhibitory effect on feed intake, but peripheral injection showed only a weak or no effect on appetite (188, 319). On the other hand, peripheral GLP-1 injection strongly decreased feed intake in Coho salmon (242), suggesting that the peripheral (GI-tract) anorexigenic Glp-1 effects might be species-specific in fish. In rainbow trout, peripheral injections of Glp-1 increase plasma glucose levels, decrease hindbrain npy and pomc mRNA levels and increase hindbrain cart expression levels, suggesting that Glp-1 regulates not only food intake but also glucose homeostasis (320). Although mammalian GLP-1 inhibits gastric emptying (321), the function of Glp-1 on digestion (speed) is still unclear in fish.
Selected Fish Adaptations in the Endocrine Regulation of Feeding
Owing to their large diversity, fishes display a wide range of interesting adaptations in the feeding biology and appetite to different environmental conditions and food availability. Research on these comparative aspects both with regards to evolution and function is still largely unexplored and only a few species, mainly with commercial interest, have been studied. Below, we provide some examples and discuss other adaptions that could be explored further.
Long-term Seasonal Fasting (The Arctic Charr)
The anadromous (sea-migrating) life-strategy of Arctic charr (Salvelinus alpinus) is characterized by substantial seasonal changes in food intake, growth, and adiposity. In the wild, most of the annual growth and energy accumulation occurs because of an intense appetite burst during the short seawater residence in summer, whereas overwintering in freshwater is characterized by anorexia and depletion of energy reserves (322–325). The seasonal cycle in food intake and growth in this species seems to be a strictly genetically programmed process as captive offspring of Arctic charr exhibit pronounced seasonal changes in food intake and growth when held at constant temperature and given food in excess (326, 327). Because of the physiologically regulated seasonal feeding cycles, Arctic charr represent an interesting model for investigation of adaptive mechanisms underlying long-term regulation of appetite and energy homeostasis (328).
It has been suggested that the seasonal feeding cycle is regulated by a lipostatic mechanism (297, 328–330). Leptin, the principal regulator of the lipostatic mechanism in mammals (331), does not appear to be involved in signaling the large variations of adiposity in the Arctic charr (297). However, hepatic leptin production increases at the end of the winter fasting period (297), when fat mobilization and increased plasma glucose occurs (325). It is possible that leptin has a role in depressing metabolism during long-term seasonal fasting, when fat stores are depleted by the suppression of liver lipolytic pathways (292, 297). It is also possible that leptin is more important as a glucostat than an adipostat in Arctic charr, as suggested in zebrafish (302).
The role of Ghrl in controlling the seasonal variation in appetite of charr has also been explored. Stomach ghrl mRNA expression seems to be negatively correlated with feed intake and growth (332), supporting that Ghrl acts as an anorexigenic factor, as suggested in one study on rainbow trout (191). The expression levels of a range of putative central appetite-controlling genes in Arctic charr such as pomc, cart, mc4r, agrp, and npy were not correlated to its annual feeding cycle (333). Further studies are needed to understand how anadromous Arctic charr can maintain an anorexic state when overwintering despite the massive loss of fat reserves.
Long-term Fasting Related with Reproduction (The Mouthbrooder)
Mouthbrooder fish hold their eggs in their mouth until their young are free-swimming. Several fish are classified as mouthbrooders, some being paternal (male holds eggs) and others maternal (most common). Eggs can be fertilized in the environment or in the female’s mouth (in the case of maternal brooding). Teleost mouthbrooder fish include cichlids (e.g., mbuna Astatotilapia burtoni) and tilapias such as Oreochromis mossambicus and Oreochromis niloticus, sea catfish (e.g., Ariopsis felis), cardinalfish (e.g., Pterapogon kauderni), and gouramis (e.g., dwarf gourami Colisa lalia). While guarding eggs, most mouthbrooders do not eat or feed less, often resulting in a weight decrease (334–338).
Very little is known about the endocrine mechanisms responsible for brooding-induced fasting. Fed mbuna females with large ovarian eggs (pre-spawning or spawning) have larger gonadotropin-releasing hormone (Gnrh1) neurons (339), which has also been observed in convict cichlid, Amatitlania nigrofasciatus (340) and higher mRNA expression levels of whole brain gnrh1 (major Gnrh form involved in reproduction), than mouthbrooding females carrying eggs, which is reflected by higher gonadosomatic indexes and higher circulating levels of sex steroids (341). However, no significant differences are seen in gnrh2, in contrast with fasting-induced changes reported for other fish species [e.g., winter flounder (342) and Ya fish (343)]. Similarly, no differences are seen in npy, pomc or mch whole-brain expression, between mbuna holding eggs in their mouths and pre-spawning females (341). However, orexin increases in fasting mbuna females, which is consistent with its stimulatory role on feeding and inhibitory actions on spawning (66). The increase in cck is more surprising, as Cck is a satiety factor that is normally secreted when the GI-tract is full. This increase in cck might be a response to long-term fasting to attenuate hunger and prevent feeding by counteracting increases in orexigenic peptides such as orexin.
Interestingly, when comparing fed and fasted mouthbrooding females from which eggs/fry have been removed, no differences in brain expressions of appetite regulators (npy, cck, orexin, pomc, and mch) were seen (341), possibly because of changes in physiology and metabolism. However, as no information is available about the effects of fasting on appetite regulators for pre-spawning females or immature fish, it is difficult to draw definitive conclusions on the changes that lead to brooding-induced fasting.
Long-term Fasting in Aquaculture (Trout and Salmon)
Like the above-mentioned Arctic charr, many other fish species, including rainbow trout and Atlantic salmon, tolerate long fasting periods. Rather than a genetically driven seasonal halt in feed intake as in charr, they adapt to long periods with low food availability in the wild. To better understand the potential role of various peptides in this process, plasma protein and/or gene expression levels of candidate appetite-regulating hormones and neuropeptides have been analyzed during variable periods of food deprivation in salmon and trout.
Leptin
The picture of leptin endocrinology dynamics in fish during fasting is not clear-cut, even within species, e.g., rainbow trout. Recent data on two lines of rainbow trout bred for either high (fat line) or low (lean line) muscle lipid content indicate that leptin response to fasting may be plastic and dependent on selective breeding, environmental factors and/or energy status and body composition (344). The two lines of trout differ in the fat deposition pattern: the fat line has higher total energy reserves, higher muscle adiposity, and lower visceral adiposity than the lean line. A 4-week fasting period decreased plasma Lep in the lean line while Lep levels and hepatic lep expression remained unchanged in the fat line (344). This contrasts previous results in rainbow trout, where leptin levels increase or remain unchanged during fasting, despite a decrease in condition factor (293, 345).
Tissue lep gene expression was also unaltered in long-term fasted fish except for an increased expression in fat rich muscle tissue (346). In the same study, the fasted fish displayed hyperphagia when they could refeed, eating as much as up to 8.4% of their body weight (346). Hence, even though the fasted fish were clearly in a catabolic state, hungry and mobilizing energy stores, leptin production and plasma levels remained unchanged.
Unlike the observation mentioned above (346), appetite does not always return immediately when food becomes available for anorectic/food-deprived salmonids (345, 347). During a 72-h refeeding period for long-term fasted rainbow trout, there was a large variability in the time to start feeding between individuals, and some did not feed at all in the beginning. This response may have been caused by high leptin levels in these individuals (345). Leptin generally did not start to decrease until some food had been ingested, raising the question of which mechanism is responsible for triggering the onset of appetite. In fine flounder (Paralichthys adspersus), leptin also decreases after, but not before refeeding (291). This fast leptin response indicates that there is a short-term meal-related regulation of leptin release (291, 345).
Available data on the relation between leptin and energy status in Atlantic salmon are still limited to those from food restriction studies or experiments using diets with different energy content (260, 290, 348, 349). Plasma leptin levels were not different between fish that were fed full or restricted (60%) rations for 10 months, although hepatic lepA2 expression was higher in the fed than in the fasted salmon (260). In a shorter trial (7 weeks), feed-restricted fish had higher plasma leptin levels and elevated hepatic lep expression levels than controls fed to satiation (290), which is consistent with some of the previous studies on rainbow trout (293, 345). Restricted feeding during several months (April–September) in Atlantic salmon parr undergoing sexual maturation showed that fish with the highest fat stores had the lowest leptin levels (349). Similarly, fish on a high-energy diet had lower leptin levels than fish on a low energy diet with less adipose stores (348). Taken together, these studies lend further support to the notion that leptin is not a long-term adiposity signal in salmonids. The results obtained from fish species are also interesting in the context of studies on wild mammals with seasonal changes in adiposity and feeding behavior, showing a large variability in the link between plasma leptin levels, fasting, and adiposity (350–353).
Ghrelin
The response of plasma Ghrl and ghrl mRNA expression to fasting in fish is highly variable between studies and fasting duration (354). There are few studies investigating the response of Ghrl to long-term fasting in Atlantic salmon and rainbow trout. In rainbow trout, plasma Ghrl levels decreased after 1–3 weeks of fasting (213). In Atlantic salmon, 2 days of fasting led to elevated plasma Ghrl levels, indicating an effect of short-term feeding status on Ghrl release, a response consistent with this “hunger hormone.” However, after 14 days of food-deprivation, Ghrl levels were unchanged in fasted salmon compared to fully fed controls (355). Whether these differences are a result of true species differences in Ghrl function (see section above about ghrelin), domestication processes or experimental design remains unclear.
Fasting-Induced Changes in Central Appetite Regulatory Neuropeptides
The recent study by Jørgensen et al. (346) is one of few that have investigated potential changes in the expression of hypothalamic appetite-regulating peptides during fasting in a salmonid species. Rainbow trout was fasted for 4 months, and among the peptides that were measured in the hypothalamus (lepa1, cart, agrp, pomca1, pomca2, pomcb, npy, mc4r, and crf), few fasting-induced effects were observed. There was an increased gene expression of pomca1 and pomcb, suggesting that increased pomc transcript levels may be a potential mechanism for a reduced appetite and foraging activity in catabolic conditions.
Peripherally injected Lep seems to increase the expression of pomc-a1 and -a2 with a concurrent transient reduction in npy gene expression (279). In rainbow trout, the leptin receptor is localized in mediobasal hypothalamic appetite centers, and it seems that Pomc and Cart mediate leptin’s acute anorexigenic effect in this species (295). It may be speculated that during long-term fasting in salmonids, increased circulating leptin levels stimulate hypothalamic Pomc neurons, suppressing appetite. Brain sensitivity (amount of receptor levels) to, e.g., leptin and Ghrl will also influence appetite. At the termination of a 7-week feeding/fasting experiment, fed Atlantic salmon parr showed an increase in lepr gene expression in the brain, while the lepr gene expression in food-deprived fish was unaltered despite increased plasma Lep levels. This was interpreted by the authors as the possible result of a negative feedback of Lep on its receptor (290).
Life-Stage Transition (First Feeding Larvae to Juveniles)
Most fish species spawn eggs, in which the developing embryo relies on yolk nutrients until it is sufficiently developed to capture, ingest, and digest feed. After onset of exogenous feeding, the larvae continue to grow and develop into juveniles—a transition triggered by environmental cues that induce a coordinated program to remodel the organism. The transition involves a wide range of changes in behavior, habitat, and physiology, and many fish larvae change food sources as they become adults; therefore, it has major consequences for feeding behavior and most likely in the control of appetite (356).
Several studies have aimed to understand the various aspects of the feeding biology and nutritional requirements of developing fish larvae to improve their performance in aquaculture. However, very few have focused on the mechanisms that control appetite and food intake (42, 357). This may be partly explained by biological and technical challenges when working with fish larvae, such as the accurate determination of food intake, the use of individual larva (instead of pools), or the handling of individual variability in growth and development.
There are several described cases where fish larvae continue to eat, despite having an apparently full GI-tract. For instance, Atlantic halibut larvae continue to ingest prey despite a full gut and with gut transit rates so high that the prey is eliminated (defecated) undigested and sometimes even alive (358). Apparently, the feedback systems and satiety signals originating in the GI-tract are not functional in these early stages. It has been argued that fish larvae have adapted to low concentrations and availability of prey in the wild. Consequently, satiety signals may not be required to prevent overfeeding. In aquaculture conditions, however, larvae are reared with constant and abundant food availability and continuous light, and therefore appetite-controlling mechanisms become crucial to avoid continuous ingestion of prey, short gut transit times of ingested food, reduced time for digestion, low digestive efficiency, and nutrient absorption (359). This is of particularly interest for altricial-gastric species, which lack a fully developed and functional stomach prior to metamorphosis (360–364).
Some studies have started to explore the ontogeny expression of several appetite regulators (71, 240, 365, 366), and their detailed spatial and differential distribution in fish larvae (159). Key factors in appetite control are present very early in fish development, such as npy at zygote stage in blunt snout bream (Megalobrama amblycephala) (367) and at blastula stages in orange-spotted grouper (170), ghrl (240) and ox (71) at cleavage stage, and gastrin (240) at blastula stage in Atlantic cod. In Atlantic halibut, only ghrl and cart mRNA expression levels were significantly modified throughout development, while ontogeny did not affect npy, pyy, and pomc-c expressions levels in the brain of the developing larvae (35). Ghrl was widely distributed in the GI-tract and present in the anterior GI-tract before the gastric glands and pepsinogen production appeared in newly Atlantic halibut hatched yolk-sac larvae (368). Notably, increased levels of ghrl in the GI-tract during metamorphosis were correlated with stomach development (360, 369). cart mRNA expression levels decreased at the initiation of halibut metamorphosis, while cart levels in whole larvae of Atlantic cod increased during the corresponding developmental phase (365). In Atlantic cod, cck, npy, and ox show a similar pattern of a moderate but consistent decrease from 3 days post-hatching (dph) until 60 dph (42, 365). The differences in cart expression between Atlantic halibut and Atlantic cod larvae are intriguing and may be a result of different factors, including the use of whole cod larvae versus halibut head and differences in developmental rate (370, 371).
Many of the neuropeptides involved in appetite control in higher vertebrates and adult teleost are present in the brain of fish larvae, suggesting a role of these genes in appetite control also in the early stages (35, 159, 168, 372–374). In the recent study of Le et al. (159), the development expression patterns of npy, cart, and ox genes were analyzed in brain regions of Atlantic cod, from start of exogenous feeding until juvenile stage. Both spatial and temporal expression patterns of orexigenic and anorexigenic factors during larval ontogeny indicated a progressive development of the brain regulatory networks that control appetite. In addition, the wide distribution and co-expression of npy, cart, and ox in hypothalamus, led the authors to propose that this is the main area for appetite control in fish larvae, comparable to mammals and adult fish (6, 374–376). However, it remains unclear to what extent these appetite-regulating genes are functional at these early developmental stages.
Few have assessed the response of these factors in terms of feed intake (35, 40) or different diets (40, 42, 377). In Atlantic cod larvae, Kortner et al. (42) showed that the expression levels of cck and npy were diet-specifically modulated and followed the same expression profile as the genes coding for digestive enzymes, suggesting a close connection between appetite control and digestion processes. Recently, two studies in Senegalese sole larvae have analyzed the effect of fatty acids ingestion in the control of food intake (378, 379). The administration of several fatty acids (leate, linoleate, α-linolenate, or eicosapentaenoate) in sole post-larvae enhanced the expression of the anorexigenic neuropeptides cart4 and pomcb and decreased the orexigenic npy, with no major discrepancies between the different fatty acids tested (378). However, the transcriptional analysis of several anorexigenic: pyya, pyyb, glp1, cckl, cart1a, cart1b, cart2a, cart4, pomc-a, pomc-b, crf; and orexigenic: gal, npy, agrp2 factors showed a dissimilar response to feeding times and dietary fatty acid composition (cod liver oil, linseed oil, soybean oil, or olive oil) that was generally not in agreement with their putative function (40). For example, the changes observed for sole npy in developmental stages 16 and 34 dph were not consistent. At 16 dph npy expression levels increased before feeding, as expected, but then continue to increase up to 3 h after feeding (40), which is counterintuitive for an orexigenic factor (1, 12). At 34 dph, npy expression was only affected by the dietary fatty acid profile. This was similar to the results obtained by Kortner et al. (42), where cod npy was diet-specifically modulated in larvae at 16 dph, but no evident changes were found at 29 dph. Furthermore, in Atlantic halibut larvae, npy levels increased 5 h after refeeding (35). The differences observed between species may suggest that the Npy is still not fully functional in appetite regulation in larvae, possibly reflecting a yet underdeveloped appetite-regulating system. Furthermore, the response of npy, pyy, pomc-c, and cart to food deprivation and refeeding in Atlantic halibut larvae did not appear to be coordinated (35), lacking a consistent expression pattern to explain their contribution to appetite control in early larvae as it was for Senegalese sole larvae (40). In addition, the differences observed between both studies in Senegalese sole larvae may be explained by the different approaches used: use of complex diets fed through the whole larval and post-larval stage (379) versus a tube-fed single meal of pure fatty acids solution (378).
Altogether, these studies support the hypothesis that a feedback signaling system from the GI-tract to the CNS is still not fully established in the early larval stages. This, however, does not rule out that developing fish larvae may have their own specific system of appetite regulation adapted to their feeding ecology or that larvae possess a rudimentary, still developing, regulatory system. Fish larvae are often considered as “feeding machines” because they can ingest food at rates above their own weight daily (357, 380–382). This suggests that larvae are constantly hungry and motivated to feed, although several studies have shown that some fish larvae exhibit a circadian prandial pattern and do not feed constantly (383–385). Given the complexity of appetite-controlling mechanisms and how difficult it is to interpret results due to the lack of specific information on the roles played by some of the potential anorexigenic and orexigenic factors in fish, it remains a challenge to elucidate the appetite-control system in fish larvae with different digestive tract morphologies and feeding strategies. A better understanding will greatly increase our basic knowledge on larval physiology and help to improve larval rearing regimes and feeding protocols in hatcheries.
The Voracious Feeders
Several species have an aggressive and voracious feeding behavior, most of them usually being carnivorous top predators. Well-known examples include Perciformes such as bluefish (Pomatomus saltatrix), bluegill (Lepomis macrochirus), cobia, groupers, tilapia and African cichlids, salmonids (e.g., rainbow trout), pikes (e.g., Northern pike Esox lucius), some characids (e.g., dourado and piranhas), as well as elasmobranchs, i.e., sharks and rays (338).
Within the teleosts, several studies have examined the effects of fasting and feeding on the expression of a few appetite regulator genes. However, there are no data on how endocrine mechanisms might regulate the increased feed intake in these voracious fish, and no comparative study has been performed between voracious species and a “gentler” herbivore/omnivore species (e.g., cyprinids, some flatfish species).
In response to fasting, it appears that most voracious fish display a similar trend to what occurs in non-aggressive species [e.g., the omnivorous goldfish and pacu (Piaractus mesopotamicus)], i.e., increases in expression of orexigenic factors [e.g., ox in dourado (73) and piranha (225), and ghrl in piranha (386)] and decreases in expression of anorexigenic factors [e.g., cart in piranha (225)]. However, few studies have examined periprandial changes in voracious fish. Taking the example of orexin, its expression appears to increase around feeding time and decrease after feeding, similar to what is seen for other fish species, such as orange grouper (70) and tilapia (387). In dourado, ox expression is similar before, during, and after feeding, suggesting a constant state of feeding/searching behavior. In addition, ox expression levels in fasted fish increase at mealtime and dramatically at post-feeding time, suggesting that dourado have a high motivation to search for food that persists after meal time (73). In contrast, pacu, a fish from the same order (Characiformes) as dourado, shows high ox levels at pre-feeding, and these tend to decrease at mealtime and post-feeding. Moreover, if pacu is not fed at the scheduled mealtime, ox levels increase at mealtime but return to basal levels within 1 h, suggesting that the fish have “given up” on searching food (388), which is reflected by their calm behavior (Volkoff, personal observation).
