Introduction
Basal cell carcinoma (BCC) represents nearly 80% of nonmelanoma primary skin malignancies in the United States. However, metastatic BCC (mBCC) is exceedingly rare, difficult to treat, and associated with high mortality, yet it's easily preventable. We report a case of mBCC in a patient with a 15-year history of a neglected primary BCC on the left upper extremity. Mismatch repair protein expression was lost in the primary tumor and metastatic deposits. In addition, p63 expression, which was present in the primary BCC, was absent in the metastatic tumor cells.
Case
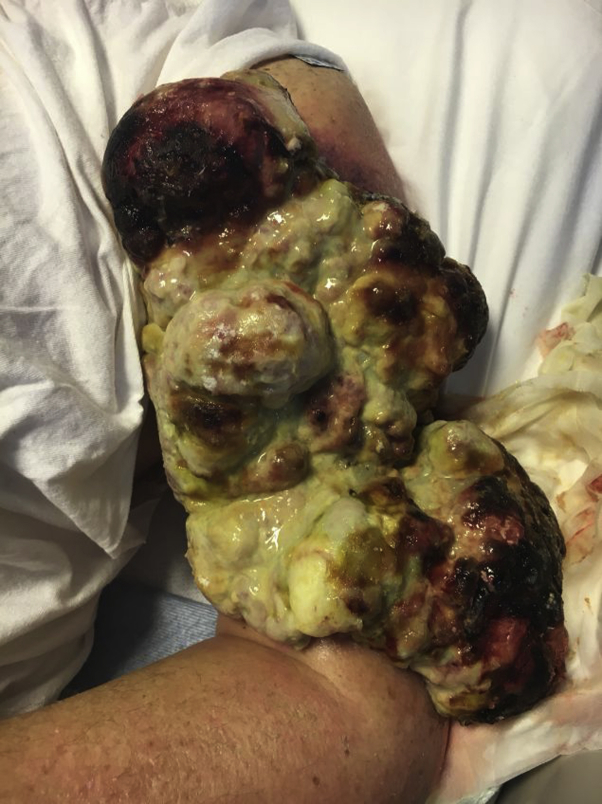
A 55-year-old white man came to the emergency department with a large, fungating, friable mass on the left upper extremity, which had been neglected for 15 years (Fig 1). Physical exam revealed a 15 × 12 cm exophytic tumor with several fixed and indurated ipsilateral axillary lymph nodes. Computed tomography (CT) scan demonstrated significant soft tissue invasion without bony erosion of the humerus.
Fig 1.
Gross image of the primary tumor in a patient with basal cell carcinoma in his left upper extremity. We appreciate John Trenton Gay, DO, for providing this clinical photo.
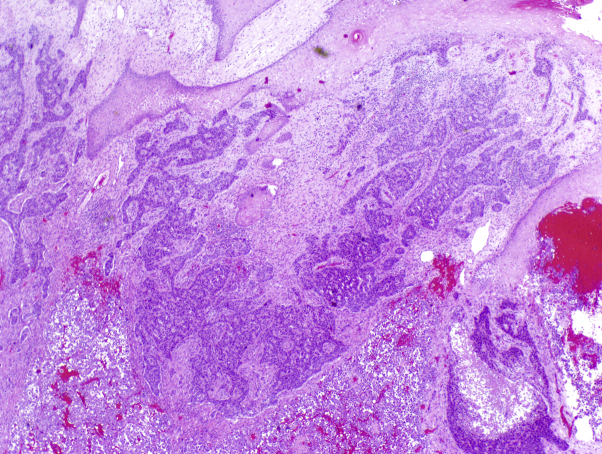
Punch biopsy was diagnostic of BCC (Fig 2). Chest CT uncovered several lung nodules, the largest measuring 2 cm, as well as a 2.5 × 2.9–cm pretracheal lymph node. The latter was sampled via endoscopic ultrasound-guided transbronchial needle biopsy; testing of this sample indicated metastatic carcinoma histologically consistent with the primary BCC.
Fig 2.
Histologic examination of a punch biopsy from a patient with basal cell carcinoma. The initial punch biopsy and amputation resection of the primary tumor showed basaloid tumor cell nests with peripheral palisading and tumor stromal clefting with an infiltrative growth pattern and foci of a poorly differentiated tumor in deeper aspects. (Hematoxylin-eosin stain; original magnification: ×20.)
