Introduction
We report a case of a fulminant cutaneous cytotoxic T-cell lymphoma nonresponsive to chemotherapy but showing durable complete response to a fast sequence of autologous and allogeneic hematopoietic stem-cell transplantation (HSCT).
Case report
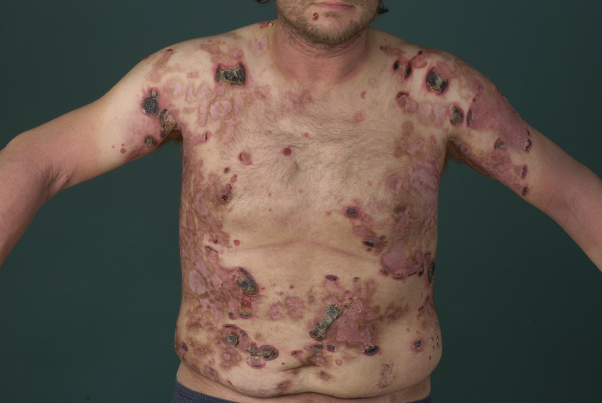
A 47-year-old man was admitted to our department with a 4-month history of initially slowly progressive disseminated erythematous plaques, of which several had ulcerated and were covered by a black crust (Fig 1). Mucous membranes were not involved (modified Severity Weighted Assessment Tool [mSWAT] score, 88.5). Previous treatments with topical steroids were ineffective. Findings from histologic analysis were compatible with lymphomatoid papulosis (LyP) type D. Two weeks later, he was re-admitted because of enlarged ulcerated plaques (no B symptoms, Eastern Cooperative Oncology Group performance status, 2).
Fig 1.
Primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma. Status before therapy with methotrexate.
Further analysis of the previous and a new biopsy found histopathologic and immunophenotypic features of a cytotoxic cutaneous T-cell lymphoma (CTCL), ie, infiltration of hyperchromatic epidermotropic pleomorphic lymphocytes, with angiocentricity in some areas, positive for T-cell receptor–βF1 and weakly for CD3, whereas CD8 was absent on most atypical cells in one biopsy and positive on approximately 40% of epidermotropic pleomorphic cells in another. CD4 was only weakly expressed in about 20% of tumor cells and CD30 was largely negative, as were CD45RO, CD45RA, and CD56. The cytotoxic markers TIA-1 and granzyme B were positive. There was no evidence for Epstein-Barr virus infection (in situ hybridization).
Computed tomography scan and lymph node sonography showed peripheral reactive lymphadenopathy and no malignant lesions in the abdomen, thorax, or central nervous system. Histopathologic analysis of a lymph node biopsy confirmed dermopathic lymphadenopathy without malignancy. Blood count showed moderate neutrophilic leukocytosis and slightly lowered lymphocyte count but no malignant cells. Bone marrow biopsy found reactive lymphocytosis without malignancy signs.
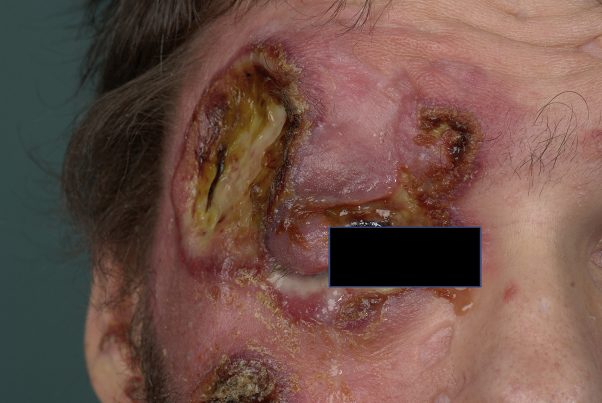
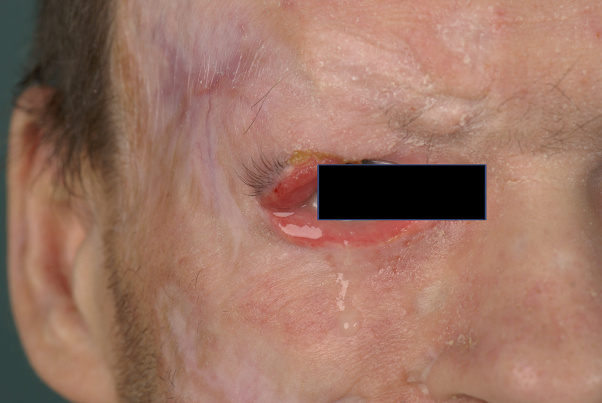
Based on the first histologic findings, which were compatible with LyP, we started treatment with methotrexate (25 mg subcutaneously weekly). However, the disease progressed rapidly, leading to several new ulcerated tumors on the legs, trunk, and face within a few weeks (mSWAT score, 124.5). One rapidly growing tumor destroyed the patient's right eyelid (Fig 2).
