Summary
Background
Bariatric surgery in patients with obesity and type 2 diabetes is associated with diabetes remission and prevention of complications. The long-term effects of bariatric surgery on microvascular complications in patients with prediabetes are unknown.
Methods
The prospective, matched Swedish Obese Subjects study examines outcomes after bariatric surgery. Patients were recruited between September 1, 1987, and January 31, 2001. Age was 37–60 years and BMI was ≥34 kg/m2 in men and ≥38 kg/m2 in women. The surgery group (n=2010) underwent gastric bypass (13·2%), banding (18·7%), or vertical banded gastroplasty (68·1%), and controls (n=2037) received usual care. After exclusion of 4 patients with suspected type 1 diabetes, and 11 patients with unknown glucose status at baseline, 4032 of the 4047 participants in the SOS study were included in the current analysis. The main outcome of this report was incidence of microvascular events (retinopathy, diabetic kidney disease, and neuropathy, whichever came first), obtained from nationwide registers, in subgroups stratified by baseline glucose status (euglycemia n=2838; prediabetes n=591; screen-detected diabetes n=246; established diabetes n=357). Data were analyzed both by intention to treat and per protocol. Median follow-up was 19 years. This study is registered with ClinicalTrials.gov, NCT01479452.
Findings
There were 374 first-time microvascular events in the control group and 224 in the surgery group (hazard ratio, 0·56; 95% CI 0·48–0·66; p<0·0001). There was a significant interaction between baseline glucose status and treatment effect (p=0.0003), and unadjusted hazard ratios for comparing the surgery group to the control group were lowest in the subgroup with prediabetes (0·18; 95% CI 0·11–0·30), followed by subgroups with screen-detected diabetes (0·39; 95% CI 0·24–0·65), established diabetes (0·54; 95% CI 0·40–0·72), and euglycemia (0·63; 95% CI 0·48–0·81). In patients with baseline prediabetes, treatment was associated with reduced incidence of microvascular events both in those who developed diabetes and in those who remained diabetes free during follow-up.
Interpretation
Bariatric surgery was associated with reduced risk of microvascular complications in all subgroups, but the greatest relative risk reduction was observed in patients with baseline prediabetes. Our results indicate that prediabetes should be treated aggressively to prevent future microvascular events.
Introduction
Type 2 diabetes is a global health problem largely caused by obesity and sedentary lifestyle.1 Worldwide, the number of adults with diabetes increased from 108 million in 1980 to 422 million in 20142 and this is predicted to increase to 592 million by 2035.3 In the US, 38% of adults have prediabetes,4 a condition with glucose levels that are higher than normal but below the threshold for type 2 diabetes. In individuals aged 45 years, prediabetes confers a 74% lifetime risk to progress to diabetes.5
Diabetes is associated with severe macrovascular complications, leading to myocardial infarction, stroke and peripheral vascular disease,6 and microvascular complications, affecting eyes, nerves, and kidneys.7 The relationship between blood glucose levels and macrovascular disease appears to be continuous with increased risk already at glucose levels defined as prediabetic.8 However, although it is well established that hyperglycemia is a risk factor for microvascular complications,9–11 the connection between prediabetes and the pathogenesis of microvascular disease is less clear.
The threshold for defining diabetes corresponds to glucose levels above which the risk of diabetic retinopathy has been shown to increase.12 According to clinical guidelines, antidiabetic treatment should be initiated to reduce the risk of microvascular complications when glucose levels are above this threshold. In patients with diabetes, the incidence and progression of microvascular disease are reduced by improved glycemic control achieved by glucose-lowering medications13–15 or intensive lifestyle intervention,16 as well as by bariatric surgery,17 which has been shown to cause diabetes remission in many patients with obesity.17–20 However, the cut-off for defining diabetes is derived from non-interventional studies and does not take into account possible differences in the magnitude of the treatment effect in patients with different stages of the disease. Studies have shown that lifestyle modification, medication or bariatric surgery can prevent progression from prediabetes to type 2 diabetes,21,22 but to our knowledge there are no longitudinal studies that have compared the effect of treatment on microvascular complications in patients with prediabetes, type 2 diabetes and normal glucose levels at baseline.
In this explorative study, we examined the effects of bariatric surgery on the incidence of microvascular complications over up to 26 years in subgroups from the Swedish Obese Subjects (SOS) study stratified by glucose status at baseline.
Methods
Study design and treatment
The SOS study has previously been described23 (for details see appendix). In brief, 2010 subjects who chose surgical treatment formed the surgery group and a non-randomized contemporaneously matched control group (n=2037) was created using 18 matching variables. The surgery and control groups had identical inclusion and exclusion criteria. The inclusion criteria were age 37 to 60 years and body mass index (BMI) of 34 kg/m2 or more for men and 38 kg/m2 or more for women, corresponding to a doubling in the rate of death in each sex.24
In the surgery group, 265 underwent gastric bypass (13·2%), 376 banding (18·7%), and 1369 vertical banded gastroplasty (68·1%). The control group received the customary treatment for obesity and diabetes at their primary health care centers. Fasting blood samples were taken at baseline, and after 2, 10, and 15 years. Self-reported diabetes and hypertension medication was obtained from SOS questionnaires. The study was approved by the relevant ethics review boards, and written or oral informed consent was obtained from all participants.