Voracious fish are often aggressive during feeding. Although aggression is often related to reproduction, in these species it also occurs outside the reproductive context (389). Interestingly, early studies in cichlid fish (Tilapia heudelotii macrocephala) and in bluegill have shown that electrical stimulation of the hypothalamic region elicited both feeding and aggressive responses (390, 391). The brain monoaminergic system, especially serotonin [5-hydroxytryptamine (5-HT)], plays a key role in controlling aggressive behavior (392). 5-HT has been reported to inhibit aggressive behavior in several voracious species, e.g., trout (393) and pikeperch (Sander lucioperca) (394). Interestingly, surface Mexican tetra (Astyanax mexicanus) species are aggressive predators, in particular during feeding episodes, whereas blind cave forms of this species exhibit reduced aggressiveness and have a tendency to continuously search for food. These differences in foraging and aggressive behaviors are related to 5-HT network modifications within hypothalamic neurons (395, 396). 5-HT also has anorexigenic actions in rainbow trout (397) and in mammals (387) and has been shown to interact with appetite regulators. For example, the behavioral effects produced by orexin administration, i.e., increased locomotion and feeding, are blocked by 5-HT antagonists (398). It would therefore be valuable to compare 5-HT levels between voracious and non-voracious fish.
Intra-species differences (sometimes referred to as personality/motivation) in basal locomotor and feeding activities are often observed between individuals. These differences might be due to different expression levels of appetite regulators or monoamines. For example, in tilapia, low serotonergic activity in the hypothalamus is correlated with a personality characterized by high feeding motivation (399). Similarly, in salmonid fish, subordinate individuals characteristically exhibit higher plasma cortisol levels than dominant ones (400). There are most likely different causes for voraciousness in fish, and more direct studies are needed to explain the underlying mechanisms of the appetite-controlling networks that result in these large differences in feeding behaviors.
How Important Is Vision? (The Blind Mexican Cavefish)
Although most fish rely in part on vision to feed (401), this sense is not essential for some species. The best example is that of fish living in cave environments, which are characterized by constant darkness and food scarcity (338, 402). Cavefish such as the Mexican tetra are often blind and have specialized anatomical features to better locate food and maximize food intake (396, 403, 404). Such adaptations include well-developed olfactory bulbs (405), taste buds (406), and lateral line neuromasts (407–409). In addition, these fish display behavioral adaptations for detecting prey and increasing feeding efficiency: they are opportunistic feeders, show increased swimming/exploratory and feeding behaviors (410), do not sleep (411), and do not exhibit schooling behavior (403, 412, 413). This enhanced food-finding efficiency is present not only in adults but also in young larvae when the yolk has been depleted (414). Overall, surface fish placed in the dark are less efficient at finding food than cavefish (415–417).
To cope with a particularly food-limited habitat compared to most surface fish, cavefish have developed behavioral (increased appetite, with ingestion of large amounts of food during feeding events) and metabolic adaptations. The latter include reduced basal metabolic rate, increased metabolic efficiency, starvation resistance (reduced weight loss during fasting), and increased body fat composition (403, 413, 418).
Peripheral injections of known orexigenic factors in cavefish, such as OX, GHRL, and apelin, increase not only food consumption but also the whole brain mRNA expressions of orexigenic factors (e.g., GHRL injections induce an increase in ox brain expression), whereas injections of CCK reduce food intake and induce a decrease in the whole brain expression orexigenic factors (e.g., apelin) (67, 79). Peripheral injections of OX greatly increase locomotor activity and ox brain mRNA levels in cavefish. Basal ox mRNA levels in whole brain are higher in cave fish than in surface fish (Buenos Aires tetra, Hyphessobrycon anisitsi, a characid surface species closely related to Astyanax) (405), suggesting that the higher overall locomotor/feeding activity in cavefish compared to the surface forms might be mediated by an increase in ox levels (67, 79). Coding mutations in mc4r also contribute to the increased appetite and starvation resistance of cavefish compared with surface fish (419).
Cavefish are avid feeders and become very active around feeding time when appetite increases (420). Brain ox mRNA expression levels increase before and decrease after a scheduled mealtime (67), suggesting that orexin acts as a short-term hunger signal and is linked to food anticipatory activity. Conversely, the brain expression of the anorexigenic pyy increases after feeding (67), suggesting a role for Pyy as a short-term satiety factor. However, cck brain expression does not display periprandial variations in cavefish (67), which might contribute to a less rapid satiety and longer bouts of feeding.
Short-term food restriction increases ox brain mRNA transcription levels in cavefish (67), indicating a role in the long-term regulation of feeding in cavefish and perhaps triggering an increased motivation to seek food. However, as opposed to most surface fish examined to date, short-term fasting does not increase brain mRNA levels of pyy and cck, suggesting that the anorexigenic systems are inhibited during fasting, perhaps to slow down digestion/gastric emptying of food in the gut or to maintain a hunger state that would favor food-seeking behavior.
Future
Many of the studies on appetite-controlling systems in teleosts are based on domesticated fish that have been bred in captivity for generations (e.g., salmon, carp, and cod). These fish, which are submitted to optimal habitat (e.g., no predators, constant optimal photoperiods and temperatures) and feeding (e.g., satiation, minimal food-seeking behavior) conditions might have present modifications in their feeding behavior and systems controlling appetite, as compared to wild fish exposed to suboptimal conditions. This phenomenon has been shown in domesticated rats that eat more than wild individuals (421). Comparisons between wild and captive populations might reveal important information on the effects of domestication on feeding behavior. Therefore, observations of feeding behavior and sampling of fish in their natural environment would be valuable.
Overall, within a few model species, only a few appetite-regulating hormones (e.g., leptin, Npy, and Cck) have been studied more in detail. In addition, there are very few studies on the mechanisms of action of these hormones, including at the level of their target cells and their receptors. Many questions related to the concepts “set-point” in energy homeostasis and stimulus for synthesis/secretion of these hormones, i.e., whether it is direct nutrient sensing by the hormone-producing cells or stimulation of these cells by another hormone/neurotransmitter or both, also remain to be answered. Also, many of these hormones are expressed both in the CNS and in peripheral tissues and the relative importance of each, as well as their interactions in controlling the appetite, are poorly understood.
One of the major limitations in the field of appetite endocrinology in fish is that the vast majority of studies have been constrained to the analyses of transcript levels. Although the existence of a proportional relationship between mRNA and protein expressions measured from a tissue have long been assumed, recent data show that this is not always the case (422). The development of fish-specific hormone assays and protein expression techniques is crucial for a better understanding of appetite-regulating mechanisms in fish. In addition, most studies analyze large portions of specific tissues (e.g., whole brain, whole hypothalamus, or whole intestine), which might also bias results, as, for example, specific regions (e.g., proximal versus distal intestine, or specific hypothalamic nuclei) might have different functions and respond differently to feeding conditions.
Although it is often observed that growth is directly related to food intake, many gaps exist on our understanding of how these two functions are connected in fish. The recent development of GH-transgenic fish is promising for the exploration of this field. Thus, the development of emerging techniques such as gene editing (CRISPR/Cas9 system) will be a great tool to study the role of appetite regulators in fish. Targeted mutagenesis using CRISPR/Cas9 system has been successfully used in several species, including zebrafish (423), salmon (424), and African cichlids (425), but so far only a few studies have used this technique to examine the role of appetite regulators on fish models, e.g., leptin receptor mutations in zebrafish (302).
Author Contributions
All authors designed, wrote and approved the final version of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
The authors acknowledge funding from EU-FP7-KBBE-2007-2A LIFECYCLE (IR, RA, EJ, and ASG), Research Council of Norway [Grants 172548/S40; 190043; 199482 (IR)], and Regional Research fund West (Grants 247978; 259919) (IR, ASG, and KM); Natural Sciences and Engineering Research Council (NSERC) Discovery Grant, 261414-03 (HV); the research center SWEMARC at the University of Gothenburg (EJ); Japan Fisheries Research and Education Agency (KM); and JSPS KAKENHI (JP15KK0288) (KM).
Terminology for Gene Names
GENE (All capitals), Mammalian protein; Gene (First letter capital), Fish Protein; Gene (First letter capital, italic), Mammalian gene; gene (small letters only and italic), Fish gene.
References
- 1.Schwartz MW, Woods SC, Porte D, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature (2000) 404:661–71. 10.1038/35007534 [DOI] [PubMed] [Google Scholar]
- 2.Friedman MI. Food intake: control, regulation and the illusion of dysregulation. In: Harris R, Mattes R, editors. Appetite and Food Intake: Behavioral and Physiological Considerations. Boca Raton: CRC Press; (2008). p. 1–19. [Google Scholar]
- 3.Kulczykowska E, Sánchez Vázquez FJ. Neurohormonal regulation of feed intake and response to nutrients in fish: aspects of feeding rhythm and stress. Aquac Res (2010) 41:654–67. 10.1111/j.1365-2109.2009.02350.x [DOI] [Google Scholar]
- 4.Lin XW, Volkoff H, Narnaware Y, Bernier NJ, Peyon P, Peter RE. Brain regulation of feeding behavior and food intake in fish. Comp Biochem Physiol A Mol Integr Physiol (2000) 126:415–34. 10.1016/S1095-6433(00)00230-0 [DOI] [PubMed] [Google Scholar]
- 5.Volkoff H, Peter RE. Feeding behavior of fish and its control. Zebrafish (2006) 3:131–40. 10.1089/zeb.2006.3.131 [DOI] [PubMed] [Google Scholar]
- 6.Volkoff H, Canosa LF, Unniappan S, Cerda-Reverter JM, Bernier NJ, Kelly SP, et al. Neuropeptides and the control of food intake in fish. Gen Comp Endocrinol (2005) 142:3–19. 10.1016/j.ygcen.2004.11.001 [DOI] [PubMed] [Google Scholar]
- 7.Volkoff H, Xu M, MacDonald E, Hoskins L. Aspects of the hormonal regulation of appetite in fish with emphasis on goldfish, Atlantic cod and winter flounder: notes on actions and responses to nutritional, environmental and reproductive changes. Comp Biochem Physiol Part A Mol Integr Physiol (2009) 153:8–12. 10.1016/j.cbpa.2008.12.001 [DOI] [PubMed] [Google Scholar]
- 8.Nelson JS. Fishes of the World. New Jersey: John Wiley and Sons; (2006). [Google Scholar]
- 9.Muroi Y, Ishii T. A novel neuropeptide Y neuronal pathway linking energy state and reproductive behavior. Neuropeptides (2016) 59:1–8. 10.1016/j.npep.2016.09.002 [DOI] [PubMed] [Google Scholar]
- 10.Kohno D, Yada T. Arcuate NPY neurons sense and integrate peripheral metabolic signals to control feeding. Neuropeptides (2012) 46:315–9. 10.1016/j.npep.2012.09.004 [DOI] [PubMed] [Google Scholar]
- 11.Lopez-Patino MA, Guijarro AI, Isorna E, Delgado MJ, Alonso-Bedate M, de Pedro N. Neuropeptide Y has a stimulatory action on feeding behavior in goldfish (Carassius auratus). Eur J Pharmacol (1999) 377:147–53. 10.1016/S0014-2999(99)00408-2 [DOI] [PubMed] [Google Scholar]
- 12.Narnaware YK, Peyon PP, Lin X, Peter RE. Regulation of food intake by neuropeptide Y in goldfish. Am J Physiol Regul Integr Comp Physiol (2000) 279:R1025–34. [DOI] [PubMed] [Google Scholar]
- 13.Zhou Y, Liang XF, Yuan XC, Li J, He Y, Fang L, et al. Neuropeptide Y stimulates food intake and regulates metabolism in grass carp, Ctenopharyngodon idellus. Aquaculture (2013) 380:52–61. 10.1016/j.aquaculture.2012.11.033 [DOI] [Google Scholar]
- 14.Silverstein JT, Plysetskaya EM. The effects of NPY and insulin on food intake regulation in fish. Am Zool (2000) 40:296–308. 10.1093/icb/40.2.296 [DOI] [Google Scholar]
- 15.Yokobori E, Azuma M, Nishiguchi R, Kang KS, Kamijo M, Uchiyama M, et al. Neuropeptide Y stimulates food intake in the zebrafish, Danio rerio. J Neuroendocrinol (2012) 24:766–73. 10.1111/j.1365-2826.2012.02281.x [DOI] [PubMed] [Google Scholar]
- 16.Aldegunde M, Mancebo M. Effects of neuropeptide Y on food intake and brain biogenic amines in the rainbow trout (Oncorhynchus mykiss). Peptides (2006) 27:719–27. 10.1016/j.peptides.2005.09.014 [DOI] [PubMed] [Google Scholar]
- 17.Kiris GA, Kumlu M, Dikel S. Stimulatory effects of neuropeptide Y on food intake and growth of Oreochromis niloticus. Aquaculture (2007) 264:383–9. 10.1016/j.aquaculture.2006.12.004 [DOI] [Google Scholar]
- 18.Carpio Y, Acosta J, Morales A, Herrera F, González LJ, Estrada MP. Cloning, expression and growth promoting action of Red tilapia (Oreochromis sp.) neuropeptide Y. Peptides (2006) 27:710–8. 10.1016/j.peptides.2005.08.013 [DOI] [PubMed] [Google Scholar]
- 19.Narnaware YK, Peter RE. Effects of food deprivation and refeeding on neuropeptide Y (NPY) mRNA levels in goldfish. Comp Biochem Physiol B Biochem Mol Biol (2001) 129:633–7. 10.1016/S1096-4959(01)00359-1 [DOI] [PubMed] [Google Scholar]
- 20.Silverstein JT, Breininger J, Baskin DG, Plisetskaya EM. Neuropeptide Y-like gene expression in the salmon brain increases with fasting. Gen Comp Endocrinol (1998) 110:157–65. 10.1006/gcen.1998.7058 [DOI] [PubMed] [Google Scholar]
- 21.Tian J, He G, Mai KS, Liu CD. Effects of postprandial starvation on mRNA expression of endocrine-, amino acid and peptide transporter-, and metabolic enzyme-related genes in zebrafish (Danio rerio). Fish Physiol Biochem (2015) 41:773–87. 10.1007/s10695-015-0045-x [DOI] [PubMed] [Google Scholar]
- 22.MacDonald E, Volkoff H. Neuropeptide Y (NPY), cocaine- and amphetamine-regulated transcript (CART) and cholecystokinin (CCK) in winter skate (Raja ocellata): cDNA cloning, tissue distribution and mRNA expression responses to fasting. Gen Comp Endocrinol (2009) 161:252–61. 10.1016/j.ygcen.2009.01.021 [DOI] [PubMed] [Google Scholar]
- 23.Kamijo M, Kojima K, Maruyama K, Konno N, Motohashi E, Ikegami T, et al. Neuropeptide Y in tiger puffer (Takifugu rubripes): distribution, cloning, characterization, and mRNA expression responses to prandial condition. Zoolog Sci (2011) 28:882–90. 10.2108/zsj.28.882 [DOI] [PubMed] [Google Scholar]
- 24.MacDonald E, Volkoff H. Cloning, distribution and effects of season and nutritional status on the expression of neuropeptide Y (NPY), cocaine and amphetamine regulated transcript (CART) and cholecystokinin (CCK) in winter flounder (Pseudopleuronectes americanus). Horm Behav (2009) 56:58–65. 10.1016/j.yhbeh.2009.03.002 [DOI] [PubMed] [Google Scholar]
- 25.Campos VF, Robaldo RB, Deschamps JC, Seixas FK, McBride AJA, Marins LF, et al. Neuropeptide Y gene expression around meal time in the Brazilian flounder Paralichthys orbignyanus. J Biosci (2012) 37:227–32. 10.1007/s12038-012-9205-7 [DOI] [PubMed] [Google Scholar]
- 26.Kehoe AS, Volkoff H. Cloning and characterization of neuropeptide Y (NPY) and cocaine and amphetamine regulated transcript (CART) in Atlantic cod (Gadus morhua). Comp Biochem Physiol A Mol Integr Physiol (2007) 146:451–61. 10.1016/j.cbpa.2006.12.026 [DOI] [PubMed] [Google Scholar]
- 27.Boonanuntanasarn S, Jangprai A, Yoshizaki G. Characterization of neuropeptide Y in snakeskin gourami and the change in its expression due to feeding status and melanocortin 4 receptor expression. Gen Comp Endocrinol (2012) 179:184–95. 10.1016/j.ygcen.2012.07.024 [DOI] [PubMed] [Google Scholar]
- 28.Peterson BC, Waldbieser GC, Riley LG, Upton KR, Kobayashi Y, Small BC. Pre- and postprandial changes in orexigenic and anorexigenic factors in channel catfish (Ictalurus punctatus). Gen Comp Endocrinol (2012) 176:231–9. 10.1016/j.ygcen.2012.01.022 [DOI] [PubMed] [Google Scholar]
