Introduction
Merkel cell carcinoma (MCC) is a rare, aggressive cutaneous malignancy with a propensity for locoregional recurrence and hematogenous spread.1, 2 MCC typically presents in elderly patients with fair complexion as a rapidly growing, firm, flesh-colored or bluish-red cutaneous nodule on sun-exposed areas, most commonly of the head and neck.3 It is traditionally thought to arise from Merkel cells, receptor cells located in the basal layer of the epidermis involved in the sense of light touch.4 Alternatively, these tumors may originate from an immature, totipotent stem cell.5 Merkel cell polyomavirus (MCPyV), a ubiquitous virus in the human skin microbiome, is a nonenveloped, double-stranded DNA virus directly involved in the pathogenesis of approximately 80% of MCCs.6, 7, 8, 9 Steps involved in the development of MCPyV+ tumors include clonal integration into the host cell genome, mutational loss of viral replication competence, expression of 2 key oncoproteins designated small tumor antigen and large tumor antigen, retinoblastoma gene suppression by large tumor antigen, and evasion of a destructive immune response.10, 11 MCPyV– tumors have the highest somatic mutation burden of any characterized malignancy with ultraviolet (UV) signature mutations predominating and exhibit high levels of T-cell–infiltrating lymphocytes and programmed death (PD)-L1 expression.12, 13, 14 Thus, MCC is an attractive target for immunotherapy because MCPyV+ tumors contain integrated viral genes expressing oncoproteins, and virus-negative tumors carry a large burden of UV signature mutations providing non–self-epitopes for immune recognition. MCC commonly presents in the context of immunosuppression from organ transplant, HIV, B-cell malignancy, or immune senescence with a median age at diagnosis of 75 years.3, 15, 16, 17
There are no randomized or prospective trials of chemotherapy in patients with distant metastasis of MCC. Retrospective series have reported median durations for complete and partial responses of 6 and 3 months, respectively, with no clear prolongation of survival.18
Talimogene laherparepvec (TVEC) is the first oncolytic viral immunotherapy approved by the US Food and Drug Administration, receiving an indication for advanced melanoma in October 2015. It consists of a herpes simplex type 1 virus genetically modified to selectively replicate in tumor cells and express human granulocyte-macrophage colony-stimulating factor to activate dendritic cells for antigen presentation.19 Viral infection causes release of pro-inflammatory and danger-associated molecules including viral and cellular DNA which, induce innate immunity, host interferon response, and T-cell infiltration of the tumor microenvironment.20 Dying tumor cells may release soluble antigens or be engulfed by antigen-presenting cells to prime tumor-specific T cells, which can destroy uninfected tumors at distant sites. This may by particularly true for MCC harboring antigenic MCPyV oncoproteins or neoepitopes produced by UV signature mutations. Thus, intratumoral injection of TVEC exerts antitumor effects through both a direct oncolytic effect in injected lesions and induction of systemic antitumor immunity.19, 21 TVEC has a favorable toxicity profile consisting primarily of mild-to-moderate transient flulike symptoms and injection-site pain. Here we report the cases of 2 elderly, frail patients with locoregionally advanced, surgically incurable MCC who refused cytotoxic chemotherapy and consented to receive intratumoral TVEC off label as first-line drug therapy.
