Abstract
Background
To prospectively investigate the incidence and relative risks of multiple sclerosis (MS) in patients with type 2 diabetes (T2DM).
Materials and methods
Patients with T2DM (n = 614,623) and age- and sex-matched controls (n = 614,021) were followed from 2000 to 2008 to identify cases of newly diagnosed MS (ICD-9-CM: 340). The person-year approach with Poisson assumption was used to evaluate the incidence density. We estimated the covariate-adjusted hazard ratio (HR) of MS incidence in relation to T2DM diabetes using a multiple Cox proportional hazard regression model.
Results
Over 9 years of follow-up, 175 T2DM patients were newly diagnosed with MS, and 114 matched controls had the same first-ever diagnosis, representing a covariate-adjusted HR of 1.44 (95% confidence interval [CI], 1.08–1.94). The sex-specific adjusted HR for both men and women with T2DM was also elevated at 1.34 (95% CI, 0.81–2.23) and 1.51 (95% CI, 1.05–2.19), respectively. Women aged ≤50 years had the greatest risk of MS (HR 2.16; 95% CI, 1.02–4.59).
Conclusion
This study demonstrated a moderate but significant association of T2DM with MS incidence, and the association was not confounded by socio-demographic characteristics or certain MS-related co-morbidities.
Keywords: Diabetes mellitus, Multiple sclerosis, Cohort studies, Cox proportional hazard model
Highlights
-
•
Risk ratio of incident multiple sclerosis (MS) in T2DM patients was 1.44.
-
•
Risk ratio of incident MS was highest in women aged <50 years.
-
•
The PAR% for T2DM in the MS incidence was estimated at 2.55%.
Introduction
Type 2 diabetes (T2DM) is the most prevalent disease in many modern societies and affects over 340 million people in the world.1 It is characterized by insulin resistance and impaired islet beta cell function, which together result in an inability to supply sufficient insulin to meet the body's demands and eventual beta cell loss.2 Recently, several studies have reported that some autoimmune diseases were associated with certain genotypes that affect autoimmunity and confer increased risk of autoantibody seroconversion, leading to damage of islet beta cells and progression of diabetes.3, 4 However, other studies have contradicted these findings.5, 6
Multiple sclerosis (MS), a chronic inflammatory and progressive immune-mediated disease of the central nervous system (CNS), is a heterogeneous disorder with variable clinical and pathologic features reflecting different pathways to tissue injury.7 Inflammation, demyelination, and axonal degeneration are the major pathologic mechanisms that cause the clinical manifestations.8, 9 Genetic and environmental factors are also thought to contribute to the pathogenesis of the disease.10 Although some evidence suggests that the predisposition of human leukocyte antigen (HLA) haplotypes in patients with T1DM protects against MS,11 most previous studies have documented similarities in immunological and epidemiological features between MS and type 1 diabetes (T1DM) through the susceptibility loci of both diseases.12, 13 Despite recent arguments that T2DM is an autoimmune disorder,14 there is still a lack of epidemiological studies examining the relationship between T2DM and MS.
The aim of this study was to examine the putative link between T2DM and risk of MS incidence. Given the rare occurrence of MS, our investigation employed a large nationwide T2DM population in Taiwan to prospectively examine whether T2DM may increase the future risk of MS. In addition, we also explored the age- and sex-specific relationships between T2DM and MS in order to identify T2DM patients at particularly high risk of MS.
Methods
Study design
A universal National Health Insurance (NHI) Program has been implemented by the NHI Administration (NHIA; previously called the Bureau of NHI) under the jurisdiction of the Ministry of Health and Welfare since March 1995. Approximately 96% of the Taiwanese population had enrolled in NHI Program and the NHI Administration had contracted 97% of hospitals and 90% of clinics all over Taiwan by the end of 1996.15 To ensure the accuracy of claim files, the NHI Administration performs quarterly expert reviews on a random sample of every 50 to 100 ambulatory and inpatient claims, and information available is considered to be complete and accurate.16 With the ethics approval from the Review Committee of the National Health Research Institutes, we used data of diabetic ambulatory care claims (1997–2008), all inpatient claims (1997–2008), and the updated registry for beneficiaries (1995–2002) for this study. The entire dataset was inter-linked through each individual's personal identification number (PIN).
