Introduction
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease characterized by abscesses, fistulas, and scarring affecting the groin, anogenital area, and axillae. HS has an estimated prevalence of 1%, and moderate-to-severe forms have a high disease burden.1 The disease is a combination of homeostasis abnormalities of the pilosebaceous unit and dysregulation of the skin immune responses. Pathogenesis involves the interaction between a person's genetic background (potentially heterozygous mutations of genes belonging to the gamma secretase complex) and environmental triggers, among which are the skin microbiome, cigarette smoking, and obesity.2 We report herein a case of radiation-induced HS and discuss its possible mechanisms.
Case
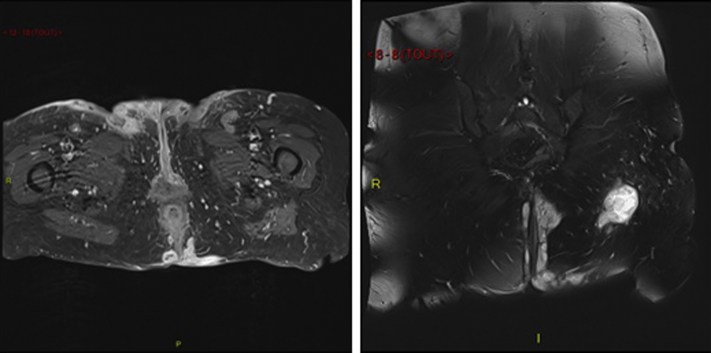
A 57-year-old active smoker, normal-weight woman (body mass index 23 kg/m2) with no personal or family history of inflammatory skin or bowel disease was referred for painful inflammatory nodules and fluctuant abscesses located in her groin and inguinal folds. The infection appeared resistant to topical clindamycin 1%, oral zinc supplementation, oral corticosteroids at a dose of 1 mg/kg/day, and several cycles of oral antibiotics (amoxicilline, tetracycline, rifampicin, and metronidazole). One year before onset of her symptoms, she was treated for uterine adenocarcinoma with a total hysterectomy and bilateral annexectomy, along with external beam radiotherapy (25 treatment sessions over 4 weeks). No chemotherapy or hormonotherapy was given and regular follow-up did not show any sign of cancer relapse. The inflammatory and painful nodules developed a few weeks after the end of radiotherapy. Upon physical examination, she had open and closed comedones along with erythematous papules, nodules, abscesses, and foul exudates from draining sinuses over her inguinal folds and groin, all typical of Hurley stage 3 HS (Fig 1). The lesions were strikingly contained within the distinct margins of the radiation treatment. Histology of skin lesions showed a heavy mixed inflammatory infiltrate around the apocrine glands with dilated lumina and inflammatory sinus tracts containing desquamated keratin and surrounded by dense fibrosis, with no granuloma formation or necrosis. Pelvic magnetic resonance imaging showed extensive subcutaneous abscesses and sinus and fistula tracts in the perineal region with deep communicating and noncommunicating rim-enhancing collections, along with marked skin thickening and subcutaneous induration after intravenous contrast (Fig 2). The patient was maintained on antibiotics and was started on adalimumab (160 mg day 1, 80 mg day 14, then 40 mg weekly from day 28). The patient's symptoms improved with no evidence of carcinoma relapse at 1-year follow-up according to clinical, biologic, and tomodensitometric follow-ups (Fig 3). Circulating adalimumab levels were within normal limits with no evidence of neutralizing antibodies.
Fig 1.
