Abstract
Difunctionalization of alkenes has become a powerful tool for quickly increasing molecular complexity in synthesis. Despite significant progress in the area of alkene difunctionalization involving the incorporation of a nitrogen atom across the C–C double bonds, approaches for the direct 1,2-carboamination of alkenes to produce linear N-containing molecules are scarce and remain a formidable challenge. Here we describe a radical-mediated oxidative intermolecular 1,2-alkylamination of alkenes with alkyl nitriles and amines involving C(sp3)–H oxidative functionalization catalysed by a combination of Ag2CO3 with iron Lewis acids. This three-component alkene 1,2-alkylamination method is initiated by the C(sp3)–H oxidative radical functionalization, which enables one-step formation of two new chemical bonds, a C–C bond and a C–N bond, to selectively produce γ-amino alkyl nitriles.
Difunctionalization of readily available alkenes is a powerful method to quickly build molecular complexity. Here the authors report a three-component, oxidative carboamination between alkenes, alkyl nitriles and amines, producing γ-amino alkyl nitriles.
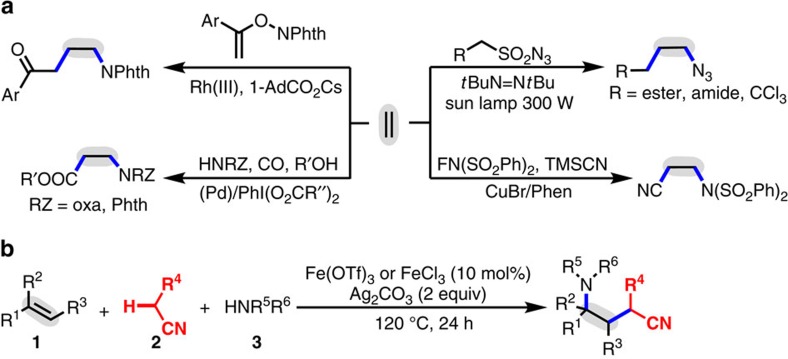
Difunctionalization of alkenes represents one of the most powerful and straightforward tools to build complex molecules via one-step construction of two chemical bonds that possess significantly synthetic utility in chemical synthesis1,2,3,4,5,6. One of the major synthetic targets for such transformations, including diamination7,8,9,10,11,12,13,14,15, aminooxygenation16,17,18,19,20,21,22,23,24, aminohalogenation25,26,27,28,29,30 and carboamination31,32,33,34,35,36, is the incorporation of a nitrogen atom (amino, amide or azide groups) across the C–C double bonds to build useful N-containing molecules through the formation of a C–N bond. Despite significant progress in the field, approaches of the alkene carboamination for producing linear N-containing molecules are scarce and remain a great challenge (Fig. 1a): available intermolecular transformations for producing linear N-containing molecules are restricted to the special amination reagents33,34,35,36. Further, to our knowledge, three-component carboamination reactions of the alkenes via C–H functionalization have never been reported.
Figure 1. 1,2-Carboamination of alkenes.
(a) Previous work for 1,2-carboamination of alkenes33,34,35,36. (b) Our radical-mediated three-component, oxidative carboamination between alkenes, alkyl nitriles and amines using a C–H oxidative functionalization.
In recent years, the C–H oxidative functionalization reaction has attracted much attention due to its inherent features, such as high step economy and atom economy1,2,3,4,5,6,37,38,39,40,41. Typical transformations include the difunctionalization of alkenes with alkyl C(sp3)–H bonds42,43,44,45,46,47,48,49,50,51,52,53,54,55 and the majority of which rely on the formation of a sp3-hybridized carbon-centred radical from the oxidative cleavage of the corresponding alkyl C(sp3)–H bond followed by addition across the C–C double bond43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59. However, such approaches are restricted to the 1,2-arylalkylation52,53,54,55,56,57,58,59,60,61,62,63, 1,2-dialkylation54 and 1,2-oxyalkylation55,56,57,58,59 of the alkenes, and the available three-component transformations are scarce53,55. In light of these literature results43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59 and our continuous interest in oxidative radical reactions60,61,62,63, we envisioned that this C–H oxidative radical functionalization strategy might be viable to achieve 1,2-carboamination of alkenes with new-conceptual, general and straightforward features.
Herein, we report an iron-catalysed oxidative three-component 1,2-carboamination of alkenes with alkyl nitriles and amines through C(sp3)–H oxidative radical functionalization to assemble γ-amino alkyl nitriles using Ag2CO3 as oxidant (Fig. 1b). The reaction enables the simultaneous formation of two new chemical bonds, a C–C bond and a C–N bond, by a sequence of C–H oxidative cleavage, radical addition across the alkenes and aminationin a highly atom-economic and selective manner64,65,66,67,68.