Stratification based on baseline glucose status and diabetes remission after 15 years
Patients were stratified into subgroups with baseline euglycemia, impaired fasting glucose (prediabetes), and type 2 diabetes that was either detected at inclusion (screen-detected) or previously diagnosed (established). Prediabetes was defined as a fasting blood glucose level of 5·0–6·0mmol/L (corresponding to fasting plasma glucose level of 5·6–6·9 mmol/L).25 Diabetes was defined by the use of diabetes medication, a fasting blood glucose level of 6·1 mmol/L or higher (corresponding to fasting plasma glucose of 7·0 mmol/L or higher). The study was initiated before repeated measurements were routinely used for the diagnosis of type 2 diabetes and single fasting glucose determinations were therefore used. For diabetes with onset before 35 years, we ruled out type 1 diabetes and latent autoimmune diabetes of adults by excluding patients positive for glutamate decarboxylase antibodies or islet cell antibodies or with C-peptide values below the detection limit at baseline. This resulted in exclusion of 2 patients in the control group and 2 in the surgery group. In addition, 11 patients had missing glucose status at baseline (4 in the control group and 7 in the surgery group) and they were excluded. Diabetes remission was defined as fasting blood glucose levels lower than 6.1 mmol/L and no diabetes medication. Patients in diabetes remission could therefore either be in partial remission (5.0–<6.1 mmol/L) or complete remission (<5.0 mmol/L). This definition has been used previously for analyzing remission rate in the SOS study17 and the glucose cut-offs are identical to those recommended by the American Diabetes Association.26
Microvascular complications
Microvascular events diagnosed during hospital or hospital-based outpatient care or that were associated with death were identified by searching the Swedish Cause of Death Register and the National Patient Register using International Classification of Diseases and intervention codes. Retinopathy was identified by codes for eye complications, diabetes retinopathy, and retinal operations; diabetic kidney disease by codes for kidney complications, diabetes nephropathy, albuminuria, renal failure, kidney transplantation, kidney biopsy, and dialysis; neuropathy by codes for neurological complications, amyothrophy, autonomous (poly)neuropathy, mononeuropathy, and polyneuropathy (appendix, Table S1). The National Patient Register has 99% coverage of inpatient care and around 80% coverage of specialist outpatient care for somatic diseases.27 There is no nationwide register for visits to general practitioners in Sweden.
Statistical methods
Mean and median values, standard deviations, and proportions were used to describe the baseline characteristics. Differences between treatment groups were evaluated with t-tests for continuous variables and a logistic regression model for dichotomous variables. Diabetes status was determined at follow-up examinations until July 1, 2015. Participants were followed in registers until the first microvascular event or December 31, 2013 (the date the registers were complete at the time of register linkage). Those without microvascular events during follow-up were censored at December 31, 2013, or at the date of emigration (n=49) or death. Two persons who withdrew consent were censored immediately after the date of inclusion to the study.
First, time to events was analyzed with Kaplan-Meier estimates of cumulative incidence, and then Cox proportional hazard models to estimate the difference between the surgery and control groups, expressed as hazard ratios with 95% confidence intervals. The primary analysis was an unadjusted analysis including a single covariate indicating the surgery/control treatment, and then complemented by an analysis with adjustment for preselected baseline risk factors; age, sex, BMI, high blood pressure, urinary albumin excretion, and smoking. The proportional hazard assumption in the Cox model was evaluated with log-log plots and a statistical test of interaction between treatment and time. No evidence of violation of this assumption was found. In addition, a propensity score approach was used in the sensitivity analyses to account for differences in characteristics of the treatment groups (appendix, Table S2).
In secondary subgroup analyses, the incidence rates were calculated in subgroups defined by risk factors at baseline. The subgroups were based on quartiles of age, BMI, and insulin, and glucose subgroups defined by baseline euglycemia, prediabetes, screen-detected diabetes, and established diabetes. We tested whether the influence of bariatric surgery on the incidence of microvascular events varied by risk factor level in these subgroups by including the corresponding interaction term [i.e. product of type of treatment (surgery or control), and the corresponding subgroup-variable] in the Cox proportional hazard models. Separate models were fitted when evaluating the interaction between the treatment variable and each of the subgroup variables.
The expected number of surgeries needed to prevent one first-time microvascular event over 10 years (number needed to treat; NNT) was calculated in different subgroups as the reciprocal of the absolute risk difference between individuals in the surgery and control groups.
All p values are two-sided and p values of less than 0·05 were considered to indicate statistical significance. The primary analysis was according to the intention-to-treat principle: controls who underwent bariatric surgery and surgery patients with reinstated anatomy during follow-up remained in their original treatment groups. Additional sensitivity analyses were done using per-protocol approach: controls who underwent bariatric surgery and surgery patients with reinstated anatomy were censored at the time of surgery. Statistical analyses were carried out using Stata statistical package 12.1 (Stata-Corp. 2011, Stata Statistical Software: Release 12, College Station, TX, USA; StataCorp LP).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. LMSC and MP had full access to all the data. LMSC had final responsibility for the decision to submit for publication.
Results
Baseline characteristics, weight changes and medication during follow-up
After exclusion of 4 patients with suspected type 1 diabetes, and 11 patients with unknown glucose status at baseline, 4032 of the 4047 participants in the SOS study were included in the current analysis (2031 controls and 2001 surgically treated patients). The median follow-up time was 19 years (interquartile range 16 to 21 years, maximum 26 years) in both the surgery and control groups.