Identification of study subjects
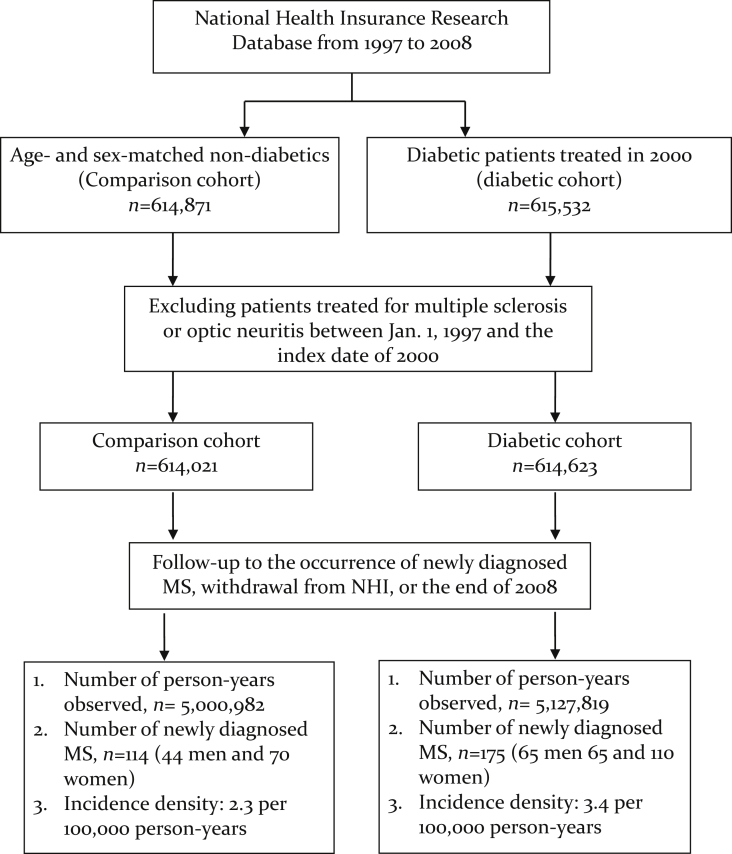
Details of the selection of patients with T2DM and control subjects have been thoroughly reported before.17, 18 Briefly, in order to be considered as a patient with T2DM, patients must have made more than one ambulatory care visit for T2DM (ICD-9-CM: 250x0 or 250x2) in 2000–2001; the first and the last visits must have been >30 days apart. A validation study surveyed a sample of 1350 patients with a diagnosis of T2DM in the NHI medical claims and noted that 1007 of them were confirmed to have accurate diagnoses, representing a concordance rate of 74.6%. The study further noted that the probability of accurate diagnosis of T2DM among patients with more than two outpatient visits was 2.67 times greater than that of patients with only one outpatient visit for T2DM.19 To ensure the accurate estimation of the incidence rate of MS, we excluded those T2DM patients who had MS diagnoses (ICD-9-CM: 340) with major illness/injury certificates between January 1, 1997 and the first clinical visit for T2DM in 2000. All MS diagnoses were verified using major illness/injury certificates. The final T2DM cohort consisted of 614,623 patients. The index date for each patient with T2DM was the date of his/her first outpatient visit for T2DM in 2000 (Fig. 1).
Fig. 1.
Flowchart for the study cohort enrollment and follow-up. MS, multiple sclerosis; NHI, National Health Insurance.
The control group was randomly selected from all NHI beneficiaries registered in 2000 by matching the T2DM group on the frequency of age (every 5 years) and sex. People included in the control group must have had no prior histories of diabetes (including T1DM and T2DM) or MS in 1997 and 1999. We selected 614,021 frequency-matched control subjects in this study; the index date for the subjects in the control group was July 1, 2000 or the date of NHI enrollment, if their first date of NHI enrollment was after July 1, 2000 (see Fig. 2).
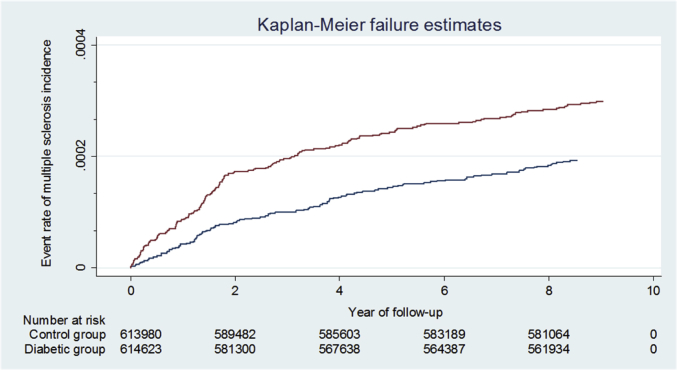
Fig. 2.
Kaplan–Meier curves and estimates of multiple sclerosis incidence in the diabetes and comparison groups.
The geographic area of each member's NHI unit, either the beneficiaries' residential area or location of their employment, was grouped into one of four geographic areas (North, Central, South, or East) and two urbanization statuses (urban or rural) according to the National Statistics of Regional Standard Classification. The information on clinical risk factors for MS, including allergy (ICD-9-CM: 995), Alzheimer's disease (ICD-9-CM: 331), anterior horn cell disease (ICD-9-CM: 335), chronic obstructive pulmonary disease (COPD) (ICD-9-CM: 410–414, 430–438), obesity (ICD-9-CM: 278), vitamin D deficiency (ICD-9-CM: 268), dyslipidemia (ICD-9-CM: 272), hypertension (ICD-9-CM: 401–405), inflammatory bowel disease (ICD-9-CM: 555–558), spinal cord injury (ICD-9-CM: 806, 952), stroke (ICD-9-CM: 430–438), thyroid disease (ICD-9-CM: 240–246), traumatic brain injury (ICD-9-CM: 801–804 or 850–854), anxiety (ICD-9-CM: 300), depression (ICD-9-CM: 296, 309, 311), stress (ICD-9-CM: 308), tonsillectomy (ICD-9-CM: 28), and appendectomy (ICD-9-CM: 47), was retrieved from inpatient and outpatient medical claims between January 1997 and the index date. Consideration of these covariates (i.e., potential confounders) in the analysis was mainly because those co-morbidities are either known clinical risk factors for MS or have been suspected of being associated with development of MS.20, 21, 22, 23, 24 We also calculated the Charlson Comorbidity Index (CCI) to indicate common comorbid conditions weighted according to mortality risk.25 The number of ambulatory care visits in 2000 for each study subject was counted.