Case 1
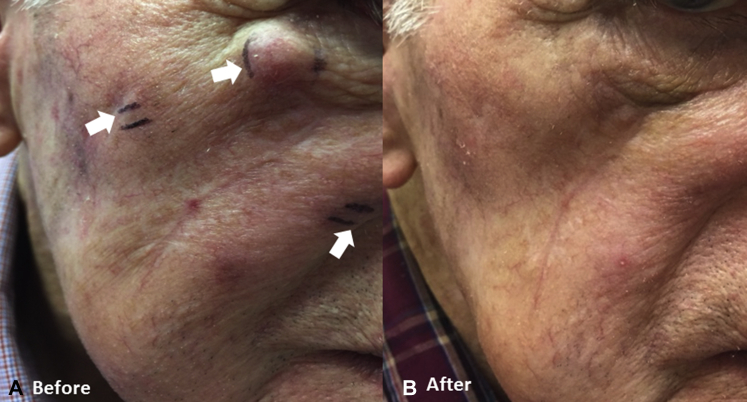
An 87-year-old white man with coronary artery disease underwent margin-negative resection of an MCC from the right cheek extending into subcutaneous fat in August 2015 followed by adjuvant radiotherapy. In March 2016, he had biopsy confirmation of locoregional recurrence with 3 firm red nodules distributed both anterior and posterior to the surgical scar as well as angiolymphatic and perineural invasion. Position emission tomography/computed tomography (PET/CT) in June found a 9-mm cutaneous nodule in the right cheek with standardized uptake value (SUV) of 2.5 but no evidence of metastases to regional lymph nodes or distant sites. Three weeks later, he had 8 palpable reddish dermal metastases up to 1.4 cm in diameter widely distributed over the right side of the face from the inferior orbital rim to the angle of the jaw (Fig 1, A). Serology testing performed at the University of Washington was negative for antibody against MCPyV oncoprotein, indicating no evidence of virus exposure, although the negative prediction value of the test is low. With the patient's consent, TVEC was administered intratumorally into all detectable metastases across the right side of the face using standard dosing according to the product insert. He received an initial dose of 2 mL of 106 PFU (plaque-forming unit)/mL TVEC on July 1, 2016 followed by maintenance doses of 1 to 2 mL of 108 PFU/mL at 2-week intervals on 3 occasions from July 25 to August 19, 2016. Toxicity was limited to mild fatigue. Two weeks after the fourth dose and 9 weeks after treatment initiation, he had a complete clinical response with no residual detectable tumor to inject (Fig 1, B). PET/CT and physical examination in January 2017 found continued complete response 5 months after the last dose.
Fig 1.
Photographs of patient 1 show multiple dermal metastases (arrows) up to 1.4 cm in diameter along the infraorbital rim before TVEC (A) and complete clinical response after 4 doses of TVEC (B).
Case 2
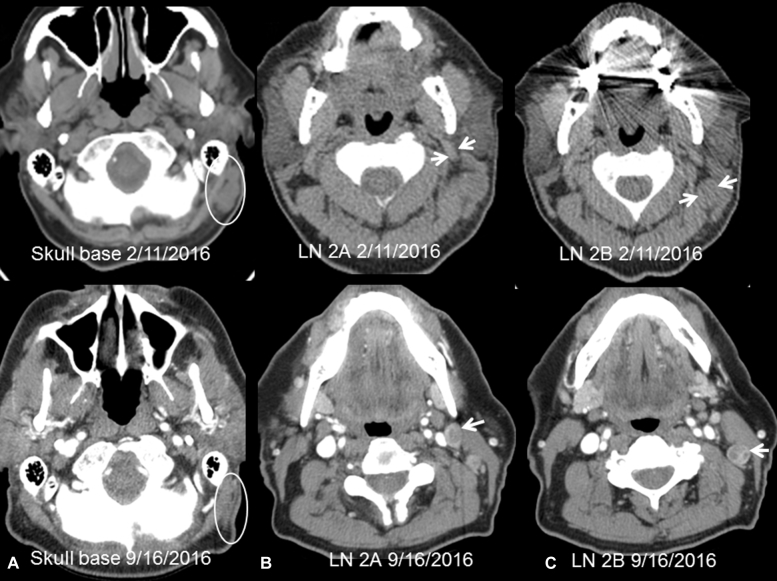
A 77-year-old white man with coronary artery disease and history of localized prostatic adenocarcinoma underwent margin-negative resection of an apical scalp MCC in January 2016. PET/CT showed no detectable metastases. Within 2 weeks, palpable left postauricular and posterior cervical lymphadenopathy developed. PET/CT in February 2016 found regional recurrence in 2 posterior mastoid lymph nodes with a maximum SUV of 9.3 and extensive left cervical lymphadenopathy involving stations IIA and IIB with a maximum SUV of 11 (Fig 2) and bidimensional measurements provided in Table I. Serology testing performed at the University of Washington was negative for antibody against MCPyV oncoprotien. The lymphadenopathy was rapidly progressive with 4 distinctly palpable left cervical lymph nodes up to 3 cm in diameter and a 2-cm left posterior mastoid node at treatment initiation. With the patient's consent, TVEC was administered intratumorally into all palpable metastases across the left side of the neck and posterior mastoid regions using standard dosing according to the product insert but with less than the maximum 4-mL dose to contain cost. He