Results
Reaction optimization
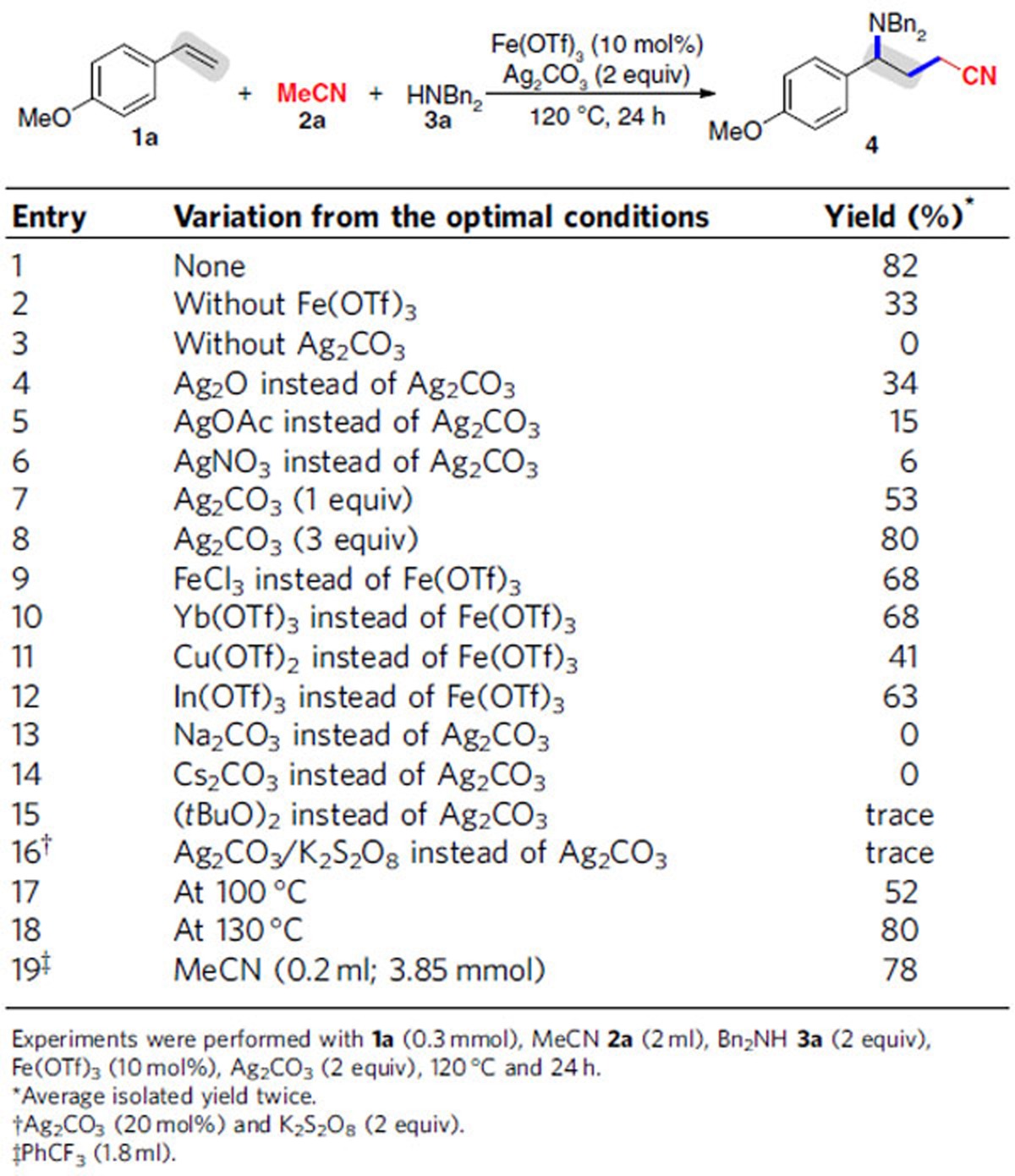
We initiated the study by investigating various reaction parameters for the three-component reaction of p-methoxystyrene (1a) with acetonitrile (2a) and dibenzylamine (3a) (Table 1). A combination of 10 mol% Fe(OTf)3, 2 equiv Ag2CO3, 120 °C and 24 h were found as the optimal reaction conditions for the conversion of alkene 1a, nitrile 2a and amine 3a to the desired product 4 in 82% yield (entry 1). The results suggest that Ag2CO3 is the real catalysts and Fe(OTf)3 only serves as a Lewis acid to promote the reaction (entries 2 and 3): although in the absence of Fe(OTf)3 transformation of alkene 2a to 4 took place albeit giving a lower yield (entry 2), no desired reaction was observed without Ag salts (entry 3). Other Ag salts, including Ag2O, AgOAc and AgNO3, had the catalytic activity for the reaction, but they were less effective than Ag2CO3 (entries 4–6). Among the amount of Ag2CO3 examined, the use of 2 equiv was turned out to be preferred (entries 1, 7 and 8). Encouraged by these, a series of other Lewis acids, such as FeCl3, Yb(OTf)3, Cu(OTf)2 and In(OTf)3, were tested (entries 9–12): they could improve the reaction, but were less effective than Fe(OTf)3. Notably, the use of other bases, Na2CO3 or Cs2CO3, instead of Ag2CO3, resulted in no formation of product 4 (entries 13 and 14), suggesting that Ag2CO3 may act as an oxidant and a catalyst, not a base. Notably, the reported efficient oxidative systems, tBuOOtBu di-tert-butyl peroxide (DTBP)42,43,44,45,46,47,48,49,50,51,52,53,54,55 or Ag2CO3/K2S2O8 (refs 60, 61, 62, 63, 64, 65, 66, 67) displayed rather lower activity for the reaction (entries 15 and 16). We found that the reaction was sensitive to the temperatures (entries 17 and 18): a lower temperature (100 °C) had a negative effect on the reaction, whereas a higher temperature (130 °C) did not improve the yield compared with the results at 120 °C. Gratifyingly, the reaction could be successfully performed in PhCF3 medium (entry 19).
Table 1. Screening of optimal reaction conditions.