Fig 2.
Primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma. Status after therapy with methotrexate and before the CHOEP regimen.
Because of this progressive course, the patient's young age, and the poor prognosis of primary cutaneous epidermotropic CD8+ CTCL,1, 2 we reached a tumor board decision to opt for HSCT, but to try to reduce tumor load and ulcerated lesions to minimize the risk of bacteremia during aplasia and immunosuppression beforehand.
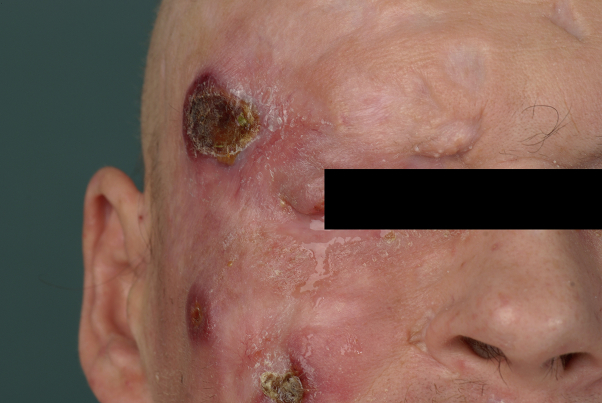
Therefore, we started 4 cycles of a polychemotherapy according to the CHOEP regimen (cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisone), leading only to partial remission (Fig 3). Second-line therapy with 2 cycles of dexamethasone, cytarabine, and cisplatin (DHAP protocol) did not halt progression. Therefore, autologous HSCT was initiated after a high-dose therapy with BEAM (carmustine, cytarabine, etoposide, melphalan) combined with alemtuzumab (CD52-directed monoclonal antibody). Response evaluation shortly after this therapy showed reduction of the disease activity but with persisting skin ulcers. After identification of a well HLA-matched unrelated stem cell donor and the patient's informed consent we applied a myeloablative conditioning using the same high-dose regimen in combination with alemtuzumab, which was used before the autologous HSCT and subsequently performed an allogeneic HSCT. The patient presented with mild complications, which were adequately treated (therapy-related pancytopenia, nausea Common Terminology Criteria grade I, and mucositis Common Terminology Criteria grade III).
Fig 3.
Primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma. Status directly after the CHOEP regimen.
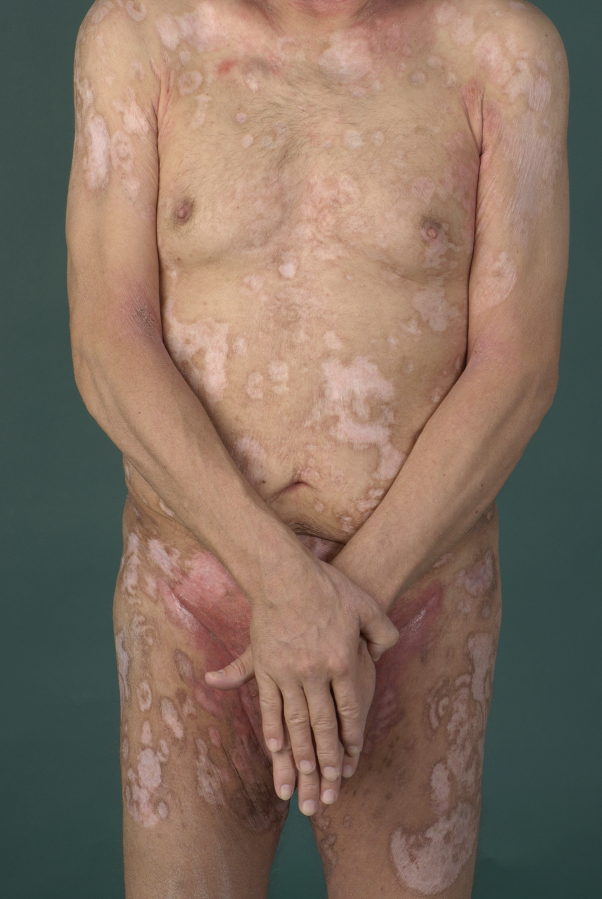
Four weeks after allogeneic HSCT, a good partial remission could be documented with regression of plaques, tumors, and ulcer size. After another 6 weeks, complete remission was achieved (mSWAT score, 6) (Figs 4 and 5), suggesting the occurrence of a relevant graft-versus-lymphoma reaction even in this aggressive disease. With a follow-up of more than 5 years (as of March 2017) our patient is still in complete remission with no relevant graft-versus-host disease and a good quality of life.
Fig 4.
Primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma. Status directly after allogeneic stem cell transplantation.
Fig 5.
Primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma. Status after allogeneic stem cell transplantation.