received an initial dose of 1 mL of 106 PFU/mL TVEC on February 19, 2016 followed by maintenance doses of 1 to 2 mL of 108 PFU/mL at 2-week intervals on 7 occasions from March 18 to June 23, 2016. Toxicity was limited to mild fatigue, nausea, and injection site tenderness. A PET/CT on May 6, 2016 showed a partial response by Response Evaluation Criteria in Solid Tumors (RECIST 1.1) criteria with 36% reduction in index lesions (Table I). A neck CT scan on September 16, 2016 found continued shrinkage of index lesions by 46% compared with baseline with residual radiographic findings in the mastoid region thought to represent linear scarring (Table I). The level IIA and IIB lymph nodes had new central necrosis on the September 2016 examination (Fig 2). PET/CT scan in late January 2017 showed resolution of hypermetabolism aside from a single left level IIA node with diminishing SUV and further shrinkage of index lesions by 62% from baseline (Table I). He was asymptomatic with normal physical examination 11 months after treatment initiation and 7 months after the last TVEC dose.
Fig 2.
Neck CT images from patient 2. Top row, Pretherapy (2/11, noncontrast). Bottom row, Three months after last dose (9/16, with contrast). A, Skull base. Conglomeration of nodes posterior to the left mastoid (oval). The nodes markedly decreased in size and showed no enhancement on 9/16 suggesting remnant scar tissue. B, Level 2A. Lymph node posterior to the left mandible (arrows). The node decreased in size and developed central necrosis. C, Level 2B. Lymph node medial to the left sternocleidomastoid muscle (arrows). The node decreased in size and on 9/16 showed central necrosis.
Table I.
Serial neck CT measurements (mm) of malignant lymphadenopathy in case 2
| Nodal station | February 2016 | May 2016 | September 2016 | January 2017 |
|---|---|---|---|---|
| Posterior mastoid | 12 × 20 and 10 × 10 | 6 × 24∗ | 4 × 17 | 4 × 20 |
| Level 2A | 11 × 16 | 13 × 21 | 11 × 17 | 10 × 16 |
| Level 2B | 17 × 21 | 13 × 13 | 12 × 14 | 5 × 7 |
| RECIST 1.1 Sum†(reduction) | 50 | 32 (36%) | 27 (46%) | 19 (62%) |
Two posterior mastoid lymph nodes coalesced to a single linear likely scar.
By RESCIST 1.1 criteria the sum of the short axes of index lymph nodes was used to determine response.
Discussion
A phase II trial of pembrolizumab, an anti–PD-1 monoclonal antibody in 26 patients with advanced MCC produced an objective response rate of 56% with 4 complete responses and 67% progression-free survival at 6 months.22 Responses occurred in 62% of virus-positive and 50% of virus-negative tumors.22 Avelumab, a monoclonal antibody binding PD-1 ligand produced an objective response rate of 32% with 8 complete responders, and progression-free survival at 6 months was 40% in 88 patients with advanced MCC.23 Although immune checkpoint inhibitors are not yet approved by the US Food and Drug Administration for MCC, these trials have provided proof of principle that immunotherapy can be very active against virus-positive and virus-negative forms of the disease. TVEC represents a promising option for immunotherapy of locoregionally advanced MCC, particularly in frail, elderly individuals considering its favorable safety profile.