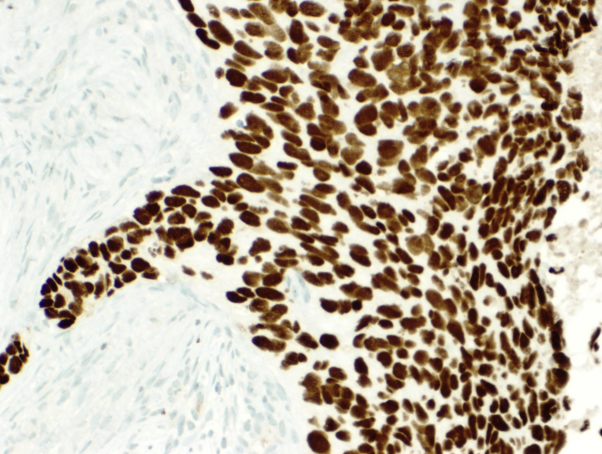
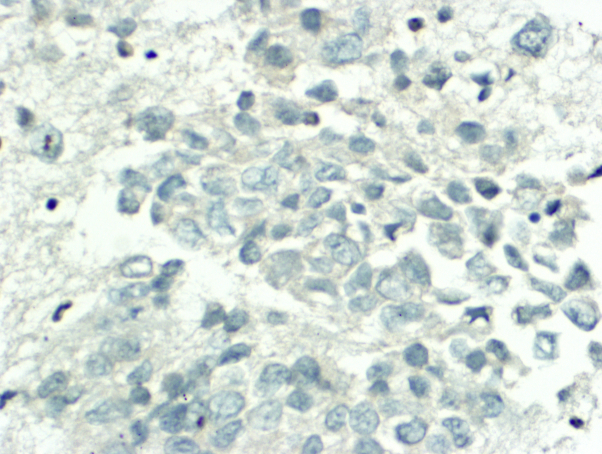
Immunohistochemistry (IHC) performed on the primary tumor and pretracheal metastases demonstrated strong positivity for epithelial cell adhesion molecule (clone Ber-EP4), B-cell lymphoma (Bcl)-2, and high molecular weight keratins, including keratin 903 and CK5/6, a pattern typical of BCC. The strong Ber-EP4 positivity in conjunction with negative adipophilin and androgen receptor staining excluded sebaceous and squamous cell carcinomas. The primary tumor and nodal metastases demonstrated loss of 2 homologous mismatch repair proteins (MLH1 and PMS2) by IHC. In addition, the primary tumor of the skin was positive for p63 expression (Fig 3), and the metastases in the nodes were negative for p63 expression (Fig 4).
Fig 3.
p63-positive primary basal cell carcinoma. (p63 staining; original magnification: ×200.)
Fig 4.
p63-negative metastatic basal cell carcinoma in the pretracheal lymph node. (p63 staining; original magnification: ×400.)
The patient was given hedgehog (HH) inhibitor therapy, but treatment was limited by poor tolerance and subsequent hospital admission for another soft tissue infection of his left arm. His hospital course was complicated by septic shock, and because his infection was unresponsive to broad-spectrum antibiotics, the oncologic surgeon opted for a palliative proximal humeral amputation. Axillary lymph nodes sampled at the time of amputation showed an identical staining pattern to the pretracheal nodes. During hospitalization, he developed renal failure and encephalopathy. CT scan of the head demonstrated intracranial masses suspicious for brain metastases. The patient's family ultimately opted for comfort care, and he died 14 weeks after diagnosis.
Discussion
BCC represents nearly 80% of nonmelanoma primary skin malignancies in the United States and is characterized by an indolent, nonaggressive course with low morbidity. Metastatic BCC occurs in <0.55% of cases; a 2014 report identified 172 cases of mBCC meeting accepted diagnostic criteria in the 30 years from 1981 through 2011.1 However, an earlier review recognized 268 cases reported in the literature from 1894 through 2004.2 Mortality is high with mBCC; those with distant spread eventually succumb to complications of the disease or treatment. Average survival for patients with isolated lymph node metastases is 3.6 years, while that of patients with hematogenous spread to distant sites, such as lung and bone, is only 8-14 months.1
The diagnosis of mBCC is based on the Lattes and Kessler criteria, which requires a documented history of BCC and histologic analysis of the proposed metastatic lesion consistent with mBCC. For cases in which metastatic disease is limited to a local lymph node, direct tumor extension must be ruled out.3 Positivity for Ber-EP4, Bcl-2, and high molecular weight keratins support a diagnosis of BCC, and negative staining for adipophilin and androgen receptor helps distinguish BCC from other carcinomas.4, 5
The tendency for BCC not to metastasize is hypothetically related to its dependence on the surrounding dermal stroma.6 Metastases occur when either tumor cells lose this dependence or when tumor emboli are large enough to include attached stroma and are transported to distant sites via lymphatics or blood vessels.6 In addition, unlike sebaceous carcinomas, most BCCs display strong expression of mismatch repair proteins, particularly in peripheral cells in tumor nests near the stromal interface, contributing to their normally indolent behavior.7 Rare reports of primary BCC with loss of mismatch repair exist, but no definitive association between aggressive behavior and mismatch repair loss has been established.8 To our knowledge, loss of mismatch repair has not been previously reported in mBCC.