Study endpoints
All study subjects were followed from the index date to the occurrence of first-time diagnosis of MS, termination of NHI policy, or the end of 2008, whichever came first. Information on the MS diagnosis was retrieved from the inpatient and outpatient claims. To ensure the accuracy of the diagnoses of MS, only MS patients with major illness/injury certificates were counted. In Taiwan, major illness/injury certificates are issued to all patients with a diagnosis of MS. The core requirement of the diagnosis is the objective demonstration of dissemination of lesions in both space and time, based upon either clinical findings alone or a combination of clinical and MRI findings. In addition, cerebrospinal fluid analyses and evoked potentials are also provided to support the diagnosis of MS. Neurologists are required to provide complete medical records, including the aforementioned clinical history/laboratory/imaging/electrophysiological data, to a committee composed of a panel of expert neurologists in the application for this approval for patents.26 In order to avoid the miscoding, we retrieved only those patients with major illness/injury certificates of this particular diagnosis.
Statistical analysis
We first described and tested the characteristics between patients with T2DM and matched controls. The age- and sex-specific incidence density rate was estimated with person-years as the denominator under the Poisson assumption. We then performed multiple Cox proportional hazard models to assess the independent effects, indicated by hazard ratios (HRs) and corresponding 95% confidence intervals (CIs), of T2DM on the risk of MS. Adjustment for both geographic area and urbanization status was made to account for possible urban–rural differences in accessibility to medical health services in Taiwan.27 Inclusion of frequency of medical visits in the multiple regression model was used to reduce the potential for surveillance bias arising from the fact that patients with T2DM might have a higher chance of having MS detected simply due to their frequent contact with clinicians.
Although we managed to adjust for potential clinical risk factors for MS, some lifestyle factors, such as smoking, remained unadjusted in the analysis, mainly due to a lack of such data in medical claims. In order to minimize potential confounding by these unmeasured risk factors for MS, we calculated propensity scores (PSs), using all variables listed in Table 1 except CCI, to predict the likelihood of having a T2DM diagnosis for all study subjects. We then conducted sensitivity analyses by treating PS as a continuous variable or categorical deciles in the Cox model to assess the robustness of the HRs estimated from the multivariate regression analysis. Treating PS as both continuous and categorical variables done in consideration that the relationship between PS and study outcome might not necessarily be linear.28 Using the prevalence rate of T2DM in 2008,29 we also calculated the overall and sex-specific population attributable risk percent (PAR%) to assess the public health impact of T2DM on MS incidence.
Table 1.
Characteristics of the study subjects.
| Characteristics | Comparison cohort n = 614,021 |
Diabetic cohort n = 614,623 |
p-value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Socio-demographics | |||||
| Age, years | <0.0001 | ||||
| ≤50 | 146,800 | 23.91 | 143,730 | 23.39 | |
| >50 | 467,221 | 76.09 | 470,893 | 76.61 | |
| Mean ± SD | |||||
| Sex | 0.980 | ||||
| Women | 318,800 | 51.9 | 319,112 | 51.9 | |
| Men | 295,221 | 48.1 | 295,511 | 48.1 | |
| Income-based insurance premium (NTD) | <0.001 | ||||
| <15,840 | 330,957 | 53.9 | 358,842 | 58.4 | |
| ≥15,840 | 283,064 | 46.1 | 255,781 | 41.6 | |
| Geographic area of living | <0.001 | ||||
| Northern | 275,695 | 44.9 | 269,819 | 43.9 | |
| Central | 151,663 | 24.7 | 143,822 | 23.4 | |
| Southern | 168,856 | 27.5 | 180,699 | 29.4 | |
| Eastern | 17,807 | 2.9 | 20,283 | 3.3 | |
| Urbanization status of living area | <0.001 | ||||
| Urban area | 406,789 | 66.3 | 414,871 | 67.5 | |
| Rural area | 206,925 | 33.7 | 199,752 | 32.5 | |
| Co-morbidities | |||||
| Anxiety | <0.0001 | ||||
| Yes | 53,884 | 8.8 | 58,927 | 9.6 | |
| No | 560,137 | 91.2 | 555,696 | 90.4 | |
| Depression | <0.0001 | ||||
| Yes | 8124 | 1.3 | 10,872 | 1.8 | |
| No | 605,897 | 98.7 | 603,751 | 98.2 | |
| Stress | <0.0001 | ||||
| Yes | 851 | 0.1 | 436 | 0.1 | |
| No | 613,170 | 99.9 | 614,187 | 99.9 | |
| Tonsillectomy | <0.001 | ||||
| Yes | 44 | 0.01 | 220 | 0.04 | |
