Introduction
Rothmund-Thomson syndrome (RTS) (OMIM #268400) is a rare autosomal recessive disorder characterized by erythema, blisters, and swelling that appears during infancy on the cheeks, spreads to the extremities, and eventually leads to poikiloderma. Additionally, a large percentage of patients have short stature and skeletal abnormalities, and some patients have hair, eyebrow, eye, and gastrointestinal problems. Osteosarcoma is reported in 30% of the patients.1 The RECQL4 gene (OMIM *603780) on 8q24.3 encoding adenosine triphosphate–dependent DNA helicase Q4 is causative of RTS. It is involved in DNA repair and is associated with increased cancer risk, but its biological function is not fully understood.2 We report a rare case of RTS with predominantly cutaneous symptoms and 2 novel compound heterozygous variants in the RECQL4 gene.
Case report
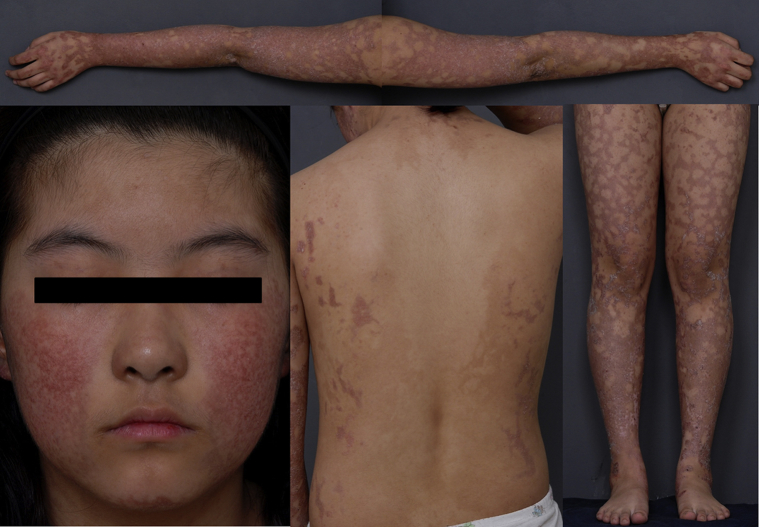
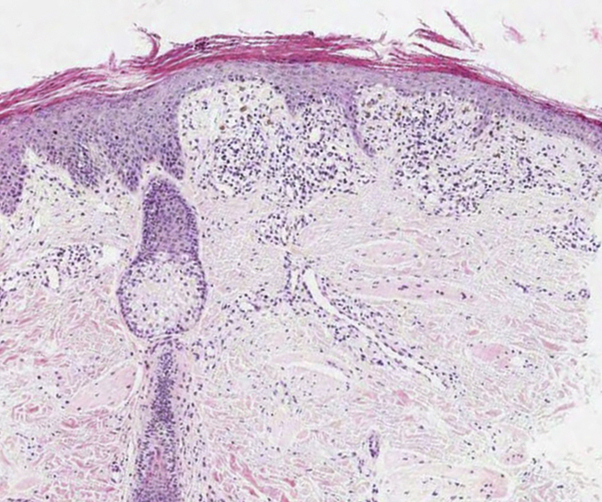
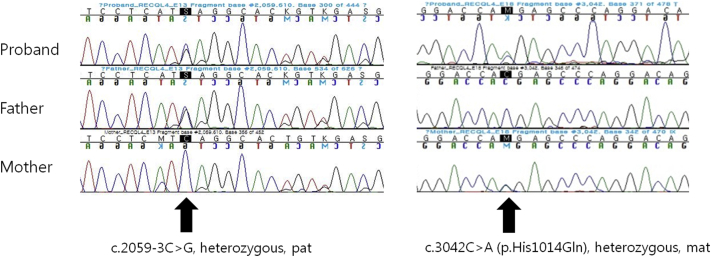
An 11-year-old girl presented with a rash on her face and extremities for more than 10 years. She was born at full term without fetal anomalies. At 6 months of age, erythematous patches and blisters developed on both cheeks followed by pigmentation and scale. The rash spread to the extremities and flanks 4 years before presentation. She also complained of photosensitivity. She did not report any hair loss or dental, musculoskeletal, ocular, or neurologic symptoms. None of her family members reported similar symptoms. Physical examination found reticulated telangiectatic erythematous patches with brownish pigmentation on the face and extremities (Fig 1). Skin biopsy found basal cell degeneration, pigmentary incontinence, telangiectasia, and moderate perivascular lymphohistiocytic infiltration, consistent with poikiloderma (Fig 2). Laboratory test results were within normal limits, including complete blood count, blood chemistry profile, urinary analysis, and urinary porphobilinogen level; however, vitamin D level was low at 16.2 ng/mL (reference range, 30-100 ng/mL). Both her height and weight were greater than the 90th percentile. She showed no intellectual impairment. For further evaluation, the patient was referred to the Department of Medical Genetics. Chromosome analysis found normal female karyotype, 46, XX, and there was no structural chromosomal aberration seen in the G-banding pattern of the metaphase chromosome. For differential molecular diagnosis of poikiloderma, targeted exome sequencing was performed. A library was prepared using the TruSight One Sequencing Panel (Illumina, Inc, San Diego, CA), which targeted a 12-Mb region spanning 4,813 genes of clinical relevance. Massively parallel