Baseline characteristics in glucose subgroups with euglycemia, prediabetes, screen-detected diabetes, and established diabetes are shown in Table 1. In total, there were 35 patients with history of microvascular disease at baseline (16 in the control group and 19 in the surgery group, p=0·61). In all subgroups, patients in the control group were slightly older, while several other risk factors were worse in the surgery group. After bariatric surgery, average maximal weight loss ranged from 25 to 32 kg in the subgroups (Figure S1). The weight changes in the control group were smaller, and varied from a few kg weight gain in the subgroup with baseline euglycemia to a weight loss of around 9 kg in the subgroup with screen-detected diabetes (Figure S1). The proportion of patients using antihypertensive, lipid-lowering and diabetes drugs during the follow-up was generally lower in the surgery group as compared to controls (appendix, Table S3).
Table 1.
Baseline characteristics of participants in the SOS study stratified by baseline glucose status.
| Euglycemia | Prediabetes | Screen-Detected Type 2 Diabetes | Established Type 2 Diabetes | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control (n=1481) | Surgery (n=1357) | Control (n=290) | Surgery (n=301) | Control (n=87) | Surgery (n=159) | Control (n=173) | Surgery (n=184) | |||||||||
| Mean | SD/ratio | Mean | SD/ratio | Mean | SD/ratio | Mean | SD/ratio | Mean | SD/ratio | Mean | SD/ratio | Mean | SD/ratio | Mean | SD/ratio | |
| Age, years | 48·2 | 6·2 | 46·7 | 5·9 | 49·7 | 6·3 | 47·5 | 6·1 | 50·1 | 6·4 | 48·9 | 5·9 | 50·6 | 6·3 | 48·4 | 6·0 |
| Men, % | 26·9 | 398/1481 | 26·5 | 359/1357 | 30·0 | 87/290 | 29·2 | 88/301 | 42·5 | 37/87 | 36·5 | 58/159 | 38·7 | 67/173 | 45·1 | 83/184 |
| Weight, kg | 113·9 | 16·4 | 120·0 | 15·9 | 117·5 | 16·6 | 122·7 | 17·2 | 122·9 | 16·9 | 126·3 | 20·8 | 113·1 | 15·7 | 120·3 | 16·2 |
| Body-Mass Index, kg/m2 | 40·0 | 4·7 | 42·3 | 4·4 | 41·2 | 4·9 | 43·1 | 4·8 | 42·0 | 4·3 | 43·5 | 5·1 | 39·0 | 4·5 | 41·0 | 3·9 |
| Waist circumference, cm | 119·3 | 11·5 | 124·6 | 10·6 | 122·6 | 10·7 | 127·4 | 10·7 | 126·6 | 11·0 | 130·8 | 13·3 | 121·1 | 8·6 | 127·1 | 10·0 |
| Waist-Hip Ratio | 0·971 | 0·074 | 0·983 | 0·076 | 0·984 | 0·069 | 1·001 | 0·076 | 1·001 | 0·060 | 1·012 | 0·078 | 1·016 | 0·068 | 1·028 | 0·079 |
| Systolic Blood Pressure, mmHg | 136·6 | 17·6 | 143·1 | 18·1 | 139·8 | 18·1 | 147·7 | 20·0 | 147·3 | 18·2 | 152·3 | 19·6 | 141·8 | 18·8 | 148·8 | 18·5 |
| Diastolic Blood Pressure, mmHg | 84·6 | 10·3 | 89·1 | 11·0 | 86·0 | 11·7 | 90·9 | 11·5 | 90·9 | 10·9 | 93·3 | 11·7 | 85·5 | 10·8 | 90·6 | 10·6 |
| Hypertension, %§ | 59·2 | 877/1481 | 74·9 | 1015/1356 | 70·7 | 205/290 | 82·1 | 247/301 | 83·9 | 73/87 | 91·2 | 145/159 | 83·2 | 144/173 | 88·0 | 162/184 |
| Serum Cholesterol, mmol/L | 5·6 | 1·0 | 5·9 | 1·1 | 5·7 | 1·1 | 5·9 | 1·0 | 5·7 | 1·2 | 5·9 | 1·3 | 5·7 | 1·2 | 5·9 | 1·2 |
| Serum HDL cholesterol, mmol/L | 1·38 | 0·34 | 1·37 | 0·32 | 1·29 | 0·29 | 1·35 | 0·31 | 1·24 | 0·27 | 1·28 | 0·31 | 1·24 | 0·30 | 1·23 | 0·30 |
| Serum Triglycerides, mmol/L | 1·80 | 0·96 | 2·09 | 1·35 | 2·32 | 1·78 | 2·26 | 1·27 | 2·74 | 2·48 | 2·85 | 2·21 | 2·96 | 2·34 | 2·96 | 2·17 |
| Blood Glucose, mmol/L | 4·18 | 0·42 | 4·24 | 0·41 | 5·42 | 0·31 | 5·42 | 0·30 | 7·98 | 2·31 | 7·88 | 2·07 | 8·95 | 2·80 | 9·33 | 2·90 |
| Serum Insulin, mU/L | 15·8 | 8·7 | 19·1 | 11·0 | 22·9 | 11·6 | 24·7 | 13·8 | 30·5 | 23·1 | 30·5 | 15·2 | 23·1 | 14·1 | 27·2 | 22·5 |
| C-Peptide, ng/mL | 3·4 | 1·2 | 3·8 | 1·5 | 4·2 | 1·3 | 4·5 | 1·5 | 5·0 | 2·2 | 5·4 | 4·3 | 3·8 | 1·5 | 4·0 | 1·4 |
| Serum Creatinine, μmol/L | 69·4 | 9·5 | 68·9 | 8·5 | 70·1 | 9·1 | 70·1 | 9·3 | 71·3 | 11·5 | 69·3 | 8·5 | 69·8 | 9·8 | 69·8 | 9·8 |
| Albumin Excretion, μgram/min* | 6·8 | 4·5–12·5 | 7·8 | 4·9–14·7 | 9·2 | 5·4–19·3 | 9·5 | 5·0–20·5 | 12·5 | 7·7–26·0 | 14·5 | 6·7–33·2 | 13·1 | 6·2–34·6 | 17·6 | 8·7–55·5 |
| Smoking, % | 20·4 | 301/1477 | 26·6 | 361/1356 | 22·8 | 66/289 | 23·3 | 70/301 | 19·3 | 16/83 | 26·6 | 42/158 | 21·5 | 37/172 | 23·4 | 43/184 |
Hypertension: diastolic blood pressure >90, or systolic blood pressure >140, or medication.