| No | 613,977 | 99.99 | 614,403 | 99.96 | |
| Appendectomy | <0.001 | ||||
| Yes | 734 | 0.12 | 1196 | 0.19 | |
| No | 613,287 | 99.88 | 613,427 | 99.81 | |
| Allergy | <0.001 | ||||
| Yes | 2579 | 0.4 | 3020 | 0.5 | |
| No | 611,442 | 99.6 | 611,603 | 99.5 | |
| Alzheimer's disease | |||||
| Yes | 364 | <0.1 | 336 | <0.1 | 0.284 |
| No | 613,657 | 99.9 | 614,287 | 99.9 | |
| Anterior horn cell disease | <0.001 | ||||
| Yes | 168 | <0.1 | 274 | <0.1 | |
| No | 613,853 | 99.9 | 614,349 | 99.9 | |
| COPD | 0.012 | ||||
| Yes | 20,654 | 3.4 | 21,177 | 3.5 | |
| No | 593,367 | 96.6 | 593,446 | 96.5 | |
| Obesity | <0.001 | ||||
| Yes | 680 | 0.11 | 2213 | 0.36 | |
| No | 613,341 | 99.89 | 612,410 | 99.64 | |
| Dyslipidemia | <0.001 | ||||
| Yes | 40,210 | 6.6 | 140,122 | 22.8 | |
| No | 573,811 | 93.4 | 474,501 | 77.2 | |
| Hypertension | <0.001 | ||||
| Yes | 149,233 | 24.3 | 274,092 | 44.6 | |
| No | 464,788 | 75.7 | 340,531 | 55.4 | |
| Inflammatory bowel disease | <0.001 | ||||
| Yes | 52,946 | 8.6 | 28,366 | 4.6 | |
| No | 561,075 | 91.4 | 586,257 | 95.4 | |
| Spinal cord injury | 0.810 | ||||
| Yes | 1758 | 0.3 | 1774 | 0.3 | |
| No | 612,263 | 99.7 | 612,849 | 99.7 | |
| Stroke | <0.001 | ||||
| Yes | 37,628 | 6.1 | 34,874 | 5.7 | |
| No | 576,393 | 93.9 | 579,749 | 94.3 | |
| Thyroid disease | <0.001 | ||||
| Yes | 9589 | 1.6 | 16,571 | 2.7 | |
| No | 604,432 | 98.4 | 598,052 | 97.3 | |
| Traumatic brain injury | <0.001 | ||||
| Yes | 10,273 | 1.7 | 11,437 | 1.9 | |
| No | 603,748 | 98.3 | 603,186 | 98.1 | |
| CCI | <0.001 | ||||
| 0 | 390,239 | 63.6 | 275,325 | 44.8 | |
| 1 | 138,995 | 22.6 | 187,413 | 30.5 | |
| ≧2 | 84,787 | 13.8 | 151,885 | 24.7 | |
| Number of ambulatory visits in 2000 | <0.001 | ||||
| Mean (SD) | 18.8 (18.2) | 31.9 (21.3) | |||
CCI, Charlson Comorbidity Index; COPD, chronic obstructive pulmonary disease; NTD, New Taiwan Dollar (1 United States Dollar = 32 NTD); SD, standard deviation.
The Cox regression model considered the following as the censoring event, whichever came first: in-hospital mortality for causes other than MS, withdrawal from NHI, or December 31, 2008. The proportional-hazard assumption for Cox regression was verified using both plots of log(−log(survival function)) versus log(time) and Schoenfeld residuals versus time; the graphs showed no indication of violation. All statistical analyses were performed using the Statistical Analysis Software (version 9.4; SAS Institute, Cary, NC, USA). A p value of <0.05 was considered statistically significant.
Results
The characteristics of study subjects are shown in Table 1. Distributions of age and sex were statistically comparable. Patients with T2DM tended to have lower income, live in the Southern and Eastern parts of the island, and were slightly more likely to reside in urban areas. With respect to the co-morbidities associated with MS, subjects with T2DM had significantly higher prevalence of anxiety, depression, tonsillectomy, appendectomy, allergy, anterior horn cell disease, COPD, dyslipidemia, hypertension, spinal cord injury, thyroid disease, and traumatic brain injury, but significantly lower prevalence of inflammatory bowel disease and stroke. Additionally, patients with T2DM had greater CCI scores, leading to an observation that patients with T2DM had significantly greater average number of ambulatory visits in 2000 (31.9 vs. 18.8).
Over 9 years of follow-up, 175 T2DM patients were newly diagnosed with MS, and 114 matched controls had the same first-ever diagnosis, representing a covariate-adjusted HR of 1.44 (95% CI, 1.08–1.94). The incidence densities for men and women with T2DM were 2.20 and 3.45 per 10,000 patient-years, respectively, while the corresponding figures for men and women in the control group were 1.49 and 2.21 per 10,000 patient-years. The incidence density decreased with age in T2DM and control groups, regardless of gender. Compared with matched controls, both men and women with T2DM showed moderately increased risks of MS, with HRs of 1.34 (95% CI, 0.81–2.23) and 1.51 (95% CI, 1.05–2.19), respectively, after adjustment for potential confounders. Although there was a small difference in sex-specific HR of MS, the difference was not significant statistically (p-interaction = 0.80). The sex- and age-specific analysis found that the only significant increase in HR was for women aged ≤50 years (HR 2.16; 95% CI, 1.02–4.59) However, the interactive effect of diabetes with age on the risk of MS was not statistically significant for men (p-interaction = 0.39) or women (p-interaction = 0.79) (Table 2).
Table 2.