Substrate scope with amines and amides
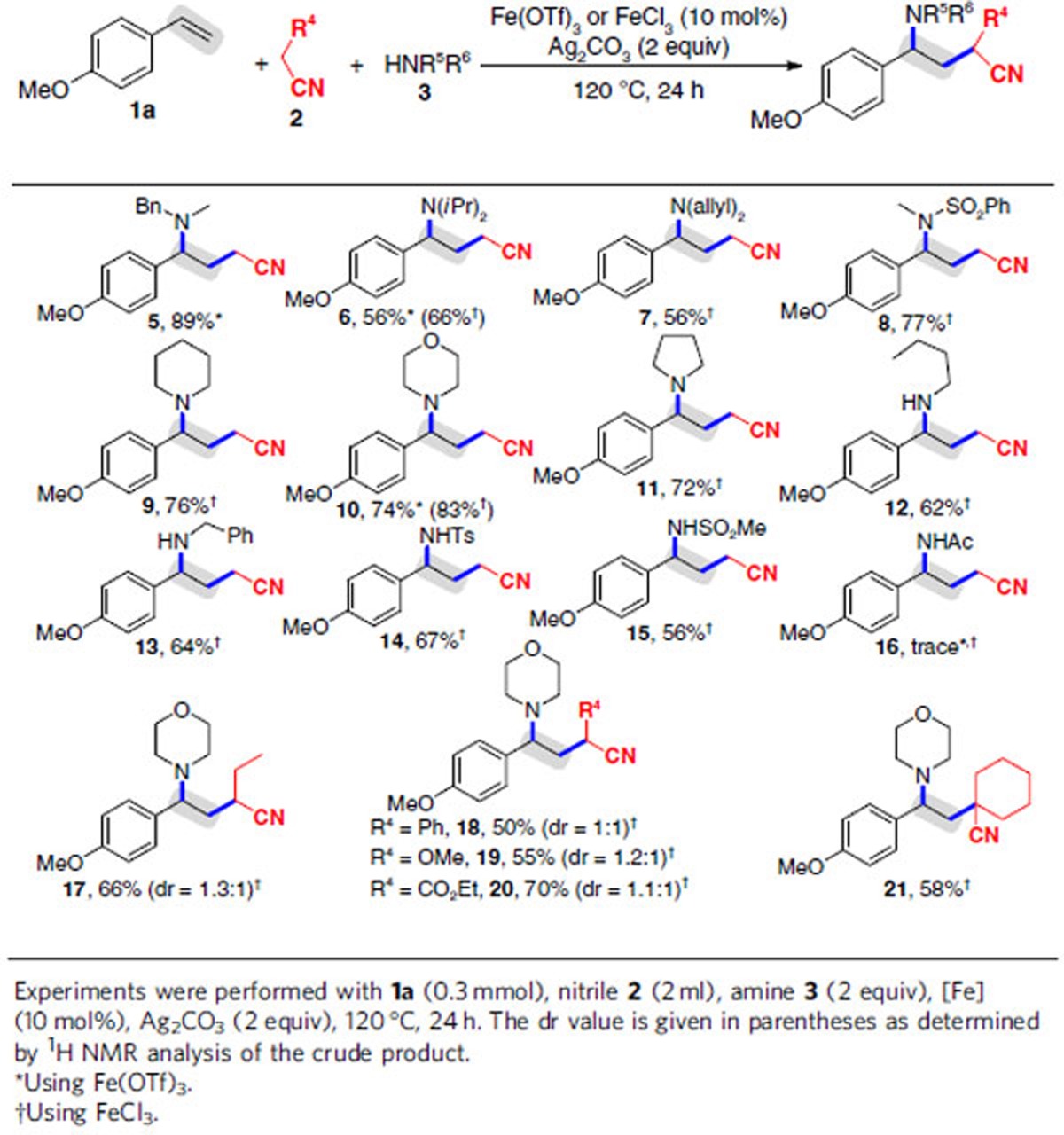
We next explored the scope of this Ag2CO3-mediated 1,2-carboamination protocol under the optimal reaction conditions with regard to alkenes 1, nitriles 2 and amines 3 (Tables 2 and 3). We first turned our attention to investigate the applicability of the optimal conditions in the reaction with various amines 3b–m in the presence of alkene 1a and acetonitrile 2a (Table 2). The resulted indicated that a wide range of secondary and primary amines 3b–j were smoothly converted to the desired products 5–13 in moderate to good yields. N-Methyl-1-phenylmethanamine (3b) was viable to furnish 5 with 89% yield in the presence of Fe(OTf)3 and Ag2CO3. For other amines 3c–j, however, Fe(OTf)3 displayed less efficient than FeCl3 (products 6–13): although treatment of alkene 1a with nitrile 2a, diisopropylamine (3c), Fe(OTf)3 and Ag2CO3 afforded 6 in 56% yield, the use of FeCl3 instead of Fe(OTf)3 enhanced the yield to 66%. Similarly, the yield of 10 from the reaction with morpholine (3g) increased from 74 to 83% when using FeCl3 instead of Fe(OTf)3. To our delight, the optimal conditions were compatible with sulfonamides 3e, 3k and 3l, giving products 8, 14 and 15 in high yields. Unfortunately, attempt to difunctionalization with acetamide 3m failed to build 1,2-carboamination product 16.
Table 2. Variation of the alkyl nitriles (2) and amines (3).
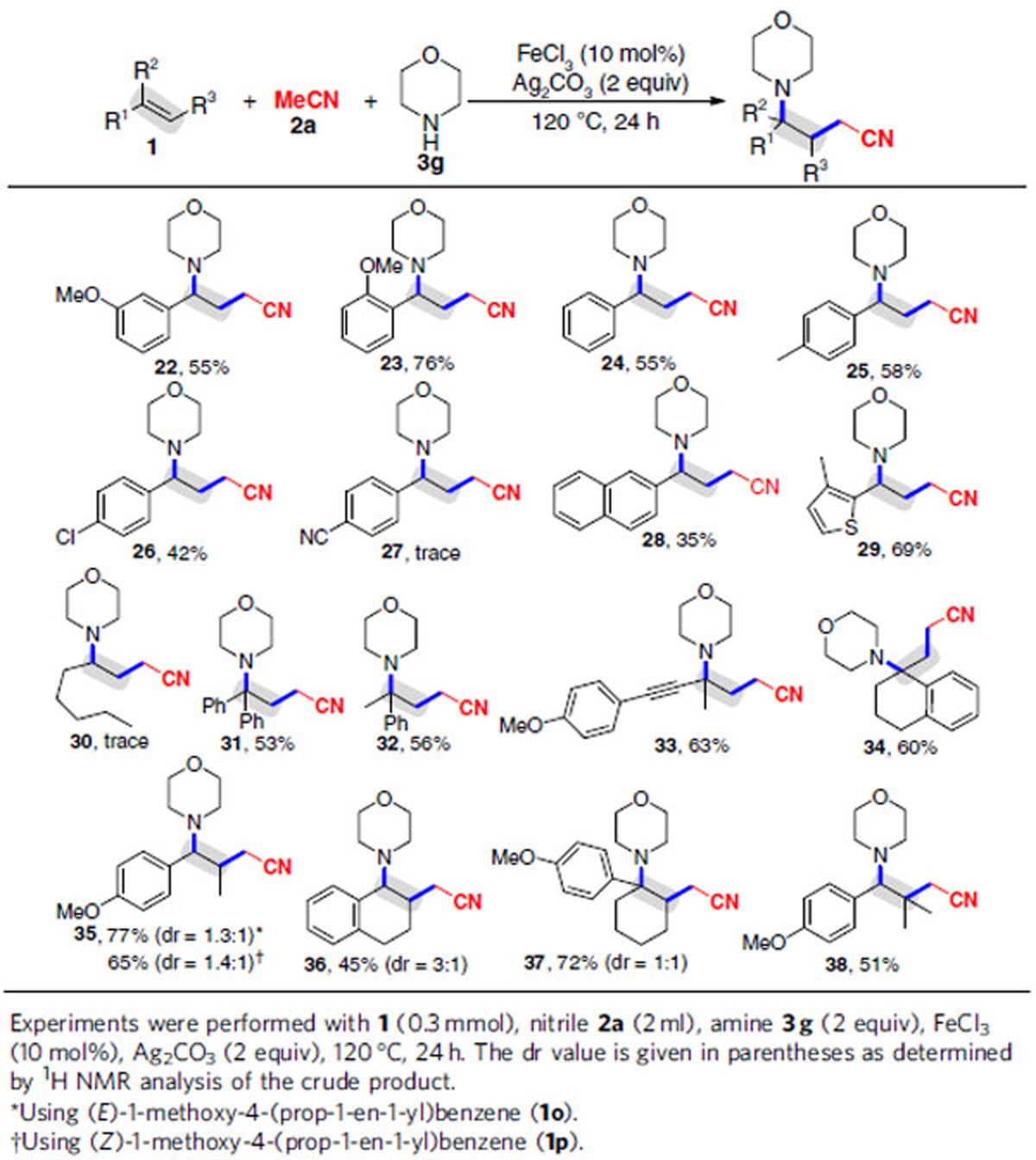
Table 3. Variation of the alkenes (1).
Subsequently, the scope of alkyl nitriles 2 was exploited in the presence of alkene 1a, morpholine 3g, FeCl3 and Ag2CO3 (Table 2). In the case of butyronitrile 2b, the reaction afforded 17 in 66% yield. Gratifyingly, the reaction was well tolerated of various acetonitriles 2c–e bearing a Ph group, a MeO group or a CO2Et group at the α position, generating 18–20 in 50–70% yields. An interesting observation was that secondary alkyl nitrile 2f containing a cyclohexyl ring also proceeded the reaction and resulted in the formation of 21 in 58% yield.
Substrate scope with alkenes