- 29.Nguyen MV, Jordal AE, Espe M, Buttle L, Lai HV, Rønnestad I. Feed intake and brain neuropeptide Y (NPY) and cholecystokinin (CCK) gene expression in juvenile cobia fed plant-based protein diets with different lysine to arginine ratios. Comp Biochem Physiol A Mol Integr Physiol (2013) 165:328–37. 10.1016/j.cbpa.2013.04.004 [DOI] [PubMed] [Google Scholar]
- 30.Peng C, Peter RE. Neuroendocrine regulation of growth hormone secretion and growth in fish. Zool Stud (1997) 36:79–89. [Google Scholar]
- 31.Carpio Y, Leon K, Acosta J, Morales R, Estrada MP. Recombinant tilapia neuropeptide Y promotes growth and antioxidant defenses in African catfish (Clarias gariepinus) fry. Aquaculture (2007) 272:649–55. 10.1016/j.aquaculture.2007.08.024 [DOI] [Google Scholar]
- 32.Wu SG, Li B, Lin HR, Li WS. Stimulatory effects of neuropeptide Y on the growth of orange-spotted grouper (Epinephelus coioides). Gen Comp Endocrinol (2012) 179:159–66. 10.1016/j.ygcen.2012.08.010 [DOI] [PubMed] [Google Scholar]
- 33.Babichuk NA, Volkoff H. Changes in expression of appetite-regulating hormones in the cunner (Tautogolabrus adspersus) during short-term fasting and winter torpor. Physiol Behav (2013) 120:54–63. 10.1016/j.physbeh.2013.06.022 [DOI] [PubMed] [Google Scholar]
- 34.Valen R, Jordal AE, Murashita K, Rønnestad I. Postprandial effects on appetite-related neuropeptide expression in the brain of Atlantic salmon, Salmo salar. Gen Comp Endocrinol (2011) 171:359–66. 10.1016/j.ygcen.2011.02.027 [DOI] [PubMed] [Google Scholar]
- 35.Gomes AS, Jordal AE, Olsen K, Harboe T, Power DM, Rønnestad I. Neuroendocrine control of appetite in Atlantic halibut (Hippoglossus hippoglossus): changes during metamorphosis and effects of feeding. Comp Biochem Physiol A Mol Integr Physiol (2015) 183:116–25. 10.1016/j.cbpa.2015.01.009 [DOI] [PubMed] [Google Scholar]
- 36.Raven PA, Uh M, Sakhrani D, Beckman BR, Cooper K, Pinter J, et al. Endocrine effects of growth hormone overexpression in transgenic Coho salmon. Gen Comp Endocrinol (2008) 159:26–37. 10.1016/j.ygcen.2008.07.011 [DOI] [PubMed] [Google Scholar]
- 37.Zhong CR, Song YL, Wang YP, Zhang TL, Duan M, Li YM, et al. Increased food intake in growth hormone-transgenic common carp (Cyprinus carpio L.) may be mediated by upregulating Agouti-related protein (AgRP). Gen Comp Endocrinol (2013) 192:81–8. 10.1016/j.ygcen.2013.03.024 [DOI] [PubMed] [Google Scholar]
- 38.Dalmolin C, Almeida DV, Figueiredo MA, Marins LF. Food intake and appetite control in a GH-transgenic zebrafish. Fish Physiol Biochem (2015) 41:1131–41. 10.1007/s10695-015-0074-5 [DOI] [PubMed] [Google Scholar]
- 39.Narnaware YK, Peter RE. Influence of diet composition on food intake and neuropeptide Y (NPY) gene expression in goldfish brain. Regul Pept (2002) 103:75–83. 10.1016/S0167-0115(01)00342-1 [DOI] [PubMed] [Google Scholar]
- 40.Bonacic K, Campoverde C, Gómez-Arbonés J, Gisbert E, Estevez A, Morais S. Dietary fatty acid composition affects food intake and gut-brain satiety signaling in Senegalese sole (Solea senegalensis, Kaup 1858) larvae and post-larvae. Gen Comp Endocrinol (2016) 228:79–94. 10.1016/j.ygcen.2016.02.002 [DOI] [PubMed] [Google Scholar]
- 41.Conde-Sieira M, Agulleiro MJ, Aguilar AJ, Miguez JM, Cerda-Reverter JM, Soengas JL. Effect of different glycaemic conditions on gene expression of neuropeptides involved in control of food intake in rainbow trout; interaction with stress. J Exp Biol (2010) 213:3858–65. 10.1242/jeb.048439 [DOI] [PubMed] [Google Scholar]
- 42.Kortner TM, Overrein I, Øie G, Kjørsvik E, Arukwe A. The influence of dietary constituents on the molecular ontogeny of digestive capability and effects on growth and appetite in Atlantic cod larvae (Gadus morhua). Aquaculture (2011) 315:114–20. 10.1016/j.aquaculture.2010.04.008 [DOI] [Google Scholar]
- 43.Tuziak SM, Rise ML, Volkoff H. An investigation of appetite-related peptide transcript expression in Atlantic cod (Gadus morhua) brain following a Camelina sativa meal-supplemented feeding trial. Gene (2014) 550:253–63. 10.1016/j.gene.2014.08.039 [DOI] [PubMed] [Google Scholar]
- 44.Riley LG, Walker AP, Dorough CP, Schwandt SE, Grau EG. Glucose regulates ghrelin, neuropeptide Y, and the GH/IGF-I axis in the tilapia, Oreochromis mossambicus. Comp Biochem Physiol A Mol Integr Physiol (2009) 154:541–6. 10.1016/j.cbpa.2009.08.018 [DOI] [PubMed] [Google Scholar]
- 45.Rogge G, Jones D, Hubert GW, Lin Y, Kuhar MJ. CART peptides: regulators of body weight, reward and other functions. Nat Rev Neurosci (2008) 9:747–58. 10.1038/nrn2493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang M, Han L, Xu Y. Roles of cocaine- and amphetamine-regulated transcript in the central nervous system. Clin Exp Pharmacol Physiol (2012) 39:586–92. 10.1111/j.1440-1681.2011.05642.x [DOI] [PubMed] [Google Scholar]
- 47.Volkoff H. The effects of amphetamine injections on feeding behavior and the brain expression of orexin, CART, tyrosine hydroxylase (TH) and thyrotropin releasing hormone (TRH) in goldfish (Carassius auratus). Fish Physiol Biochem (2013) 39:979–91. 10.1007/s10695-012-9756-4 [DOI] [PubMed] [Google Scholar]
- 48.Kristensen P, Judge ME, Thim L, Ribel U, Christjansen KN, Wulff BS, et al. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature (1998) 393:72–6. 10.1038/29993 [DOI] [PubMed] [Google Scholar]
- 49.Vrang N, Larsen PJ, Kristensen P, Tang-Christensen M. Central administration of cocaine-amphetamine-regulated transcript activates hypothalamic neuroendocrine neurons in the rat. Endocrinology (2000) 141:794–801. 10.1210/endo.141.2.7295 [DOI] [PubMed] [Google Scholar]
- 50.Kuhar MJ, Adams S, Dominguez G, Jaworski J, Balkan B. CART peptides. Neuropeptides (2002) 36:1–8. 10.1054/npep.2002.0887 [DOI] [PubMed] [Google Scholar]
- 51.Tachibana T, Takagi T, Tomonaga S, Ohgushi A, Ando R, Denbow DM, et al. Central administration of cocaine- and amphetamine-regulated transcript inhibits food intake in chicks. Neurosci Lett (2003) 337:131–4. 10.1016/S0304-3940(02)01321-6 [DOI] [PubMed] [Google Scholar]
- 52.Volkoff H, Peter RE. Effects of CART peptides on food consumption, feeding and associated behaviors in the goldfish, Carassius auratus: actions on neuropeptide Y- and orexin A-induced feeding. Brain Res (2000) 887:125–33. 10.1016/S0006-8993(00)03001-8 [DOI] [PubMed] [Google Scholar]
- 53.Volkoff H, Peter RE. Characterization of two forms of cocaine- and amphetamine-regulated transcript (CART) peptide precursors in goldfish: molecular cloning and distribution, modulation of expression by nutritional status, and interactions with leptin. Endocrinology (2001) 142:5076–88. 10.1210/endo.142.12.8519 [DOI] [PubMed] [Google Scholar]
- 54.Akash G, Kaniganti T, Tiwari NK, Subhedar NK, Ghose A. Differential distribution and energy status-dependent regulation of the four CART neuropeptide genes in the zebrafish brain. J Comp Neurol (2014) 522:2266–85. 10.1002/cne.23532 [DOI] [PubMed] [Google Scholar]
- 55.Murashita K, Kurokawa T. Multiple cocaine- and amphetamine-regulated transcript (CART) genes in medaka, Oryzias latipes: cloning, tissue distribution and effect of starvation. Gen Comp Endocrinol (2011) 170:494–500. 10.1016/j.ygcen.2010.11.005 [DOI] [PubMed] [Google Scholar]
- 56.Bonacic K, Martinez A, Martin-Robles AJ, Munoz-Cueto JA, Morais S. Characterization of seven cocaine- and amphetamine-regulated transcripts (CARTs) differentially expressed in the brain and peripheral tissues of Solea senegalensis (Kaup). Gen Comp Endocrinol (2015) 224:260–72. 10.1016/j.ygcen.2015.08.017 [DOI] [PubMed] [Google Scholar]
- 57.Murashita K, Kurokawa T, Ebbesson LOE, Stefansson SO, Rønnestad I. Characterization, tissue distribution, and regulation of agouti-related protein (AgRP), cocaine- and amphetamine-regulated transcript (CART) and neuropeptide Y (NPY) in Atlantic salmon (Salmo salar). Gen Comp Endocrinol (2009) 162:160–71. 10.1016/j.ygcen.2009.03.015 [DOI] [PubMed] [Google Scholar]
- 58.Nishio S, Gibert Y, Berekelya L, Bernard L, Brunet F, Guillot E, et al. Fasting induces CART down-regulation in the zebrafish nervous system in a cannabinoid receptor 1-dependent manner. Mol Endocrinol (2012) 26:1316–26. 10.1210/me.2011-1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Libran-Perez M, Velasco C, Lopez-Patino MA, Miguez JM, Soengas JL. Counter-regulatory response to a fall in circulating fatty acid levels in rainbow trout. Possible involvement of the hypothalamus-pituitary-interrenal axis. PLoS One (2014) 9:e113291. 10.1371/journal.pone.0113291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Conde-Sieira M, Bonacic K, Velasco C, Valente LMP, Morais S, Soengas JL. Hypothalamic fatty acid sensing in Senegalese sole (Solea senegalensis): response to long-chain saturated, monounsaturated, and polyunsaturated (n-3) fatty acids. Am J Physiol Regul Integr Comp Physiol (2015) 309:R1521–31. 10.1152/ajpregu.00386.2015 [DOI] [PubMed] [Google Scholar]
- 61.Matsuki T, Sakurai T. Orexins and orexin receptors: from molecules to integrative physiology. In: Civelli O, Zhou Q-Y, editors. Orphan G Protein-Coupled Receptors and Novel Neuropeptides. Berlin, Heidelberg: Springer; (2008). p. 27–55. [DOI] [PubMed] [Google Scholar]
- 62.Nunez A, Rodrigo-Angulo ML, Andres ID, Garzon M. Hypocretin/orexin neuropeptides: participation in the control of sleep-wakefulness cycle and energy homeostasis. Curr Neuropharmacol (2009) 7:50–9. 10.2174/157015909787602797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li S-B, Jones JR, de Lecea L. Hypocretins, neural systems, physiology, and psychiatric disorders. Curr Psychiatry Rep (2016) 18:1–12. 10.1007/s11920-015-0639-0 [DOI] [PubMed] [Google Scholar]
- 64.Teske JA, Mavanji V. Energy expenditure: role of orexin. Vitam Horm (2012) 89:91–109. 10.1016/B978-0-12-394623-2.00006-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nixon JP, Kotz CM, Novak CM, Billington CJ, Teske JA. Neuropeptides controlling energy balance: orexins and neuromedins. Handb Exp Pharmacol (2012) 209:77–109. 10.1007/978-3-642-24716-3_4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hoskins LJ, Xu M, Volkoff H. Interactions between gonadotropin-releasing hormone (GnRH) and orexin in the regulation of feeding and reproduction in goldfish (Carassius auratus). Horm Behav (2008) 54:379–85. 10.1016/j.yhbeh.2008.04.011 [DOI] [PubMed] [Google Scholar]
- 67.Wall A, Volkoff H. Effects of fasting and feeding on the brain mRNA expressions of orexin, tyrosine hydroxylase (TH), PYY and CCK in the Mexican blind cavefish (Astyanax fasciatus mexicanus). Gen Comp Endocrinol (2013) 183:44–52. 10.1016/j.ygcen.2012.12.011 [DOI] [PubMed] [Google Scholar]
- 68.Novak CM, Jiang XL, Wang CF, Teske JA, Kotz CM, Levine JA. Caloric restriction and physical activity in zebrafish (Danio rerio). Neurosci Lett (2005) 383:99–104. 10.1016/j.neulet.2005.03.048 [DOI] [PubMed] [Google Scholar]
- 69.Amiya N, Mizusawa K, Kobayashi Y, Yamanome T, Amano M, Takahashi A. Food deprivation increases the expression of the prepro-orexin gene in the hypothalamus of the barfin flounder, Verasper moseri. Zoolog Sci (2012) 29:43–8. 10.2108/zsj.29.43 [DOI] [PubMed] [Google Scholar]
- 70.Yan A, Zhang L, Tang Z, Zhang Y, Qin C, Li B, et al. Orange-spotted grouper (Epinephelus coioides) orexin: molecular cloning, tissue expression, ontogeny, daily rhythm and regulation of NPY gene expression. Peptides (2011) 32:1363–70. 10.1016/j.peptides.2011.05.004 [DOI] [PubMed] [Google Scholar]
- 71.Xu MY, Volkoff H. Molecular characterization of prepro-orexin in Atlantic cod (Gadus morhua): cloning, localization, developmental profile and role in food intake regulation. Mol Cell Endocrinol (2007) 271:28–37. 10.1016/j.mce.2007.03.003 [DOI] [PubMed] [Google Scholar]
- 72.Buckley C, MacDonald EE, Tuziak SM, Volkoff H. Molecular cloning and characterization of two putative appetite regulators in winter flounder (Pleuronectes americanus): preprothyrotropin-releasing hormone (TRH) and preproorexin (OX). Peptides (2010) 31:1737–47. 10.1016/j.peptides.2010.05.017 [DOI] [PubMed] [Google Scholar]
- 73.Volkoff H, Sabioni RE, Cyrino JEP. Appetite regulating factors in dourado, Salminus brasiliensis: cDNA cloning and effects of fasting and feeding on gene expression. Gen Comp Endocrinol (2016) 237:34–42. 10.1016/j.ygcen.2016.07.022 [DOI] [PubMed] [Google Scholar]
- 74.Tsujino N, Sakurai T. Orexin/hypocretin: a neuropeptide at the interface of sleep, energy homeostasis, and reward system. Pharmacol Rev (2009) 61:162–76. 10.1124/pr.109.001321 [DOI] [PubMed] [Google Scholar]
- 75.Volkoff H, Bjorklund JM, Peter RE. Stimulation of feeding behavior and food consumption in the goldfish, Carassius auratus, by orexin-A and orexin-B. Brain Res (1999) 846:204–9. 10.1016/S0006-8993(99)02052-1 [DOI] [PubMed] [Google Scholar]
- 76.Facciolo RM, Crudo M, Zizza M, Giusi G, Canonaco M. Feeding behaviors and ORXR-beta-GABA A R subunit interactions in Carassius auratus. Neurotoxicol Teratol (2011) 33:641–50. 10.1016/j.ntt.2011.09.008 [DOI] [PubMed] [Google Scholar]
- 77.Nakamachi T, Matsuda K, Maruyama K, Miura T, Uchiyama M, Funahashi H, et al. Regulation by orexin of feeding behaviour and locomotor activity in the goldfish. J Neuroendocrinol (2006) 18:290–7. 10.1111/j.1365-2826.2006.01415.x [DOI] [PubMed] [Google Scholar]
- 78.Matsuda K, Kang KS, Sakashita A, Yahashi S, Vaudry H. Behavioral effect of neuropeptides related to feeding regulation in fish. Ann N Y Acad Sci (2011) 1220:117–26. 10.1111/j.1749-6632.2010.05884.x [DOI] [PubMed] [Google Scholar]
- 79.Penney CC, Volkoff H. Peripheral injections of cholecystokinin, apelin, ghrelin and orexin in cavefish (Astyanax fasciatus mexicanus): effects on feeding and on the brain expression levels of tyrosine hydroxylase, mechanistic target of rapamycin and appetite-related hormones. Gen Comp Endocrinol (2014) 196:34–40. 10.1016/j.ygcen.2013.11.015 [DOI] [PubMed] [Google Scholar]
- 80.Yokobori E, Kojima K, Azuma M, Kang KS, Maejima S, Uchiyama M, et al. Stimulatory effect of intracerebroventricular administration of orexin A on food intake in the zebrafish, Danio rerio. Peptides (2011) 32:1357–62. 10.1016/j.peptides.2011.05.010 [DOI] [PubMed] [Google Scholar]
- 81.Panula P. Hypocretin/orexin in fish physiology with emphasis on zebrafish. Acta Physiol (Oxf) (2010) 198:381–6. 10.1111/j.1748-1716.2009.02038.x [DOI] [PubMed] [Google Scholar]
- 82.Abbott M, Volkoff H. Thyrotropin releasing hormone (TRH) in goldfish (Carassius auratus): role in the regulation of feeding and locomotor behaviors and interactions with the orexin system and cocaine- and amphetamine-regulated transcript (CART). Horm Behav (2011) 59:236–45. 10.1016/j.yhbeh.2010.12.008 [DOI] [PubMed] [Google Scholar]
- 83.Hoskins LJ, Volkoff H. Daily patterns of mRNA expression of two core circadian regulatory proteins, Clock2 and Per1, and two appetite-regulating peptides, OX and NPY, in goldfish (Carassius auratus, Linnaeus). Comp Biochem Physiol A Physiol (2012) 163:127–36. 10.1016/j.cbpa.2012.05.197 [DOI] [PubMed] [Google Scholar]
- 84.Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou M-X, et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun (1998) 251:471–6. 10.1006/bbrc.1998.9489 [DOI] [PubMed] [Google Scholar]
- 85.Fang P, Yu M, Guo L, Bo P, Zhang Z, Shi M. Galanin and its receptors: a novel strategy for appetite control and obesity therapy. Peptides (2012) 36:331–9. 10.1016/j.peptides.2012.05.016 [DOI] [PubMed] [Google Scholar]
- 86.Lang R, Gundlach AL, Holmes FE, Hobson SA, Wynick D, Hökfelt T, et al. Physiology, signaling, and pharmacology of galanin peptides and receptors: three decades of emerging diversity. Pharmacol Rev (2014) 67:118. 10.1124/pr.112.006536 [DOI] [PubMed] [Google Scholar]
- 87.Mensah ET, Volkoff H, Unniappan S. Galanin systems in non-mammalian vertebrates with special focus on fishes. EXS (2010) 102:243–62. 10.1007/978-3-0346-0228-0_17 [DOI] [PubMed] [Google Scholar]
- 88.de Pedro N, Cespedes MV, Delgado MJ, Alonso-Bedate M. The galanin-induced feeding stimulation is mediated via alpha-adrenergic receptors in goldfish. Regul Pept (1995) 57:77–84. 10.1016/0167-0115(95)91255-4 [DOI] [PubMed] [Google Scholar]
- 89.Guijarro AI, Delgado MJ, Pinillos ML, López-Patiño MA, Alonso-Bedate M, De Pedro N. Galanin and β-endorphin as feeding regulators in cyprinids: effect of temperature. Aquac Res (1999) 30:483–9. 10.1046/j.1365-2109.1999.00360.x [DOI] [Google Scholar]
- 90.Unniappan S, Cerda-Reverter JM, Peter RE. In situ localization of preprogalanin mRNA in the goldfish brain and changes in its expression during feeding and starvation. Gen Comp Endocrinol (2004) 136:200–7. 10.1016/j.ygcen.2003.12.010 [DOI] [PubMed] [Google Scholar]