Median and interquartile range
Incidence of microvascular events in subgroups defined by baseline glucose status
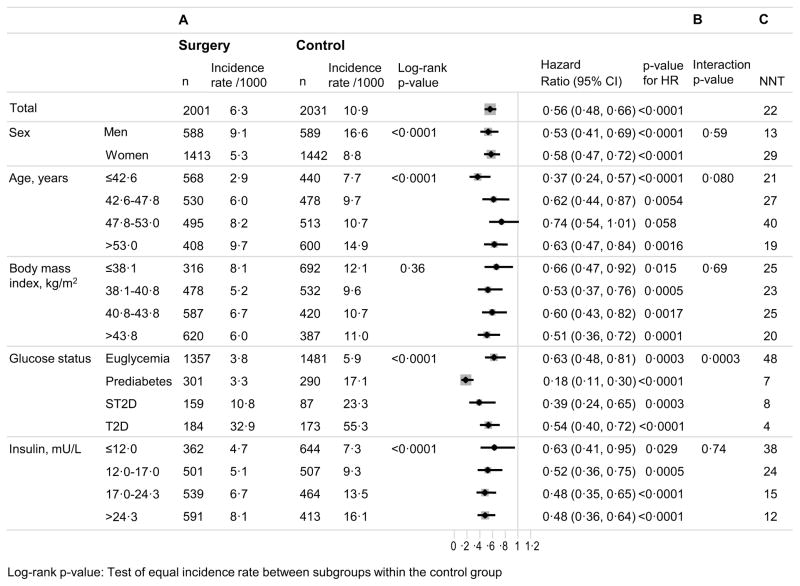
In the entire cohort, there were 224 events first-time microvascular events (complications of the eyes, kidneys, or nerves, whichever came first) in the surgery group and 374 events in the control group, corresponding to incidence rates of 6·3 and 10·9 events per 1000 person-years, respectively (hazard ratio, 0·56; 95% CI 0·48–0·66; p<0·0001). In the control group, the incidence of microvascular events was higher in men than in women, increased with aging, severity of glucose dysregulation, and insulin concentrations but was similar across subgroups defined by baseline BMI (Figure 1). Bariatric surgery was associated with reduced incidence of microvascular events in all subgroups except for those aged 47·8–53·0 years (p=0·058).
Figure 1. Risk factor-treatment interaction analyses for incidence of microvascular events in the SOS study.
A: Incidence of first time microvascular events (retinopathy, nephropathy and neuropathy, whichever came first) in high-risk and low-risk subgroups. For continuous variables, subgrouping is based on quartiles of baseline values. B: Risk factor-treatment interactions for microvascular events in subgroups. C: Number needed to treat (NNT) over 10 years to prevent one microvascular event.
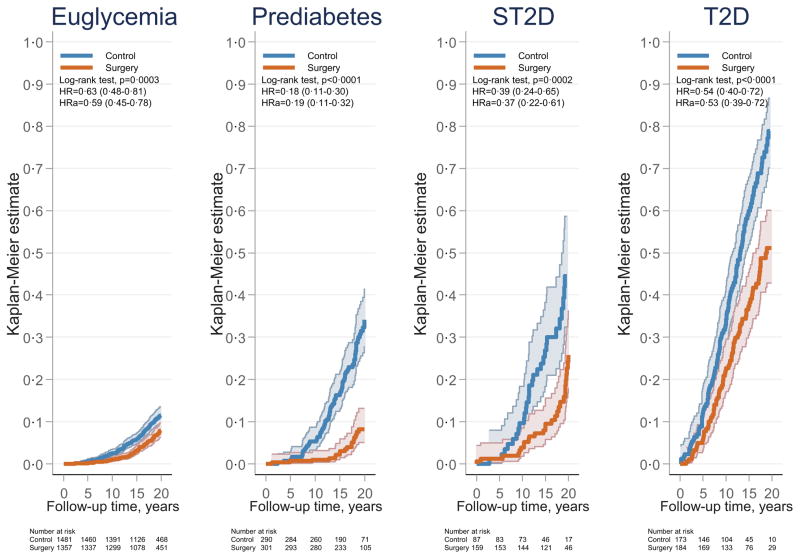
The only statistically significant interaction between treatment effect and baseline risk factors was observed for baseline glucose status (p for interaction 0·0003) (Figure 1). In the glucose subgroups, the lowest hazard ratio was observed for those with prediabetes (0·18, 95% CI 0·11–0·30; p<0·0001), followed by screen-detected diabetes (0·39, 95% CI 0·24–0·65; p=0·0002), established diabetes (0·54, 95% CI 0·40–0·72; p<0·0001), and euglycemia (0·63, 95% CI 0·48–0·81; p=0·0003) (Figure 1). These associations persisted after multivariable adjustment for baseline risk factors (age, sex, BMI, blood pressure, urinary albumin excretion, and smoking) (Figure 2). The treatment benefit was greater in patients with prediabetes compared to that in patients with screen-detected diabetes (p=0·0256), established diabetes (p=0·00010), and normal glucose status (p<0·0001). Results remained essentially unchanged after analyses based on per-protocol approaches and with different adjustments/matching (appendix, Table S2).