The optimal conditions were applicable to an array of alkenes 1b–f, 1h–i and 1k–s (products 22–26, 28–29 and 31–38), but electron-withdrawing aryl alkene 1g and simple aliphatic alkene, namely oct-1-ene (1j), had no reactivity (products 27 and 30; Table 3). Initially, the substitution effect of the aryl ring at the terminal alkenes were examined: several substituted aryl rings, such as m-MeOC6H4, o-MeOC6H4, C6H5, p-MeC6H4, m-MeC6H4, naphthalen-2-yl and 3-methylthiophen-2-yl, were perfectly tolerated, and both the electronic nature of the aryl group and the substituent position on the aryl group had an impact on the reactivity (products 22–29). Using m-methoxystyrene (1b), for example, afforded 22 in 55% yield, whereas bulky o-methoxystyrene (1c) furnished 23 in 76% yield. Alkene 1f having a weak electron-deficient 4-ClC6H4 group successfully underwent the 1,2-alkylamination reaction to offer 26, albeit in a diminished yield. However, alkene 1g having a strong electron-deficient 4-CNC6H4 group had no reactivity (product 27). Gratifyingly, the optimal conditions were consistent with 1,1-disubstituted alkenes, including 1,1-diphenylethylene (1k), prop-1-en-2-ylbenzene (1l), 1-methoxy-4-(3-methylbut-3-en-1-yn-1-yl)benzene (1m) and 1-methylene-1,2,3,4-tetrahydronaphthalene (1n), generating 31–34 with concomitant formation of a quaternary carbon centre. A particularly attractive feature of this 1,2-alkylamination is the ability to enable the conversion of di- and trisubstituted internal alkenes 1o–s to diverse complex products 35–38 in moderate to good yields. It was noted that the reaction of (E)-1-methoxy-4-(prop-1-en-1-yl)benzene (1o) or (Z)-1-methoxy-4-(prop-1-en-1-yl)benzene (1p) had no retention of geometrical selectivity in the double bond (product 35), which supported a radical process.
Control experiments and mechanistic studies
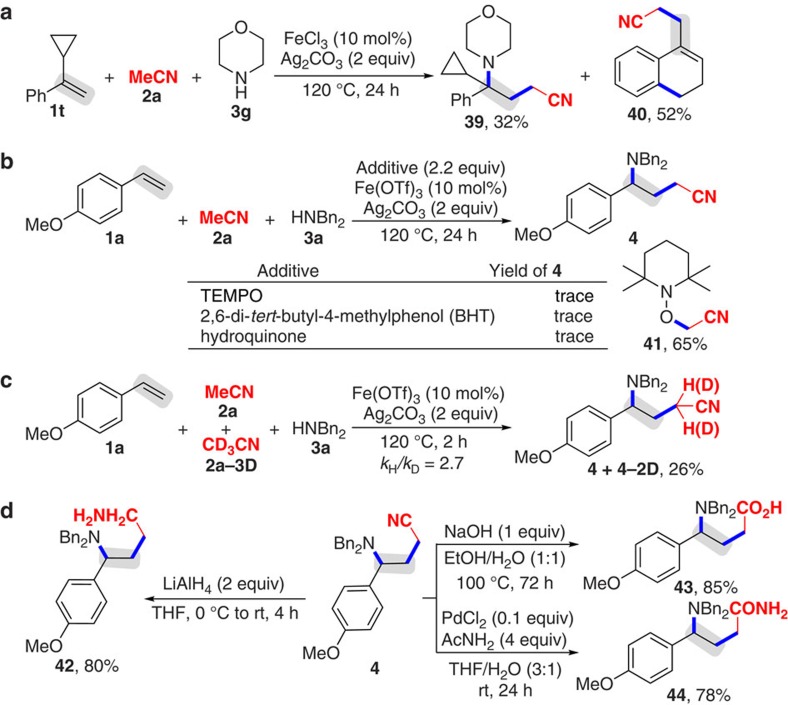
Using (1-cyclopropylvinyl)benzene (1t) to react with nitrile 2a and amine 3g, the 1,2-alkylarmination product 39 along with the mono alkylation/ring-opening/cyclization product 40 was observed (Fig. 2a)55. Notably, the reaction of alkene 1a with nitrile 2a and amine 3a could not take place in the presence of a stoichiometric amount of radical inhibitors, such as 2,2,6,6-Tetramethyl-1-piperidinyloxy (TEMPO), 2,6-di-tert-butyl-4-methylphenol and hydroquinone (Fig. 2b).