sequencing was performed on the Illumina NextSeq platform. Average depth of coverage of the entire panel was 97×, and 97% of the targeted bases were covered at 10× sequence reads. Targeted exome sequencing found 2 novel heterozygous variants in the RECQL4 gene, confirmed by Sanger sequencing: an intronic variant, NG_016430.1 (NM_004260.3):c.2059-3C>G and a missense variant, NM_004260.3:c.3042C>A (p.His1014Gln) (Fig 3). Through targeted Sanger sequencing of samples from her parents, a transconfiguration of the 2 detected variants was confirmed. In silico analysis of the c.2059-3C>G variant found a possible damaging effect on the protein structure, confirmed by LoFtool. Moreover, using targeted exome sequencing, a novel heterozygous missense variant c.1627G>A (p.Val543Met) in the FERMT1 gene was also identified.
Fig 1.
Poikiloderma in a patient with RTS. Reticulated telangiectatic erythematous patches with brownish pigmentation are seen on the face and extremities, sparing the trunk.
Fig 2.
Skin biopsy specimen findings consistent with poikiloderma. Hyperkeratosis and parakeratosis with basal cell liquefactive degeneration, pigmentary incontinence, telangiectasia, and moderate lymphohistiocytic infiltrate around the dermal blood vessels are seen. (Hematoxylin-eosin stain, virtual slide.)
Fig 3.
Two novel heterozygous variants in the RECQL4 gene confirmed by Sanger sequencing. One was an intronic variant, c.2059-3C>G from her father, and the other was a missense variant, c.3042C>A (p.His1014Gln) from her mother.
Unfortunately, further functional studies to confirm the protein damaging effect were refused by her parents. RTS was diagnosed, and the patient was advised to avoid sun exposure and undergo annual checkups for the eyes, skin, and bones. Calcium/vitamin D supplements were also recommended.
Discussion
To our knowledge, in about 400 cases reported, typical poikiloderma was associated with various other symptoms and signs.3, 4 Erythema and bullae typically develop on both cheeks at age 3 to 6 months. The rash subsequently spreads to the extremities and buttocks, and poikiloderma develops on the affected areas. The characteristic findings in this case strongly suggest RTS, even without hair, dental, or bone anomalies. Genetic analysis confirmed RTS. Few cases of RTS have presented with mild symptoms,5 but no reported case only had cutaneous symptoms. Several possible mechanism of this rare presentation include tissue differential expression of RECQL4 variants, incomplete penetrance of the gene, or presence of pathogenic variants in genes modulating RECQL4 gene function or expression. Further investigations for clarification of the pathophysiology are needed.