Figure 2. Cumulative incidence of microvascular events after bariatric surgery or usual care in subgroups stratified by baseline glucose status.
The x-axes are truncated at 20 years but all observations after 20 years were included in the analyses. Euglycemia, normal fasting glucose; Prediabetes, impaired fasting glucose; ST2D, screen-detected type 2 diabetes; T2D, established type 2 diabetes. HR, hazard ratio; HRa, adjusted hazard ratio.
In the entire cohort, one microvascular event was prevented for every 22 patients who underwent surgery (Figure 1). After stratification by baseline glucose status, the NNT was similar in subgroups with prediabetes, screen-detected diabetes, and established diabetes (7, 8, and 4, respectively) but higher (48) in the subgroup with baseline euglycemia.
Incidence of microvascular events affecting eyes, kidneys, and nerves
Retinopathy was the most common microvascular complication, and the incidence was reduced after bariatric surgery in all subgroups stratified by baseline glucose status with hazard ratios ranging from 0·18 (95% CI 0·09–0·36; p<0·0001) in the subgroup with prediabetes to 0·51 (95% CI 0·37–0·70; p<0·0001) in the subgroup with established diabetes (Figure S2a). Bariatric surgery was also associated with reduced incidence of diabetic kidney disease in the subgroup with prediabetes (hazard ratio 0·29 (95% CI 0·15–0·56), p<0·0001) and the subgroup with established diabetes (hazard ratio 0·47 (95% CI 0·29–0·77), p=0·0019) but not in the subgroups with baseline euglycemia (p=0·1697) or screen-detected diabetes (p=0·0506) (Figure S2b). Microvascular complications affecting nerves were few and bariatric surgery was only associated with reduced incidence in the subgroup with prediabetes (p=0.0012) (Figure S2c).
Incidence of microvascular events in patients with diabetes at baseline in relation to diabetes remission during follow-up
In the surgery group, 30·2% (n=39) of the patients with baseline diabetes were in remission at the 15-year follow-up. Patients in remission had a significantly lower incidence of microvascular events compared with those who were not in remission after 15 years (8·0 versus 25·2 events per 1000 person-years, hazard ratio 0·21, 95% CI 0·08–0·56; p=0·0005, Figure S3).
Incidence of microvascular events in patients with prediabetes at baseline in relation to diabetes status during follow-up
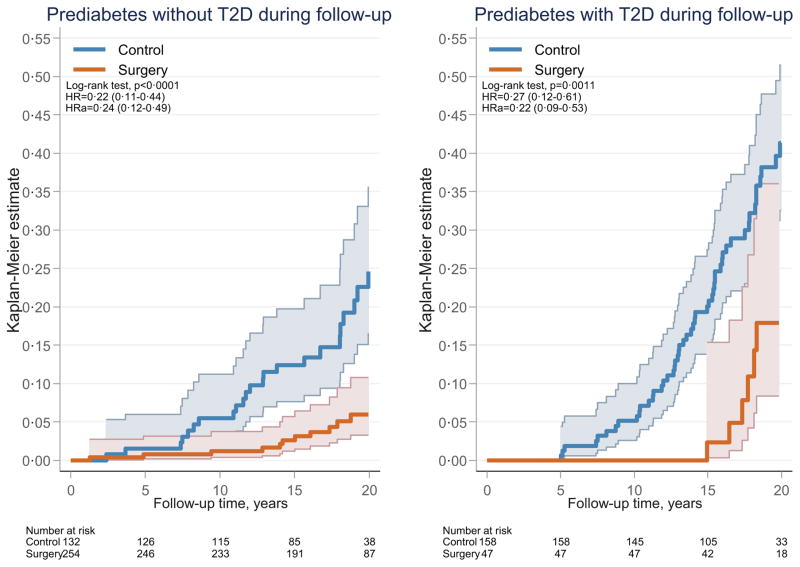
In the subgroup with baseline prediabetes, 54·5% (n=158) of the controls and 15·6% (n=47) of the patients in the surgery group had developed diabetes at or before the 15-year follow-up. The incidence of microvascular events was higher in the patients who developed diabetes compared with those who remained diabetes free during follow-up (p<0·0001). Among patients with baseline prediabetes, bariatric surgery was associated with reduced incidence of microvascular events in those who developed diabetes (hazard ratio 0·27 (95% CI 0·12–0·61), p=0·0011) and also in those who remained free from diabetes during follow-up (hazard ratio 0·22 (95% CI 0·11–0·44), p<0·0001) (Figure 3). In addition, bariatric surgery was associated with lower fasting glucose levels at the 2-year follow-up in those with baseline prediabetes who remained free from diabetes during long-term follow-up (blood glucose: 4·18±0·55 and 4·86±0·65 mmol/L in the surgery and control groups, respectively; p<0·0001).