Figure 2. Control experiments and utilizations of product 4.
(a) Radical testing experiment based on the selectivity. (b) Trapping experiments with a stoichiometric amount of radical inhibitors. (c) Kinetic isotopic effect (KIE) study. (d) Synthetic utilizations.
In addition, under the optimal conditions nitrile 2a reacted with TEMPO afforded product 41. These results suggested that the current reaction is triggered by a free-radical process. The kinetic isotope effect experiment gave a large kinetic isotope effect value (kH/kD=2.7), implying that the cleavage of the C(sp3)–H bond may be rate-limiting (Fig. 2c and for the detailed information, see Supplementary Fig. 39)37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55. Gratifyingly, product 4 were easily converted to 1,4-diamine 42, γ-amino acid 43 and γ-amino amide 44 in good yields (Fig. 2d)69,70.
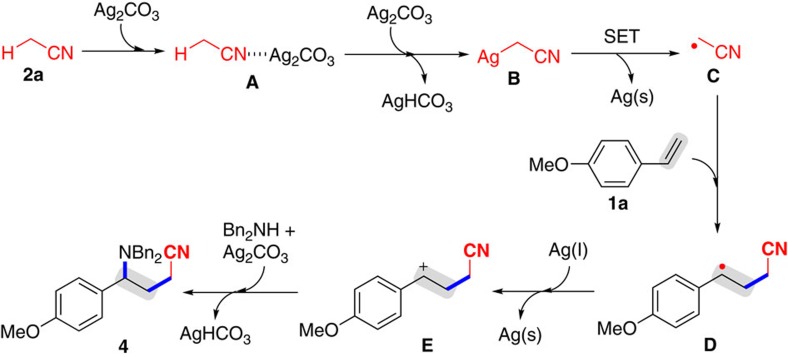
Consequently, the mechanisms for the Ag2CO3-mediated 1,2-alkylamination reaction was proposed (Fig. 3)31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68. Coordination of the nitrogen atom in MeCN 2a with AgCO3 gives the intermediate A, which sequentially reacts with AgCO3 to afford the AgCH2CN intermediate B and AgHCO3. The decomposition the AgCH2CN intermediate B readily takes place under heating to form the alkyl radical C (supported by the results of Fig. 2b), AgHCO3 and the Ag0 species [Ag(s)] through single electron transfer42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65. Subsequently, addition of the alkyl radical C across the C–C double bond in alkene 1a produces the alkyl radical intermediate D (supported by the reaction of alkene 1t; Fig. 2a). Intermediate D is converted into the carbon-centered cation E, followed by reaction with amine 3a affords the product 4, AgHCO3 and the Ag0 species through a sequence of oxidation and nucleophilic addition64,65,66,67,68. Notably, the radical intermediates C and D can be stabilized by Lewis acids, thus improving the yields.
Figure 3. Possible mechanism.
The alkyl radical C is generated from decomposition of the AgCH2CN intermediate B via single-electron transfer. Subsequently, addition of the alkyl radical C across the C–C double and oxidative amination afford product 4.
In summary, we have developed a silver-mediated intermolecular 1,2-alkylamination of alkenes with alkyl nitriles and amines involving C(sp3)–H oxidative radical functionalization for producing γ-amino alkyl nitriles. The generality of such an intermolecular 1,2-alkylamination reaction is demonstrated by a wide scope with respect to alkenes, alkyl nitriles and amines. The radical mechanism was also discussed according to the control experiments. Importantly, applications of the products, γ-amino alkyl nitriles, to prepare other valuable synthons have been examined. Currently, our laboratory is working to apply this C–H oxidative radical functionalization strategy in synthesis.
Methods
General procedure for 1,2-carboamination of alkenes
To a Schlenk tube were added Fe(OTf)3 or FeCl3 (10 mol%), Ag2CO3 (0.6 mmol), alkene 1 (0.3 mmol), amine 2 (0.6 mmol) and MeCN (2 ml). Then the tube was recharged with argon and the mixture was stirred at 120 °C for 24 h. After cooling to room temperature, the mixture was filtered through a small plug of silica gel to remove the precipitate and washed with with EtOAc (3 × 10 ml). The solvent was then removed in vacuo and the residue was further purified by silica gel flash column chromatography (10–40% ethyl acetate/hexane+0.1% Et3N) to afford the desired product.
Data availability
The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre under deposition number 1453224 (4). These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. All other data supporting the findings of this study are available within the article and its Supplementary Information file or from the authors upon reasonable request. For NMR spectra of the compounds in this article, see Supplementary Figs 1–39.
Additional information
How to cite this article: Liu, Y.-Y. et al. Oxidative 1,2-carboamination of alkenes with alkyl nitriles and amines toward γ-amino alkyl nitriles. Nat. Commun. 8, 14720 doi: 10.1038/ncomms14720 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Supplementary figures, supplementary tables, supplementary methods, and supplementary references.
Acknowledgments
We thank the Natural Science Foundation of China (numbers 21472039 and 21625203) and the Hunan Provincial Natural Science Foundation of China (number 13JJ2018).
Footnotes
The authors declare no competing financial interests.