Overall and gender- and age-specific incidence densities and relative hazards of multiple sclerosis in the diabetes and comparison cohorts.
| Variables | Comparison cohort |
Diabetic cohort |
Crude HR (95% CI) in association with diabetic group | Adjusted HRb (95% CI)b in association with diabetic group | ||||
|---|---|---|---|---|---|---|---|---|
| n | Number of events | ID (per 10,000 patient-years) (95% CI)a | n | Number of events | ID (per 10,000 patient-years) (95% CI) | |||
| Men | ||||||||
| ≤50 years | 82,321 | 20 | 2.43 (1.48–3.75) | 81,204 | 35 | 4.31 (3.00–6.01) | 1.73 (1.00–3.05) | 1.66 (0.64–4.35) |
| >50 years | 212,884 | 24 | 1.13 (0.72–1.68) | 214,282 | 30 | 1.40 (0.95–2.00) | 1.26 (0.74–2.15) | 1.26 (0.69–2.26) |
| Total | 295,205 | 44 | 1.49 (1.08–2.03) | 295,486 | 65 | 2.20 (1.70–2.81) | 1.48 (1.01–2.17) | 1.34 (0.81–2.23) |
| Women | ||||||||
| ≤50 years | 64,479 | 30 | 4.65 (3.14–6.64) | 62,505 | 49 | 7.85 (5.8–10.37) | 1.65 (1.04–2.6) | 2.16 (1.02–4.59) |
| >50 years | 254,337 | 40 | 1.57 (1.12–2.14) | 256,632 | 61 | 2.38 (1.82–3.05) | 1.52 (1.02–2.26) | 1.24 (0.80–1.91) |
| Total | 318,816 | 70 | 2.21 (1.71–2.77) | 319,137 | 110 | 3.45 (2.83–4.16) | 1.57 (1.16–2.12) | 1.51 (1.05–2.19) |
| Overall | 614,021 | 114 | 1.86 (1.53–2.23) | 614,623 | 175 | 2.85 (2.44–3.30) | 1.53 (1.21–1.94) | 1.44 (1.08–1.94) |
CI, confidence interval; HR, hazard ratio; ID, incidence density.
p value for the interaction between diabetes and gender was 0.80.
p value for the interaction between diabetes and age was 0.39 and 0.79 for men and women, respectively.
Based on Poisson assumption.
Based on Cox proportional hazard regression, with adjustment for age; sex; income-based insurance premium; geographic area of living; urbanization status of living area; Charlson Comorbidity Index; frequency of medical visit; and selected co-morbidities, including allergy, Alzheimer's disease, anterior horn cell disease, COPD, obesity, vitamin D deficiency, dyslipidemia, hypertension, inflammatory bowel disease, spinal cord injury, stroke, thyroid disease, and traumatic brain injury, anxiety, depression, stress, tonsillectomy, and appendectomy.
The regression model that treated PS as a continuous variable showed a HR of 1.36 (95% CI, 1.00–1.86), and that using deciles to indicate PS showed a HR of 1.40 (95% CI, 1.02–1.91). Although the results from PS adjustment models and those reported from multivariate regression models adjusted for individual co-morbidities (HR 1.44; 95% CI, 1.08–1.94) showed slight differences, they all suggested an increased risk of multiple sclerosis in relation to T2DM. The overall PAR% of T2DM in the development of MS was estimated at 2.55%; the PAR%s for men and women were estimated to be 2.00% and 2.94%, respectively.
Discussions
In this large population-based study over a 9-year period, we found that T2DM was significantly associated with an increased risk of MS incidence. Such elevated risk was more evident in women than in men, especially in women aged ≤50 years. The higher apparent risk noted in women might be attributable to a greater number of MS cases occurring among women. These results were unlikely to be confounded by socio-demographic characteristics and MS related co-morbidities. The loss of statistical significance in the HRs associated with most age-sex stratifications might be due to the small number of events.
The putative association between diabetes and MS has been argued for more than 15 years. However, all current evidence of increased MS incidence among diabetics came from studies of T1DM. Previous studies have shown that T1DM and MS might share some common immune pathogenetic mechanisms, and that the interaction of T1DM and certain environmental factors could contribute to the increased risk of MS.12, 13, 30 Nonetheless, the evidence linking T2DM and MS is still scant, even though T2DM has recently been recognized as an autoimmune disease.14 To the best of our knowledge, our study is the first population-based cohort study that provides reliable epidemiological evidence suggesting a significant association of T2DM with elevated risk of MS.
Although both diabetes and MS have genetic susceptibility, no common gene has been found to be shared by MS and diabetes (either T1DM or T2DM). However, some evidence has suggested that the activation of macrophages, TH1 CD4+ T cells, or b cell-cytotoxic CD8+ T cells might act synergistically to destroy b cells, resulting in T1DM.31 Another study revealed that interferon gamma-induced protein 10 is associated with insulin resistance and incident diabetes in patients with nonalcoholic fatty liver disease.32 Previous studies have also reported potential targets for IgG antibodies associated with insulin resistance33 and autoantibodies against pancreatic islet antigens in patients with T2DM.34 Therefore, T2DM might lead to the occurrence of MS through the HLA-DR genetic susceptibility or environmental trigger factors. For example, viral infection, to which T2DM patients might be more prone than controls, is also considered as potential risk factor of MS.22 Therefore, our results tended to provide support for the recent argument that T2DM, like T1DM, might be an autoimmune disease, and that patients with T2DM might be prone to the development of MS due to the interactions of immunity and environment.