Figure 3. Cumulative incidence of microvascular events after bariatric surgery or usual care in patients with baseline prediabetes stratified by development of type 2 diabetes at or before the 15-year follow-up.
The x-axes are truncated at 20 years but all observations after 20 years were included in the analyses. Prediabetes, impaired fasting glucose; Without diabetes, type 2 diabetes not present at or before the 15-year follow-up; With diabetes, type 2 diabetes diagnosed at or before the 15-year follow-up. HR, hazard ratio; HRa, adjusted hazard ratio.
Discussion
In this exploratory study we show that bariatric surgery, compared with usual care, is associated with reduced incidence of microvascular diabetes complications in patients with obesity and glucose status ranging from euglycemia to established diabetes, and that the relative risk reduction is greatest in patients with prediabetes. Our results illustrate the importance of durable diabetes remission for prevention of microvascular events. Patients with diabetes who were treated by bariatric surgery and experienced long-lasting remission, had markedly reduced risk of microvascular diabetes complications compared with those who were not in remission at the 15-year follow-up, however, it is unknown if patients cycled between remission and relapse before this time point. There have been similar findings in the literature; fewer microvascular complications in patients with diabetes who experienced remission after gastric bypass28 and an additional reduction of the risk for microvascular disease for every year spent in remission after bariatric surgery, even if the patients eventually experienced a relapse.29 Importantly, we also show that bariatric surgery reduces the risk of future microvascular events in those with prediabetes at baseline regardless of whether they had progressed to diabetes or remained diabetes free at the 15-year follow-up.
In an earlier report, we showed that prevention of diabetes complications was greater when bariatric surgery was performed in patients with recently diagnosed diabetes than in those with longer diabetes duration.17 This observation, together with the reduced incidence of diabetes after bariatric surgery in patients with obesity,22 led us to speculate that intervention even before diabetes has been diagnosed may prevent diabetes complications. In our current study, we showed that bariatric surgery reduced the risk of microvascular events in patients with obesity and baseline prediabetes or euglycemia. With the exception of a report from the Diabetes Prevention Outcome Study in Da Qing, China, showing that lifestyle intervention (diet, exercise, or diet plus exercise for 6 years) reduces the 20-year incidence of severe retinopathy in individuals with prediabetes (when the three intervention groups were combined),30 no previous intervention study has shown a reduced incidence of microvascular events in patients without diabetes.
In a recent 15-year follow-up of the Diabetes Prevention Program in the US, those who remained diabetes free had 28% lower prevalence of microvascular complications compared with those who developed diabetes.21 Similarly, in our study, we observed a greater reduction of microvascular events in patients with baseline prediabetes who remained diabetes free for 15 years compared to those who developed diabetes during follow-up, emphasizing the importance of successful diabetes prevention to reduce microvascular complications. Importantly, we also found that the incidence of microvascular events in patients with baseline prediabetes who remained diabetes free was lower in those treated by bariatric surgery compared with usual care. At the 2-year follow-up, we showed that fasting glucose levels in participants with baseline prediabetes who remained free from diabetes at the 15-year follow-up were significantly lower in the surgery group compared with the control group. Thus, the reduced risk for microvascular events after bariatric surgery cannot merely be explained by prevention of diabetes but may also be related to reduction of slightly elevated glucose levels in patients with baseline prediabetes who remained diabetes free during follow-up.
Prediabetes and diabetes represent different stages of the same progressive disease, only distinguished by diagnostic criteria that have been described as quite arbitrary31 but that greatly influence patient care. When diabetes is diagnosed, treatment is immediately started and the goal, defined by leading organizations,25,32 is to achieve the best possible glucose control in order to prevent future vascular complications. In contrast, treatment of prediabetes is much less aggressive, although in recent years the American Diabetes Association has started to recommend lifestyle treatment and, for some groups, metformin to prevent diabetes development.33 Because the glycemic cut-off for diabetes is based on risk for microvascular complications, our result showing the benefit of treating prediabetes is perhaps unexpected. However, this cut-off was originally chosen on the basis of cross-sectional data12 whereas our study examines longitudinal interventional data. Our findings emphasize the importance of treating prediabetes by demonstrating that even without progression to diabetes, long-term exposure to slightly elevated glucose levels below the diabetes threshold increases the risk for diabetes complications.
The SOS study has some limitations, including the lack of randomization due to ethical reasons related to the high risk of bariatric surgery in the 1980s. The majority of patients underwent surgical procedures that are not used today and due to sample size and number of events it was not feasible to stratify the analysis by type procedure. Our study is also limited by the lack of HbA1c data and the fact that the nationwide health registers used to trace microvascular events do not capture visits to general practitioners. Although the development of microvascular complications was not a pre-specified endpoint in the original study plan, the SOS study is to our knowledge the only available study allowing evaluation of the long-term effects of an intervention on microvascular disease in patients with obesity and glucose status ranging from euglycemia to established type 2 diabetes. Ideally, randomized studies should be performed to confirm our results and verify the large treatment benefit in patients with prediabetes, i.e. patients who are not currently prioritized for bariatric surgery. It has been suggested that the definition of success of bariatric surgery should focus on improvement of obesity related comorbidities34 and our results suggest that this should include prevention of microvascular events.