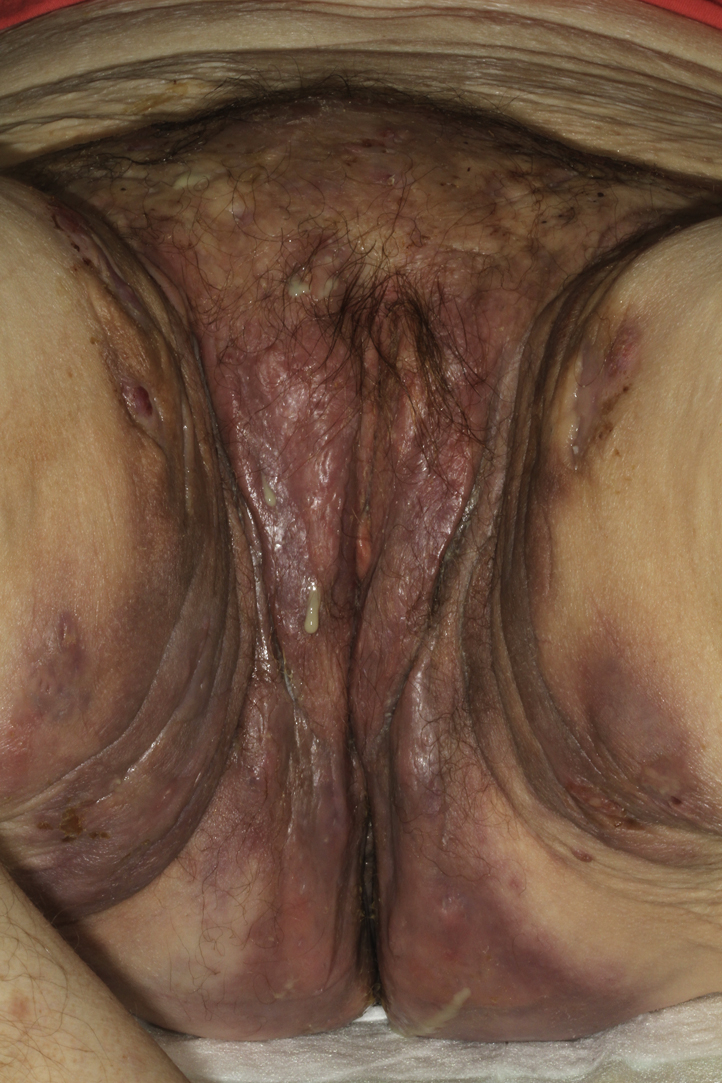
Hurley stage 3 hidradenitis suppurativa with open and closed comedones, papules, nodules, and communicating abscesses strictly limited to the inguinal folds and groin.
Fig 2.
T1 (left) and T2 (right) gadolinium-injected magnetic resonance images showing skin induration and extensive subcutaneous abscesses and sinus and fistula tracts in the perineal region. There is no communication with the anal canal.
Fig 3.
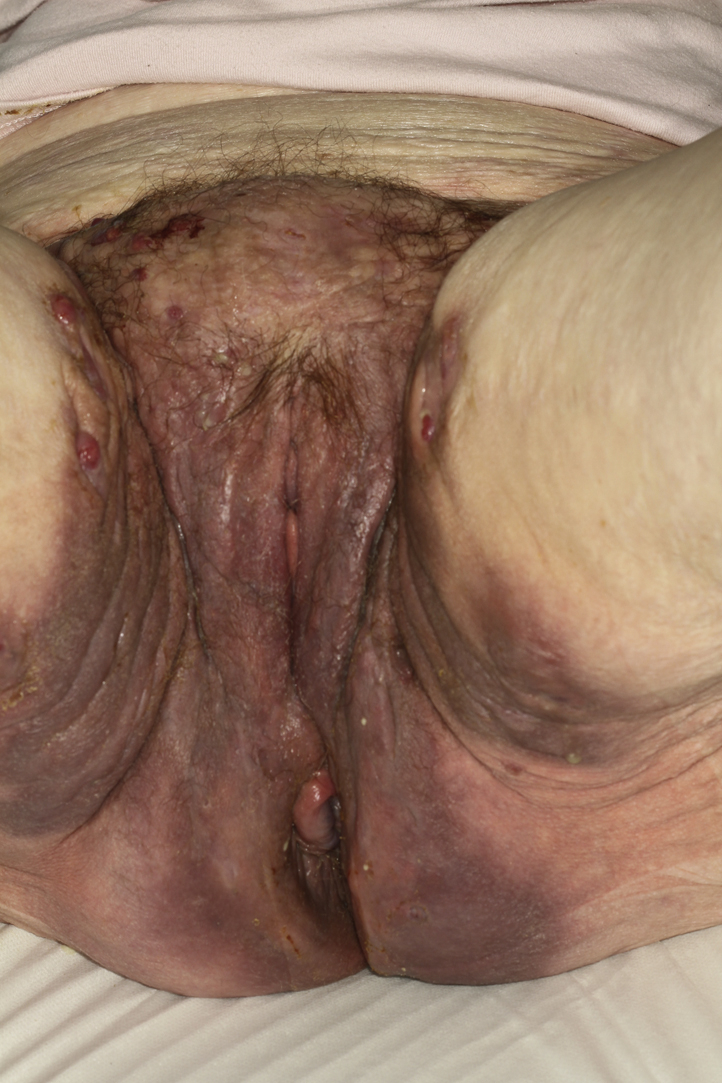
Clinical improvement after 2 months of treatment with adalimumab.