No clear evidence of autoimmunity has been described in animal models of T2DM either, although some studies did show that targeting components of the adaptive immune system, such as IFN-γ-expressing type 1 T helper cells and B lymphocytes, can increase insulin resistance.33, 35 Moreover, a clinical trial reported that antibodies against G-protein-coupled receptors have been detected in sera from a subgroup of T2DM patients at a greater risk of hypertension and cardiovascular complications.36 Rho-kinase-activating autoantibodies are also present in sera of T2DM patients with maculopathy and macroalbuminuria,35 and autoantibodies against IL-6 have been detected in sera from 2.5% of Danish patients with T2DM.37 In addition, evidence also suggests that both T1DM and T2DM share a common autoimmune response in the presence of pancreatic β cells in adults with latent autoimmune diabetes mellitus.38 Collectively, these studies suggest that the increased risk of MS might occur in not only patients with T1DM but also those with T2DM.
This study had the following strengths. First, it was a large, nationwide, population-based study with high representativeness of the T2DM patient population of Taiwan in the year 2000. Second, the advantage of using insurance claims data in clinical research is that longitudinal records for a wide sample of demographically diverse patients are easily accessible39 and the size of the data set enabled stratified analyses according to age and sex. Third, this diabetes cohort was formed using the NHI database, and all research information was retrieved from NHI claims, which has little possibility of recall bias, little likelihood of non-response, and little loss to follow-up of cohort members. Fourth, the adjustment of geographic area, urbanization status, and the frequency of outpatient visits for each study subject has minimized confounding by disease surveillance bias.
Despite the above methodological strengths, several limitations should be noted. First, diagnoses of T2DM and MS that are completely dependent upon ICD-9-CM codes are subject to inaccuracy, which is a major limitation of this study compared to those studies based on the standardized clinical examinations of patients. However, the NHIA of the Ministry of Health and Wealth conducts quarterly expert reviews of any hospital with outlier charges or outlier practice patterns. In addition, we used at least two diabetes-related diagnoses with the first and the last visits >30 days apart, which may largely reduce the likelihood of disease misclassification.40 As for the diagnosis of MS, some inconsistent information on MS prevalence was noted in previous studies using data from the NHIA.41, 42, 43 Therefore, we included only those patients with major illness/injury certificates of MS diagnosis, which are reconfirmed by an expert committee, to avoid miscoding. These methods are believed to greatly reduce the potential for disease misclassification bias. Still, the medical claims may not be able to capture all patients with T2DM, indicating that some study subjects selected as controls in our analysis might in fact have undiagnosed T2DM. Such exposure misclassification, however, is likely to be non-differential (i.e., the degree of exposure misclassification is independent of the subsequent risk of multiple sclerosis); and non-differential misclassification of exposure would tend to underestimate rather than overestimate the true relative risk estimates of multiple sclerosis in relation to T2DM. Second, although we have tried to adjust for some potential confounders in the analysis,22 we were unable to consider a number of known risk factor for MS, including severity, duration, and treatment regimens of T2DM, smoking, alcohol consumption, vitamin intake, and certain occupational/environmental hazard in our study,20 which might result in residual confounding in our study results.
Conclusion
In summary, the results of this population-based cohort study suggested an increased risk of MS among male and female patients with T2DM; the elevated risk of MS was especially high in female T2DM patients aged 50 years or less. Although the biological mechanisms underlying the association of T2DM with the risk of MS have not been fully understood, further studies are warranted to see if our study findings can be reproduced. Given the high prevalence of T2DM, even a weak to moderate association between T2DM and MS could still signify a large number of people affected. In addition, although a large-scale screen for MS in patients with T2DM may not be cost-effective, both patients with T2DM and clinicians should be informed of the relationship between T2DM and MS in order to facilitate early detection and appropriate management of MS in patients with T2DM.
Author contributions
W-H Hou, C-Y Li, and C-C Tsai designed the study, did statistical analyses, drafted the initial manuscript, and revised important content. Y Sun participated in study design, interpretation of results, and revising the submitted work. H-H Chang analyzed the data and drafted the statistical parts of the manuscript. C-Y Li is the guarantor of this work, has full access to all the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflicts of interest
None declared.
Acknowledgements
This study was supported by a grant from the Ministry of Science and Technology, (MOST 104-2314-B-006 -020 -MY2). This study is also based in part on data from the NHIRD provided by the Bureau of National Health Insurance and the Department of Health and managed by National Health Research Institutes. The interpretation and conclusions contained herein do not represent those of Bureau of National Health Insurance, Department of Health, or National Health Research Institutes.
Footnotes
Peer review under responsibility of the Japan Epidemiological Association.