Author contributions Y.-Y.L. and X.-H.Y. contributed equally to this work. Y.-Y.L., X.-H.Y., S.L. and J.-H.L. conceived the project and wrote the manuscript. Y.-Y.L. and X.-H.Y performed the experiments. Y.-Y.L., X.-H.Y. and R.-J.S. analysed the data.
References
- Patai S. The Chemistry of Alkenes Wiley Interscience (1964). [Google Scholar]
- Li G., Chang T.-T. & Sharpless B. K. Catalytic asymmetric aminohydroxylation (AA) of olefins. Angew. Chem. Int. Ed. Engl. 35, 451–454 (1996). [Google Scholar]
- Wolfe J. P. Stereoselective synthesis of saturated heterocycles via palladium-catalyzed alkene carboetherification and carboamination reactions. Synlett 2008, 2913–2937 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe J. P. Intramolecular alkoxycyanation and alkoxyacylation reactions: new types of alkene difunctionalizations for the construction of oxygen heterocycles. Angew. Chem. Int. Ed. 51, 10224–10225 (2012). [DOI] [PubMed] [Google Scholar]
- Romero R. M., Woste T. H. & Muniz K. Vicinal difunctionalization of alkenes with iodine(III) reagents and catalysts. Chem. Asian J. 9, 972–983 (2014). [DOI] [PubMed] [Google Scholar]
- Francesca C. & Goti A. Metal-catalysed 1,2-diamination reactions. Nat. Chem. 1, 269–275 (2009). [DOI] [PubMed] [Google Scholar]
- Lucet D., Gall T. L. & Mioskowski C. The chemistry of vicinal diamines. Angew. Chem. Int. Ed. 37, 2580–2627 (1998). [DOI] [PubMed] [Google Scholar]
- de Figueiredo R. M. Transition-metal-catalyzed diamination of olefins. Angew. Chem. Int. Ed. 48, 1190–1193 (2009). [DOI] [PubMed] [Google Scholar]
- Zhu Y., Cornwall R. G., Du H., Zhao B. & Shi Y. Catalytic diamination of olefins via N-N bond activation. Acc. Chem. Res. 47, 3665–3678 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuff J., Höelmann C. H., Nieger M. & Muñiz K. Palladium(II)-catalyzed intramolecular diamination of unfunctionalized alkenes. J. Am. Chem. Soc. 127, 14586–14587 (2005). [DOI] [PubMed] [Google Scholar]
- Iglesias A., Pérez E. & Muñiz K. An Intermolecular palladium-catalyzed diamination of unactivated alkenes. Angew. Chem. Int. Ed. 49, 8109–8111 (2010). [DOI] [PubMed] [Google Scholar]
- Röben C., Souto J. A., González Y., Lishchynskyi A. & Muñiz K. Enantioselective metal-free diamination of styrenes. Angew. Chem. Int. Ed. 50, 9478–9482 (2011). [DOI] [PubMed] [Google Scholar]
- Martínez C. & Muñiz K. Palladium-catalyzed vicinal difunctionalization of internal alkenes: diastereoselective synthesis of diamines. Angew. Chem. Int. Ed. 51, 7031–7034 (2012). [DOI] [PubMed] [Google Scholar]
- Zhang B. & Studer A. Copper-catalyzed intermolecular aminoazidation of alkenes. Org. Lett. 16, 1790–1793 (2014). [DOI] [PubMed] [Google Scholar]
- Yuan Y.-A., Lu D.-F., Chen Y.-R. & Xu H. Iron-catalyzed direct diazidation for a broad range of olefins. Angew. Chem. Int. Ed. 55, 534–538 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexanian E. J., Lee C. & Sorensen E. J. Palladium-catalyzed ring-forming aminoacetoxylation of alkenes. J. Am. Chem. Soc. 127, 7690–7691 (2005). [DOI] [PubMed] [Google Scholar]
- Liu G. & Stahl S. S. Highly regioselective Pd-catalyzed intermolecular aminoacetoxylation of alkenes and evidence for cis-aminopalladation and SN2 C-O bond formation. J. Am. Chem. Soc. 128, 7179–7181 (2006). [DOI] [PubMed] [Google Scholar]
- Desai L. V. & Sanford M. S. Construction of tetrahydrofurans by PdII/PdIV-catalyzed aminooxygenation of alkenes. Angew. Chem. Int. Ed. 46, 5737–5740 (2007). [DOI] [PubMed] [Google Scholar]
- Zhu H., Chen P. & Liu G. Pd-catalyzed intramolecular aminohydroxylation of alkenes with hydrogen peroxide as oxidant and water as nucleophile. J. Am. Chem. Soc. 136, 1766–1769 (2014). [DOI] [PubMed] [Google Scholar]
- Zhu H., Chen P. & Liu G. Palladium-catalyzed intramolecular aminoacetoxylation of unactivated alkenes with hydrogen peroxide as oxidant. Org. Lett. 17, 1485–1488 (2015). [DOI] [PubMed] [Google Scholar]
- Chen C., Chen P. & Liu G. Palladium-catalyzed intramolecular aminotrifluoromethoxylation of alkenes. J. Am. Chem. Soc. 137, 15648–15651 (2015). [DOI] [PubMed] [Google Scholar]
- Sun X. et al. Mn-catalyzed highly efficient aerobic oxidative hydroxyazidation of olefins: a direct approach to β-azido alcohols. J. Am. Chem. Soc. 137, 6059–6066 (2015). [DOI] [PubMed] [Google Scholar]
- Rao W.-H., Yin X.-S. & Shi B.-F. Catalyst-controlled amino-versus oxy-acetoxylation of urea-tethered alkenes: efficient synthesis of cyclic ureas and isoureas. Org. Lett. 17, 3758–3761 (2015). [DOI] [PubMed] [Google Scholar]
- Legnani L. & Morandi B. Direct catalytic synthesis of unprotected 2-amino-1-phenylethanols from alkenes by using iron(II) phthalocyanine. Angew. Chem. Int. Ed. 55, 2248–2251 (2016). [DOI] [PubMed] [Google Scholar]
- Wu T., Yin G. & Liu G. Palladium-catalyzed intramolecular aminofluorination of unactivated alkenes. J. Am. Chem. Soc. 131, 16354–16355 (2009). [DOI] [PubMed] [Google Scholar]