In conclusion, our results show that bariatric surgery reduces the incidence of microvascular complications in patients with obesity with or without diabetes at the time of surgery and that the treatment benefit is greater in patients with prediabetes compared with those with diabetes or normal glucose status at baseline. The fact that bariatric surgery prevented microvascular events in patients with prediabetes who remained diabetes free shows that exposure to glucose levels that appear to be harmless in cross-sectional cohorts can cause significant damage if they persist over a long time. Our data therefore indicate that prediabetes is a condition that should be treated more aggressively rather than waiting until glucose levels reach the diabetic range.
Supplementary Material
Research in context.
Evidence before this study
Type 2 diabetes is a progressive disease causing considerable morbidity and mortality due to micro- and macro-vascular damage. Hyperglycemia is a strong risk factor for microvascular complications such as retinopathy, diabetic kidney disease and neuropathy, and it is known that these complications are reduced in patients with diabetes by improved glycemic control. Prediabetes is a condition with glucose levels that are below the threshold for type 2 diabetes but higher than normal, and with a markedly increased risk of future type 2 diabetes. Several studies have shown that lifestyle modification or medication can prevent progression from prediabetes to type 2 diabetes but it is less clear if improved glycemic control results in prevention of microvascular complications in individuals with prediabetes. We searched PubMed.gov and ClinicalTrials.gov up to November 7, 2016 using the key words: microvascular complications, intervention, prevention, prediabetes, and diabetes. We did not find any studies in which prevention of microvascular complications was compared in patients with diabetes, prediabetes and normal glucose levels.
Added value of this study
We examined the treatment benefit of bariatric surgery, an effective anti-diabetic treatment, in prevention of microvascular complications over up to 26 years in patients with obesity stratified by baseline glucose status. Bariatric surgery was associated with reduced incidence of microvascular complications in subgroups of patients with glucose status ranging from euglycemia to established type 2 diabetes, but the largest relative risk reduction was obtained in individuals with baseline prediabetes. We also showed that bariatric surgery reduced the risk of future microvascular events in those with baseline prediabetes, both in those who progressed to diabetes and in those who remained diabetes free at follow-up.
Implications of all the available evidence
Although it is well established that type 2 diabetes is a progressive disease, there is limited knowledge about when to start interventions to achieve the greatest benefit in terms of preventing microvascular complications. The current study suggests that risk reduction in response to improved glycemic control is greatest in patients with prediabetes. Further research is therefore urgently needed to determine how patients with prediabetes should be monitored and treated to reduce the risk for microvascular damage.
Acknowledgments
Funding: US National Institutes of Health, Swedish Research Council, Sahlgrenska University Hospital ALF research grant, Swedish Diabetes Foundation.
This project was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number R01DK105948 (the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health), the Swedish Research Council K2013-99X-22279-01, K2013-54X-11285-19, Sahlgrenska University Hospital ALF research grant and the Swedish Diabetes Foundation. We thank the staff members at the SOS Secretariat and at 480 primary health-care centers and 25 surgical departments in Sweden that participated in the study. We thank Rosie Perkins (Institute of Medicine, University of Gothenburg) for editing the manuscript.
Footnotes
Contributors
LMSC, KS, MP, BC designed the study. LMSC wrote the paper with contributions from all authors. MP did the statistical analysis and all authors contributed to data interpretation. LMSC, KS, PJ, MN and JAA organized register linkage with the Swedish authorities. All authors critically reviewed the content and contributed to manuscript revision, approved the final version and agree to be accountable for all aspects of the work.
