Abstract
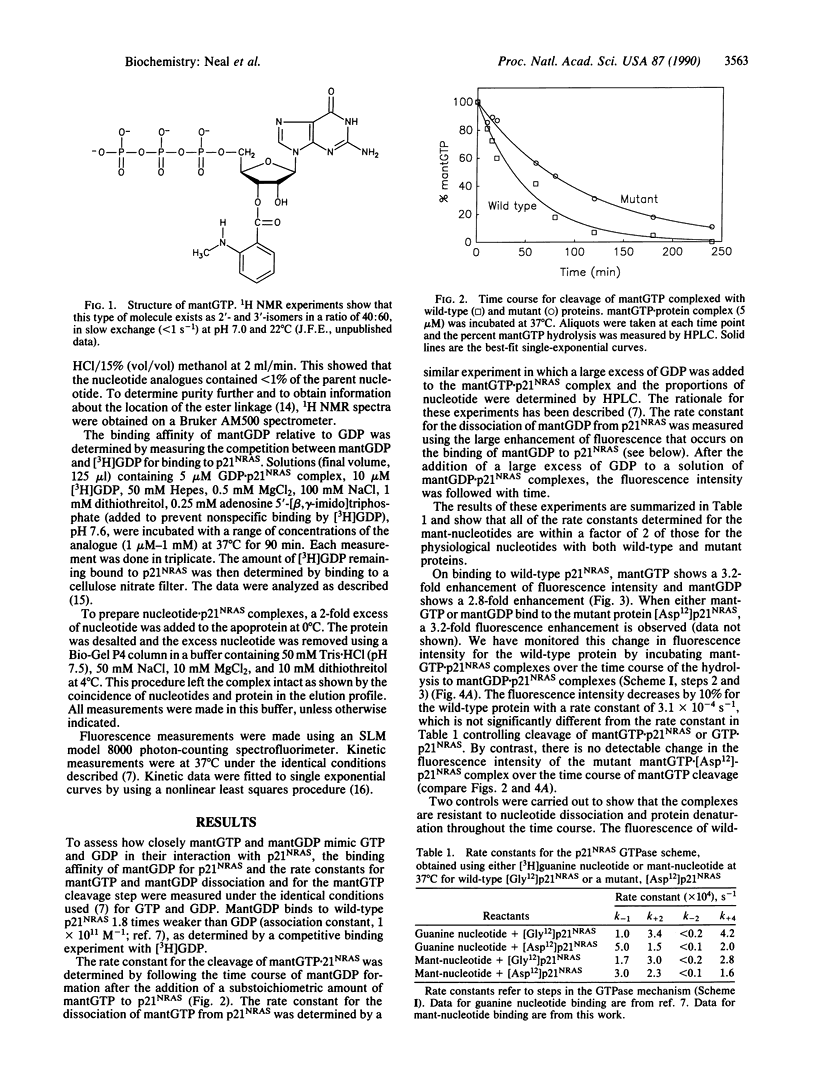
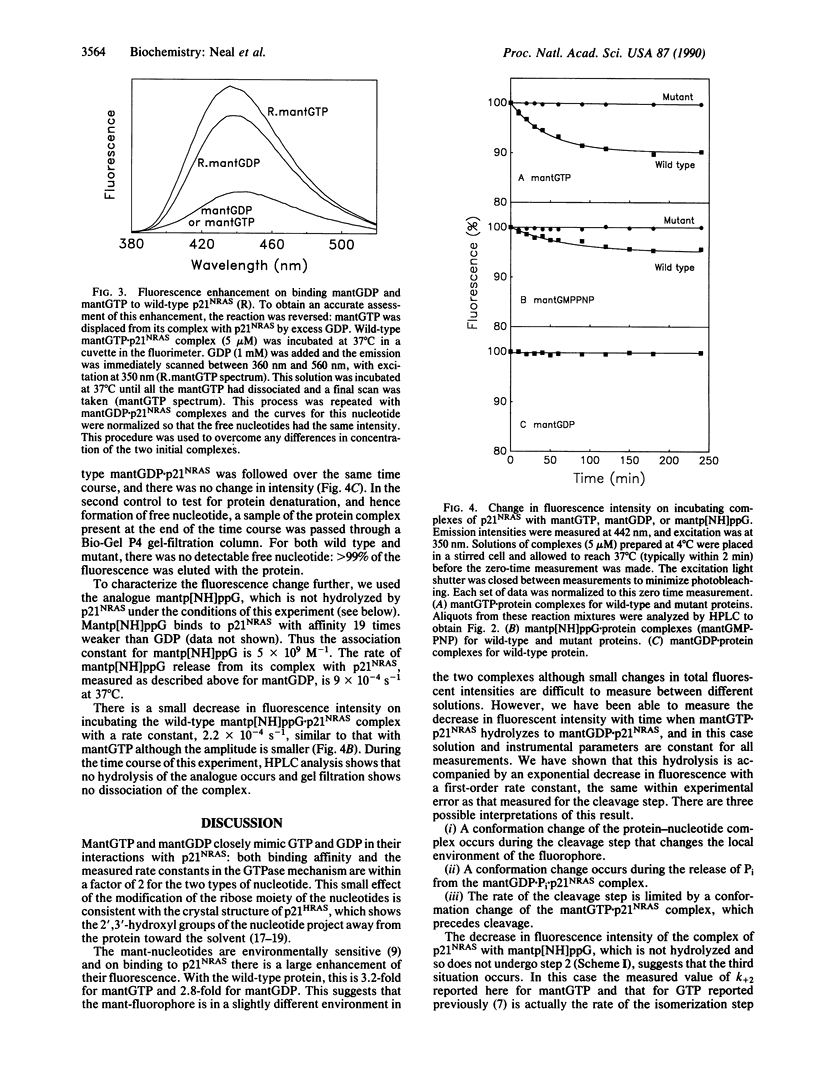
2'(3')-O-(N-Methyl)anthraniloylguanosine 5'-triphosphate (mantGTP) is a fluorescent analogue of GTP that has similar properties to the physiological substrate in terms of its binding constant and the kinetics of its interactions with p21NRAS, the NRAS protooncogene product. There is a 3-fold increase in fluorescence intensity when mantGTP binds to p21NRAS. The rate constant for the cleavage of mantGTP complexed with the protein is similar to that of GTP, and cleavage is accompanied by a fluorescence intensity change in the wild-type protein complex. A two-phase fluorescence change also occurs when the nonhydrolyzable analogue 2'(3')-O-(N-methyl)anthraniloylguanosine 5'-[beta, gamma-imido]triphosphate (mantp[NH]ppG) binds to wild-type p21NRAS. The second phase occurs at the same rate as the second phase observed after mantGTP binding. Thus this second phase is probably a conformation change of the p21NRAS nucleotiside triphosphate complex and that the change controls the rate of GTP hydrolysis on the protein. With a transforming mutant, [Asp12]-p21NRAS, there is no second phase of the fluorescence change after mantGTP or mantp[NH]ppG binding, even though mantGTP is hydrolyzed. This shows that an equivalent conformational change does not occur and thus the mutant may stay in a "GTP-like" conformation throughout the GTPase cycle. These results are discussed in terms of the proposed role of p21NRAS in signal transduction and the transforming properties of the mutant.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adari H., Lowy D. R., Willumsen B. M., Der C. J., McCormick F. Guanosine triphosphatase activating protein (GAP) interacts with the p21 ras effector binding domain. Science. 1988 Apr 22;240(4851):518–521. doi: 10.1126/science.2833817. [DOI] [PubMed] [Google Scholar]
- Burk S. C., Papastavros M. Z., McCormick F., Redfield A. G. Identification of resonances from an oncogenic activating locus of human N-RAS-encoded p21 protein using isotope-edited NMR. Proc Natl Acad Sci U S A. 1989 Feb;86(3):817–820. doi: 10.1073/pnas.86.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calés C., Hancock J. F., Marshall C. J., Hall A. The cytoplasmic protein GAP is implicated as the target for regulation by the ras gene product. Nature. 1988 Apr 7;332(6164):548–551. doi: 10.1038/332548a0. [DOI] [PubMed] [Google Scholar]