A phase Ib trial in advanced melanoma of TVEC combined with anti–CTLA-4 immune checkpoint blockade by standard-dose ipilimumab found an objective response rate of 50%, considerably higher than historical results with ipilimumab alone.24 Similarly, a phase Ib trial in advanced melanoma of TVEC combined with pembrolizumab produced an objective response rate of 48% and an acceptable toxicity profile consisting predominantly of fatigue, fever, and chills.25
In the 2 cases of regionally advanced MCC reported herein, disease was rapidly progressive with appearance of multiple new nodules within a few weeks. Intratumoral TVEC not only led to regression of injected nodules but prevented development of new metastases locoregionally and distantly over periods of 6 to 11 months in cases 1 and 2, respectively. Patient 1 achieved a clinical complete response and patient 2 partial responses persisting for more than 5 and 7 months after the last TVEC dose, respectively. Serologic testing failed to confirm MCPyV exposure in both patients, but a negative result does not exclude virus-associated MCC, and insufficient tissue was available for immunohistochemical viral large T-antigen detection. Thus, whether the cases of MCC here reported were virus positive or negative is uncertain. However, MCPyV shares no homology with herpes simplex type 1 comprising TVEC, and recent clinical experience indicates comparable response to immunotherapy among viral-positive and viral-negative MCC. These observations coupled with the emerging role for immunotherapy in advanced MCC indicate the need for prospective trials of TVEC alone or combined with blockade of the PD-1/PD-L1 immune checkpoint in both viral-positive and viral-negative advanced MCC. A phase II trial of TVEC with or without hypofractionated radiotherapy for melanoma, MCC, or other solid tumors with skin metastasis has recently opened to accrual (NCT02819843).
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Tothill R., Estall V., Rischin D. Merkel cell carcinoma: emerging biology, current approaches, and future directions. Am Soc Clin Oncol Educ Book. 2015:e519–e526. doi: 10.14694/EdBook_AM.2015.35.e519. [DOI] [PubMed] [Google Scholar]
- 2.Zhan F.Q., Packianathan V.S., Zeitouni N.C. Merkel cell carcinoma: a review of current advances. J Natl Compr Canc Netw. 2009;7:333–339. doi: 10.6004/jnccn.2009.0025. [DOI] [PubMed] [Google Scholar]
- 3.Albores-Saavedra J., Batich K., Chable-Montero F. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population based study. J Cutan Pathol. 2010;37:20–27. doi: 10.1111/j.1600-0560.2009.01370.x. [DOI] [PubMed] [Google Scholar]
- 4.Ratner D., Nelson B.R., Brown M.D. Merkel cell carcinoma. J Am Acad Dermatol. 1993;29:143–156. doi: 10.1016/0190-9622(93)70159-q. [DOI] [PubMed] [Google Scholar]
- 5.Tilling T., Moll I. Which are the cells of origin in merkel cell carcinoma? J Skin Cancer. 2012;2012:680410. doi: 10.1155/2012/680410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng H., Shuda M., Chang Y. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096–1100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leroux-Kozal V., Leveque N., Brodard V. Merkel cell carcinoma: histopathologic and prognostic features according to the immunohistochemical expression of Merkel cell polyomavirus large T antigen correlated with viral load. Hum Pathol. 2015;46:443–453. doi: 10.1016/j.humpath.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Santos-Juanes J., Fernandez-Vega I., Fuentes N. Merkel cell carcinoma and Merkel cell polyomavirus: a systematic review and meta-analysis. Br J Dermatol. 2015;173:42–49. doi: 10.1111/bjd.13870. [DOI] [PubMed] [Google Scholar]