Discussion
We report a case of radiation-induced HS and discuss its possible mechanisms. Whereas the median age of onset of HS is 20 years, the late onset of HS in the present case and its exclusive localization at the site of radiotherapy for uterine carcinoma raised the suspicion either of a paraneoplastic origin or of radiation exposure as the key triggering event. Since the close, regular and careful search for any sign of cancer relapse was negative, we believe skin irradiation is likely to have initiated skin changes leading to an inflammatory form of HS.
This suspicion is reinforced by a previous report by Maher et al who described a patient with a preexisting HS who had a flare of HS over the entire radiation site after treatment for ductal in situ carcinoma; the authors suggested that radiation-induced apoptosis and the enhanced skin immune response were the main factors of HS exacerbation.3, 4
In addition to genetic, endocrine, bacterial and hormonal influences, the innate immune system is in fact found to play an important role in the development of HS. A reduction in natural killer cells, an increased production of free oxygen radicals by neutrophils, an increase in inflammatory cytokines, an enhanced expression of toll-like receptor 2 in macrophages and dendritic cells, and an aberrant expression of antimicrobial peptides have all been incriminated in its pathogenesis.2, 5, 6, 7 High-dose radiation therapy (RT), which is used for the treatment of endometrial adenocarcinoma, can lead to the local production of proinflammatory anaphylatoxins C3a and C5a, to the influx of immune cells in skin, to an increase in apoptosis, and to an increase in the generation of free radicals, providing a hypothesis to the potential mechanism responsible.8 This is not in contradiction with previous evidence that low-dose RT might destroy the hair follicle and is a potentially useful treatment for early lesions of HS because the actual dose regimen used in our case was much higher.9 As was seen in our patient, RT could therefore lead to abnormal proinflammatory and immune responses to bacterial commensal flora and induce HS de novo in predisposed individuals. This raises a specific challenge in the context of cancer, especially regarding the use of adalimumab, which has recently been approved for the treatment of moderate-to-severe HS.10 Hence, in addition to the previously established immunologic and microbiologic hypothesis on the pathophysiology of HS, this case presents an alternative mechanism of HS; further research needs to be done to better understand all potential mechanisms leading to this debilitating disease.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Revuz J. Hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2009;23(9):985–998. doi: 10.1111/j.1468-3083.2009.03356.x. [DOI] [PubMed] [Google Scholar]
- 2.Prens E., Deckers I. Pathophysiology of hidradenitis suppurativa: an update. J Am Acad Dermatol. 2015;73:8–11. doi: 10.1016/j.jaad.2015.07.045. [DOI] [PubMed] [Google Scholar]
- 3.Goldschmidt H., Breneman J.C., Breneman D.L. Ionizing radiation therapy in dermatology. J Am Acad Dermatol. 1994 Feb;30(2 Pt 1):157–182. doi: 10.1016/s0190-9622(94)70014-1. quiz 183-186. [DOI] [PubMed] [Google Scholar]
- 4.Maher M., Larsen L. A case of radiation induced localized exacerbation of hidradenitis suppurativa. JAAD Case Rep. 2016;2(1):44–46. doi: 10.1016/j.jdcr.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelly G., Prens E.P. Inflammatory mechanisms in hidradenitis suppurativa. Dermatol Clin. 2016;34(1):51–58. doi: 10.1016/j.det.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Dréno B., Khammari A., Brocard A. Hidradenitis suppurativa: the role of deficient cutaneous innate immunity. Arch Dermatol. 2012;148(2):182–186. doi: 10.1001/archdermatol.2011.315. [DOI] [PubMed] [Google Scholar]
- 7.Giamarellos-Bourboulis E.J., Antonopoulou A., Petropoulou C. Altered innate and adaptive immune responses in patients with hidradenitis suppurativa. Br J Dermatol. 2007;156:51–56. doi: 10.1111/j.1365-2133.2006.07556.x. [DOI] [PubMed] [Google Scholar]
- 8.Surace L., Lysenko V., Fontana A.O. Complement is a central mediator of radiotherapy-induced tumor-specific immunity and clinical response. Immunity. 2015;42(4):767–777. doi: 10.1016/j.immuni.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Trombetta M., Werts E.D., Parda D. The role of radiotherapy in the treatment of hidradenitis suppurativa: case report and review of the literature. Dermatol Online J. 2010;16(2):16. [PubMed] [Google Scholar]
- 10.Kimball A.B., Okun M.M., Williams D.A. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375(5):422–434. doi: 10.1056/NEJMoa1504370. [DOI] [PubMed] [Google Scholar]