Although the c.2059-3C>G variant in the RECQL4 gene has not been reported thus far, the possible effect on the protein through missplicing can be assumed. In addition to the in silico analysis result of this variant, particularly short introns in the RECQL4 gene may increase the chance of missplicing of such an intronic mutation. The other variant c.3042C>A is known to be rare with a low minor allele frequency (MAF, A=0.0001/15), which tends toward mutation rather than polymorphism. Thus, these mutations probably are pathogenic for RTS in this case.
Mutations of the RECQL4 gene are also responsible for the RAPADILINO and Baller-Gerold syndrome. Unlike RTS, RAPADILINO syndrome does not manifest ectodermal symptoms, and Baller-Gerold syndrome is characterized by craniosynostosis. However, they all may share features such as radial ray defects and short stature.1 The varying phenotypes of the syndromes imply functional diversity of the RECQL4 protein, and the domain of the protein affected by the mutation may determine predominant symptoms and signs of the disease. Investigating genetic causes other than the RECQL4 gene in these syndromes is necessary for further understanding.
The differential diagnoses of hereditary poikiloderma and photosensitivity include Kindler syndrome, dyskeratosis congenita, and xeroderma pigmentosum. Kindler syndrome, a rare autosomal recessive genodermatosis related to FERMT1 gene mutation, is characterized by congenital acral blisters, marked skin atrophy, and mucosal stenosis.6 It also presents with photosensitivity that tends to lessen with age, which was not true in this case. In addition, the c.1627G>A variant, which is a novel, heterozygous missense variant, is less likely to be pathogenic. Poikiloderma in dyskeratosis congenita usually appears after 5 years of age with nail dystrophy. The features of xeroderma pigmentosum are nonmelanoma skin cancers and precancerous lesions by the second decade, with ocular or neurologic involvement.7 Earlier onset of poikiloderma and absence of nail changes, skin cancer, and other precancerous lesions in this case are more suggestive of RTS, which was also confirmed by targeted exome sequencing.
For cutaneous lesions of RTS, pulsed dye laser is used to treat telangiectasia. Sunscreens should be used to prevent skin cancer. Standard therapies to treat cancer are available for affected patients. Close monitoring of skin areas, osteosarcomas, and visual impairment is necessary for early detection and treatment.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Sznajer Y., Siitonen H.A., Roversi G. Atypical Rothmund-Thomson syndrome in a patient with compound heterozygous mutations in RECQL4 gene and phenotypic features in RECQL4 syndromes. Eur J Pediatr. 2008;167:175–181. doi: 10.1007/s00431-007-0447-6. [DOI] [PubMed] [Google Scholar]
- 2.Larizza L., Roversi G., Volpi L. Rothmund-Thomson syndrome. Orphanet J Rare Dis. 2010;5:2. doi: 10.1186/1750-1172-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang L.L., Levy M.L., Lewis R.A. Clinical manifestations in a cohort of 41 Rothmund-Thomson syndrome patients. Am J Med Genet. 2001;102:11–17. doi: 10.1002/1096-8628(20010722)102:1<11::aid-ajmg1413>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 4.Larizza L., Roversi G., Verloes A. Clinical utility gene card for: Rothmund-Thomson syndrome. Eur J Hum Genet. 2013;21 doi: 10.1038/ejhg.2012.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colombo E.A., Fontana L., Roversi G. Novel physiological RECQL4 alternative transcript disclosed by molecular characterisation of Rothmund-Thomson Syndrome sibs with mild phenotype. Eur J Hum Genet. 2014;22:1298–1304. doi: 10.1038/ejhg.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Has C., Bruckner-Tuderman L. Molecular and diagnostic aspects of genetic skin fragility. J Dermatol Sci. 2006;44:129–144. doi: 10.1016/j.jdermsci.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Lipsker D. What is poikiloderma? Dermatology. 2003;207:243–245. doi: 10.1159/000073082. [DOI] [PubMed] [Google Scholar]