- 91.Sterling ME, Karatayev O, Chang GQ, Algava DB, Leibowitz SF. Model of voluntary ethanol intake in zebrafish: effect on behavior and hypothalamic orexigenic peptides. Behav Brain Res (2015) 278:29–39. 10.1016/j.bbr.2014.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Oshima N, Kasukawa H, Fujii R, Wilkes BC, Hruby VJ, Hadley ME. Action of melanin-concentrating hormone (MCH) on teleost chromatophores. Gen Comp Endocrinol (1986) 64:381–8. 10.1016/0016-6480(86)90072-9 [DOI] [PubMed] [Google Scholar]
- 93.Kawauchi H, Kawazoe I, Tsubokawa M, Kishida M, Baker BI. Characterization of melanin-concentrating hormone in chum salmon pituitaries. Nature (1983) 305:321–3. 10.1038/305321a0 [DOI] [PubMed] [Google Scholar]
- 94.Joost HG. Appetite Control. Heidelberg: Springer; (2012). [Google Scholar]
- 95.Matsuda K, Shimakura S, Maruyama K, Miura T, Uchiyama M, Kawauchi H, et al. Central administration of melanin-concentrating hormone (MCH) suppresses food intake, but not locomotor activity, in the goldfish, Carassius auratus. Neurosci Lett (2006) 399:259–63. 10.1016/j.neulet.2006.02.005 [DOI] [PubMed] [Google Scholar]
- 96.Matsuda K, Shimakura S, Miura T, Maruyama K, Uchiyama M, Kawauchi H, et al. Feeding-induced changes of melanin-concentrating hormone (MCH)-like immunoreactivity in goldfish brain. Cell Tissue Res (2007) 328:375–82. 10.1007/s00441-006-0347-5 [DOI] [PubMed] [Google Scholar]
- 97.Shimakura S, Miura T, Maruyama K, Nakamachi T, Uchiyama M, Kageyama H, et al. Alpha-melanocyte-stimulating hormone mediates melanin-concentrating hormone-induced anorexigenic action in goldfish. Horm Behav (2008) 53:323–8. 10.1016/j.yhbeh.2007.10.009 [DOI] [PubMed] [Google Scholar]
- 98.Tuziak SM, Volkoff H. A preliminary investigation of the role of melanin-concentrating hormone (MCH) and its receptors in appetite regulation of winter flounder (Pseudopleuronectes americanus). Mol Cell Endocrinol (2012) 348:281–96. 10.1016/j.mce.2011.09.015 [DOI] [PubMed] [Google Scholar]
- 99.Takahashi A, Tsuchiya K, Yamanome T, Amano M, Yasuda A, Yamamori K, et al. Possible involvement of melanin-concentrating hormone in food intake in a teleost fish, barfin flounder. Peptides (2004) 25:1613–22. 10.1016/j.peptides.2004.02.022 [DOI] [PubMed] [Google Scholar]
- 100.Berman JR, Skariah G, Maro GÂS, Mignot E, Mourrain P. Characterization of two melanin-concentrating hormone genes in zebrafish reveals evolutionary and physiological links with the mammalian MCH system. J Comp Neurol (2009) 517:695–710. 10.1002/cne.22171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tuziak SM, Volkoff H. Melanin-concentrating hormone (MCH) and gonadotropin-releasing hormones (GnRH) in Atlantic cod, Gadus morhua: tissue distributions, early ontogeny and effects of fasting. Peptides (2013) 50:109–18. 10.1016/j.peptides.2013.10.005 [DOI] [PubMed] [Google Scholar]
- 102.Lovejoy DA. Chapter 101 – CRH family A2 – kastin. Second ed In: Abba J, editor. Handbook of Biologically Active Peptides. Boston, MA: Academic Press; (2013). p. 752–9. [Google Scholar]
- 103.Bernier NJ, Peter RE. The hypothalamic-pituitary-interrenal axis and the control of food intake in teleost fish. Comp Biochem Physiol B Biochem Mol Biol (2001) 129:639–44. 10.1016/S1096-4959(01)00360-8 [DOI] [PubMed] [Google Scholar]
- 104.De Pedro N, Alonso-Gomez AL, Gancedo B, Delgado MJ, Alonso-Bedate M. Role of corticotropin-releasing factor (CRF) as a food intake regulator in goldfish. Physiol Behav (1993) 53:517–20. 10.1016/0031-9384(93)90146-7 [DOI] [PubMed] [Google Scholar]
- 105.Matsuda K. Regulation of feeding behavior and psychomotor activity by corticotropin-releasing hormone (CRH) in fish. Front Neurosci (2013) 7:91. 10.3389/fnins.2013.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ortega VA, Lovejoy DA, Bernier NJ. Appetite-suppressing effects and interactions of centrally administered corticotropin-releasing factor, urotensin I and serotonin in rainbow trout (Oncorhynchus mykiss). Front Neurosci (2013) 7:196. 10.3389/fnins.2013.00196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang T, Zhou C, Yuan D, Lin F, Chen H, Wu H, et al. Schizothorax prenanti corticotropin-releasing hormone (CRH): molecular cloning, tissue expression, and the function of feeding regulation. Fish Physiol Biochem (2014) 40:1407–15. 10.1007/s10695-014-9935-6 [DOI] [PubMed] [Google Scholar]
- 108.Metz JR, Peters JJM, Flik G. Molecular biology and physiology of the melanocortin system in fish: a review. Gen Comp Endocrinol (2006) 148:150–62. 10.1016/j.ygcen.2006.03.001 [DOI] [PubMed] [Google Scholar]
- 109.Klovins J, Haitina T, Fridmanis D, Kilianova Z, Kapa I, Fredriksson R, et al. The melanocortin system in Fugu: determination of POMC/AGRP/MCR gene repertoire and synteny, as well as pharmacology and anatomical distribution of the MCRs. Mol Biol Evol (2004) 21:563–79. 10.1093/molbev/msh050 [DOI] [PubMed] [Google Scholar]
- 110.Boswell T, Takeuchi S. Recent developments in our understanding of the avian melanocortin system: its involvement in the regulation of pigmentation and energy homeostasis. Peptides (2005) 26:1733–43. 10.1016/j.peptides.2004.11.039 [DOI] [PubMed] [Google Scholar]
- 111.Gantz I, Fong TM. The melanocortin system. Am J Physiol Endocrinol Metab (2003) 284:E468–74. 10.1152/ajpendo.00434.2002 [DOI] [PubMed] [Google Scholar]
- 112.Cone RD. Studies on the physiological functions of the melanocortin system. Endocr Rev (2006) 27:736–49. 10.1210/er.2006-0034 [DOI] [PubMed] [Google Scholar]
- 113.Gonzalez-Nunez V, Gonzalez-Sarmiento R, Rodriguez RE. Identification of two proopiomelanocortin genes in zebrafish (Danio rerio). Brain Res Mol Brain Res (2003) 120:1–8. 10.1016/j.molbrainres.2003.09.012 [DOI] [PubMed] [Google Scholar]
- 114.Arends RJ, Vermeer H, Martens GJ, Leunissen JA, Wendelaar Bonga SE, Flik G. Cloning and expression of two proopiomelanocortin mRNAs in the common carp (Cyprinus carpio L.). Mol Cell Endocrinol (1998) 143:23–31. 10.1016/S0303-7207(98)00139-7 [DOI] [PubMed] [Google Scholar]
- 115.Takahashi A, Amano M, Itoh T, Yasuda A, Yamanome T, Amemiya Y, et al. Nucleotide sequence and expression of three subtypes of proopiomelanocortin mRNA in barfin flounder. Gen Comp Endocrinol (2005) 141:291–303. 10.1016/j.ygcen.2005.01.010 [DOI] [PubMed] [Google Scholar]
- 116.Cardoso JC, Laiz-Carrion R, Louro B, Silva N, Canario AV, Mancera JM, et al. Divergence of duplicate POMC genes in gilthead sea bream Sparus auratus. Gen Comp Endocrinol (2011) 173:396–404. 10.1016/j.ygcen.2010.12.001 [DOI] [PubMed] [Google Scholar]
- 117.Okuta A, Ando H, Ueda H, Urano A. Two types of cDNAs encoding proopiomelanocortin of sockeye salmon, Oncorhynchus nerka. Zoolog Sci (1996) 13:421–7. 10.2108/zsj.13.421 [DOI] [PubMed] [Google Scholar]
- 118.Takahashi A, Kobayashi Y, Amano M, Yamanome T. Structural and functional diversity of proopiomelanocortin in fish with special reference to barfin flounder. Peptides (2009) 30:1374–82. 10.1016/j.peptides.2009.04.014 [DOI] [PubMed] [Google Scholar]
- 119.Leder EH, Silverstein JT. The pro-opiomelanocortin genes in rainbow trout (Oncorhynchus mykiss): duplications, splice variants, and differential expression. J Endocrinol (2006) 188:355–63. 10.1677/joe.1.06283 [DOI] [PubMed] [Google Scholar]
- 120.Murashita K, Jordal A-EO, Nilsen TO, Stefansson SO, Kurokawa T, Björnsson BT, et al. Leptin reduces Atlantic salmon growth through the central pro-opiomelanocortin pathway. Comp Biochem Physiol Part A Mol Integr Physiol (2011) 158:79–86. 10.1016/j.cbpa.2010.09.001 [DOI] [PubMed] [Google Scholar]
- 121.Jørgensen EH, Bernier NJ, Maule AG, Vijayan MM. Effect of long-term fasting and a subsequent meal on mRNA abundances of hypothalamic appetite regulators, central and peripheral leptin expression and plasma leptin levels in rainbow trout. Peptides (2016) 86:162–70. 10.1016/j.peptides.2015.08.010 [DOI] [PubMed] [Google Scholar]
- 122.Kang DY, Kim HC. Functional relevance of three proopiomelanocortin (POMC) genes in darkening camouflage, blind-side hypermelanosis, and appetite of Paralichthys olivaceus. Comp Biochem Physiol B Biochem Mol Biol (2015) 179:44–56. 10.1016/j.cbpb.2014.09.002 [DOI] [PubMed] [Google Scholar]
- 123.Cerdá-Reverter JM, Ringholm A, Schiöth HB, Peter RE. Molecular cloning, pharmacological characterization, and brain mapping of the melanocortin 4 receptor in the goldfish: involvement in the control of food intake. Endocrinology (2003) 144:2336–49. 10.1210/en.2002-0213 [DOI] [PubMed] [Google Scholar]
- 124.Song Y, Golling G, Thacker T, Cone R. Agouti-related protein (AGRP) is conserved and regulated by metabolic state in the zebrafish, Danio rerio. Endocrine (2003) 22:257–65. 10.1385/ENDO:22:3:257 [DOI] [PubMed] [Google Scholar]
- 125.Forlano PM, Cone RD. Conserved neurochemical pathways involved in hypothalamic control of energy homeostasis. J Comp Neurol (2007) 505:235–48. 10.1002/cne.21447 [DOI] [PubMed] [Google Scholar]
- 126.Cerda-Reverter JM, Schioth HB, Peter RE. The central melanocortin system regulates food intake in goldfish. Regul Pept (2003) 115:101–13. 10.1016/S0167-0115(03)00144-7 [DOI] [PubMed] [Google Scholar]
- 127.Ringholm A, Fredriksson R, Poliakova N, Yan YL, Postlethwait JH, Larhammar D, et al. One melanocortin 4 and two melanocortin 5 receptors from zebrafish show remarkable conservation in structure and pharmacology. J Neurochem (2002) 82:6–18. 10.1046/j.1471-4159.2002.00934.x [DOI] [PubMed] [Google Scholar]
- 128.Li J-T, Yang Z, Chen H-P, Zhu C-H, Deng S-P, Li G-L, et al. Molecular cloning, tissue distribution, and pharmacological characterization of melanocortin-4 receptor in spotted scat, Scatophagus argus. Gen Comp Endocrinol (2016) 230–231:143–52. 10.1016/j.ygcen.2016.04.010 [DOI] [PubMed] [Google Scholar]
- 129.Jangprai A, Boonanuntanasarn S, Yoshizaki G. Characterization of melanocortin 4 receptor in snakeskin gourami and its expression in relation to daily feed intake and short-term fasting. Gen Comp Endocrinol (2011) 173:27–37. 10.1016/j.ygcen.2011.04.021 [DOI] [PubMed] [Google Scholar]
- 130.Wan Y, Zhang Y, Ji P, Li Y, Xu P, Sun X. Molecular characterization of CART, AgRP, and MC4R genes and their expression with fasting and re-feeding in common carp (Cyprinus carpio). Mol Biol Rep (2012) 39:2215–23. 10.1007/s11033-011-0970-4 [DOI] [PubMed] [Google Scholar]
- 131.Wei R, Yuan D, Wang T, Zhou C, Lin F, Chen H, et al. Characterization, tissue distribution and regulation of agouti-related protein (AgRP) in a cyprinid fish (Schizothorax prenanti). Gene (2013) 527:193–200. 10.1016/j.gene.2013.06.003 [DOI] [PubMed] [Google Scholar]
- 132.Schiöth HB, Haitina T, Ling MK, Ringholm A, Fredriksson R, Cerdá-Reverter JM, et al. Evolutionary conservation of the structural, pharmacological, and genomic characteristics of the melanocortin receptor subtypes. Peptides (2005) 26:1886–900. 10.1016/j.peptides.2004.11.034 [DOI] [PubMed] [Google Scholar]
- 133.Klovins J, Haitina T, Ringholm A, Löwgren M, Fridmanis D, Slaidina M, et al. Cloning of two melanocortin (MC) receptors in spiny dogfish. Eur J Biochem (2004) 271:4320–31. 10.1111/j.1432-1033.2004.04374.x [DOI] [PubMed] [Google Scholar]
- 134.Kobayashi Y, Tsuchiya K, Yamanome T, Schioth HB, Kawauchi H, Takahashi A. Food deprivation increases the expression of melanocortin-4 receptor in the liver of barfin flounder, Verasper moseri. Gen Comp Endocrinol (2008) 155:280–7. 10.1016/j.ygcen.2007.05.010 [DOI] [PubMed] [Google Scholar]
- 135.Sanchez E, Rubio VC, Thompson D, Metz J, Flik G, Millhauser GL, et al. Phosphodiesterase inhibitor-dependent inverse agonism of agouti-related protein on melanocortin 4 receptor in sea bass (Dicentrarchus labrax). Am J Physiol Regul Integr Comp Physiol (2009) 296:R1293–306. 10.1152/ajpregu.90948.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schjolden J, Schioth HB, Larhammar D, Winberg S, Larson ET. Melanocortin peptides affect the motivation to feed in rainbow trout (Oncorhynchus mykiss). Gen Comp Endocrinol (2009) 160:134–8. 10.1016/j.ygcen.2008.11.003 [DOI] [PubMed] [Google Scholar]
- 137.Cerdá-Reverter JM, Peter RE. Endogenous melanocortin antagonist in fish: structure, brain mapping, and regulation by fasting of the goldfish agouti-related protein gene. Endocrinology (2003) 144:4552–61. 10.1210/en.2003-0453 [DOI] [PubMed] [Google Scholar]
- 138.Kallman KD, Borkoski V. Sex-linked gene controlling onset of sexual maturity in female and male platyfish (Xiphophorus maculatus), fecundity in females and adult size in males. Genetics (1978) 89:79–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lampert KP, Schmidt C, Fischer P, Volff JN, Hoffmann C, Muck J, et al. Determination of onset of sexual maturation and mating behavior by melanocortin receptor 4 polymorphisms. Curr Biol (2010) 20:1729–34. 10.1016/j.cub.2010.08.029 [DOI] [PubMed] [Google Scholar]
- 140.Valassi E, Scacchi M, Cavagnini F. Neuroendocrine control of food intake. Nutr Metab Cardiovasc Dis (2008) 18:158–68. 10.1016/j.numecd.2007.06.004 [DOI] [PubMed] [Google Scholar]
- 141.Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen YR, Gantz I, et al. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science (1997) 278:135–8. 10.1126/science.278.5335.135 [DOI] [PubMed] [Google Scholar]
- 142.Klovins J, Schioth HB. Agouti-related proteins (AGRPs) and agouti-signaling peptide (ASIP) in fish and chicken. Ann N Y Acad Sci (2005) 1040:363–7. 10.1196/annals.1327.063 [DOI] [PubMed] [Google Scholar]
- 143.Kurokawa T, Murashita K, Uji S. Characterization and tissue distribution of multiple agouti-family genes in pufferfish, Takifugu rubripes. Peptides (2006) 27:3165–75. 10.1016/j.peptides.2006.09.013 [DOI] [PubMed] [Google Scholar]
- 144.Agulleiro MJ, Cortés R, Leal E, Ríos D, Sánchez E, Cerdá-Reverter JM. Characterization, tissue distribution and regulation by fasting of the agouti family of peptides in the sea bass (Dicentrarchus labrax). Gen Comp Endocrinol (2014) 205:251–9. 10.1016/j.ygcen.2014.02.009 [DOI] [PubMed] [Google Scholar]
- 145.Song Y, Cone RD. Creation of a genetic model of obesity in a teleost. FASEB J (2007) 21:2042–9. 10.1096/fj.06-7503com [DOI] [PubMed] [Google Scholar]
- 146.Kalra SP, Dube MG, Pu SY, Xu B, Horvath TL, Kalra PS. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr Rev (1999) 20:68–100. 10.1210/er.20.1.68 [DOI] [PubMed] [Google Scholar]
- 147.Wynne K, Stanley S, McGowan B, Bloom S. Appetite control. J Endocrinol (2005) 184:291–318. 10.1677/joe.1.05866 [DOI] [PubMed] [Google Scholar]
- 148.Bouret SG, Draper SJ, Simerly RB. Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice. J Neurosci (2004) 24:2797–805. 10.1523/JNEUROSCI.5369-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.King BA. The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiol Behav (2006) 87:221–44. 10.1016/j.physbeh.2005.10.007 [DOI] [PubMed] [Google Scholar]
- 150.Cerdá-Reverter JM, Canosa LF. Chapter 1 Neuroendocrine systems of the fish brain. In: Bernier N, Kraak GVD, Farrell A, Brauner C, editors. Fish Physiology. Amsterdam: Academic Press; (2009). p. 3–74. [Google Scholar]
- 151.Machluf Y, Gutnick A, Levkowitz G. Development of the zebrafish hypothalamus. Ann N Y Acad Sci (2011) 1220:93–105. 10.1111/j.1749-6632.2010.05945.x [DOI] [PubMed] [Google Scholar]
- 152.Suarez MD, Martinez TF, Saez MI, Morales AE, Garcia-Gallego M. Effects of dietary restriction on post-mortem changes in white muscle of sea bream (Sparus aurata). Aquaculture (2010) 307:49–55. 10.1016/j.aquaculture.2010.07.006 [DOI] [Google Scholar]
- 153.Biran J, Tahor M, Wircer E, Levkowitz G. Role of developmental factors in hypothalamic function. Front Neuroanat (2015) 9:47. 10.3389/fnana.2015.00047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liu Q, Chen Y, Copeland D, Ball H, Duff RJ, Rockich B, et al. Expression of leptin receptor gene in developing and adult zebrafish. Gen Comp Endocrinol (2010) 166:346–55. 10.1016/j.ygcen.2009.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cerdá-Reverter JM, Anglade I, Martínez-Rodríguez G, Mazurais D, Muñoz-Cueto JA, Carrillo M, et al. Characterization of neuropeptide Y expression in the brain of a perciform fish, the sea bass (Dicentrarchus labrax). J Chem Neuroanat (2000) 19:197–210. 10.1016/S0891-0618(00)00063-6 [DOI] [PubMed] [Google Scholar]
- 156.Traverso JM, Ravaglia MA, Vissio PG, Maggese MC, Paz DA. Localization of neuropeptide Y-like immunoreactive structures in the brain of the pejerrey, Odontesthes bonariensis (Teleostei, Atheriniformes). Anat Histol Embryol (2003) 32:29–35. 10.1046/j.1439-0264.2003.00434.x [DOI] [PubMed] [Google Scholar]
- 157.Chiba A, Honma Y. Neuropeptide Y-immunoreactive structures in the telencephalon and diencephalon of the white sturgeon, Acipenser transmontanus, with special regard to the hypothalamo-hypophyseal system. Arch Histol Cytol (1994) 57:77–86. 10.1679/aohc.57.77 [DOI] [PubMed] [Google Scholar]