- Cremo C. R., Yount R. G. 2'-Deoxy-3'-O-(4-benzoylbenzoyl)- and 3'(2')-O-(4-benzoylbenzoyl)-1,N6-ethenoadenosine 5'-diphosphate, fluorescent photoaffinity analogues of adenosine 5'-diphosphate. Synthesis, characterization, and interaction with myosin subfragment 1. Biochemistry. 1987 Nov 17;26(23):7524–7534. doi: 10.1021/bi00397a048. [DOI] [PubMed] [Google Scholar]
- Eccleston J. F. Spectroscopic studies of the nucleotide binding site of elongation factor Tu from Escherichia coli. An approach to characterizing the elementary steps of the elongation cycle of protein biosynthesis. Biochemistry. 1981 Oct 13;20(21):6265–6272. doi: 10.1021/bi00524a055. [DOI] [PubMed] [Google Scholar]
- Feuerstein J., Goody R. S., Webb M. R. The mechanism of guanosine nucleotide hydrolysis by p21 c-Ha-ras. The stereochemical course of the GTPase reaction. J Biol Chem. 1989 Apr 15;264(11):6188–6190. [PubMed] [Google Scholar]
- Feuerstein J., Kalbitzer H. R., John J., Goody R. S., Wittinghofer A. Characterisation of the metal-ion-GDP complex at the active sites of transforming and nontransforming p21 proteins by observation of the 17O-Mn superhyperfine coupling and by kinetic methods. Eur J Biochem. 1987 Jan 2;162(1):49–55. doi: 10.1111/j.1432-1033.1987.tb10540.x. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Grand R. J., Levine B. A., Byrd P. J., Gallimore P. H. The binding of guanine nucleotide to N-ras p21--a phosphorous and proton magnetic resonance study. Oncogene. 1989 Mar;4(3):355–361. [PubMed] [Google Scholar]
- Hall A., Self A. J. The effect of Mg2+ on the guanine nucleotide exchange rate of p21N-ras. J Biol Chem. 1986 Aug 25;261(24):10963–10965. [PubMed] [Google Scholar]
- Hiratsuka T. New ribose-modified fluorescent analogs of adenine and guanine nucleotides available as substrates for various enzymes. Biochim Biophys Acta. 1983 Feb 15;742(3):496–508. doi: 10.1016/0167-4838(83)90267-4. [DOI] [PubMed] [Google Scholar]