Discussion
We classified this fulminant CTCL as a primary aggressive cutaneous cytotoxic T-cell non-Hodgkin lymphoma (non–Sezary syndrome, non–mycosis fungoides),3 most likely aggressive epidermotropic CD8+ cytotoxic CTCL, because it showed marked epidermotropism by many T cells with α/β receptor and expression of TIA-1 and granzyme B, of which at least some expressed CD8. Unusual was that not all malignant T cells were CD8+ and that they were negative for CD45RA; however, this finding is not incompatible with this type of lymphoma.
Contrary to other cases1, 4 of primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphomas, our patient presented with no mucosal or extracutaneous involvement when we decided to perform HSCT, but one might speculate that visceralization was prevented by the good response to HSCT. Additionally, there were no clinical or histologic signs sufficiently suggestive of another lymphoma. The development of ulcerated lesions was more rapid than in CD8+ mycosis fungoides, and there was no waxing and waning as in LyP or negativity for CD30 (albeit staining for CD30 may be weak in LyP type D).
The prognosis of aggressive epidermotropic CD8+ CTCL is poor, with median survival of 12 months5 and average 5-year survival rate estimated to be 0%1 to 18%,6 mainly because of high aggressiveness; rapid spread to the lungs, testes, and central nervous system; and low response rate to chemotherapy. Nevertheless, systemic polychemotherapy is usually attempted but often without lasting response.6 In our case, because of the multiple skin ulcerations, with imminent suspected bacterial superinfection and, consequently, with high risk for severe infectious complications, allogeneic HSCT was not considered as the first salvage option after conventional chemotherapy. However, having not achieved complete remission after high-dose chemotherapy with autologous HSCT, but improvement of the skin ulceration, allogeneic HSCT was performed. This sequential approach with autologous HSCT, shortly followed by allogeneic HSCT, might be beneficial in patients with high tumor burden. In other patients, direct allogeneic HSCT is probably sufficient to allow long-term disease control.
There are only a few case reports on allogeneic transplantation in primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphomas.7 The clinical outcome varies, but complete remission after allogeneic HSCT is possible, even in heavily pretreated patients.8 We show that a fast sequence of autologous and allogeneic HSCT might be a curative option in patients with therapy-resistant aggressive disease, especially when performed early and should therefore be included in the initial therapeutic considerations.
Footnotes
Drs Plachouri, Weishaupt, Sunderkötter, and Stelljes contributed equally to this article.
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Berti E., Tomasini D., Vermeer M.H., Meijer C.J., Alessi E., Willemze R. Primary cutaneous CD8-positive epidermotropic cytotoxic T cell lymphomas. Am J Pathol. 1999;155:483–492. doi: 10.1016/S0002-9440(10)65144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Willemze R.1., Jaffe E.S., Burg G. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768–3785. doi: 10.1182/blood-2004-09-3502. [DOI] [PubMed] [Google Scholar]
- 3.Burg G., Kempf W., Cozzio A. WHO/EORTC classification of cutaneous lymphomas: histological and molecular aspects. J Cutan Pathol. 2005;32:647–674. doi: 10.1111/j.0303-6987.2005.00495.x. [DOI] [PubMed] [Google Scholar]
- 4.Nofal A., Abdel-Mawla M.Y., Assaf M., Salah E. Primary cutaneous aggressive epidermotropic CD8+ T-cell lymphoma: proposed diagnostic criteria and therapeutic evaluation. J Am Acad Dermatol. 2012;67:748–759. doi: 10.1016/j.jaad.2011.07.043. [DOI] [PubMed] [Google Scholar]
- 5.Robson A., Assaf C., Bagot M. Aggressive epidermotropic cutaneous CD8+ lymphoma: a cutaneous lymphoma with distinct clinical and pathological features. Report of an EORTC Cutaneous Lymphoma Task Force Workshop. Histopathology. 2015;67:425–441. doi: 10.1111/his.12371. [DOI] [PubMed] [Google Scholar]
- 6.Gormley R.H., Hess S.D., Anand D., Junkins-Hopkins J., Rook A.H., Kim E.J. Primary cutaneous aggressive epidermotropic CD8+ T-cell lymphoma. J Am Acad Dermatol. 2010;62:300–307. doi: 10.1016/j.jaad.2009.02.035. [DOI] [PubMed] [Google Scholar]
- 7.Liu V., Cutler C.S., Young A.Z. Case records of the Massachusetts General Hospital. Case 38-2007. A 44-year-old woman with generalized, painful, ulcerated skin lesions. N Engl J Med. 2007;357:2496–2505. doi: 10.1056/NEJMcpc0706687. [DOI] [PubMed] [Google Scholar]
- 8.Introcaso C.E., Leber B., Greene K., Ubriani R., Rook A.H., Kim E.J. Stem cell transplantation in advanced cutaneous T-cell lymphoma. J Am Acad Dermatol. 2008;58:645–649. doi: 10.1016/j.jaad.2007.12.021. [DOI] [PubMed] [Google Scholar]