- 158.Vallarino M, Danger JM, Fasolo A, Pelletier G, Saint-Pierre S, Vaudry H. Distribution and characterization of neuropeptide Y in the brain of an elasmobranch fish. Brain Res (1988) 448:67–76. 10.1016/0006-8993(88)91102-X [DOI] [PubMed] [Google Scholar]
- 159.Le HTMD, Angotzi AR, Ebbesson LOE, Karlsen Ø, Rønnestad I. The ontogeny and brain distribution dynamics of the appetite regulators npy, cart and pox in larval Atlantic cod (Gadus morhua L.). PLoS One (2016) 11:e0153743. 10.1371/journal.pone.0153743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Herget U, Wolf A, Wullimann MF, Ryu S. Molecular neuroanatomy and chemoarchitecture of the neurosecretory preoptic-hypothalamic area in zebrafish larvae. J Comp Neurol (2014) 522:1542–64. 10.1002/cne.23480 [DOI] [PubMed] [Google Scholar]
- 161.Olivereau M, Olivereau J. Localization of crf-like immunoreactivity in the brain and pituitary of teleost fish. Peptides (1988) 9:13–21. 10.1016/0196-9781(88)90004-6 [DOI] [PubMed] [Google Scholar]
- 162.Matz SP, Hofeldt GT. Immunohistochemical localization of corticotropin-releasing factor in the brain and corticotropin-releasing factor and thyrotropin-stimulating hormone in the pituitary of Chinook salmon (Oncorhynchus tshawytscha). Gen Comp Endocrinol (1999) 114:151–60. 10.1006/gcen.1999.7253 [DOI] [PubMed] [Google Scholar]
- 163.Ando H, Ando J, Urano A. Localization of mRNA encoding thyrotropin-releasing hormone precursor in the brain of sockeye salmon. Zoolog Sci (1998) 15:945–53. 10.2108/zsj.15.945 [DOI] [Google Scholar]
- 164.Grone BP, Maruska KP. Divergent evolution of two corticotropin-releasing hormone (CRH) genes in teleost fishes. Front Neurosci (2015) 9:365. 10.3389/fnins.2015.00365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Frankish HM, Dryden S, Hopkins D, Wang Q, Williams G. Neuropeptide-Y, the hypothalamus, and diabetes – insights into the central control of metabolism. Peptides (1995) 16:757–71. 10.1016/0196-9781(94)00200-P [DOI] [PubMed] [Google Scholar]
- 166.Perez Sirkin DI, Suzuki H, Canepa MM, Vissio PG. Orexin and neuropeptide Y: tissue specific expression and immunoreactivity in the hypothalamus and preoptic area of the cichlid fish Cichlasoma dimerus. Tissue Cell (2013) 45:452–9. 10.1016/j.tice.2013.09.001 [DOI] [PubMed] [Google Scholar]
- 167.Williams G, Bing C, Cai XJ, Harrold JA, King PJ, Liu XH. The hypothalamus and the control of energy homeostasis: different circuits, different purposes. Physiol Behav (2001) 74:683–701. 10.1016/S0031-9384(01)00612-6 [DOI] [PubMed] [Google Scholar]
- 168.Faraco JH, Appelbaum L, Marin W, Gaus SE, Mourrain P, Mignot E. Regulation of hypocretin (orexin) expression in embryonic zebrafish. J Biol Chem (2006) 281:29753–61. 10.1074/jbc.M605811200 [DOI] [PubMed] [Google Scholar]
- 169.Kaslin J, Nystedt JM, Ostergard M, Peitsaro N, Panula P. The orexin/hypocretin system in zebrafish is connected to the aminergic and cholinergic systems. J Neurosci (2004) 24:2678–89. 10.1523/JNEUROSCI.4908-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chen R, Li W, Lin H. cDNA cloning and mRNA expression of neuropeptide Y in orange spotted grouper, Epinephelus coioides. Comp Biochem Physiol B Biochem Mol Biol (2005) 142:79–89. 10.1016/j.cbpc.2005.06.003 [DOI] [PubMed] [Google Scholar]
- 171.Kojima K, Kamijo M, Kageyama H, Uchiyama M, Shioda S, Matsuda K. Neuronal relationship between orexin-A- and neuropeptide Y-induced orexigenic actions in goldfish. Neuropeptides (2009) 43:63–71. 10.1016/j.npep.2009.01.004 [DOI] [PubMed] [Google Scholar]
- 172.Brothers SP, Wahlestedt C. Therapeutic potential of neuropeptide Y (NPY) receptor ligands. EMBO Mol Med (2010) 2:429–39. 10.1002/emmm.201000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mendieta-Zerón H, López M, Dieguez C. Gastrointestinal peptides controlling body weight homeostasis. Gen Comp Endocrinol (2008) 155:481–95. 10.1016/j.ygcen.2007.11.009 [DOI] [PubMed] [Google Scholar]
- 174.Jönsson E, Holmgren S. Integrated function and control of the gut endocrine | Endocrine systems of the gut. Encycl Fish Physiol (2011) 2:1341–7. [Google Scholar]
- 175.Murphy KG, Bloom SR. Gut hormones in the control of appetite. Exp Physiol (2004) 89:507–16. 10.1113/expphysiol.2004.027789 [DOI] [PubMed] [Google Scholar]
- 176.Johansson V. Behavioural Effects and Central Nervous System Actions of Growth Hormone in Salmonid Fish, Zoologiska Institutionen. Göteborg, Sweden: University of Gothenburg; (2004). [Google Scholar]
- 177.Matsuda K, Miura T, Kaiya H, Maruyama K, Shimakura S-I, Uchiyama M, et al. Regulation of food intake by acyl and des-acyl ghrelins in the goldfish. Peptides (2006) 27:2321–5. 10.1016/j.peptides.2006.03.028 [DOI] [PubMed] [Google Scholar]
- 178.Grove DJ, Loizides LG, Nott J. Satiation amount, frequency of feeding and gastric emptying rate in Salmo gairdneri. J Fish Biol (1978) 12:507–16. 10.1111/j.1095-8649.1978.tb04195.x [DOI] [Google Scholar]
- 179.Camilleri M. Peripheral mechanisms in appetite regulation. Gastroenterology (2015) 148:1219–33. 10.1053/j.gastro.2014.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kaiya H, Miyazato M, Kangawa K. Recent advances in the phylogenetic study of ghrelin. Peptides (2011) 32:2155–74. 10.1016/j.peptides.2011.04.027 [DOI] [PubMed] [Google Scholar]
- 181.Miura T, Maruyama K, Shimakura S-I, Kaiya H, Uchiyama M, Kangawa K, et al. Regulation of food intake in the goldfish by interaction between ghrelin and orexin. Peptides (2007) 28:1207–13. 10.1016/j.peptides.2007.03.023 [DOI] [PubMed] [Google Scholar]
- 182.Riley LG, Fox BK, Kaiya H, Hirano T, Grau EG. Long-term treatment of ghrelin stimulates feeding, fat deposition, and alters the GH/IGF-I axis in the tilapia, Oreochromis mossambicus. Gen Comp Endocrinol (2005) 142:234–40. 10.1016/j.ygcen.2005.01.009 [DOI] [PubMed] [Google Scholar]
- 183.Tinoco AB, Näslund J, Delgado MJ, de Pedro N, Johnsson JI, Jönsson E. Ghrelin increases food intake, swimming activity and growth in juvenile brown trout (Salmo trutta). Physiol Behav (2014) 124:15–22. 10.1016/j.physbeh.2013.10.034 [DOI] [PubMed] [Google Scholar]
- 184.Yuan X, Cai W, Liang X-F, Su H, Yuan Y, Li A, et al. Obestatin partially suppresses ghrelin stimulation of appetite in “high-responders” grass carp, Ctenopharyngodon idellus. Comp Biochem Physiol A Mol Integr Physiol (2015) 184:144–9. 10.1016/j.cbpa.2015.02.019 [DOI] [PubMed] [Google Scholar]
- 185.Date Y, Nakazato M, Murakami N, Kojima M, Kangawa K, Matsukura S. Ghrelin acts in the central nervous system to stimulate gastric acid secretion. Biochem Biophys Res Commun (2001) 280:904–7. 10.1006/bbrc.2000.4212 [DOI] [PubMed] [Google Scholar]
- 186.Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, et al. A role for ghrelin in the central regulation of feeding. Nature (2001) 409:194–8. 10.1038/35051587 [DOI] [PubMed] [Google Scholar]
- 187.Velasco C, Librán-Pérez M, Otero-Rodiño C, López-Patiño MA, Míguez JM, Cerdá-Reverter JM, et al. Ghrelin modulates hypothalamic fatty acid-sensing and control of food intake in rainbow trout. J Endocrinol (2016) 228:25–37. 10.1530/JOE-15-0391 [DOI] [PubMed] [Google Scholar]
- 188.Schroeter JC, Fenn CM, Small BC. Elucidating the roles of gut neuropeptides on channel catfish feed intake, glycemia, and hypothalamic NPY and POMC expression. Comp Biochem Physiol A Mol Integr Physiol (2015) 188:168–74. 10.1016/j.cbpa.2015.06.031 [DOI] [PubMed] [Google Scholar]
- 189.Miura T, Maruyama K, Shimakura S-I, Kaiya H, Uchiyama M, Kangawa K, et al. Neuropeptide Y mediates ghrelin-induced feeding in the goldfish, Carassius auratus. Neurosci Lett (2006) 407:279–83. 10.1016/j.neulet.2006.08.071 [DOI] [PubMed] [Google Scholar]
- 190.Polakof S, Miguez JM, Soengas JL. Ghrelin effects on central glucosensing and energy homeostasis-related peptides in rainbow trout. Domest Anim Endocrinol (2011) 41:126–36. 10.1016/j.domaniend.2011.05.006 [DOI] [PubMed] [Google Scholar]
- 191.Jönsson E, Kaiya H, Björnsson BT. Ghrelin decreases food intake in juvenile rainbow trout (Oncorhynchus mykiss) through the central anorexigenic corticotropin-releasing factor system. Gen Comp Endocrinol (2010) 166:39–46. 10.1016/j.ygcen.2009.11.001 [DOI] [PubMed] [Google Scholar]
- 192.Olsson C, Holbrook JD, Bompadre G, Jonsson E, Hoyle CHV, Sanger GJ, et al. Identification of genes for the ghrelin and motilin receptors and a novel related gene in fish, and stimulation of intestinal motility in zebrafish (Danio rerio) by ghrelin and motilin. Gen Comp Endocrinol (2008) 155:217–26. 10.1016/j.ygcen.2007.05.016 [DOI] [PubMed] [Google Scholar]
- 193.Olsson C, Holmberg A, Holmgren S. Development of enteric and vagal innervation of the zebrafish (Danio rerio) gut. J Comp Neurol (2008) 508:756–70. 10.1002/cne.21705 [DOI] [PubMed] [Google Scholar]
- 194.Kitazawa T, Itoh K, Yaosaka N, Maruyama K, Matsuda K, Teraoka H, et al. Ghrelin does not affect gastrointestinal contractility in rainbow trout and goldfish in vitro. Gen Comp Endocrinol (2012) 178:539–45. 10.1016/j.ygcen.2012.06.025 [DOI] [PubMed] [Google Scholar]
- 195.Chan CB, Cheng CHK. Identification and functional characterization of two alternatively spliced growth hormone secretagogue receptor transcripts from the pituitary of black seabream Acanthopagrus schlegeli. Mol Cell Endocrinol (2004) 214:81–95. 10.1016/j.mce.2003.11.020 [DOI] [PubMed] [Google Scholar]
- 196.Kaiya H, Mori T, Miyazato M, Kangawa K. Ghrelin receptor (GHS-R)-like receptor and its genomic organisation in rainbow trout, Oncorhynchus mykiss. Comp Biochem Physiol A Mol Integr Physiol (2009) 153:438–50. 10.1016/j.cbpa.2009.04.612 [DOI] [PubMed] [Google Scholar]
- 197.Raybould HE. Mechanisms of CCK signaling from gut to brain. Curr Opin Pharmacol (2007) 7:570–4. 10.1016/j.coph.2007.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Johnsen AH, Rehfeld JF. The phylogeny of the cholecystokinin gastrin family. Regul Pept (1992) 39:256–256. 10.1016/0167-0115(92)90560-H [DOI] [Google Scholar]
- 199.Chandra R, Liddle RA. Cholecystokinin. Curr Opin Endocrinol Diabetes Obes (2007) 14:63–7. 10.1097/MED.0b013e3280122850 [DOI] [PubMed] [Google Scholar]
- 200.Himick BA, Peter RE. Bombesin acts to suppress feeding behavior and alter serum growth hormone in goldfish. Physiol Behav (1994) 55:65–72. 10.1016/0031-9384(94)90011-6 [DOI] [PubMed] [Google Scholar]
- 201.Rubio VC, Sanchez-Vazquez FJ, Madrid JA. Role of cholecystokinin and its antagonist proglumide on macronutrient selection in European sea bass Dicentrarchus labrax, L. Physiol Behav (2008) 93:862–9. 10.1016/j.physbeh.2007.12.001 [DOI] [PubMed] [Google Scholar]
- 202.Gelineau A, Boujard T. Oral administration of cholecystokinin receptor antagonists increase feed intake in rainbow trout. J Fish Biol (2001) 58:716–24. 10.1111/j.1095-8649.2001.tb00524.x [DOI] [Google Scholar]
- 203.Murashita K, Fukada H, Hosokawa H, Masumoto T. Cholecystokinin and peptide Y in yellowtail (Seriola quinqueradiata): molecular cloning, real-time quantitative RT-PCR, and response to feeding and fasting. Gen Comp Endocrinol (2006) 145:287–97. 10.1016/j.ygcen.2005.09.008 [DOI] [PubMed] [Google Scholar]
- 204.Kamisaka Y, Drivenes O, Kurokawa T, Tagawa M, Rønnestad I, Tanaka M, et al. Cholecystokinin mRNA in Atlantic herring, Clupea harengus – molecular cloning, characterization, and distribution in the digestive tract during the early life stages. Peptides (2005) 26:385–93. 10.1016/j.peptides.2004.10.018 [DOI] [PubMed] [Google Scholar]
- 205.Volkoff H. Cloning and tissue distribution of appetite-regulating peptides in pirapitinga (Piaractus brachypomus). J Anim Physiol Anim Nutr (2015) 99:987–1001. 10.1111/jpn.12318 [DOI] [PubMed] [Google Scholar]
- 206.Kurokawa T, Suzuki T, Hashimoto H. Identification of gastrin and multiple cholecystokinin genes in teleost. Peptides (2003) 24:227–35. 10.1016/S0196-9781(03)00034-2 [DOI] [PubMed] [Google Scholar]
- 207.Murashita K, Kurokawa T, Nilsen TO, Rønnestad I. Ghrelin, cholecystokinin, and peptide YY in Atlantic salmon (Salmo salar): molecular cloning and tissue expression. Gen Comp Endocrinol (2009) 160:223–35. 10.1016/j.ygcen.2008.11.024 [DOI] [PubMed] [Google Scholar]
- 208.Micale V, Campo S, D’Ascola A, Guerrera MC, Levanti MB, Germana A, et al. Cholecystokinin in white sea bream: molecular cloning, regional expression, and immunohistochemical localization in the gut after feeding and fasting. PLoS One (2012) 7:e52428. 10.1371/journal.pone.0052428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Jensen H, Rourke IJ, Moller M, Jonson L, Johnsen AH. Identification and distribution of CCK-related peptides and mRNAs in the rainbow trout, Oncorhynchus mykiss. Biochim Biophys Acta (2001) 1517:190–201. 10.1016/S0167-4781(00)00263-3 [DOI] [PubMed] [Google Scholar]
- 210.Jönsson E, Forsman A, Einarsdottir IE, Egner B, Ruohonen K, Björnsson BT. Circulating levels of cholecystokinin and gastrin-releasing peptide in rainbow trout fed different diets. Gen Comp Endocrinol (2006) 148:187–94. 10.1016/j.ygcen.2006.02.016 [DOI] [PubMed] [Google Scholar]
- 211.Murashita K, Fukada H, Rønnestad I, Kurokawa T, Masumoto T. Nutrient control of release of pancreatic enzymes in yellowtail (Seriola quinqueradiata): involvement of CCK and PY in the regulatory loop. Comp Biochem Physiol A Mol Integr Physiol (2008) 150:438–43. 10.1016/j.cbpa.2008.05.003 [DOI] [PubMed] [Google Scholar]
- 212.Murashita K, Fukada H, Hosokawa H, Masumoto T. Changes in cholecystokinin and peptide Y gene expression with feeding in yellowtail (Seriola quinqueradiata): relation to pancreatic exocrine regulation. Comp Biochem Physiol B Biochem Mol Biol (2007) 146:318–25. 10.1016/j.cbpb.2006.11.009 [DOI] [PubMed] [Google Scholar]
- 213.Jönsson E, Forsman A, Einarsdottir IE, Kaiya H, Ruohonen K, Bjornsson BT. Plasma ghrelin levels in rainbow trout in response to fasting, feeding and food composition, and effects of ghrelin on voluntary food intake. Comp Biochem Physiol A Mol Integr Physiol (2007) 147:1116–24. 10.1016/j.cbpa.2007.03.024 [DOI] [PubMed] [Google Scholar]
- 214.Lohmus M, Raven P, Sundstrom L, Devlin R. Disruption of seasonality in growth hormone-transgenic Coho salmon (Oncorhynchus kisutch) and the role of cholecystokinin in seasonal feeding behavior. Horm Behav (2008) 54:506–13. 10.1016/j.yhbeh.2008.02.010 [DOI] [PubMed] [Google Scholar]
- 215.Kamisaka Y, Jordal A-EO, Edvardsen RB, Kryvi H, Otterlei E, Rønnestad I. A case report on the distended gut syndrome (DGS) in cultured larvae of Atlantic cod (Gadus morhua). Aquaculture (2010) 309:38–48. 10.1016/j.aquaculture.2010.09.006 [DOI] [Google Scholar]
- 216.Webb KA, Jr, Khan IA, Nunez BS, Rønnestad I, Holt GJ. Cholecystokinin: molecular cloning and immunohistochemical localization in the gastrointestinal tract of larval red drum, Sciaenops ocellatus (L.). Gen Comp Endocrinol (2010) 166:152–9. 10.1016/j.ygcen.2009.10.010 [DOI] [PubMed] [Google Scholar]
- 217.Furutani T, Masumoto T, Fukada H. Molecular cloning and tissue distribution of cholecystokinin-1 receptor (CCK-1R) in yellowtail Seriola quinqueradiata and its response to feeding and in vitro CCK treatment. Gen Comp Endocrinol (2013) 186:1–8. 10.1016/j.ygcen.2013.02.003 [DOI] [PubMed] [Google Scholar]
- 218.Rathore RM, Angotzi AR, Jordal A-EO, Rønnestad I. Cholecystokinin receptors in Atlantic salmon: molecular cloning, gene expression, and structural basis. Physiol Rep (2013) 1:e00069. 10.1002/phy2.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Tinoco AB, Valenciano AI, Gómez-Boronat M, Blanco AM, Nisembaum LG, de Pedro N, et al. Two cholecystokinin receptor subtypes are identified in goldfish, being the CCKAR involved in the regulation of intestinal motility. Comp Biochem Physiol A Mol Integr Physiol (2015) 187:193–201. 10.1016/j.cbpa.2015.05.027 [DOI] [PubMed] [Google Scholar]
- 220.Woods SC. Gastrointestinal satiety signals I. An overview of gastrointestinal signals that influence food intake. Am J Physiol Gastrointest Liver Physiol (2004) 286:G7–13. 10.1152/ajpgi.00448.2003 [DOI] [PubMed] [Google Scholar]
- 221.Lundberg JM, Tatemoto K, Terenius L, Hellström PM, Mutt V, Hökfelt T, et al. Localization of peptide YY (PYY) in gastrointestinal endocrine cells and effects on intestinal blood flow and motility. Proc Natl Acad Sci U S A (1982) 79:4471–5. 10.1073/pnas.79.14.4471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, et al. Gut hormone PYY3-36 physiologically inhibits food intake. Nature (2002) 418:650–4. 10.1038/nature00887 [DOI] [PubMed] [Google Scholar]
- 223.Grandt D, Schimiczek M, Beglinger C, Layer P, Goebell H, Eysselein VE, et al. Two molecular forms of Peptide YY (PYY) are abundant in human blood: characterization of a radioimmunoassay recognizing PYY 1–36 and PYY 3–36. Regul Pept (1994) 51:151–9. 10.1016/0167-0115(94)90204-6 [DOI] [PubMed] [Google Scholar]