- 9.Schowalter R.M., Pastrana D.V., Pumphrey K.A. Merkel cell polyomavirus and two previously unknown polyomaviruses are chronically shed from human skin. Cell Host Microbe. 2010;7:509–515. doi: 10.1016/j.chom.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sastre-Garau X., Peter M., Avril M.F. Merkel cell carcinoma of the skin: pathological and molecular evidence for a causative role of MCV in oncogenesis. J Pathol. 2009;218:48–56. doi: 10.1002/path.2532. [DOI] [PubMed] [Google Scholar]
- 11.Church C.D., Nghiem P. How does the Merkel polyomavirus lead to a lethal cancer? Many answers, many questions, and a new mouse model. J Invest Dermatol. 2015;135:1221–1224. doi: 10.1038/jid.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harms P.W., Patel R.M., Verhaegen M.E. Distinct gene expression profiles of viral- and nonviral-associated merkel cell carcinoma revealed by transcriptome analysis. J Invest Dermatol. 2013;133:936–945. doi: 10.1038/jid.2012.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harms P.W., Vats P., Verhaegen M.E. The distinctive mutational spectra of polyomavirus-negative Merkel cell carcinoma. Cancer Res. 2015;75:3720–3727. doi: 10.1158/0008-5472.CAN-15-0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong S.Q., Waldeck K., Vergara I.A. UV-associated mutations underlie the etiology of MCV-negative Merkel cell carcinomas. Cancer Res. 2015;75:5228–5234. doi: 10.1158/0008-5472.CAN-15-1877. [DOI] [PubMed] [Google Scholar]
- 15.Clarke C.A., Robbins H.A., Tatalovich Z. Risk of merkel cell carcinoma after solid organ transplantation. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/dju382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tadmor T., Aviv A., Polliack A. Merkel cell carcinoma, chronic lymphocytic leukemia and other lymphoproliferative disorders: an old bond with possible new viral ties. Ann Oncol. 2011;22:250–256. doi: 10.1093/annonc/mdq308. [DOI] [PubMed] [Google Scholar]
- 17.Manganoni M.A., Farisoglio C., Tucci G. Merkel cell carcinoma and HIV infection: a case report and review of the literature. AIDS Patient Care STDS. 2007;21:447–451. doi: 10.1089/apc.2006.0152. [DOI] [PubMed] [Google Scholar]
- 18.Tai P.T., Yu E., Tonita J. Merkel cell carcinoma of the skin. J Cutan Med Surg. 2000;4:186–195. doi: 10.1177/120347540000400403. [DOI] [PubMed] [Google Scholar]
- 19.Andtbacka R.H., Kaufman H.L., Collichio F. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33:2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 20.Bommareddy P.K., Patel A., Hossain S., Kaufman H.L. Talimogene laherparepvec (T-VEC) and other oncolytic viruses for the treatment of melanoma. Am J Clin Dermatol. 2017;18:1–15. doi: 10.1007/s40257-016-0238-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaufman H.L., Amatruda T., Reid T. Systemic versus local responses in melanoma patients treated with talimogene laherparepvec from a multi-institutional phase II study. J Immunother Cancer. 2016;4:12. doi: 10.1186/s40425-016-0116-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nghiem P.T., Bhatia S., Lipson E.J. PD-1 Blockade with pembrolizumab in advanced Merkel-cell carcinoma. N Engl J Med. 2016;374:2542–2552. doi: 10.1056/NEJMoa1603702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaufman H.L., Russell J., Hamid O. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17:1374–1385. doi: 10.1016/S1470-2045(16)30364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puzanov I., Milhem M.M., Minor D. Talimogene laherparepvec in combination with ipilimumab in previously untreated, unresectable stage IIIB-IV melanoma. J Clin Oncol. 2016;34:2619–2626. doi: 10.1200/JCO.2016.67.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Long GV. Efficacy analysis of MASTERKEY-265 phase 1b study of talimogene laherparepvec (TVEC) and pembrolizumab (pembro) for unresectable stage IIIB-IV melanoma. J Clin Oncol. 2016;34(suppl):abstract 9568