- Qiu S., Xu T., Zhou J., Guo Y. & Liu G. Palladium-catalyzed intermolecular aminofluorination of styrene. J. Am. Chem. Soc. 132, 2856–2857 (2010). [DOI] [PubMed] [Google Scholar]
- Yin G., Wu T. & Liu G. Highly selective palladium-catalyzed intramolecular chloroamination of unactivated alkenes by using hydrogen peroxide as an oxidant. Chem. Eur. J. 18, 451–455 (2012). [DOI] [PubMed] [Google Scholar]
- Bovino M. T. & Chemler S. R. Catalytic enantioselective alkene aminohalogenation/cyclization involving atom transfer. Angew. Chem. Int. Ed. 51, 3923–3927 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong W., Feige P., de Haro T. & Nevado C. Regio- and enantioselective aminofluorination of alkenes. Angew. Chem. Int. Ed. 52, 2469–2473 (2013). [DOI] [PubMed] [Google Scholar]
- Zhang H., Song Y., Zhao J., Zhang J. & Zhang Q. Regioselective radical aminofluorination of styrenes. Angew. Chem. Int. Ed. 53, 11079–11083 (2014). [DOI] [PubMed] [Google Scholar]
- Mai D. N. & Wolfe J. P. Asymmetric palladium-catalyzed carboamination reactions for the synthesis of enantiomerically enriched 2-(arylmethyl)- and 2-(alkenylmethyl)pyrrolidines. J. Am. Chem. Soc. 132, 12157–12159 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner A., Scott J. S. & Bower J. F. An umpolung approach to alkene carboamination: palladium catalyzed 1,2-amino-acylation, -carboxylation, -arylation, -vinylation, and -alkynylation. J. Am. Chem. Soc. 137, 7224–7230 (2015). [DOI] [PubMed] [Google Scholar]
- Piou T. & Rovis T. Rhodium-catalysed syn-carboamination of alkenes via a transient directing group. Nature 527, 86–90 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J., Qi X., Li M., Chen P. & Liu G. Palladium-catalyzed intermolecular aminocarbonylation of alkenes: efficient access of β-amino acid derivatives. J. Am. Chem. Soc. 137, 2480–2483 (2015). [DOI] [PubMed] [Google Scholar]
- Weidner K., Giroult A., Panchaud P. & Renaud P. Efficient carboazidation of alkenes using a radical desulfonylative azide transfer process. J. Am. Chem. Soc. 132, 17511–17515 (2010). [DOI] [PubMed] [Google Scholar]
- Zhang H. et al. Copper-catalyzed intermolecular aminocyanation and diamination of alkenes. Angew. Chem. Int. Ed. 52, 2529–2533 (2013). [DOI] [PubMed] [Google Scholar]
- Liu C., Zhang H., Shi W. & Lei A. Bond formations between two nucleophiles: transition metal catalyzed oxidative cross-coupling reactions. Chem. Rev. 111, 1780–1824 (2011). [DOI] [PubMed] [Google Scholar]
- Xie Y.-X., Song R.-J., Xiang J.-N. & Li J.-H. Transition metal-catalyzed C-H oxidation reactions. Chin. J. Org. Chem. 32, 1555–1567 (2012). [Google Scholar]
- Zhang C., Tang C. & Jiao N. Recent advances in copper-catalyzed dehydrogenative functionalization via a single electron transfer (SET) process. Chem. Soc. Rev. 41, 3464–3484 (2012). [DOI] [PubMed] [Google Scholar]
- Girard S. A., Knauber T. & Li C.-J. The cross-dehydrogenative coupling of Csp 3-bonds: a versatile strategy for C-C bond formations. Angew. Chem. Int. Ed. 53, 74–100 (2014). [DOI] [PubMed] [Google Scholar]
- Song R.-J., Liu Y., Xie Y.-X. & Li J.-H. Difunctionalization of acrylamides through C-H oxidative radical coupling: new approaches to oxindoles. Synthesis 47, 1195–1209 (2015). [Google Scholar]
- Wu T., Mu X. & Liu G.-S. Palladium-catalyzed oxidative arylalkylation of activated alkenes: dual C-H bond cleavage of an arene and acetonitrile. Angew. Chem. Int. Ed. 50, 12578–12581 (2011). [DOI] [PubMed] [Google Scholar]
- Wei W.-T. et al. Synthesis of oxindoles by iron-catalyzed oxidative 1,2-alkylarylation of activated alkenes with an aryl C(sp2)-H bond and a C(sp3)-H bond adjacent to a heteroatom. Angew. Chem. Int. Ed. 52, 3638–3641 (2013). [DOI] [PubMed] [Google Scholar]
- Zhou M.-B. et al. Oxidative 1,2-difunctionalization of activated alkenes with benzylic C(sp3)–H bonds and aryl C(sp2)-H bonds. Chem. Commun. 49, 10817–10819 (2013). [DOI] [PubMed] [Google Scholar]
- Zhou S.-L., Guo L.-N., Wang H. & Duan X.-H. Copper-catalyzed oxidative benzylarylation of acrylamides by benzylic C-H bond functionalization for the synthesis of oxindoles. Chem. Eur. J. 19, 12970–12973 (2013). [DOI] [PubMed] [Google Scholar]
- Wang H., Guo L.-N. & Duan X.-H. Silver-catalyzed oxidative coupling/cyclization of acrylamides with 1,3-dicarbonyl compounds. Chem. Commun. 49, 10370–10373 (2013). [DOI] [PubMed] [Google Scholar]
- Li Z.-J., Zhang Y., Zhang L.-Z. & Liu Z.-Q. Free-radical cascade alkylarylation of alkenes with simple alkanes: highly efficient access to oxindoles via selective (sp3)C-H and (sp2)C-H bond functionalization. Org. Lett. 16, 382–385 (2014). [DOI] [PubMed] [Google Scholar]
- Li J., Wang Z., Wu N., Gao G. & You J. Radical cascade cyanomethylation of activated alkenes to construct cyano substituted oxindoles. Chem. Commun. 50, 15049–15051 (2014). [DOI] [PubMed] [Google Scholar]