- 224.Sundstrom G, Larsson TA, Brenner S, Venkatesh B, Larhammar D. Evolution of the Neuropeptide Y family: new genes by chromosome duplications in early vertebrates and in teleost fishes. Gen Comp Endocrinol (2008) 155:705–16. 10.1016/j.ygcen.2007.08.016 [DOI] [PubMed] [Google Scholar]
- 225.Volkoff H. Appetite regulating peptides in red-bellied piranha, Pygocentrus nattereri: cloning, tissue distribution and effect of fasting on mRNA expression levels. Peptides (2014) 56:116–24. 10.1016/j.peptides.2014.03.022 [DOI] [PubMed] [Google Scholar]
- 226.Kurokawa T, Suzuki T. Development of neuropeptide Y-related peptides in the digestive organs during the larval stage of Japanese flounder, Paralichthys olivaceus. Gen Comp Endocrinol (2002) 126:30–8. 10.1006/gcen.2001.7774 [DOI] [PubMed] [Google Scholar]
- 227.Chen Y, Pandit NP, Fu J, Li D, Li J. Identification, characterization and feeding response of peptide YYb (PYYb) gene in grass carp (Ctenopharyngodon idellus). Fish Physiol Biochem (2013) 40:45–55. 10.1007/s10695-013-9822-6 [DOI] [PubMed] [Google Scholar]
- 228.Gonzalez R, Unniappan S. Molecular characterization, appetite regulatory effects and feeding related changes of peptide YY in goldfish. Gen Comp Endocrinol (2009) 166:273–9. 10.1016/j.ygcen.2009.09.008 [DOI] [PubMed] [Google Scholar]
- 229.Fällmar H, Sundstro¨m GR, Lundell I, Mohell N, Larhammar D. Neuropeptide Y/peptide YY receptor Y2 duplicate in zebrafish with unique introns displays distinct peptide binding properties. Comp Biochem Physiol B Biochem Mol Biol (2011) 160:166–73. 10.1016/j.cbpb.2011.08.001 [DOI] [PubMed] [Google Scholar]
- 230.Bauer PV, Hamr SC, Duca FA. Regulation of energy balance by a gut-brain axis and involvement of the gut microbiota. Cell Mol Life Sci (2016) 73:737–55. 10.1007/s00018-015-2083-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Merali Z, McIntosh J, Anisman H. Role of bombesin-related peptides in the control of food intake. Neuropeptides (1999) 33:376–86. 10.1054/npep.1999.0054 [DOI] [PubMed] [Google Scholar]
- 232.McColl KE, el-Omar E. Review article: gastrin releasing peptide and its value in assessing gastric secretory function. Aliment Pharmacol Ther (1995) 9:341–7. 10.1111/j.1365-2036.1995.tb00392.x [DOI] [PubMed] [Google Scholar]
- 233.Holmgren S, Jönsson AC. Occurrence and effects on motility of bombesin related peptides in the gastrointestinal tract of the Atlantic cod, Gadus morhua. Comp Biochem Physiol C (1988) 89:249–56. 10.1016/0742-8413(88)90219-8 [DOI] [PubMed] [Google Scholar]
- 234.Holstein B, Humphrey CS. Stimulation of gastric acid secretion and suppression of VIP-like immunoreactivity by bombesin in the Atlantic codfish, Gadus morhua. Acta Physiol Scand (1980) 109:217–23. 10.1111/j.1748-1716.1980.tb06589.x [DOI] [PubMed] [Google Scholar]
- 235.Thorndyke M, Holmgren S. Bombesin potentiates the effect of acetylcholine on isolated strips of fish stomach. Regul Pept (1990) 30:125–35. 10.1016/0167-0115(90)90053-Y [DOI] [PubMed] [Google Scholar]
- 236.Jensen J, Conlon JM. Isolation and primary structure of gastrin-releasing peptide from a teleost fish, the trout (Oncorhynchus mykiss). Peptides (1992) 13:995–9. 10.1016/0196-9781(92)90061-7 [DOI] [PubMed] [Google Scholar]
- 237.Bosi G, Di Giancamillo A, Arrighi S, Domeneghini C. An immunohistochemical study on the neuroendocrine system in the alimentary canal of the brown trout, Salmo trutta, L., 1758. Gen Comp Endocrinol (2004) 138:166–81. 10.1016/j.ygcen.2004.06.003 [DOI] [PubMed] [Google Scholar]
- 238.Volkoff H, Peyon P, Lin X, Peter RE. Molecular cloning and expression of cDNA encoding a brain bombesin/gastrin-releasing peptide-like peptide in goldfish. Peptides (2000) 21:639–48. 10.1016/S0196-9781(00)00199-6 [DOI] [PubMed] [Google Scholar]
- 239.Koven W, Schulte P. The effect of fasting and refeeding on mRNA expression of PepT1 and gastrointestinal hormones regulating digestion and food intake in zebrafish (Danio rerio). Fish Physiol Biochem (2012) 38:1–11. 10.1007/s10695-012-9649-6 [DOI] [PubMed] [Google Scholar]
- 240.Xu M, Volkoff H. Molecular characterization of ghrelin and gastrin-releasing peptide in Atlantic cod (Gadus morhua): cloning, localization, developmental profile and role in food intake regulation. Gen Comp Endocrinol (2009) 160:250–8. 10.1016/j.ygcen.2008.12.004 [DOI] [PubMed] [Google Scholar]
- 241.Canosa LF, Unniappan S, Peter RE. Periprandial changes in growth hormone release in goldfish: role of somatostatin, ghrelin, and gastrin-releasing peptide. Am J Physiol Regul Integr Comp Physiol (2005) 289:R125–33. 10.1152/ajpregu.00759.2004 [DOI] [PubMed] [Google Scholar]
- 242.White SL, Volkoff H, Devlin RH. Regulation of feeding behavior and food intake by appetite-regulating peptides in wild-type and growth hormone-transgenic Coho salmon. Horm Behav (2016) 84:18–28. 10.1016/j.yhbeh.2016.04.005 [DOI] [PubMed] [Google Scholar]
- 243.Zhang YY, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homolog. Nature (1994) 372:425–32. 10.1038/372425a0 [DOI] [PubMed] [Google Scholar]
- 244.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature (2006) 444:840–6. 10.1038/nature05482 [DOI] [PubMed] [Google Scholar]
- 245.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature (2006) 444:875–80. 10.1038/nature05487 [DOI] [PubMed] [Google Scholar]
- 246.Kurokawa T, Uji S, Suzuki T. Identification of cDNA coding for a homologue to mammalian leptin from pufferfish, Takifugu rubripes. Peptides (2005) 26:745–50. 10.1016/j.peptides.2004.12.017 [DOI] [PubMed] [Google Scholar]
- 247.Londraville RL, Macotela Y, Duff RJ, Easterling MR, Liu Q, Crespi EJ. Comparative endocrinology of leptin: assessing function in a phylogenetic context. Gen Comp Endocrinol (2014) 203:146–57. 10.1016/j.ygcen.2014.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Gorissen M, Flik G. Leptin in teleostean fish, towards the origins of leptin physiology. J Chem Neuroanat (2014) 61–62:200–6. 10.1016/j.jchemneu.2014.06.005 [DOI] [PubMed] [Google Scholar]
- 249.Gorissen M, Bernier N, Nabuurs S, Flik G, Huising M. Two divergent leptin paralogues in zebrafish (Danio rerio) that originate early in teleostean evolution. J Endocrinol (2009) 201(3):329–39. 10.1677/JOE-09-0034 [DOI] [PubMed] [Google Scholar]
- 250.Kurokawa T, Murashita K. Genomic characterization of multiple leptin genes and a leptin receptor gene in the Japanese medaka, Oryzias latipes. Gen Comp Endocrinol (2009) 161:229–37. 10.1016/j.ygcen.2009.01.008 [DOI] [PubMed] [Google Scholar]
- 251.Zhang H, Chen H, Zhang Y, Li S, Lu D, Zhang H, et al. Molecular cloning, characterization and expression profiles of multiple leptin genes and a leptin receptor gene in orange-spotted grouper (Epinephelus coioides). Gen Comp Endocrinol (2013) 181:295–305. 10.1016/j.ygcen.2012.09.008 [DOI] [PubMed] [Google Scholar]
- 252.Shpilman M, Hollander-Cohen L, Ventura T, Gertler A, Levavi-Sivan B. Production, gene structure and characterization of two orthologs of leptin and a leptin receptor in tilapia. Gen Comp Endocrinol (2014) 207:74–85. 10.1016/j.ygcen.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 253.Ohga H, Matsumori K, Kodama R, Kitano H, Nagano N, Yamaguchi A, et al. Two leptin genes and a leptin receptor gene of female chub mackerel (Scomber japonicus): molecular cloning, tissue distribution and expression in different obesity indices and pubertal stages. Gen Comp Endocrinol (2015) 222:88–98. 10.1016/j.ygcen.2015.06.002 [DOI] [PubMed] [Google Scholar]
- 254.Morini M, Pasquier J, Dirks R, van den Thillart G, Tomkiewicz J, Rousseau K, et al. Duplicated leptin receptors in two species of eel bring new insights into the evolution of the leptin system in vertebrates. PLoS One (2015) 10:31. 10.1371/journal.pone.0126008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Huising MO, Kruiswijk CP, Flik G. Phylogeny and evolution of class-I helical cytokines. J Endocrinol (2006) 189:1–25. 10.1677/joe.1.06591 [DOI] [PubMed] [Google Scholar]
- 256.Belen Tinoco A, Gabriela Nisembaum L, Isorna E, Jesus Delgado M, de Pedro N. Leptins and leptin receptor expression in the goldfish (Carassius auratus). Regulation by food intake and fasting/overfeeding conditions. Peptides (2012) 34:329–35. 10.1016/j.peptides.2012.02.001 [DOI] [PubMed] [Google Scholar]
- 257.Yan A-F, Chen T, Chen S, Ren C-H, Hu C-Q, Cai Y-M, et al. Goldfish leptin-AI and leptin-AII: function and central mechanism in feeding control. Int J Mol Sci (2016) 17:783. 10.3390/ijms17060783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Li JT, Hou GY, Kong XF, Li CY, Zeng JM, Li HD, et al. The fate of recent duplicated genes following a fourth-round whole genome duplication in a tetraploid fish, common carp (Cyprinus carpio). Sci Rep (2015) 5:8199. 10.1038/srep08199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Lien S, Koop BF, Sandve SR, Miller JR, Kent MP, Nome T, et al. The Atlantic salmon genome provides insights into rediploidization. Nature (2016) 533:200. 10.1038/nature17164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Rønnestad I, Nilsen TO, Murashita K, Angotzi AR, Moen A-GG, Stefansson SO, et al. Leptin and leptin receptor genes in Atlantic salmon: cloning, phylogeny, tissue distribution and expression correlated to long-term feeding status. Gen Comp Endocrinol (2010) 168:55–70. 10.1016/j.ygcen.2010.04.010 [DOI] [PubMed] [Google Scholar]
- 261.Angotzi AR, Stefansson SO, Nilsen TO, Rathore RM, Rønnestad I. Molecular cloning and genomic characterization of novel leptin-like genes in salmonids provide new insight into the evolution of the leptin gene family. Gen Comp Endocrinol (2013) 187:48–59. 10.1016/j.ygcen.2013.03.022 [DOI] [PubMed] [Google Scholar]
- 262.Zabeau L, Defeau D, Van der Heyden J, Iserentant H, Vandekerckhove J, Tavernier J. Functional analysis of leptin receptor activation using a Janus kinase/signal transducer and activator of transcription complementation assay. Mol Endocrinol (2004) 18:150–61. 10.1210/me.2003-0078 [DOI] [PubMed] [Google Scholar]
- 263.Fruhbeck G. Intracellular signalling pathways activated by leptin. Biochem J (2006) 393:7–20. 10.1042/BJ20051578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Cao YB, Xue JL, Wu LY, Jiang W, Hu PN, Zhu J. The detection of 3 leptin receptor isoforms in crucian carp gill and the influence of fasting and hypoxia on their expression. Domest Anim Endocrinol (2011) 41:74–80. 10.1016/j.domaniend.2011.04.002 [DOI] [PubMed] [Google Scholar]
- 265.Gong N, Bjornsson BT. Leptin signaling in the rainbow trout central nervous system is modulated by a truncated leptin receptor isoform. Endocrinology (2014) 155:2445–55. 10.1210/en.2013-2131 [DOI] [PubMed] [Google Scholar]
- 266.Tartaglia LA. The leptin receptor. J Biol Chem (1997) 272:6093–6. 10.1074/jbc.272.10.6093 [DOI] [PubMed] [Google Scholar]
- 267.Kurokawa T, Okamoto T, Gen K, Uji S, Murashita K, Unuma T, et al. Influence of water temperature on morphological deformities in cultured larvae of Japanese eel, Anguilla japonica, at completion of yolk resorption. J World Aquac Soc (2008) 39:726–35. 10.1111/j.1749-7345.2008.00208.x [DOI] [Google Scholar]
- 268.Tinoco AB, Nisembaum LG, Isorna E, Delgado MJ, de Pedro N. Leptins and leptin receptor expression in the goldfish (Carassius auratus). Regulation by food intake and fasting/overfeeding conditions. Peptides (2012) 34:329–35. 10.1016/j.peptides.2012.02.001 [DOI] [PubMed] [Google Scholar]
- 269.Angotzi AR, Stefansson SO, Nilsen TO, Øvrebø JI, Andersson E, Taranger GL, et al. Identification of a novel leptin receptor duplicate in Atlantic salmon: expression analyses in different life stages and in response to feeding status. Gen Comp Endocrinol (2016) 235:108–19. 10.1016/j.ygcen.2016.06.004 [DOI] [PubMed] [Google Scholar]
- 270.Gong Y, Luo Z, Zhu Q-L, Zheng J-L, Tan X-Y, Chen Q-L, et al. Characterization and tissue distribution of leptin, leptin receptor and leptin receptor overlapping transcript genes in yellow catfish Pelteobagrus fulvidraco. Gen Comp Endocrinol (2013) 182:1–6. 10.1016/j.ygcen.2012.11.006 [DOI] [PubMed] [Google Scholar]
- 271.Gong N, Einarsdottir IE, Johansson M, Björnsson BT. Alternative splice variants of the rainbow trout leptin receptor encode multiple circulating leptin-binding proteins. Endocrinology (2013) 154:2331–40. 10.1210/en.2012-2082 [DOI] [PubMed] [Google Scholar]
- 272.Uotani S, Bjorbaek C, Tornoe J, Flier JS. Functional properties of leptin receptor isoforms internalization and degradation of leptin and ligand-induced receptor downregulation. Diabetes (1999) 48:279–86. 10.2337/diabetes.48.2.279 [DOI] [PubMed] [Google Scholar]
- 273.Oswal A, Yeo G. Leptin and the control of body weight: a review of its diverse central targets, signaling mechanisms, and role in the pathogenesis of obesity. Obesity (2010) 18:221–9. 10.1038/oby.2009.228 [DOI] [PubMed] [Google Scholar]
- 274.Harris RBS. Is leptin the parabiotic “satiety” factor? Past and present interpretations. Appetite (2013) 61:111–8. 10.1016/j.appet.2012.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Keen-Rhinehart E, Ondek K, Schneider JE. Neuroendocrine regulation of appetitive ingestive behavior. Front Neurosci (2013) 7:213. 10.3389/fnins.2013.00213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Friedman J. Leptin at 20: an overview. J Endocrinol (2014) 223:T1–8. 10.1530/JOE-14-0405 [DOI] [PubMed] [Google Scholar]
- 277.Ahima RS, Flier JS. Leptin. Annu Rev Physiol (2000) 62:413–37. 10.1146/annurev.physiol.62.1.413 [DOI] [PubMed] [Google Scholar]
- 278.Bagnasco M, Kalra PS, Kalra SP. Ghrelin and leptin pulse discharge in fed and fasted rats. Endocrinology (2002) 143:726–9. 10.1210/endo.143.2.8743 [DOI] [PubMed] [Google Scholar]
- 279.Murashita K, Uji S, Yamamoto T, Rønnestad I, Kurokawa T. Production of recombinant leptin and its effects on food intake in rainbow trout (Oncorhynchus mykiss). Comp Biochem Physiol B Biochem Mol Biol (2008) 150:377–84. 10.1016/j.cbpb.2008.04.007 [DOI] [PubMed] [Google Scholar]
- 280.Chisada S-I, Kurokawa T, Murashita K, Rønnestad I, Taniguchi Y, Toyoda A, et al. Leptin receptor-deficient (knockout) medaka, Oryzias latipes, show chronical up-regulated levels of orexigenic neuropeptides, elevated food intake and stage specific effects on growth and fat allocation. Gen Comp Endocrinol (2014) 195:9–20. 10.1016/j.ygcen.2013.10.008 [DOI] [PubMed] [Google Scholar]
- 281.Pfundt B, Sauerwein H, Mielenz M. Leptin mRNA and protein immunoreactivity in adipose tissue and liver of rainbow trout (Oncorhynchus mykiss) and immunohistochemical localization in liver. Anat Histol Embryol (2009) 38:406–10. 10.1111/j.1439-0264.2009.00951.x [DOI] [PubMed] [Google Scholar]
- 282.Won ET, Baltzegar DA, Picha ME, Borski RJ. Cloning and characterization of leptin in a Perciform fish, the striped bass (Morone saxatilis): control of feeding and regulation by nutritional state. Gen Comp Endocrinol (2012) 178:98–107. 10.1016/j.ygcen.2012.04.019 [DOI] [PubMed] [Google Scholar]
- 283.Douros JD, Baltzegar DA, Breves JP, Lerner DT, Seale AP, Gordon Grau E, et al. Prolactin is a major inhibitor of hepatic leptin A synthesis and secretion: studies utilizing a homologous leptin A ELISA in the tilapia. Gen Comp Endocrinol (2014) 207:86–93. 10.1016/j.ygcen.2014.03.007 [DOI] [PubMed] [Google Scholar]
- 284.Salmerón C, Johansson M, Angotzi AR, Rønnestad I, Jonsson E, Bjornsson BT, et al. Effects of nutritional status on plasma leptin levels and in vitro regulation of adipocyte leptin expression and secretion in rainbow trout. Gen Comp Endocrinol (2015) 210:114–23. 10.1016/j.ygcen.2014.10.016 [DOI] [PubMed] [Google Scholar]
- 285.Salmerón C, Johansson M, Asaad M, Angotzi AR, Rønnestad I, Stefansson SO, et al. Roles of leptin and ghrelin in adipogenesis and lipid metabolism of rainbow trout adipocytes in vitro. Comp Biochem Physiol Part A Mol Integr Physiol (2015) 188:40–8. 10.1016/j.cbpa.2015.06.017 [DOI] [PubMed] [Google Scholar]
- 286.Volkoff H, Eykelbosh AJ, Peter RE. Role of leptin in the control of feeding of goldfish Carassius auratus: interactions with cholecystokinin, neuropeptide Y and orexin A, and modulation by fasting. Brain Res (2003) 972:90–109. 10.1016/S0006-8993(03)02507-1 [DOI] [PubMed] [Google Scholar]
- 287.Li G-G, Liang X-F, Xie Q, Li G, Yu Y, Lai K. Gene structure, recombinant expression and functional characterization of grass carp leptin. Gen Comp Endocrinol (2010) 166:117–27. 10.1016/j.ygcen.2009.10.009 [DOI] [PubMed] [Google Scholar]
- 288.Rønnestad I, Søyland MA, Hansen T, Jordal A-EO, Nilsen TO, Gomes AS, et al. Effects of intraperitoneal administration of leptin on voluntary feed intake, appetite signaling pathways and metabolism in Atlantic salmon, Salmo salar. FASEB J (2016) 30:lb644. [Google Scholar]