References
- 1.Danaei G., Finucane M.M., Lu Y. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27(1):S5–S10. doi: 10.2337/diacare.27.2007.s5. [DOI] [PubMed] [Google Scholar]
- 3.Han X., Luo Y., Ren Q. Implication of genetic variants near SLC30A8, HHEX, CDKAL1, CDKN2A/B, IGF2BP2, FTO, TCF2, KCNQ1, and WFS1 in type 2 diabetes in a Chinese population. BMC Med Genet. 2010;11:81. doi: 10.1186/1471-2350-11-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qi Q., Hu F.B. Genetics of type 2 diabetes in European populations. J Diabetes. 2012;4:203–212. doi: 10.1111/j.1753-0407.2012.00224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rafiq S., Melzer D., Weedon M.N. Gene variants influencing measures of inflammation or predisposing to autoimmune and inflammatory diseases are not associated with the risk of type 2 diabetes. Diabetologia. 2008;51:2205–2213. doi: 10.1007/s00125-008-1160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winkler C., Raab J., Grallert H., Ziegler A.G. Lack of association of type 2 diabetes susceptibility genotypes and body weight on the development of islet autoimmunity and type 1 diabetes. PLoS One. 2012;7:e35410. doi: 10.1371/journal.pone.0035410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiner H.L. Multiple sclerosis is an inflammatory T-cell-mediated autoimmune disease. Arch Neurol. 2004;61:1613–1615. doi: 10.1001/archneur.61.10.1613. [DOI] [PubMed] [Google Scholar]
- 8.Compston A., Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 9.Dendrou C.A., Fugger L., Friese M.A. Immunopathology of multiple sclerosis. Nat Rev Immunol. 2015;15:545–558. doi: 10.1038/nri3871. [DOI] [PubMed] [Google Scholar]
- 10.Sadovnick A.D., Ebers G.C., Dyment D.A., Risch N.J. Evidence for genetic basis of multiple sclerosis. The Canadian Collaborative Study Group. Lancet. 1996;347:1728–1730. doi: 10.1016/s0140-6736(96)90807-7. [DOI] [PubMed] [Google Scholar]
- 11.Lernmark A. Multiple sclerosis and type 1 diabetes: an unlikely alliance. Lancet. 2002;359:1450–1451. doi: 10.1016/S0140-6736(02)08464-7. [DOI] [PubMed] [Google Scholar]
- 12.Knip M., Kukko M., Kulmala P. Humoral beta-cell autoimmunity in relation to HLA-defined disease susceptibility in preclinical and clinical type 1 diabetes. Am J Med Genet. 2002;115:48–54. doi: 10.1002/ajmg.10343. [DOI] [PubMed] [Google Scholar]
- 13.Buzzetti R., Pozzilli P., Di Mario U., Ballerini C., Massacesi L. Multiple sclerosis and type I diabetes. Diabetologia. 2002;45:1735–1736. doi: 10.1007/s00125-002-0967-6. [DOI] [PubMed] [Google Scholar]
- 14.Velloso L.A., Eizirik D.L., Cnop M. Type 2 diabetes mellitus – an autoimmune disease? Nat Rev Endocrinol. 2013;9:750–755. doi: 10.1038/nrendo.2013.131. [DOI] [PubMed] [Google Scholar]
- 15.Lu J.F., Hsiao W.C. Does universal health insurance make health care unaffordable? Lessons from Taiwan. Health Aff (Millwood) 2003;22:77–88. doi: 10.1377/hlthaff.22.3.77. [DOI] [PubMed] [Google Scholar]
- 16.Chen H.F., Chang Y.H., Ko M.C., Li C.Y. A large scale population-based cohort study on the risk of ovarian neoplasm in patients with type 2 diabetes mellitus. Gynecol Oncol. 2014;134:576–580. doi: 10.1016/j.ygyno.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Chen H.F., Chen P., Li C.Y. Risk of malignant neoplasm of the pancreas in relation to diabetes: a population-based study in Taiwan. Diabetes Care. 2011;34:1177–1179. doi: 10.2337/dc10-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun Y., Lu C.J., Chen R.C., Hou W.H., Li C.Y. Risk of amyotrophic lateral sclerosis in patients with diabetes: a nationwide population-based cohort study. J Epidemiol. 2015;25:445–451. doi: 10.2188/jea.JE20140176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin C.C., Lai M.S., Syu C.Y., Chang S.C., Tseng F.Y. Accuracy of diabetes diagnosis in health insurance claims data in Taiwan. J Formos Med Assoc. 2005;104:157–163. [PubMed] [Google Scholar]
- 20.Ramagopalan S.V., Dobson R., Meier U.C., Giovannoni G. Multiple sclerosis: risk factors, prodromes, and potential causal pathways. Lancet Neurol. 2010;9:727–739. doi: 10.1016/S1474-4422(10)70094-6. [DOI] [PubMed] [Google Scholar]
- 21.Young C.A. Factors predisposing to the development of multiple sclerosis. Q J Med. 2011;104:383–386. doi: 10.1093/qjmed/hcr012. [DOI] [PubMed] [Google Scholar]
- 22.Wens I., Dalgas U., Stenager E., Eijnde B.O. Risk factors related to cardiovascular diseases and the metabolic syndrome in multiple sclerosis – a systematic review. Mult Scler. 2013;19:1556–1564. doi: 10.1177/1352458513504252. [DOI] [PubMed] [Google Scholar]
- 23.Belbasis L., Bellou V., Evangelou E., Ioannidis J.P., Tzoulaki I. Environmental risk factors and multiple sclerosis: an umbrella review of systematic reviews and meta-analyses. Lancet Neurol. 2015;14:263–273. doi: 10.1016/S1474-4422(14)70267-4. [DOI] [PubMed] [Google Scholar]