- Marshall C. J. Oncogenes. J Cell Sci Suppl. 1986;4:417–430. doi: 10.1242/jcs.1986.supplement_4.22. [DOI] [PubMed] [Google Scholar]
- Neal S. E., Eccleston J. F., Hall A., Webb M. R. Kinetic analysis of the hydrolysis of GTP by p21N-ras. The basal GTPase mechanism. J Biol Chem. 1988 Dec 25;263(36):19718–19722. [PubMed] [Google Scholar]
- Pai E. F., Kabsch W., Krengel U., Holmes K. C., John J., Wittinghofer A. Structure of the guanine-nucleotide-binding domain of the Ha-ras oncogene product p21 in the triphosphate conformation. Nature. 1989 Sep 21;341(6239):209–214. doi: 10.1038/341209a0. [DOI] [PubMed] [Google Scholar]
- Pingoud A., Wehrmann M., Pieper U., Gast F. U., Urbanke C., Alves J., Feuerstein J., Wittinghofer A. Spectroscopic and hydrodynamic studies reveal structural differences in normal and transforming H-ras gene products. Biochemistry. 1988 Jun 28;27(13):4735–4740. doi: 10.1021/bi00413a023. [DOI] [PubMed] [Google Scholar]
- Stryer L., Bourne H. R. G proteins: a family of signal transducers. Annu Rev Cell Biol. 1986;2:391–419. doi: 10.1146/annurev.cb.02.110186.002135. [DOI] [PubMed] [Google Scholar]
- Tong L. A., de Vos A. M., Milburn M. V., Jancarik J., Noguchi S., Nishimura S., Miura K., Ohtsuka E., Kim S. H. Structural differences between a ras oncogene protein and the normal protein. Nature. 1989 Jan 5;337(6202):90–93. doi: 10.1038/337090a0. [DOI] [PubMed] [Google Scholar]
- Tong L., Milburn M. V., de Vos A. M., Kim S. H. Structure of ras proteins. Science. 1989 Jul 21;245(4915):244–244. doi: 10.1126/science.2665078. [DOI] [PubMed] [Google Scholar]
- Trahey M., McCormick F. A cytoplasmic protein stimulates normal N-ras p21 GTPase, but does not affect oncogenic mutants. Science. 1987 Oct 23;238(4826):542–545. doi: 10.1126/science.2821624. [DOI] [PubMed] [Google Scholar]
- de Vos A. M., Tong L., Milburn M. V., Matias P. M., Jancarik J., Noguchi S., Nishimura S., Miura K., Ohtsuka E., Kim S. H. Three-dimensional structure of an oncogene protein: catalytic domain of human c-H-ras p21. Science. 1988 Feb 19;239(4842):888–893. doi: 10.1126/science.2448879. [DOI] [PubMed] [Google Scholar]