- 289.Yuan X, Li A, Liang X-F, Huang W, Song Y, He S, et al. Leptin expression in mandarin fish Siniperca chuatsi (Basilewsky): regulation by postprandial and short-term fasting treatment. Comp Biochem Physiol A Mol Integr Physiol (2016) 194:8–18. 10.1016/j.cbpa.2016.01.014 [DOI] [PubMed] [Google Scholar]
- 290.Trombley S, Maugars G, Kling P, Bjornsson BT, Schmitz M. Effects of long-term restricted feeding on plasma leptin, hepatic leptin expression and leptin receptor expression in juvenile Atlantic salmon (Salmo salar L.). Gen Comp Endocrinol (2012) 175:92–9. 10.1016/j.ygcen.2011.10.001 [DOI] [PubMed] [Google Scholar]
- 291.Fuentes EN, Kling P, Einarsdottir IE, Alvarez M, Antonio Valdes J, Molina A, et al. Plasma leptin and growth hormone levels in the fine flounder (Paralichthys adspersus) increase gradually during fasting and decline rapidly after refeeding. Gen Comp Endocrinol (2012) 177:120–7. 10.1016/j.ygcen.2012.02.019 [DOI] [PubMed] [Google Scholar]
- 292.Jørgensen EH, Martinsen M, Strøm V, Hansen KER, Ravuri CS, Gong N, et al. Long-term fasting in the anadromous Arctic charr is associated with downregulation of metabolic enzyme activity and upregulation of leptin A1 and SOCS expression in the liver. J Exp Biol (2013) 216:3222–30. 10.1242/jeb.088344 [DOI] [PubMed] [Google Scholar]
- 293.Kling P, Rønnestad I, Stefansson SO, Murashita K, Kurokawa T, Bjornsson BT. A homologous salmonid leptin radioimmunoassay indicates elevated plasma leptin levels during fasting of rainbow trout. Gen Comp Endocrinol (2009) 162:307–12. 10.1016/j.ygcen.2009.04.003 [DOI] [PubMed] [Google Scholar]
- 294.Douros JD, Baltzegar DA, Mankiewicz J, Taylor J, Yamaguchi Y, Lerner DT, et al. Control of leptin by metabolic state and its regulatory interactions with pituitary growth hormone and hepatic growth hormone receptors and insulin like growth factors in the tilapia (Oreochromis mossambicus). Gen Comp Endocrinol (2017) 240:227–37. 10.1016/j.ygcen.2016.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Gong N, Jönsson E, Bjornsson BT. Acute anorexigenic action of leptin in rainbow trout is mediated by the hypothalamic Pi3k pathway. J Mol Endocrinol (2015) 56:227–38. 10.1530/JME-15-0279 [DOI] [PubMed] [Google Scholar]
- 296.Nieminen P, Mustonen A-M, Hyvärinen H. Fasting reduces plasma leptin-and ghrelin-immunoreactive peptide concentrations of the burbot (Lota lota) at 2 degrees C but not at 10 degrees C. Zoolog Sci (2003) 20:1109–15. 10.2108/zsj.20.1109 [DOI] [PubMed] [Google Scholar]
- 297.Frøiland E, Jobling M, Björnsson BT, Kling P, Ravuri CS, Jørgensen EH. Seasonal appetite regulation in the anadromous Arctic charr: evidence for a role of adiposity in the regulation of appetite but not for leptin in signalling adiposity. Gen Comp Endocrinol (2012) 178:330–7. 10.1016/j.ygcen.2012.06.017 [DOI] [PubMed] [Google Scholar]
- 298.Huising MO, Geven EJW, Kruiswijk CP, Nabuurs SB, Stolte EH, Spanings FAT, et al. Increased leptin expression in common carp (Cyprinus carpio) after food intake but not after fasting or feeding to satiation. Endocrinology (2006) 147:5786–97. 10.1210/en.2006-0824 [DOI] [PubMed] [Google Scholar]
- 299.Londraville RL, Duvall CS. Murine leptin injections increase intracellular fatty acid-binding protein in green sunfish (Lepomis cyanellus). Gen Comp Endocrinol (2002) 129:56–62. 10.1016/S0016-6480(02)00510-5 [DOI] [PubMed] [Google Scholar]
- 300.de Pedro N, Martinez-Alvarez R, Delgado MJ. Acute and chronic leptin reduces food intake and body weight in goldfish (Carassius auratus). J Endocrinol (2006) 188:513–20. 10.1677/joe.1.06349 [DOI] [PubMed] [Google Scholar]
- 301.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, et al. Effects of the obese gene-product on body-weight regulation in ob/ob mice. Science (1995) 269:540–3. 10.1126/science.7624776 [DOI] [PubMed] [Google Scholar]
- 302.Michel M, Page-McCaw PS, Chen WB, Cone RD. Leptin signaling regulates glucose homeostasis, but not adipostasis, in the zebrafish. Proc Natl Acad Sci U S A (2016) 113:3084–9. 10.1073/pnas.1513212113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Aguilar AJ, Conde-Sieira M, Polakof S, Miguez JM, Soengas JL. Central leptin treatment modulates brain glucosensing function and peripheral energy metabolism of rainbow trout. Peptides (2010) 31:1044–54. 10.1016/j.peptides.2010.02.026 [DOI] [PubMed] [Google Scholar]
- 304.Baltzegar DA, Reading BJ, Douros JD, Borski RJ. Role for leptin in promoting glucose mobilization during acute hyperosmotic stress in teleost fishes. J Endocrinol (2014) 220:61–72. 10.1530/JOE-13-0292 [DOI] [PubMed] [Google Scholar]
- 305.Won ET, Douros JD, Hurt DA, Borski RJ. Leptin stimulates hepatic growth hormone receptor and insulin-like growth factor gene expression in a teleost fish, the hybrid striped bass. Gen Comp Endocrinol (2016) 229:84–91. 10.1016/j.ygcen.2016.02.003 [DOI] [PubMed] [Google Scholar]
- 306.Mullur R, Liu Y-Y, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev (2014) 94:355–82. 10.1152/physrev.00030.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Fliers E, Klieverik LP, Kalsbeek A. Novel neural pathways for metabolic effects of thyroid hormone. Trends Endocrinol Metab (2010) 21:230–6. 10.1016/j.tem.2009.11.008 [DOI] [PubMed] [Google Scholar]
- 308.Goodyear K. Effects of Thyroid Hormone Injections on Feeding and Appetite-Regulating Hormones in Goldfish (Carassius auratus), Biology. St John’s: Memorial University of Newfoundland; (2012). 35 p. [Google Scholar]
- 309.Zhang J, Sun P, Yang F, Kong T, Zhang R. Tributyltin disrupts feeding and energy metabolism in the goldfish (Carassius auratus). Chemosphere (2016) 152:221–8. 10.1016/j.chemosphere.2016.02.127 [DOI] [PubMed] [Google Scholar]
- 310.Li DP, Liu ZD, Xie CX. Effect of stocking density on growth and serum concentrations of thyroid hormones and cortisol in Amur sturgeon, Acipenser schrenckii. Fish Physiol Biochem (2012) 38:511–20. 10.1007/s10695-011-9531-y [DOI] [PubMed] [Google Scholar]
- 311.Navarro I, Rojas P, Capilla E, Albalat A, Castillo J, Montserrat N, et al. Insights into insulin and glucagon responses in fish. Fish Physiol Biochem (2002) 27:205–16. 10.1023/B:FISH.0000032726.78074.04 [DOI] [Google Scholar]
- 312.Kelley KM. Experimental diabetes mellitus in a teleost fish. I. Effect of complete isletectomy and subsequent hormonal treatment on metabolism in the goby, Gillichthys mirabilis. Endocrinology (1993) 132:2689–95. 10.1210/en.132.6.2689 [DOI] [PubMed] [Google Scholar]
- 313.Libran-Perez M, Velasco C, Otero-Rodino C, Lopez-Patino MA, Miguez JM, Soengas JL. Effects of insulin treatment on the response to oleate and octanoate of food intake and fatty acid-sensing systems in rainbow trout. Domest Anim Endocrinol (2015) 53:124–35. 10.1016/j.domaniend.2015.06.004 [DOI] [PubMed] [Google Scholar]
- 314.Busby ER, Mommsen TP. Proglucagons in vertebrates: expression and processing of multiple genes in a bony fish. Comp Biochem Physiol B Biochem Mol Biol (2016) 199:58–66. 10.1016/j.cbpb.2016.02.004 [DOI] [PubMed] [Google Scholar]
- 315.Tang-Christensen M, Larsen PJ, Thulesen J, Rømer J, Vrang N. The proglucagon-derived peptide, glucagon-like peptide-2, is a neurotransmitter involved in the regulation of food intake. Nat Med (2000) 6:802–7. 10.1038/77535 [DOI] [PubMed] [Google Scholar]
- 316.Turton MD, O’Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature (1996) 379:69–72. 10.1038/379069a0 [DOI] [PubMed] [Google Scholar]
- 317.Plisetskaya EM, Mommsen TP. Glucagon and glucagon-like peptides in fishes. Int Rev Cytol (1996) 168:187–257. 10.1016/S0074-7696(08)60885-2 [DOI] [PubMed] [Google Scholar]
- 318.Roch GJ, Wu S, Sherwood NM. Hormones and receptors in fish: do duplicates matter? Gen Comp Endocrinol (2009) 161:3–12. 10.1016/j.ygcen.2008.10.017 [DOI] [PubMed] [Google Scholar]
- 319.Silverstein JT, Bondareva VM, Leonard JBK, Plisetskaya EM. Neuropeptide regulation of feeding in catfish, Ictalurus punctatus: a role for glucagon-like peptide-1 (GLP-1)? Comp Biochem Physiol B Biochem Mol Biol (2001) 129:623–31. 10.1016/S1096-4959(01)00357-8 [DOI] [PubMed] [Google Scholar]
- 320.Polakof S, Miguez JM, Soengas JL. Evidence for a gut-brain axis used by glucagon-like peptide-1 to elicit hyperglycaemia in fish. J Neuroendocrinol (2011) 23:508–18. 10.1111/j.1365-2826.2011.02137.x [DOI] [PubMed] [Google Scholar]
- 321.Imeryüz N, Yeğen BC, Bozkurt A, Coşkun T, Villanueva-Peñacarrillo ML, Ulusoy NB. Glucagon-like peptide-1 inhibits gastric emptying via vagal afferent-mediated central mechanisms. Am J Physiol (1997) 273:G920–7. [DOI] [PubMed] [Google Scholar]
- 322.Boivin TG, Power G. Winter condition and proximate composition of anadromous Arctic charr (Salvelinus alpinus) in eastern Ungava Bay, Quebec. Can J Zool (1990) 68:2284–89. 10.1139/z90-319 [DOI] [Google Scholar]
- 323.Dutil JD. Energetic constraints and spawning interval in the anadromous Arctic charr (Salvelinus alpinus). Copeia (1986) 1986:945. 10.2307/1445291 [DOI] [Google Scholar]
- 324.Jobling M, Koskela J, Pirhonen J. Feeding time, feed intake and growth of baltic salmon, Salmo salar, and brown trout, Salmo trutta, reared in monoculture and duoculture at constant low temperature. Aquaculture (1998) 163:73–84. 10.1016/S0044-8486(98)00224-5 [DOI] [Google Scholar]
- 325.Jørgensen EH, Johansen SJS, Jobling M. Seasonal patterns of growth, lipid deposition and lipid depletion in anadromous Arctic charr. J Fish Biol (1997) 51:312–26. 10.1111/j.1095-8649.1997.tb01668.x [DOI] [Google Scholar]
- 326.Saether BS, Johnsen HK, Jobling M. Seasonal changes in food consumption and growth of Arctic charr exposed to either simulated natural or a 12:12 LD photoperiod at constant water temperature. J Fish Biol (1996) 48:1113–22. 10.1006/jfbi.1996.0114 [DOI] [Google Scholar]
- 327.Tveiten H, Johnsen HK, Jobling M. Influence of maturity status on the annual cycles of feeding and growth in Arctic charr reared at constant temperature. J Fish Biol (1996) 48:910–24. 10.1111/j.1095-8649.1996.tb01486.x [DOI] [Google Scholar]
- 328.Jørgensen EH, Johnsen HK. Rhythmic life of the Arctic charr: adaptations to life at the edge. Mar Genomics (2014) 14:71–81. 10.1016/j.margen.2013.10.005 [DOI] [PubMed] [Google Scholar]
- 329.Jobling M. Are compensatory growth and catch-up growth two sides of the same coin? Aquac Int (2010) 18:501–10. 10.1007/s10499-009-9260-8 [DOI] [Google Scholar]
- 330.Jobling M, Miglavs I. The size of lipid depots – a factor contributing to the control of food intake in Arctic charr, Salvelinus alpinus? J Fish Biol (1993) 43:487–9. 10.1111/j.1095-8649.1993.tb00583.x [DOI] [Google Scholar]
- 331.Rosenbaum M, Leibel RL. Role of leptin in energy homeostasis in humans. J Endocrinol (2014) 223:T83–96. 10.1530/JOE-14-0358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Frøiland E, Murashita K, Jørgensen EH, Kurokawa T. Leptin and ghrelin in anadromous Arctic charr: cloning and change in expressions during a seasonal feeding cycle. Gen Comp Endocrinol (2010) 165:136–43. 10.1016/j.ygcen.2009.06.010 [DOI] [PubMed] [Google Scholar]
- 333.Striberny A, Ravuri CS, Jobling M, Jorgensen EH. Seasonal differences in relative gene expression of putative central appetite regulators in Arctic charr (Salvelinus alpinus) do not reflect its annual feeding cycle. PLoS One (2015) 10:e0138857. 10.1371/journal.pone.0138857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Bone Q, Moore RH. Biology of Fishes. New York: Taylor & Francis; (2008). [Google Scholar]
- 335.Keenleyside MH, editor. Cichlid Fishes: Behaviour, Ecology and Evolution. Chapman and Hall; (1991). [Google Scholar]
- 336.Oppenheimer JR. Mouthbreeding in fishes. Anim Behav (1970) 18(Pt 3):493–503. 10.1016/0003-3472(70)90045-X [DOI] [PubMed] [Google Scholar]
- 337.Wolfgang M, Schierwater B. Energy expenditure for mouthbrooding in a cichlid fish. Behav Ecol Sociobiol (1988) 22:161–4. 10.1007/BF00300565 [DOI] [Google Scholar]
- 338.Helfman GF, Collette BB, Facey DE, Bowen BW. The Diversity of Fishes: Biology, Evolution and Ecology. Hoboken, NJ: Wiley-Blackwell; (2009). [Google Scholar]
- 339.White SA, Fernald RD. Gonadotropin-releasing-hormone containing neurons change size with reproductive state in female Haplochromis burtoni. J Neurosci (1993) 13:434–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Nesjan E, Gutierrez-Ibanez C, Cameron JR, Merrigan S, Wylie DR, Hurd PL. Social status and GnRH soma size in female convict cichlids (Amatitlania nigrofasciatus). Behav Brain Res (2014) 272:205–8. 10.1016/j.bbr.2014.06.028 [DOI] [PubMed] [Google Scholar]
- 341.Grone BP, Carpenter RE, Lee M, Maruska KP, Fernald RD. Food deprivation explains effects of mouthbrooding on ovaries and steroid hormones, but not brain neuropeptide and receptor mRNAs, in an African cichlid fish. Horm Behav (2012) 62:18–26. 10.1016/j.yhbeh.2012.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Tuziak SM, Volkoff H. Gonadotrophin-releasing hormone in winter flounder (Pseudopleuronectes americanus): molecular characterization, distribution and effects of fasting. Gen Comp Endocrinol (2013) 184:9–21. 10.1016/j.ygcen.2012.12.010 [DOI] [PubMed] [Google Scholar]
- 343.Wang T, Yuan D, Zhou C, Lin F, Chen H, Wu H, et al. Characterization of Schizothorax prenanti cgnrhII gene: fasting affects cgnrhII expression. J Fish Biol (2014) 85:407–20. 10.1111/jfb.12430 [DOI] [PubMed] [Google Scholar]
- 344.Johansson M, Morgenroth D, Einarsdottir IE, Gong N, Bjornsson BT. Energy stores, lipid mobilization and leptin endocrinology of rainbow trout. J Comp Physiol B (2016) 186:759–73. 10.1007/s00360-016-0988-y [DOI] [PubMed] [Google Scholar]
- 345.Johansson M, Bjornsson BT. Elevated plasma leptin levels of fasted rainbow trout decrease rapidly in response to feed intake. Gen Comp Endocrinol (2015) 214:24–9. 10.1016/j.ygcen.2015.02.020 [DOI] [PubMed] [Google Scholar]
- 346.Jørgensen EH, Bernier NJ, Maule AG, Vijayan MM. Effect of long-term fasting and a subsequent meal on mRNA abundances of hypothalamic appetite regulators, central and peripheral leptin expression and plasma leptin levels in rainbow trout. Peptides (2015) 86:162–170. 10.1016/j.peptides.2015.08.010 [DOI] [PubMed] [Google Scholar]
- 347.Crim LW, Wilson CE, So YP, Idler DR, Johnston CE. Feeding, reconditioning, and rematuration responses of captive Atlantic salmon (Salmo salar) kelt. Can J Fish Aquat Sci (1992) 49:1835–42. 10.1139/f92-203 [DOI] [Google Scholar]
- 348.Johnsen CA, Hagen O, Adler M, Jonsson E, Kling P, Bickerdike R, et al. Effects of feed, feeding regime and growth rate on flesh quality, connective plasma hormones in farmed Atlantic salmon (Salmo salar L.). Aquaculture (2011) 318:343–54. 10.1016/j.aquaculture.2011.05.040 [DOI] [Google Scholar]
- 349.Trombley S, Mustafa A, Schmitz M. Regulation of the seasonal leptin and leptin receptor expression profile during early sexual maturation and feed restriction in male Atlantic salmon, Salmo salar L., parr. Gen Comp Endocrinol (2014) 204:60–70. 10.1016/j.ygcen.2014.04.033 [DOI] [PubMed] [Google Scholar]
- 350.Schneider JE, Blum RM, Wade GN. Metabolic control of food intake and estrous cycles in Syrian hamsters. I. Plasma insulin and leptin. Am J Physiol Regul Integr Comp Physiol (2000) 278:R476–85. [DOI] [PubMed] [Google Scholar]
- 351.Kronfeld-Schor N, Richardson C, Silvia BA, Kunz TH, Widmaier EP. Dissociation of leptin secretion and adiposity during prehibernatory fattening in little brown bats. Am J Physiol Regul Integr Comp Physiol (2000) 279:R1277–81. [DOI] [PubMed] [Google Scholar]
- 352.Nieminen P, Mustonen AM, Asikainen J, Hyvarinen H. Seasonal weight regulation of the raccoon dog (Nyctereutes procyonoides): interactions between melatonin, leptin, ghrelin, and growth hormone. J Biol Rhythms (2002) 17:155–63. 10.1177/074873002129002447 [DOI] [PubMed] [Google Scholar]
- 353.Arnould JPY, Morris MJ, Rawlins DR, Boyd IL. Variation in plasma leptin levels in response to fasting in Antarctic fur seals (Arctocephalus gazella). J Comp Physiol B (2002) 172:27–34. 10.1007/s003600100224 [DOI] [PubMed] [Google Scholar]
- 354.Jönsson E. The role of ghrelin in energy balance regulation in fish. Gen Comp Endocrinol (2013) 187:79–85. 10.1016/j.ygcen.2013.03.013 [DOI] [PubMed] [Google Scholar]
- 355.Hevrøy EM, Azpeleta C, Shimizu M, Lanzen A, Kaiya H, Espe M, et al. Effects of short-term starvation on ghrelin, GH-IGF system, and IGF-binding proteins in Atlantic salmon. Fish Physiol Biochem (2011) 37:217–32. 10.1007/s10695-010-9434-3 [DOI] [PubMed] [Google Scholar]
- 356.McMenamin SK, Parichy DM. Chapter five – Metamorphosis in teleosts. In: Yun-Bo S, editor. Current Topics in Developmental Biology. Amsterdam: Academic Press; (2013). p. 127–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Rønnestad I, Yúfera M, Ueberschär B, Ribeiro L, Saele Ø, Boglione C. Feeding behaviour and digestive physiology in larval fish: current knowledge, and gaps and bottlenecks in research. Rev Aquac (2013) 5:S59–98. 10.1111/raq.12010 [DOI] [Google Scholar]