- 24.Marrie R.A., Miller A., Sormani M.P., attendees of the International Workshop on Comorbidity in Multiple Sclerosis Recommendations for observational studies of comorbidity in multiple sclerosis. Neurology. 2016 doi: 10.1212/WNL.0000000000002474. pii: 10.1212/WNL.0000000000002474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charlson M., Szatrowski T.P., Peterson J., Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 26.Tsai C.P., Yuan C.L., Yu H.Y., Chen C., Guo Y.C., Shan D.E. Multiple sclerosis in Taiwan. J Chin Med Assoc. 2004;67:500–505. [PubMed] [Google Scholar]
- 27.Tan H.F., Tseng H.F., Chang C.K., Lin W., Hsiao S.H. Accessibility assessment of the Health Care Improvement Program in rural Taiwan. J Rural Health. 2005;21:372–377. doi: 10.1111/j.1748-0361.2005.tb00110.x. [DOI] [PubMed] [Google Scholar]
- 28.Kurth T., Walker A.M., Glynn R.J. Results of multivariable logistic regression, propensity matching, propensity adjustment, and propensity-based weighting under conditions of nonuniform effect. Am J Epidemiol. 2006;163:262–270. doi: 10.1093/aje/kwj047. [DOI] [PubMed] [Google Scholar]
- 29.Jiang Y.D., Chang C.H., Tai T.Y., Chen J.F., Chuang L.M. Incidence and prevalence rates of diabetes mellitus in Taiwan: analysis of the 2000–2009 Nationwide Health Insurance database. J Formos Med Assoc. 2012;111:599–604. doi: 10.1016/j.jfma.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 30.Marrosu M.G., Cocco E., Lai M., Spinicci G., Pischedda M.P., Contu P. Patients with multiple sclerosis and risk of type 1 diabetes mellitus in Sardinia, Italy: a cohort study. Lancet. 2002;359:1461–1465. doi: 10.1016/S0140-6736(02)08431-3. [DOI] [PubMed] [Google Scholar]
- 31.Yoon J.W., Jun H.S. Autoimmune destruction of pancreatic beta cells. Am J Ther. 2005;12:580–591. doi: 10.1097/01.mjt.0000178767.67857.63. [DOI] [PubMed] [Google Scholar]
- 32.Chang C.C., Wu C.L., Su W.W. Interferon gamma-induced protein 10 is associated with insulin resistance and incident diabetes in patients with nonalcoholic fatty liver disease. Sci Rep. 2015;5:10096. doi: 10.1038/srep10096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winer D.A., Winer S., Shen L. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med. 2011;17:610–617. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brooks-Worrell B.M., Juneja R., Minokadeh A., Greenbaum C.J., Palmer J.P. Cellular immune responses to human islet proteins in antibody-positive type 2 diabetic patients. Diabetes. 1999;48:983–988. doi: 10.2337/diabetes.48.5.983. [DOI] [PubMed] [Google Scholar]
- 35.Winer S., Chan Y., Paltser G. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hempel P., Karczewski P., Kohnert K.D. Sera from patients with type 2 diabetes contain agonistic autoantibodies against G protein-coupled receptors. Scand J Immunol. 2009;70:159–160. doi: 10.1111/j.1365-3083.2009.02280.x. [DOI] [PubMed] [Google Scholar]
- 37.Fosgerau K., Galle P., Hansen T. Interleukin-6 autoantibodies are involved in the pathogenesis of a subset of type 2 diabetes. J Endocrinol. 2010;204:265–273. doi: 10.1677/JOE-09-0413. [DOI] [PubMed] [Google Scholar]
- 38.Tuomi T., Groop L.C., Zimmet P.Z., Rowley M.J., Knowles W., Mackay I.R. Antibodies to glutamic acid decarboxylase reveal latent autoimmune diabetes mellitus in adults with a non-insulin-dependent onset of disease. Diabetes. 1993;42:359–362. doi: 10.2337/diab.42.2.359. [DOI] [PubMed] [Google Scholar]
- 39.Remick R.A. Diagnosis and management of depression in primary care: a clinical update and review. CMAJ. 2002;167:1253–1260. [PMC free article] [PubMed] [Google Scholar]
- 40.Sun Y., Chang Y.H., Chen H.F., Su Y.H., Su H.F., Li C.Y. Risk of Parkinson disease onset in patients with diabetes: a 9-year population-based cohort study with age and sex stratifications. Diabetes Care. 2012;35:1047–1049. doi: 10.2337/dc11-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang J.H., Chen Y.H., Lin H.C. Comorbidities amongst patients with multiple sclerosis: a population-based controlled study. Eur J Neurol. 2010;17:1215–1219. doi: 10.1111/j.1468-1331.2010.02971.x. [DOI] [PubMed] [Google Scholar]
- 42.Chen Y.H., Lin H.L., Lin H.C. Does multiple sclerosis increase risk of adverse pregnancy outcomes? A population-based study. Mult Scler. 2009;15:606–612. doi: 10.1177/1352458508101937. [DOI] [PubMed] [Google Scholar]
- 43.Sheu J.J., Lin H.C. Association between multiple sclerosis and chronic periodontitis: a population-based pilot study. Eur J Neurol. 2013;20:1053–1059. doi: 10.1111/ene.12103. 38. [DOI] [PubMed] [Google Scholar]