A homologue to the tumor suppressor protein p53, p63 plays an important role in epidermal development and differentiation.9 There are 6 distinct isoforms of p63 that result from transcription of various promoters and alternative C-terminal splicing.10 Some evidence suggests that p63 inhibits metastasis because loss of p63 expression leads to upregulation of genes associated with changing morphology and invasion.11 p63 is a regulator of the HH pathway, mutations in which affect BCC pathogenesis. Constitutive activation of the HH pathway results in resistance to apoptosis via the overexpression of the Gli target gene, Bcl-2. Also, suppressor of fused (SUFU) is a cytoplasmic protein that modulates the HH pathway by binding to Gli and prevents Gli translocation into the nucleus. Overexpression of p63 results in increased SUFU expression and decreased Bcl-2. Normally, Gli activation increases p63, providing feedback that downregulates the HH pathway. p63 levels can be increased in BCC, likely secondary to the constitutive activity of Gli.10 This raises the question of whether loss of p63 was a critical step in the development of metastatic disease in this patient or an incidental finding in a poorly differentiated metastatic tumor.
Metastatic BCC is a potentially fatal disease, and this case reinforces the value of timely diagnosis and treatment of localized BCC. In addition, it documents mismatch repair protein loss in mBCC and suggests that p63 is an inhibitor of invasion and metastasis.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.McCusker M., Basset-Sequin N., Dummer R. Metastatic basal cell carcinoma: prognosis dependent on anatomic site and spread of disease. Eur J Cancer. 2014;50(4):774–783. doi: 10.1016/j.ejca.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 2.Ionesco D.N., Arida M., Jukic D.M. Metastatic basal cell carcinoma: four case reports, review of literature, and immunohistochemical evaluation. Arch Pathol Lab Med. 2006;130(1):45–51. doi: 10.5858/2006-130-45-MBCCFC. [DOI] [PubMed] [Google Scholar]
- 3.Lattes R., Kessler R.W. Metastasizing basal-cell epithelioma of the skin: report of two cases. Cancer. 1951;4:866–878. doi: 10.1002/1097-0142(195107)4:4<866::aid-cncr2820040424>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 4.Beer T.W., Shepherd P., Theaker J.M. Ber EP4 and epithelial membrane antigen aid distinction of basal cell, squamous cell, and basosquamous carcinomas of the skin. Histopathology. 2000;37(3):218–223. doi: 10.1046/j.1365-2559.2000.00999.x. [DOI] [PubMed] [Google Scholar]
- 5.Mulay K., White V.A., Shah S.J. Sebaceous carcinoma: clinicopathologic features and diagnostic role of immunohistochemistry (including androgen receptor) Can J Ophthalmol. 2014;49(4):326–332. doi: 10.1016/j.jcjo.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Blewitt R.W. Why does basal cell carcinoma metastasize so rarely? Int J Dermatol. 1980;19:144–146. doi: 10.1111/j.1365-4362.1980.tb00281.x. [DOI] [PubMed] [Google Scholar]
- 7.Mosterd K., Nellen R., Van Engeland M. Defect in DNA mismatch repair do not account for early-onset basal cell carcinoma. Br J Dermatol. 2008;159(3):751–753. doi: 10.1111/j.1365-2133.2008.08721.x. [DOI] [PubMed] [Google Scholar]
- 8.Young L.C., Listgarten J., Trotter M.J. Evidence that dysregulated DNA mismatch repair characterizes human nonmelanoma skin cancer. Br J Dermatol. 2008;158(1):59–69. doi: 10.1111/j.1365-2133.2007.08249.x. [DOI] [PubMed] [Google Scholar]
- 9.Reis-Filho J.S., Torio B., Albergaria A., Schmitt F.C. p63 expression in normal skin and usual cutaneous carcinomas. J Cutan Pathol. 2002;29(9):517–523. doi: 10.1034/j.1600-0560.2002.290902.x. [DOI] [PubMed] [Google Scholar]
- 10.Chari N.S., Romano R.A., Koster M.I. Interaction between the TP63 and SHH pathways is an important determinant of epidermal homeostasis. Cell Death Differ. 2013;20:1080–1088. doi: 10.1038/cdd.2013.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbieri C.E., Tang L.J., Brown K.A., Pietenpol J.A. Loss of p63 leads to increased cell migration and up-regulation of genes involved in invasion and metastasis. Cancer Res. 2006;66(15):7589–7597. doi: 10.1158/0008-5472.CAN-06-2020. [DOI] [PubMed] [Google Scholar]