- 358.Harboe T, Mangor-Jensen A, Moren M, Hamre K, Rønnestad I. Control of light condition affects the feeding regime and enables successful eye migration in Atlantic halibut juveniles. Aquaculture (2009) 290:250–5. 10.1016/j.aquaculture.2009.02.032 [DOI] [Google Scholar]
- 359.Rønnestad I, Kamisaka Y, Conceição LEC, Morais S, Tonheim SK. Digestive physiology of marine fish larvae: hormonal control and processing capacity for proteins, peptides and amino acids. Aquaculture (2007) 268:82–97. 10.1016/j.aquaculture.2007.04.031 [DOI] [Google Scholar]
- 360.Gomes A, Kamisaka Y, Harboe T, Power D, Rønnestad I. Functional modifications associated with gastrointestinal tract organogenesis during metamorphosis in Atlantic halibut (Hippoglossus hippoglossus). BMC Dev Biol (2014) 14:11. 10.1186/1471-213X-14-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Darias MJ, Murray HM, Gallant JW, Douglas SE, Yúfera M, Martínez-Rodríguez G. Ontogeny of pepsinogen and gastric proton pump expression in red porgy (Pagrus pagrus): determination of stomach functionality. Aquaculture (2007) 270:369–78. 10.1016/j.aquaculture.2007.04.045 [DOI] [Google Scholar]
- 362.Douglas SE, Gawlicka A, Mandla S, Gallant JW. Ontogeny of the stomach in winter flounder: characterization and expression of the pepsinogen and proton pump genes and determination of pepsin activity. J Fish Biol (1999) 55:897–915. 10.1111/j.1095-8649.1999.tb00729.x [DOI] [Google Scholar]
- 363.Murray HM, Gallant JW, Johnson SC, Douglas SE. Cloning and expression analysis of three digestive enzymes from Atlantic halibut (Hippoglossus hippoglossus) during early development: predicting gastrointestinal functionality. Aquaculture (2006) 252:394–408. 10.1016/j.aquaculture.2005.03.030 [DOI] [Google Scholar]
- 364.Yúfera M, Moyano FJ, Astola A, Pousão-Ferreira P, Martínez-Rodríguez G. Acidic digestion in a teleost: postprandial and circadian pattern of gastric pH, pepsin activity, and pepsinogen and proton pump mRNAs expression. PLoS One (2012) 7:e33687. 10.1371/journal.pone.0033687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Kortner TM, Overrein I, Øie G, Kjorsvik E, Bardal T, Wold PA, et al. Molecular ontogenesis of digestive capability and associated endocrine control in Atlantic cod (Gadus morhua) larvae. Comp Biochem Physiol Part A Mol Integr Physiol (2011) 160:190–9. 10.1016/j.cbpa.2011.05.033 [DOI] [PubMed] [Google Scholar]
- 366.Kamisaka Y, Totland GK, Tagawa M, Kurokawa T, Suzuki T, Tanaka M, et al. Ontogeny of cholecystokinin-immunoreactive cells in the digestive tract of Atlantic halibut, Hippoglossus hippoglossus, larvae. Gen Comp Endocrinol (2001) 123:31–7. 10.1006/gcen.2001.7653 [DOI] [PubMed] [Google Scholar]
- 367.Ping HC, Feng K, Zhang GR, Wei KJ, Zou GW, Wang WM. Ontogeny expression of ghrelin, neuropeptide Y and cholecystokinin in blunt snout bream, Megalobrama amblycephala. J Anim Physiol Anim Nutr (2014) 98:338–46. 10.1111/jpn.12084 [DOI] [PubMed] [Google Scholar]
- 368.Einarsdóttir I, Power D, Jönsson E, Björnsson B. Occurrence of ghrelin-producing cells, the ghrelin receptor and Na+,K+-ATPase in tissues of Atlantic halibut (Hippoglossus hippoglossus) during early development. Cell Tissue Res (2011) 344:481–98. 10.1007/s00441-011-1158-x [DOI] [PubMed] [Google Scholar]
- 369.Manning AJ, Murray HM, Gallant JW, Matsuoka MP, Radford E, Douglas SE. Ontogenetic and tissue-specific expression of preproghrelin in the Atlantic halibut, Hippoglossus hippoglossus L. J Endocrinol (2008) 196:181–92. 10.1677/JOE-07-0517 [DOI] [PubMed] [Google Scholar]
- 370.Kvåle A, Mangor-Jensen A, Moren M, Espe M, Hamre K. Development and characterisation of some intestinal enzymes in Atlantic cod (Gadus morhua L.) and Atlantic halibut (Hippoglossus hippoglossus L.) larvae. Aquaculture (2007) 264:457–68. 10.1016/j.aquaculture.2006.12.024 [DOI] [Google Scholar]
- 371.Kamisaka Y, Rønnestad I. Reconstructed 3D models of digestive organs of developing Atlantic cod (Gadus morhua) larvae. Mar Biol (2011) 158:233–43. 10.1007/s00227-010-1554-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Mukherjee A, Subhedar NK, Ghose A. Ontogeny of the cocaine- and amphetamine-regulated transcript (CART) neuropeptide system in the brain of zebrafish, Danio rerio. J Comp Neurol (2012) 520:770–97. 10.1002/cne.22779 [DOI] [PubMed] [Google Scholar]
- 373.Mathieu M, Tagliafierro G, Bruzzone F, Vallarino M. Neuropeptide tyrosine-like immunoreactive system in the brain, olfactory organ and retina of the zebrafish, Danio rerio, during development. Brain Res Dev Brain Res (2002) 139:255–65. 10.1016/S0165-3806(02)00577-1 [DOI] [PubMed] [Google Scholar]
- 374.Demski LS, Northcutt RG. The terminal nerve: a new chemosensory system in vertebrates? Science (1983) 220:435–7. 10.1126/science.6836287 [DOI] [PubMed] [Google Scholar]
- 375.Dietrich MO, Horvath TL. Feeding signals and brain circuitry. Eur J Neurosci (2009) 30:1688–96. 10.1111/j.1460-9568.2009.06963.x [DOI] [PubMed] [Google Scholar]
- 376.Williams KW, Elmquist JK. Lighting up the hypothalamus: coordinated control of feeding behavior. Nat Neurosci (2011) 14:277–8. 10.1038/nn0311-277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Moguel-Hernández I, Peña R, Andree KB, Tovar-Ramirez D, Bonacic K, Dumas S, et al. Ontogeny changes and weaning effects in gene expression patterns of digestive enzymes and regulatory digestive factors in spotted rose snapper (Lutjanus guttatus) larvae. Fish Physiol Biochem (2016) 42:1319–34. 10.1007/s10695-016-0220-8 [DOI] [PubMed] [Google Scholar]
- 378.Velasco C, Bonacic K, Soengas JL, Morais S. Orally administered fatty acids enhance anorectic potential but do not activate central fatty acid sensing in Senegalese sole post-larvae. J Exp Biol (2017) 220:677–85. 10.1242/jeb.150979 [DOI] [PubMed] [Google Scholar]
- 379.Bonacic K, Campoverde C, Gomez-Arbones J, Gisbert E, Estevez A, Morais S. Dietary fatty acid composition affects food intake and gut-brain satiety signaling in Senegalese sole (Solea senegalensis, Kaup 1858) larvae and post-larvae. Gen Comp Endocrinol (2016) 228:79–94. 10.1016/j.ygcen.2016.02.002 [DOI] [PubMed] [Google Scholar]
- 380.Barahona-Fernandes MH, Conan G. Daily food intake of reared larvae of the European seabass (Dicentrarchus labrax L.) Statistical analysis and modelling. ICES Symposium on the Early Life History of Fish Woods Hole (1981). p. 9–12. [Google Scholar]
- 381.Govoni J, Boehlert G, Watanabe Y. The physiology of digestion in fish larvae. Environ Biol Fishes (1986) 16:59–77. 10.1007/BF00005160 [DOI] [Google Scholar]
- 382.Parra G, Yúfera M. Comparative energetics during early development of two marine fish species, Solea senegalensis (Kaup) and Sparus aurata (L.). J Exp Biol (2001) 204:2175–83. [DOI] [PubMed] [Google Scholar]
- 383.Ai-Jun M, Xue-Zhou L, Yong-Jiang X, You L, Zhi-Meng Z. Feeding rhythm and growth of the tongue sole, Cynoglossus semilaevis Günther, during its early life stages. Aquac Res (2006) 37:586–93. 10.1111/j.1365-2109.2006.01466.x [DOI] [Google Scholar]
- 384.Mata-Sotres JA, Martinez-Rodriguez G, Perez-Sanchez J, Sanchez-Vazquez FJ, Yufera M. Daily rhythms of clock gene expression and feeding behavior during the larval development in gilthead seabream, Sparus aurata. Chronobiol Int (2015) 32:1061–74. 10.3109/07420528.2015.1058271 [DOI] [PubMed] [Google Scholar]
- 385.Kotani T, Fushimi H. Determination of appropriate feeding schedules from diel feeding rhythms in finfish larviculture. Aquaculture (2011) 315:104–13. 10.1016/j.aquaculture.2010.10.032 [DOI] [Google Scholar]
- 386.Volkoff H. Cloning, tissue distribution and effects of fasting on mRNA expression levels of leptin and ghrelin in red-bellied piranha (Pygocentrus nattereri). Gen Comp Endocrinol (2015) 217:20–7. 10.1016/j.ygcen.2015.05.004 [DOI] [PubMed] [Google Scholar]
- 387.Chen W-B, Wang X, Zhou Y-L, Dong H-Y, Lin H-R, Li W-S. Molecular cloning, tissue distribution and the expression in the regulation of food intake of prepro-orexin in Nile tilapia (Oreochromis niloticus). Zoolog Res (2011) 32:285–92. 10.3724/SP.J.1141.2011.03285 [DOI] [PubMed] [Google Scholar]
- 388.Volkoff H, Esatevan Sabioni R, Coutinho LL, Cyrino JEP. Appetite regulating factors in pacu (Piaractus mesopotamicus): tissue distribution and effects of food quantity and quality on gene expression. Comp Biochem Physiol Part A Mol Integr Physiol (2017) 203:241–54. 10.1016/j.cbpa.2016.09.022 [DOI] [PubMed] [Google Scholar]
- 389.Villars TA. Hormones and aggressive behavior in teleost fishes. In: Svare BB, editor. Hormones and Aggressive Behavior. Boston, MA: Springer US; (1983). p. 407–33. [Google Scholar]
- 390.Demski LS. Feeding and aggressive behavior evoked by hypothalamic stimulation in a cichlid fish. Comp Biochem Physiol A Physiol (1973) 44:685–92. 10.1016/0300-9629(73)90134-5 [DOI] [PubMed] [Google Scholar]
- 391.Demski LS, Knigge KM. The telencephalon and hypothalamus of the bluegill (Lepomis macrochirus): evoked feeding, aggressive and reproductive behavior with representative frontal sections. J Comp Neurol (1971) 143:1–16. 10.1002/cne.901430102 [DOI] [PubMed] [Google Scholar]
- 392.Backstrom T, Pettersson A, Johansson V, Winberg S. CRF and urotensin I effects on aggression and anxiety-like behavior in rainbow trout. J Exp Biol (2011) 214:907. 10.1242/jeb.045070 [DOI] [PubMed] [Google Scholar]
- 393.Winberg S, Øverli Ø, Lepage O. Suppression of aggression in rainbow trout (Oncorhynchus mykiss) by dietary l-tryptophan. J Exp Biol (2001) 204:3867. [DOI] [PubMed] [Google Scholar]
- 394.Krol J, Zakes Z. Effect of dietary l-tryptophan on cannibalism, survival and growth in pikeperch Sander lucioperca (L.) post-larvae. Aquac Int (2016) 24:441–51. 10.1007/s10499-015-9936-1 [DOI] [Google Scholar]
- 395.Hinaux H, Retaux S, Elipot Y. Chapter 17 – Social behavior and aggressiveness in Astyanax A2 – Keene, Alex C. In: Yoshizawa M, McGaugh SE, editors. Biology and Evolution of the Mexican Cavefish. Amsterdam: Academic Press; (2016). p. 335–59. [Google Scholar]
- 396.Elipot Y, Hinaux H, Callebert J, Retaux S. Evolutionary shift from fighting to foraging in blind cavefish through changes in the serotonin network. Curr Biol (2013) 23:1–10. 10.1016/j.cub.2012.10.044 [DOI] [PubMed] [Google Scholar]
- 397.Perez Maceira JJ, Mancebo MJ, Aldegunde M. The involvement of 5-HT-like receptors in the regulation of food intake in rainbow trout (Oncorhynchus mykiss). Comp Biochem Physiol C Toxicol Pharmacol (2014) 161:1–6. 10.1016/j.cbpc.2013.12.003 [DOI] [PubMed] [Google Scholar]
- 398.Donovan M, Tecott L. Serotonin and the regulation of mammalian energy balance. Front Neurosci (2013) 7:36. 10.3389/fnins.2013.00036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Silva PIM, Martins CIM, Hoglund E, Gjøen HM, Øverli Ø. Feeding motivation as a personality trait in Nile tilapia (Oreochromis niloticus): role of serotonergic neurotransmission. Fish Physiol Biochem (2014) 40:1547–57. 10.1007/s10695-014-9947-2 [DOI] [PubMed] [Google Scholar]
- 400.Gilmour KM, DiBattista JD, Thomas JB. Physiological causes and consequences of social status in salmonid fish. Integr Comp Biol (2005) 45:263–73. 10.1093/icb/45.2.263 [DOI] [PubMed] [Google Scholar]
- 401.Wagner HJ. Vision in Fishes: An Introduction Encyclopedia of Fish Physiology. San Diego: Academic Press; (2011). p. 98–101. [Google Scholar]
- 402.Trajano E, Bichuette ME, Kapoor BG. Biology of Subterranean Fishes. Enfield, NH; Boca Raton, FL: Science Publishers, CRC Press; (2010). [Google Scholar]
- 403.Yoshizawa M. Behaviors of cavefish offer insight into developmental evolution. Mol Reprod Dev (2015) 82:268–80. 10.1002/mrd.22471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Yoshizawa M. Chapter 13 – The evolution of sensory adaptation in Astyanax mexicanus. In: Keene AC, Yoshizawa M, McGaugh SE, editors. Biology and Evolution of the Mexican Cavefish. Amsterdam: Academic Press; (2016). p. 247–67. [Google Scholar]
- 405.Menuet A, Alunni A, Joly JS, Jeffery WR, Retaux S. Expanded expression of Sonic Hedgehog in Astyanax cavefish: multiple consequences on forebrain development and evolution. Development (2007) 134:845–55. 10.1242/dev.02780 [DOI] [PubMed] [Google Scholar]
- 406.Parzefall J, Trajano E. Behavioral patterns in subterranean fishes. In: Trajano E, Bichuette ME, Kapoor BG, editors. Biology of Subterranean Fishes. Boca Raton, FL: Science Publishers; (2010). p. 81–114. [Google Scholar]
- 407.Montgomery J, Coombs S, Baker C. The mechanosensory lateral line system of the hypogean form of Astyanax fasciatus. Environ Biol Fishes (2001) 62:87–96. 10.1023/A:1011873111454 [DOI] [Google Scholar]
- 408.Yoshizawa M, Goriçki S, Soares D, Jeffery WR. Evolution of a behavioral shift mediated by superficial neuromasts helps cavefish find food in darkness. Curr Biol (2010) 20:1631–6. 10.1016/j.cub.2010.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Jeffery W, Strickler A, Guiney S, Heyser D, Tomarev S. Prox 1 in eye degeneration and sensory organ compensation during development and evolution of the cavefish Astyanax. Dev Genes Evol (2000) 210:223–30. 10.1007/s004270050308 [DOI] [PubMed] [Google Scholar]
- 410.Retaux S, Elipot Y. Feed or fight: a behavioral shift in blind cavefish. Commun Integr Biol (2013) 6:e23166. 10.4161/cib.23166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Duboue ER, Keene AC, Borowsky RL. Evolutionary convergence on sleep loss in cavefish populations. Curr Biol (2011) 21:671–6. 10.1016/j.cub.2011.03.020 [DOI] [PubMed] [Google Scholar]
- 412.Gregson JNS, Burt de Perera T. Shoaling in eyed and blind morphs of the characin Astyanax fasciatus under light and dark conditions. J Fish Biol (2007) 70:1615–9. 10.1111/j.1095-8649.2007.01430.x [DOI] [Google Scholar]
- 413.Niemiller ML, Soares D. Cave environments. In: Riesch R, Tobler M, Plath M, editors. Extremophile Fishes: Ecology, Evolution, and Physiology of Teleosts in Extreme Environments. Cham: Springer International Publishing; (2015). p. 161–91. [Google Scholar]
- 414.Espinasa L, Bibliowicz J, Jeffery WR, Retaux S. Enhanced prey capture skills in Astyanax cavefish larvae are independent from eye loss. Evodevo (2014) 5:1–7. 10.1186/2041-9139-5-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Hüppop K. Food-finding ability in cave fish (Astyanax fasciatus). Int J Speleol (1987) 16:59–66. 10.5038/1827-806X.16.1.4 [DOI] [Google Scholar]
- 416.Mitchell RW, Russell WH, Elliott WR. Mexican Eyeless Characin Fishes, Genus Astyanax: Environment, Distribution, and Evolution. Lubbock, TX: Texas Tech Press; (1977). [Google Scholar]
- 417.Volkoff H. Feeding behavior, starvation response, and endocrine regulation of feeding in Mexican blind cavefish (Astyanax fasciatus mexicanus). In: Keene AC, Yoshizawa M, EcGaugh SE, editors. Biology and Evolution of the Mexican Cavefish. Amsterdam: Academic Press; (2015). p. 269–90. [Google Scholar]
- 418.Salin K, Voituron Y, Mourin J, Hervant F. Cave colonization without fasting capacities: an example with the fish Astyanax fasciatus mexicanus. Comp Biochem Physiol A Mol Integr Physiol (2010) 156:451–7. 10.1016/j.cbpa.2010.03.030 [DOI] [PubMed] [Google Scholar]
- 419.Aspiras AC, Rohner N, Martineau B, Borowsky RL, Tabin CJ. Melanocortin 4 receptor mutations contribute to the adaptation of cavefish to nutrient-poor conditions. Proc Natl Acad Sci U S A (2015) 112:9668–73. 10.1073/pnas.1510802112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Aranda A, Madrid JA, Sanchez-Vazquez EJ. Influence of light on feeding anticipatory activity in goldfish. J Biol Rhythms (2001) 16:50–7. 10.1177/074873040101600106 [DOI] [PubMed] [Google Scholar]
- 421.Shepherd DS. Feeding patterns and operant responding by wild and domesticated rats in self-maintenance conditions. Behav Brain Res (1986) 19:83–7. 10.1016/0166-4328(86)90050-1 [DOI] [PubMed] [Google Scholar]
- 422.Haider S, Pal R. Integrated analysis of transcriptomic and proteomic data. Curr Genomics (2013) 14:91–110. 10.2174/1389202911314020003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Hwang WY, Fu Y, Reyon D, Maeder ML, Tsai SQ, Sander JD, et al. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat Biotechnol (2013) 31:227–9. 10.1038/nbt.2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Edvardsen RB, Leininger S, Kleppe L, Skaftnesmo KO, Wargelius A. Targeted mutagenesis in Atlantic salmon (Salmo salar L.) using the CRISPR/Cas9 system induces complete knockout individuals in the F0 generation. PLoS One (2014) 9:e108622. 10.1371/journal.pone.0108622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Juntti SA, Hilliard AT, Kent KR, Kumar A, Nguyen A, Jimenez MA, et al. A neural basis for control of cichlid female reproductive behavior by prostaglandin F2alpha. Curr Biol (2016) 26:943–9. 10.1016/j.cub.2016.01.067 [DOI] [PMC free article] [PubMed] [Google Scholar]