- Chu X.-Q., Xing Z.-H., Meng H., Xu X.-P. & Ji S.-J. Copper-mediated radical alkylarylation of unactivated alkenes with acetonitrile leading to fluorenes and pyrroloindoles. Org. Chem. Front. 3, 165–169 (2016). [Google Scholar]
- Li Y., Liu B., Li H.-B., Wang Q. & Li J.-H. Oxidative radical 1,2-alkylarylation of alkenes with α-C(sp3)-H bonds of acetonitriles involving 1,2-aryl migration. Chem. Commun. 51, 1024–1026 (2015). [DOI] [PubMed] [Google Scholar]
- Chu X.-Q., Meng H., Zi Y., Xu X.-P. & Ji S.-J. Oxidative C(sp3)–H functionalization of acetonitrile and alkanes with allylic alcohols under metal-free conditions. Org. Chem. Front. 2, 216–220 (2015). [Google Scholar]
- Bunescu A., Wang Q. & Zhu J. Copper-catalyzed cyanomethylation of allylic alcohols with concomitant 1,2-aryl migration: efficient synthesis of functionalized ketones containing an α-quaternary center. Angew. Chem. Int. Ed. 54, 3132–3135 (2015). [DOI] [PubMed] [Google Scholar]
- Bunescu A., Wang Q. & Zhu J. Synthesis of functionalized epoxides by copper-catalyzed alkylative epoxidation of allylic alcohols with alkyl nitriles. Org. Lett. 17, 1890–1893 (2015). [DOI] [PubMed] [Google Scholar]
- Liang W., Chen P. & Liu G. AgF-mediated dialkylation of activate alkenes: an efficient access to nitrile-containing spirooxindoles. Chin. J. Chem. 32, 681–684 (2014). [Google Scholar]
- Bunescu A., Wang Q. & Zhu J. Copper-mediated/catalyzed oxyalkylation of alkenes with alkylnitriles. Chem. Eur. J. 20, 14633–14636 (2014). [DOI] [PubMed] [Google Scholar]
- Chatalova-Sazepin C., Wang Q., Sammis G. M. & Zhu J. Copper-catalyzed intermolecular carboetherification of unactivated alkenes by alkyl nitriles and alcohols. Angew. Chem. Int. Ed. 54, 5443–5446 (2015). [DOI] [PubMed] [Google Scholar]
- Liao Z. et al. Copper-catalyzed radical carbooxygenation: alkylation and alkoxylation of styrenes. Chem. Asian J. 10, 96–99 (2015). [DOI] [PubMed] [Google Scholar]
- Ha T. M., Chatalova-Sazepin C., Wang Q. & Zhu J. Copper-catalyzed formal [2+2+1] heteroannulation of alkenes, alkylnitriles, and water: method development and application to a total synthesis of (±)-Sacidumlignan D. Angew. Chem. Int. Ed. 55, 9249–9252 (2016). [DOI] [PubMed] [Google Scholar]
- Ha T. M., Wang Q. & Zhu J. Copper-catalysed cyanoalkylative cycloetherification of alkenes to 1,3-dihydroisobenzofurans: development and application to the synthesis of citalopram. Chem. Commun. 52, 11100–11103 (2016). [DOI] [PubMed] [Google Scholar]
- Zhou M.-B. et al. Metal-free oxidative tandem coupling of activated alkenes with carbonyl C(sp2)-H bonds and aryl C(sp2)-H bonds using TBHP. Chem. Sci. 4, 2690–2694 (2013). [Google Scholar]
- Fan J.-H. et al. Iron-catalyzed oxidative arylmethylation of activated alkenes using a peroxide as the methyl source. Synlett 25, 657–660 (2014). [Google Scholar]
- Hu M. et al. Metal-free radical [2+2+1] carbocyclization of benzene-linked 1,n-enynes: dual C(sp3)-H functionalization adjacent to a heteroatom. Angew. Chem. Int. Ed. 54, 9577–9580 (2015). [DOI] [PubMed] [Google Scholar]
- Ouyang X.-H., Song R.-J., Hu M., Yang Y. & Li J.-H. Silver-mediated intermolecular 1,2-alkylarylation of styrenes with α-carbonyl alkyl bromides and indoles. Angew. Chem. Int. Ed. 55, 3187–3191 (2016). [DOI] [PubMed] [Google Scholar]
- Harmata M. Silver in Organic Chemistry John Wiley & Sons Inc. (2010). [Google Scholar]
- He C. et al. Silver-mediated oxidative C-H/C-H functionalization: a strategy to construct polysubstituted furans. J. Am. Chem. Soc. 134, 5766–5769 (2012). [DOI] [PubMed] [Google Scholar]
- Li Z., Song L. & Li C. Silver-catalyzed radical aminofluorination of unactivated alkenes in aqueous media. J. Am. Chem. Soc. 135, 4640–4643 (2013). [DOI] [PubMed] [Google Scholar]
- Wei X.-H., Li Y.-M., Zhou A.-X., Yang T.-T. & Yang S.-D. Silver-catalyzed carboazidation of arylacrylamides. Org. Lett. 15, 4158–4161 (2013). [DOI] [PubMed] [Google Scholar]
- Liu J. et al. Silver-catalyzed cross-coupling of isocyanides and active methylene compounds by a radical process. Angew. Chem. Int. Ed. 54, 10618–10622 (2015). [DOI] [PubMed] [Google Scholar]
- Rappoport Z. The Chemistry of the Cyano Group Wiley (1970). [Google Scholar]
- Maffioli S. I., Marzorati E. & Marazzi A. Mild and reversible dehydration of primary amides with PdCl2 in aqueous acetonitrile. Org. Lett. 7, 5237–5239 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures, supplementary tables, supplementary methods, and supplementary references.
Data Availability Statement
The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre under deposition number 1453224 (4). These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. All other data supporting the findings of this study are available within the article and its Supplementary Information file or from the authors upon reasonable request. For NMR spectra of the compounds in this article, see Supplementary Figs 1–39.