Declaration of interests
LMSC has obtained lecture fees from AstraZeneca, Johnson&Johnson, and MSD. KS holds stocks in Pfizer. BC and CK are and SH previously was employed by AstraZeneca and BC, CK and SH all hold stocks in the same company. No other conflict of interest relevant to this study was reported. MN has received lecture and/or consulting fees from Abbott, Sanofi-Aventis, Roche, Itrim International, and Strategic Health Resources; research grants from AstraZeneca and Pfizer (last 5 years).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nathan DM. Diabetes: Advances in Diagnosis and Treatment. JAMA. 2015;314(10):1052–62. doi: 10.1001/jama.2015.9536. [DOI] [PubMed] [Google Scholar]
- 2.N C. D. Risk Factor Collaboration. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4. 4 million participants. Lancet. 2016;387(10027):1513–30. doi: 10.1016/S0140-6736(16)00618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103(2):137–49. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and Trends in Diabetes Among Adults in the United States, 1988–2012. JAMA. 2015;314(10):1021–9. doi: 10.1001/jama.2015.10029. [DOI] [PubMed] [Google Scholar]
- 5.Ligthart S, van Herpt TT, Leening MJ, et al. Lifetime risk of developing impaired glucose metabolism and eventual progression from prediabetes to type 2 diabetes: a prospective cohort study. Lancet Diabetes Endocrinol. 2016;4(1):44–51. doi: 10.1016/S2213-8587(15)00362-9. [DOI] [PubMed] [Google Scholar]
- 6.Rahman S, Rahman T, Ismail AA, Rashid AR. Diabetes-associated macrovasculopathy: pathophysiology and pathogenesis. Diabetes Obes Metab. 2007;9(6):767–80. doi: 10.1111/j.1463-1326.2006.00655.x. [DOI] [PubMed] [Google Scholar]
- 7.Nathan DM. Long-term complications of diabetes mellitus. N Engl J Med. 1993;328(23):1676–85. doi: 10.1056/NEJM199306103282306. [DOI] [PubMed] [Google Scholar]
- 8.Ryden L, Mellbin L. Glucose perturbations and cardiovascular risk: challenges and opportunities. Diab Vasc Dis Res. 2012;9(3):170–6. doi: 10.1177/1479164112451581. [DOI] [PubMed] [Google Scholar]
- 9.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–89. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 10.Holman RR, Sourij H, Califf RM. Cardiovascular outcome trials of glucose-lowering drugs or strategies in type 2 diabetes. Lancet. 2014;383(9933):2008–17. doi: 10.1016/S0140-6736(14)60794-7. [DOI] [PubMed] [Google Scholar]
- 11.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–12. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20(7):1183–97. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 13.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–53. [PubMed] [Google Scholar]
- 14.Ismail-Beigi F, Craven T, Banerji MA, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376(9739):419–30. doi: 10.1016/S0140-6736(10)60576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–72. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 16.Look Ahead Research Group. Effect of a long-term behavioural weight loss intervention on nephropathy in overweight or obese adults with type 2 diabetes: a secondary analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 2014;2(10):801–9. doi: 10.1016/S2213-8587(14)70156-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sjöström L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA. 2014;311(22):2297–304. doi: 10.1001/jama.2014.5988. [DOI] [PubMed] [Google Scholar]
- 18.Dixon JB, O’Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2008;299(3):316–23. doi: 10.1001/jama.299.3.316. [DOI] [PubMed] [Google Scholar]
- 19.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2015;386(9997):964–73. doi: 10.1016/S0140-6736(15)00075-6. [DOI] [PubMed] [Google Scholar]
- 20.Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. N Engl J Med. 2014;370(21):2002–13. doi: 10.1056/NEJMoa1401329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015;3(11):866–75. doi: 10.1016/S2213-8587(15)00291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carlsson LM, Peltonen M, Ahlin S, et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med. 2012;367(8):695–704. doi: 10.1056/NEJMoa1112082. [DOI] [PubMed] [Google Scholar]
- 23.Sjöström L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351(26):2683–93. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 24.Waaler HT. Height, weight and mortality. The Norwegian experience. Acta Med Scand Suppl. 1984;679:1–56. doi: 10.1111/j.0954-6820.1984.tb12901.x. [DOI] [PubMed] [Google Scholar]
- 25.Chamberlain JJ, Rhinehart AS, Shaefer CF, Jr, Neuman A. Diagnosis and Management of Diabetes: Synopsis of the 2016 American Diabetes Association Standards of Medical Care in Diabetes. Ann Intern Med. 2016;164(8):542–52. doi: 10.7326/M15-3016. [DOI] [PubMed] [Google Scholar]
- 26.Buse JB, Caprio S, Cefalu WT, et al. How do we define cure of diabetes? Diabetes Care. 2009;32(11):2133–5. doi: 10.2337/dc09-9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. doi: 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y, Corsino L, Shantavasinkul PC, et al. Gastric Bypass Surgery Leads to Long-term Remission or Improvement of Type 2 Diabetes and Significant Decrease of Microvascular and Macrovascular Complications. Ann Surg. 2016;263(6):1138–42. doi: 10.1097/SLA.0000000000001509. [DOI] [PubMed] [Google Scholar]
- 29.Coleman KJ, Haneuse S, Johnson E, et al. Long-term Microvascular Disease Outcomes in Patients With Type 2 Diabetes After Bariatric Surgery: Evidence for the Legacy Effect of Surgery. Diabetes Care. 2016;39(8):1400–7. doi: 10.2337/dc16-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gong Q, Gregg EW, Wang J, et al. Long-term effects of a randomised trial of a 6-year lifestyle intervention in impaired glucose tolerance on diabetes-related microvascular complications: the China Da Qing Diabetes Prevention Outcome Study. Diabetologia. 2011;54(2):300–7. doi: 10.1007/s00125-010-1948-9. [DOI] [PubMed] [Google Scholar]
- 31.DeFronzo RA, Abdul-Ghani MA. Preservation of beta-cell function: the key to diabetes prevention. J Clin Endocrinol Metab. 2011;96(8):2354–66. doi: 10.1210/jc.2011-0246. [DOI] [PubMed] [Google Scholar]
- 32.International Diabetes Federation Guideline Development Group. Global guideline for type 2 diabetes. Diabetes Res Clin Pract. 2014;104(1):1–52. doi: 10.1016/j.diabres.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 33.American Diabetes Association. 4 Prevention or Delay of Type 2 Diabetes. Diabetes Care. 2016;39(Suppl 1):S36–8. doi: 10.2337/dc16-S007. [DOI] [PubMed] [Google Scholar]
- 34.Fruhbeck G. Bariatric and metabolic surgery: a shift in eligibility and success criteria. Nat Rev Endocrinol. 2015;11(8):465–77. doi: 10.1038/nrendo.2015.84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.